Acetylation of Phenylalanine Hydroxylase and Tryptophan 2,3-Dioxygenase Alters Hepatic Aromatic Amino Acid Metabolism in Weaned Piglets
Abstract
:1. Introduction
2. Results
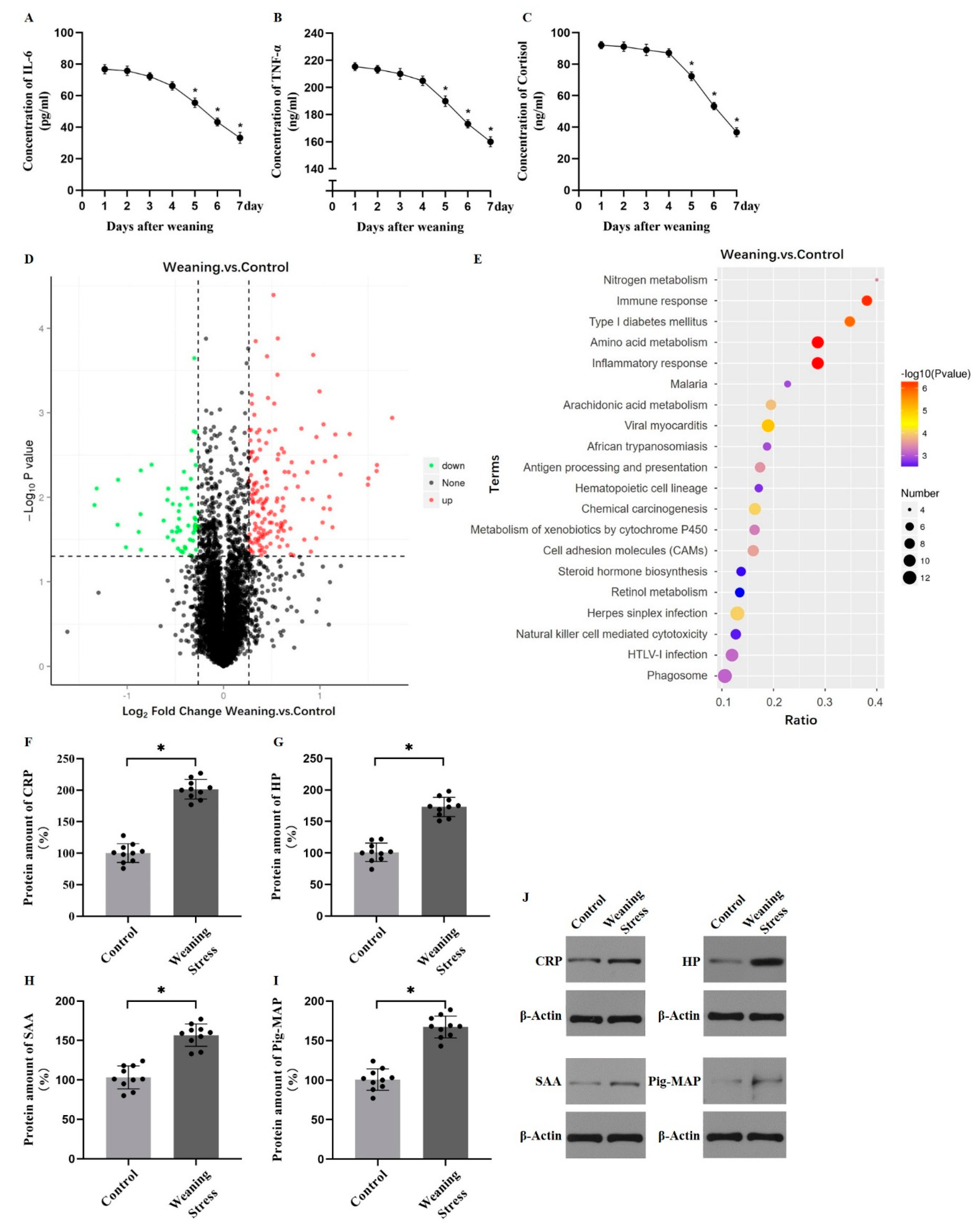
2.1. Weaning Stress Reduces the Content of Hepatic Aromatic Amino Acids in Piglets
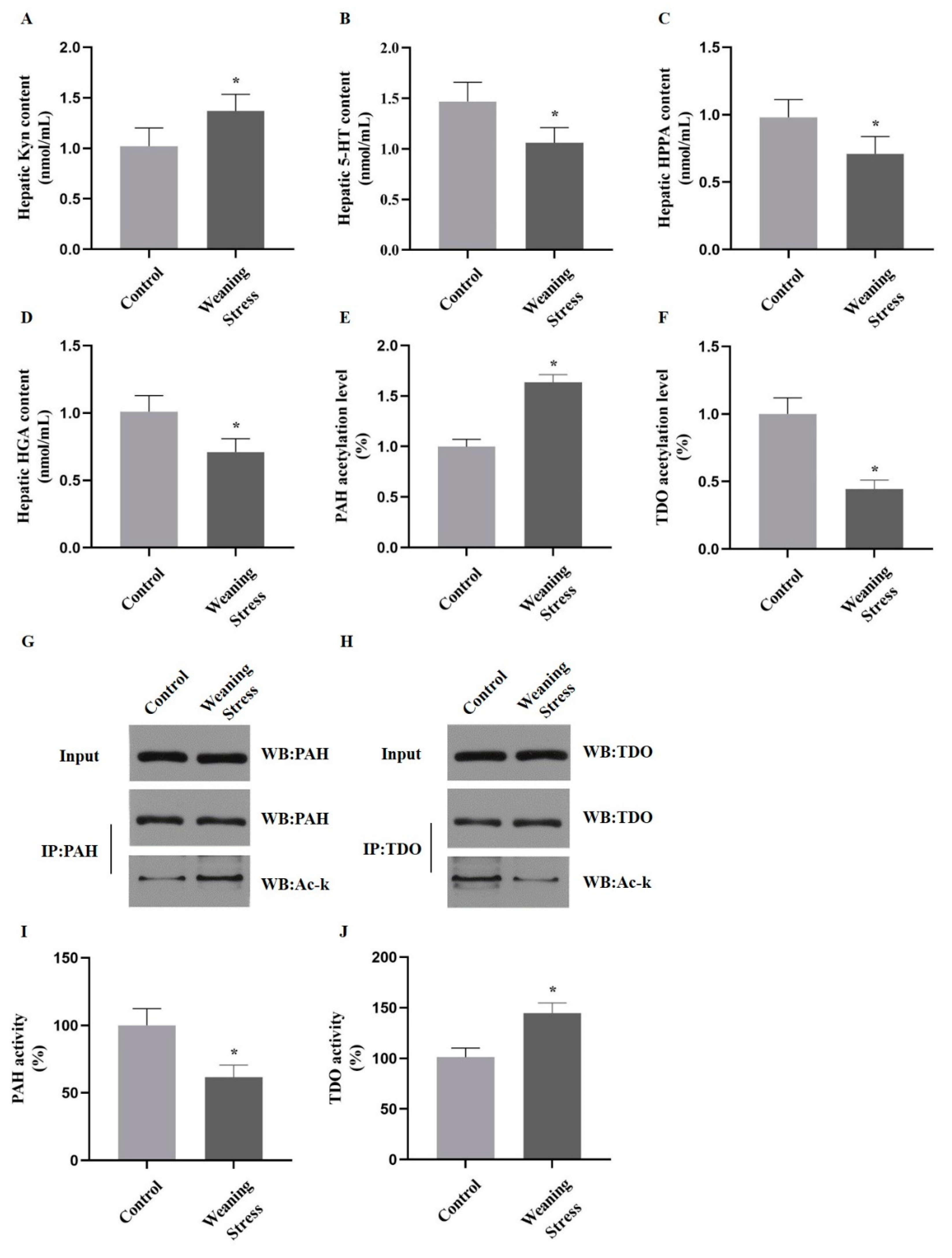
2.2. Weaning Stress Inhibits Phenylalanine and Tyrosine Catabolism and Promotes Hepatic Tryptophan Catabolism
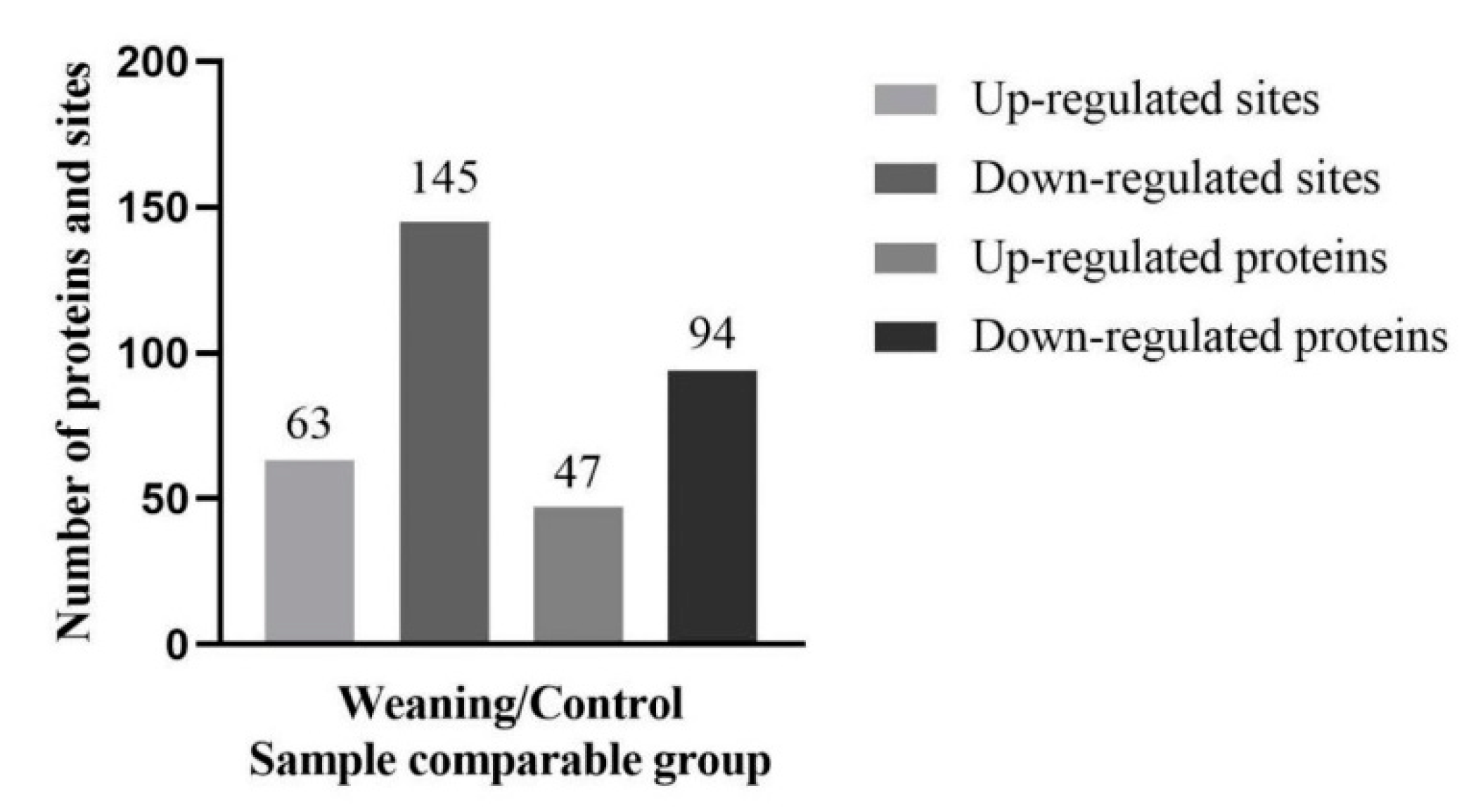
2.3. Lysine Acetylation Modifies the Key Enzyme of Hepatic AAAs Metabolism, thus Altering the Utilization of AAAs
2.4. Acetylation of PAH and TDO Modulates Its Activity
2.5. Acetylation of PAH Inhibiting Phenylalanine Catabolism and Deacetylation of TDO Promote Tryptophan Catabolism in Primary Hepatocytes
3. Discussion
4. Methods and Materials
4.1. Experimental Design
4.2. Sample Collection
4.3. Protein Preparation
4.4. Biochemical Analyses
4.5. Affinity Enrichment
4.6. LC-MS/MS Analysis
4.7. Database Search and Pathway Analysis
4.8. Hepatocyte Isolation and Cell Culture
4.9. Cells Treatment and Transient Transfection
4.10. Isotope Tracer Experiments and GC-MS Analysis
4.11. Western Blots and Immunoprecipitation
4.12. Enzymatic Activity Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Abbreviation | Explanation |
|---|---|
| AAA | aromatic amino acid |
| Tyr | tyrosine |
| Trp | tryptophan |
| Phe | phenylalanine |
| APPs | acute-phase proteins |
| PAH | phenylalanine hydroxylase |
| TDO | tryptophan 2,3-dioxygenase |
| Glu | glutamic acid |
| Gly | glycine |
| Asp | aspartic acid |
| Ala | alanine |
| Lys | lysine |
| PTMs | posttranslational modifications |
| GDH | glutamate dehydrogenase |
| CPS | carbamoyl phosphate synthetase |
| AST | aspartate aminotransferase |
| OTC | ornithine transcarbamylase |
| TPH2 | tryptophan hydroxylase 2 |
| TNF-α | tumor necrosis factor-α |
| Ack | acetyl-lysine |
| Pig-MAP | pig-major acute phase protein |
| CRP | C-reactive protein |
| HP | haptoglobin |
| IL-6 | interleukin 6 |
| Kyn | kynurenine |
| SAA | serum amyloid A |
| 5-HT | 5-hydroxytryptamine |
| HPPA | 4-hydroxyphenylpyruvate |
| HGA | homogentisic acid |
| PWSD | postweaning stress syndrome |
| FDR | false discovery rate |
| TSA | trichostatin A |
| NAM | nicotinamide |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ANOVA | analysis of variance |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
| WT | wild-type |
| TCA | tricarboxylic acid |
| SIRT1 | sirtuin 1 |
| HPLC | high-performance liquid chromatography |
| NSI | NanoSpray Ionization |
| NCE | normalized collision energy |
| AGC | automatic gain control |
| DMEM | dulbecco’s modified eagle medium |
| PBS | phosphate buffer saline |
| SIM | selected ion monitoring |
References
- Dejong, C.H.C.; van de Poll, M.C.G.; Soeters, P.B.; Jalan, R.; Olde Damink, S.W.M. Aromatic Amino Acid Metabolism during Liver Failure. J. Nutr. 2007, 137, 1579S–1585S. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Hurren, N.M.; Herndon, D.N.; Børsheim, E. Whole body and skeletal muscle protein turnover in recovery from burns. Int. J. Burns Trauma 2013, 3, 9. [Google Scholar]
- Antonides, A.; Schoonderwoerd, A.C.; Scholz, G.; Berg, B.M.; Nordquist, R.E.; van der Staay, F.J. Pre-weaning dietary iron deficiency impairs spatial learning and memory in the cognitive holeboard task in piglets. Front. Behav. Neurosci. 2015, 9, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manary, M.J.; Yarasheski, K.E.; Hart, C.A.; Broadhead, R.L. Plasma urea appearance rate is lower when children with kwashiorkor and infection are fed egg white-tryptophan rather than milk protein. J. Nutr. 2000, 130, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.W.; Badaloo, A.; Wilson, L.; Taylor-Bryan, C.; Chambers, B.; Reid, M.; Forrester, T.; Jahoor, F. Dietary supplementation with aromatic amino acids increases protein synthesis in children with severe acute malnutrition. J. Nutr. 2014, 144, 660–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeds, P.J.; Fjeld, C.R.; Jahoor, F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J. Nutr. 1994, 124, 906–910. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, L.; Chen, R.; Hu, H.; Pan, Y.; Jiang, H.; Wan, X.; Jin, H.; Gong, Y. Acetylome profiling reveals extensive lysine acetylation of the fatty acid metabolism pathway in the diatom Phaeodactylum tricornutum. Mol. Cell. Prot. 2018, 17, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Pacella-Ince, L.; Zander-Fox, D.; Lane, M. Mitochondrial SIRT3 and its target glutamate dehydrogenase are altered in follicular cells of women with reduced ovarian reserve or advanced maternal age. Hum. Reprod. 2014, 29, 1490–1499. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, T.; Lomb, D.J.; Haigis, M.C.; Guarente, L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 2009, 137, 560–570. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Lu, Y.; Hu, Y.; Zhai, X.; Xu, W.; Jing, H.; Tian, X.; Lin, Y.; Gao, D.; Yao, J. Salvianolic acid B protects against acute ethanol-induced liver injury through SIRT1-mediated deacetylation of p53 in rats. Toxicol. Lett. 2014, 228, 67–74. [Google Scholar] [CrossRef]
- Li, L.; Zhang, P.; Bao, Z.; Wang, T.; Liu, S.; Huang, F. PGC-1α promotes ureagenesis in mouse periportal hepatocytes through SIRT3 and SIRT5 in response to glucagon. Sci. Rep. 2016, 6, 24156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, S.; Lu, Y.; Su, Q.; Lin, T.; Zhang, X.; Zhang, H.; Zhang, J.; Zhang, L.; Zhu, Z.; Li, H. H3K9 acetylation of Tph2 involved in depression-like behavior in male, but not female, juvenile offspring rat induced by prenatal stress. Neuroscience 2018, 381, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Carroll, J.; Matteri, R.; Touchette, K.; Allee, G. Effects of weaning and weaning weight on neuroendocrine regulators of feed intake in pigs. J. Anim. Sci. 2007, 85, 2133–2139. [Google Scholar] [CrossRef]
- Hampson, D. Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 1986, 40, 32–40. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaysen, G.A.; Chertow, G.M.; Adhikarla, R.; Young, B.; Ronco, C.; Levin, N.W. Inflammation and dietary protein intake exert competing effects on serum albumin and creatinine in hemodialysis patients. Kidney Int. 2001, 60, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotech. 2013, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Moeser, A.J.; Klok, C.V.; Ryan, K.A.; Wooten, J.G.; Little, D.; Cook, V.L.; Blikslager, A.T. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G173–G181. [Google Scholar] [CrossRef]
- Arrieta-Cruz, I.; Gutiérrez-Juárez, R. The role of circulating amino acids in the hypothalamic regulation of liver glucose metabolism. Adv. Nutr. 2016, 7, 790S–797S. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning induced hepatic oxidative stress, apoptosis, and aminotransferases through MAPK signaling pathways in piglets. Oxid. Med. Cell. Longev. 2016, 2016, 4768541. [Google Scholar] [CrossRef]
- Larsen, M.; Kristensen, N.B. Precursors for liver gluconeogenesis in periparturient dairy cows. Animal 2013, 7, 1640–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis: Type 2 diabetes. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Chen, J.; Wu, L.; Li, G.; Xie, P. Metabolic response to oral microcystin-LR exposure in the rat by NMR-based metabonomic study. J. Prot. Res. 2012, 11, 5934–5946. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, B.; Klasing, K. Modulation of nutrient metabolism and homeostasis by the immune system. World’s Poult. Sci. J. 2004, 60, 90–100. [Google Scholar] [CrossRef]
- Kopple, J.D. Phenylalanine and tyrosine metabolism in chronic kidney failure. J. Nutr. 2007, 137, 1586S–1590S. [Google Scholar] [CrossRef]
- Hsu, J.W.; Ball, R.O.; Pencharz, P.B. Evidence that phenylalanine may not provide the full needs for aromatic amino acids in children. Pediatr. Res. 2007, 61, 361. [Google Scholar] [CrossRef] [Green Version]
- Strasser, B.; Sperner-Unterweger, B.; Fuchs, D.; Gostner, J.M. Mechanisms of inflammation-associated depression: Immune influences on tryptophan and phenylalanine metabolisms. In Inflammation-Associated Depression: Evidence, Mechanisms and Implications; Springer: Berlin, Germany, 2016; pp. 95–115. [Google Scholar]
- Strasser, B.; Becker, K.; Fuchs, D.; Gostner, J.M. Kynurenine pathway metabolism and immune activation: Peripheral measurements in psychiatric and co-morbid conditions. Neuropharmacology 2017, 112, 286–296. [Google Scholar] [CrossRef]
- Widner, B.; Laich, A.; Sperner-Unterweger, B.; Ledochowski, M.; Fuchs, D. Neopterin production, tryptophan degradation, and mental depression—What is the link? Brain Behav. Immun. 2002, 16, 590–595. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amin. Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef]
- Gostner, J.M.; Becker, K.; Sperner-Unterweger, B.; Überall, F.; Fuchs, D.; Strasser, B. Role of tryptophan metabolism in mood, behavior, and cognition. In Targeting the Broadly Pathogenic Kynurenine Pathway; Springer: Berlin, Germany, 2015; pp. 75–89. [Google Scholar]
- Domingues, P.; González-Tablas, M.; Otero, Á.; Pascual, D.; Miranda, D.; Ruiz, L.; Sousa, P.; Ciudad, J.; Gonçalves, J.M.; Lopes, M.C. Tumor infiltrating immune cells in gliomas and meningiomas. Brain Behav. Immun. 2016, 53, 1–15. [Google Scholar] [CrossRef]
- Strasser, B.; Fuchs, D. Role of physical activity and diet on mood, behavior, and cognition. Neurol. Psychiat. Brain Res. 2015, 21, 118–126. [Google Scholar] [CrossRef]
- Gleissenthall, G.v.; Geisler, S.; Malik, P.; Kemmler, G.; Benicke, H.; Fuchs, D.; Mechtcheriakov, S. Tryptophan metabolism in post-withdrawal alcohol-dependent patients. Alcohol Alcohol. 2014, 49, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Yao, W.; Li, J.; He, Q.; Shao, Y.; Huang, F. Acetyl-CoA from inflammation-induced fatty acids oxidation promotes hepatic malate-aspartate shuttle activity and glycolysis. Am. J. Physiol-Endoc. Metab. 2018, 315, E496–E510. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yao, W.; Shao, Y.; Zheng, R.; Huang, F. PCAF fine-tunes hepatic metabolic syndrome, inflammatory disease, and cancer. J. Cell. Mol. Med. 2018, 22, 5787–5800. [Google Scholar] [CrossRef] [PubMed]
- Zaini, M.A.; Müller, C.; de Jong, T.V.; Ackermann, T.; Hartleben, G.; Kortman, G.; Gührs, K.-H.; Fusetti, F.; Krämer, O.H.; Guryev, V. A p300 and SIRT1 regulated acetylation switch of C/EBPα controls mitochondrial function. Cell Rep. 2018, 22, 497–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flydal, M.I.; Martinez, A. Phenylalanine hydroxylase: Function, structure, and regulation. IUBMB Life 2013, 65, 341–349. [Google Scholar] [CrossRef]
- Fitzpatrick, P.F. The aromatic amino acid hydroxylases. Adv. Enzymol. Relat. Areas. Mol. Biol. 2000, 74, 235–294. [Google Scholar]
- Olsson, E.; Teigen, K.; Martinez, A.; Jensen, V.R. The aromatic amino acid hydroxylase mechanism: A perspective from computational chemistry. Adv. Inorg. Chem. 2010, 62, 437–500. [Google Scholar]
- Zhao, S.; Xu, W.; Jiang, W.; Yu, W.; Lin, Y.; Zhang, T.; Yao, J.; Zhou, L.; Zeng, Y.; Li, H. Regulation of cellular metabolism by protein lysine acetylation. Science 2010, 327, 1000–1004. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Bazer, F.; Datta, S.; Johnson, G.; Li, P.; Satterfield, M.; Spencer, T. Proline metabolism in the conceptus: Implications for fetal growth and development. Amino Acids 2008, 35, 691–702. [Google Scholar] [CrossRef]
- Wang, X.; Wu, W.; Lin, G.; Li, D.; Wu, G.; Wang, J. Temporal proteomic analysis reveals continuous impairment of intestinal development in neonatal piglets with intrauterine growth restriction. J. Prot. Res. 2009, 9, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wu, Z.; Jia, S.; Wu, G. Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J. Chromatogr. B 2014, 964, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 272, G1382–G1390. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Meininger, C.J. Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Meth. Enzymol. 2008, 440, 177–189. [Google Scholar] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2011, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronemeyer, T.; Wiese, S.; Ofman, R.; Bunse, C.; Pawlas, M.; Hayen, H.; Eisenacher, M.; Stephan, C.; Meyer, H.E.; Waterham, H.R. The proteome of human liver peroxisomes: Identification of five new peroxisomal constituents by a label-free quantitative proteomics survey. PLoS ONE 2013, 8, e57395. [Google Scholar] [CrossRef]
- Soria, L.R.; Marrone, J.; Molinas, S.M.; Lehmann, G.L.; Calamita, G.; Marinelli, R.A. Lipopolysaccharide impairs hepatocyte ureagenesis from ammonia: Involvement of mitochondrial aquaporin-8. FEBS Lett. 2014, 588, 1686–1691. [Google Scholar] [CrossRef] [Green Version]
- Leighty, R.W.; Antoniewicz, M.R. COMPLETE-MFA: Complementary parallel labeling experiments technique for metabolic flux analysis. Metab. Eng. 2013, 20, 49–55. [Google Scholar] [CrossRef]
- Nielsen, KH. Rat liver phenylalanine hydroxylase. A method for the measurement of activity, with particular reference to the distinctive features of the enzyme and the pteridine cofactor. Eur. J. Biochem. 1969, 7, 360–369. [Google Scholar] [CrossRef]
- Dairam, A.; Antunes, E.; Saravanan, K.; Daya, S. Non-steroidal anti-inflammatory agents, tolmetin and sulindac, inhibit liver tryptophan 2,3-dioxygenase activity and alter brain neurotransmitter levels. Life Sci. 2006, 79, 2269–2274. [Google Scholar] [CrossRef]


| Item | Weaning Group | Control Group | SEM | p-Value |
|---|---|---|---|---|
| Asp | 439.56 | 277.20 | 78.36 | 0.106 |
| Glu | 2340.47 a | 1170.86 b | 115.32 | 0.026 |
| Asn | 398.06 | 343.26 | 37.39 | 0.125 |
| Ser | 798.58 | 702.48 | 55.62 | 0.132 |
| Cit | 15.10 | 6.250 | 2.60 | 0.078 |
| Thr | 195.25 | 102.64 | 33.40 | 0.069 |
| Tau | 362.53 | 261.74 | 71.51 | 0.083 |
| Ala | 2540.13 a | 1250.07 b | 98.49 | 0.035 |
| Tyr | 651.67 a | 891.59 b | 56.40 | 0.046 |
| Trp | 161.44 a | 361.26 b | 36.30 | 0.062 |
| Met | 589.79 | 779.20 | 63.39 | 0.105 |
| Val | 1080.71 | 980.56 | 154.57 | 0.074 |
| Phe | 677.56 a | 913.59 b | 93.34 | 0.039 |
| His | 445.62 | 562.35 | 112.26 | 0.076 |
| Ile | 596.79 | 766.27 | 146.61 | 0.068 |
| Leu | 1210.28 | 1310.93 | 182.19 | 0.078 |
| Orn | 654.05 | 750.79 | 109.25 | 0.059 |
| Lys | 1100.94 | 625.02 | 178.14 | 0.087 |
| Pro | 1110.50 | 994.83 | 249.50 | 0.065 |
| Protein Accession | Position | Gene Name | Protein Description | Weaning vs. Control Ratio | Regulated Type | Amino Acid |
|---|---|---|---|---|---|---|
| F1SRJ8 | 38 | PAH | Phenylalanine-4-hydroxylase OS = Homo sapiens | 1.53 | Up | K |
| F1SRJ8 | 204 | PAH | Phenylalanine-4-hydroxylase OS = Homo sapiens | 1.49 | Up | K |
| F1SRJ8 | 282 | PAH | Phenylalanine-4-hydroxylase OS = Homo sapiens | 1.42 | Up | K |
| F1RWA8 | 94 | TDO | Tryptophan 2,3-dioxygenase OS = Homo sapiens | 0.68 | Down | K |
| F1RWA8 | 212 | TDO | Tryptophan 2,3-dioxygenase OS = Homo sapiens | 0.73 | Down | K |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Yao, W.; Wang, T.; Li, J.; He, Q.; Huang, F. Acetylation of Phenylalanine Hydroxylase and Tryptophan 2,3-Dioxygenase Alters Hepatic Aromatic Amino Acid Metabolism in Weaned Piglets. Metabolites 2020, 10, 146. https://doi.org/10.3390/metabo10040146
Huang L, Yao W, Wang T, Li J, He Q, Huang F. Acetylation of Phenylalanine Hydroxylase and Tryptophan 2,3-Dioxygenase Alters Hepatic Aromatic Amino Acid Metabolism in Weaned Piglets. Metabolites. 2020; 10(4):146. https://doi.org/10.3390/metabo10040146
Chicago/Turabian StyleHuang, Lu, Weilei Yao, Tongxin Wang, Juan Li, Qiongyu He, and Feiruo Huang. 2020. "Acetylation of Phenylalanine Hydroxylase and Tryptophan 2,3-Dioxygenase Alters Hepatic Aromatic Amino Acid Metabolism in Weaned Piglets" Metabolites 10, no. 4: 146. https://doi.org/10.3390/metabo10040146
APA StyleHuang, L., Yao, W., Wang, T., Li, J., He, Q., & Huang, F. (2020). Acetylation of Phenylalanine Hydroxylase and Tryptophan 2,3-Dioxygenase Alters Hepatic Aromatic Amino Acid Metabolism in Weaned Piglets. Metabolites, 10(4), 146. https://doi.org/10.3390/metabo10040146





