Comparative Cerebroprotective Potential of d- and l-Carnosine Following Ischemic Stroke in Mice
Abstract
:1. Introduction
2. Results
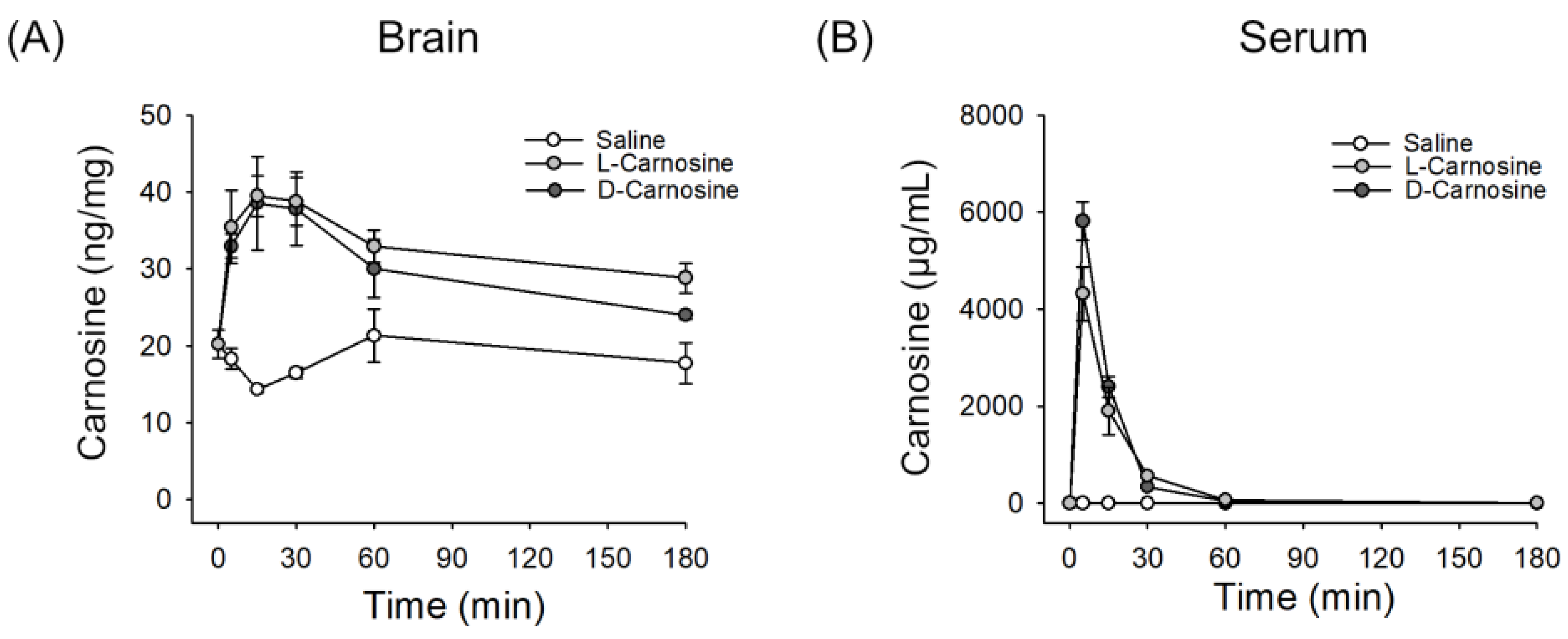
2.1. l- and d-Carnosine in Serum and Brain Levels Postintravenous Administration
2.2. Both d- and l-Carnosine Exhibit Protection against Mouse Transient Focal Ischemia
2.3. Effect of l- and d-Carnosine on ROS Accumulation in Primary Neurons
2.4. Neuroprotection in Primary Cortical Neuronal Cultures
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Isolation of Serum and Brain Samples for Kinetic Studies of l- or d-Carnosine in Mice
4.3. Determination of Carnosine Levels in Serum or Brain Using LC-MS/MS
4.4. Transient Focal Cerebral Ischemia
4.5. Drug Treatment in the MCAO Model
4.6. 2, 3, 5-Triphenyltetrazolium Chloride (TTC) Staining
4.7. In Vitro Culture of Primary Cortical Neurons
4.8. Oxidative Stress Assay
4.9. Excitotoxicity Assay
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MCAO | Middle Cerebral Artery Occlusion |
| TTC | 2, 3, 5-Triphenyltetrazolium chloride |
| AUC | Area under curve |
| Cmax | Peak serum concentration |
| ROS | Reactive oxygen species |
| ICA | Internal carotid artery |
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akpan, N.; Troy, C.M. Caspase inhibitors: Prospective therapies for stroke. Neuroscientist 2013, 19, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.D.; Al-Khoury, L.; Zivin, J.A. Neuroprotection for ischemic stroke: Two decades of success and failure. NeuroRx 2004, 1, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Chollet, F.; Rigal, J.; Marque, P.; Barbieux-Guillot, M.; Raposo, N.; Fabry, V.; Albucher, J.F.; Pariente, J.; Loubinoux, I. Serotonin Selective Reuptake Inhibitors (SSRIs) and Stroke. Curr. Neurol. Neurosci. Rep. 2018, 18, 100. [Google Scholar] [CrossRef]
- Fisher, M. New approaches to neuroprotective drug development. Stroke 2011, 42 (Suppl. 1), S24–S27. [Google Scholar] [CrossRef] [Green Version]
- Hess, D.C. NXY-059: A hopeful sign in the treatment of stroke. Stroke 2006, 37, 2649–2650. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.; Buchan, A.M. C-EPO: Ready for prime-time preconditioning? Cerebrovasc. Dis. 2005, 19, 272–273. [Google Scholar] [CrossRef]
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–415. [Google Scholar] [CrossRef]
- Macrae, I.M.; Allan, S.M. Stroke: The past, present and future. Brain Neurosci. Adv. 2018, 2. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Bae, O.N.; Serfozo, K.; Baek, S.H.; Lee, K.Y.; Dorrance, A.; Rumbeiha, W.; Fitzgerald, S.D.; Farooq, M.U.; Naravelta, B.; Bhatt, A.; et al. Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke 2013, 44, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Majid, A. Neuroprotection in stroke: Past, present, and future. ISRN Neurol. 2014, 2014, 515716. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Senut, M.C.; Rajanikant, K.; Greenberg, E.; Bandagi, R.; Zemke, D.; Mousa, A.; Kassab, M.; Farooq, M.U.; Gupta, R.; et al. Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J. Neurosci. Res. 2008, 86, 2984–2991. [Google Scholar] [CrossRef] [Green Version]
- Rajanikant, G.K.; Zemke, D.; Senut, M.C.; Frenkel, M.B.; Chen, A.F.; Gupta, R.; Majid, A. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke 2007, 38, 3023–3031. [Google Scholar] [CrossRef] [Green Version]
- Boldyrev, A.; Bulygina, E.; Leinsoo, T.; Petrushanko, I.; Tsubone, S.; Abe, H. Protection of neuronal cells against reactive oxygen species by carnosine and related compounds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 137, 81–88. [Google Scholar] [CrossRef]
- Fontana, M.; Pinnen, F.; Lucente, G.; Pecci, L. Prevention of peroxynitrite-dependent damage by carnosine and related sulphonamido pseudodipeptides. Cell Mol. Life Sci. 2002, 59, 546–551. [Google Scholar] [CrossRef]
- Abe, H.; Dobson, G.P.; Hoeger, U.; Parkhouse, W.S. Role of histidine-related compounds to intracellular buffering in fish skeletal muscle. Am. J. Physiol. 1985, 249, R449–R454. [Google Scholar] [CrossRef]
- Guiotto, A.; Calderan, A.; Ruzza, P.; Borin, G. Carnosine and carnosine-related antioxidants: A review. Curr. Med. Chem. 2005, 12, 2293–2315. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Carnosine and its possible roles in nutrition and health. Adv. Food. Nutr. Res. 2009, 57, 87–154. [Google Scholar]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.H.; Noh, A.R.; Kim, K.A.; Akram, M.; Shin, Y.J.; Kim, E.S.; Yu, S.W.; Majid, A.; Bae, O.N. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke 2014, 45, 2438–2443. [Google Scholar] [CrossRef] [Green Version]
- Aldini, G.; Orioli, M.; Rossoni, G.; Savi, F.; Braidotti, P.; Vistoli, G.; Yeum, K.J.; Negrisoli, G.; Carini, M. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J. Cell Mol. Med. 2011, 15, 1339–1354. [Google Scholar] [CrossRef]
- Bellia, F.; Vecchio, G.; Rizzarelli, E. Carnosine derivatives: New multifunctional drug-like molecules. Amino Acids 2012, 43, 153–163. [Google Scholar] [CrossRef]
- Riedl, E.; Koeppel, H.; Brinkkoetter, P.; Sternik, P.; Steinbeisser, H.; Sauerhoefer, S.; Janssen, B.; van der Woude, F.J.; Yard, B.A. A CTG polymorphism in the CNDP1 gene determines the secretion of serum carnosinase in Cos-7 transfected cells. Diabetes 2007, 56, 2410–2413. [Google Scholar] [CrossRef] [Green Version]
- Janssen, B.; Hohenadel, D.; Brinkkoetter, P.; Peters, V.; Rind, N.; Fischer, C.; Rychlik, I.; Cerna, M.; Romzova, M.; de Heer, E.; et al. Carnosine as a protective factor in diabetic nephropathy: Association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 2005, 54, 2320–2327. [Google Scholar] [CrossRef] [Green Version]
- Vistoli, G.; Orioli, M.; Pedretti, A.; Regazzoni, L.; Canevotti, R.; Negrisoli, G.; Carini, M.; Aldini, G. Design, synthesis, and evaluation of carnosine derivatives as selective and efficient sequestering agents of cytotoxic reactive carbonyl species. ChemMedChem 2009, 4, 967–975. [Google Scholar] [CrossRef]
- Margolis, F.L. Carnosine in the primary olfactory pathway. Science 1974, 184, 909–911. [Google Scholar] [CrossRef]
- Cararo, J.H.; Streck, E.L.; Schuck, P.F.; Ferreira Gda, C. Carnosine and Related Peptides: Therapeutic Potential in Age-Related Disorders. Aging Dis. 2015, 6, 369–379. [Google Scholar]
- Teufel, M.; Saudek, V.; Ledig, J.P.; Bernhardt, A.; Boularand, S.; Carreau, A.; Cairns, N.J.; Carter, C.; Cowley, D.J.; Duverger, D.; et al. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J. Biol. Chem. 2003, 278, 6521–6531. [Google Scholar] [CrossRef] [Green Version]
- Unno, H.; Yamashita, T.; Ujita, S.; Okumura, N.; Otani, H.; Okumura, A.; Nagai, K.; Kusunoki, M. Structural basis for substrate recognition and hydrolysis by mouse carnosinase CN2. J. Biol. Chem. 2008, 283, 27289–27299. [Google Scholar] [CrossRef] [Green Version]
- Otani, H.; Okumura, N.; Hashida-Okumura, A.; Nagai, K. Identification and characterization of a mouse dipeptidase that hydrolyzes L-carnosine. J. Biochem. 2005, 137, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Pandya, V.; Ekka, M.K.; Dutta, R.K.; Kumaran, S. Mass spectrometry assay for studying kinetic properties of dipeptidases: Characterization of human and yeast dipeptidases. Anal. Biochem. 2011, 418, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I. Naive pooled-data approach for pharmacokinetic studies in pediatrics with a very small sample size. Am. J. Ther. 2014, 21, 269–274. [Google Scholar] [CrossRef] [PubMed]
- KuKanich, B.; Huff, D.; Riviere, J.E.; Papich, M.G. Naive averaged, naive pooled, and population pharmacokinetics of orally administered marbofloxacin in juvenile harbor seals. J. Am. Vet. Med. Assoc. 2007, 230, 390–395. [Google Scholar] [CrossRef]
- Xie, Z.; Baba, S.P.; Sweeney, B.R.; Barski, O.A. Detoxification of aldehydes by histidine-containing dipeptides: From chemistry to clinical implications. Chem. Biol. Interact. 2013, 202, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.J.; Vistoli, G.; Katunga, L.A.; Funai, K.; Regazzoni, L.; Monroe, T.B.; Gilardoni, E.; Cannizzaro, L.; Colzani, M.; De Maddis, D.; et al. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J. Clin. Investig. 2018, 128, 5280–5293. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O′Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages. Antioxidants (Basel) 2019, 8, 281. [Google Scholar] [CrossRef] [Green Version]
- Peters, V.; Calabrese, V.; Forsberg, E.; Volk, N.; Fleming, T.; Baelde, H.; Weigand, T.; Thiel, C.; Trovato, A.; Scuto, M.; et al. Protective Actions of Anserine Under Diabetic Conditions. Int. J. Mol. Sci. 2018, 19, 2751. [Google Scholar] [CrossRef] [Green Version]
- Schön, M.; Mousa, A.; Berk, M.; Chia, W.L.; Ukropec, J.; Majid, A.; Ukropcová, B.; de Courten, B. The Potential of Carnosine in Brain-Related Disorders: A Comprehensive Review of Current Evidence. Nutrients 2019, 11, 1196. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yao, K.; Fan, Y.; He, P.; Wang, X.; Hu, W.; Chen, Z. Carnosine protects brain microvascular endothelial cells against rotenone-induced oxidative stress injury through histamine H1and H2receptors in vitro. Clin. Exp. Pharmacol. Physiol. 2012, 39, 1019–1025. [Google Scholar] [CrossRef]
- Khama-Murad, A.; Mokrushin, A.; Pavlinova, L. Neuroprotective properties of l-carnosine in the brain slices exposed to autoblood in the hemorrhagic stroke model in vitro. Regul. Pept. 2011, 167, 65–69. [Google Scholar] [CrossRef]
- Bae, O.N.; Majid, A. Role of histidine/histamine in carnosine-induced neuroprotection during ischemic brain damage. Brain Res. 2013, 1527, 246–254. [Google Scholar] [CrossRef]
- Orioli, M.; Aldini, G.; Beretta, G.; Facino, R.M.; Carini, M. LC-ESI-MS/MS determination of 4-hydroxy-trans-2-nonenal Michael adducts with cysteine and histidine-containing peptides as early markers of oxidative stress in excitable tissues. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 827, 109–118. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Medana, C.; Visentin, S.; Giancotti, V.; Zunino, V.; Meineri, G. Determination of carnosine, anserine, homocarnosine, pentosidine and thiobarbituric acid reactive substances contents in meat from different animal species. Food. Chem. 2011, 126, 1939–1947. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar]
- Hasan, M.M.; Islam, M.S.; Hoque, K.M.F.; Haque, A.; Reza, M.A. Effect of Citrus macroptera Fruit Pulp Juice on Alteration of Caspase Pathway Rendering Anti-Proliferative Activity against Ehrlich’s Ascites Carcinoma in Mice. Toxicol. Res. 2019, 35, 271–277. [Google Scholar] [CrossRef] [Green Version]



| Half-Life (min) | Cmax (5 min) (μg/mL) | AUC (μg/mL min) | CL (mL/min/kg) | Vss (mL/kg) | |
|---|---|---|---|---|---|
| D-carnosine | 75 | 5818 | 108112 | 9.23 | 120 |
| L-carnosine | 78 | 4011 | 89802 | 11.08 | 223 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, S.; Kim, E.-S.; Kim, D.; Burrows, D.; De Felice, M.; Kim, M.; Baek, S.-H.; Ali, A.; Redgrave, J.; Doeppner, T.R.; et al. Comparative Cerebroprotective Potential of d- and l-Carnosine Following Ischemic Stroke in Mice. Int. J. Mol. Sci. 2020, 21, 3053. https://doi.org/10.3390/ijms21093053
Jain S, Kim E-S, Kim D, Burrows D, De Felice M, Kim M, Baek S-H, Ali A, Redgrave J, Doeppner TR, et al. Comparative Cerebroprotective Potential of d- and l-Carnosine Following Ischemic Stroke in Mice. International Journal of Molecular Sciences. 2020; 21(9):3053. https://doi.org/10.3390/ijms21093053
Chicago/Turabian StyleJain, Saurabh, Eun-Sun Kim, Donghyun Kim, David Burrows, Milena De Felice, Minyeong Kim, Seung-Hoon Baek, Ali Ali, Jessica Redgrave, Thorsten R. Doeppner, and et al. 2020. "Comparative Cerebroprotective Potential of d- and l-Carnosine Following Ischemic Stroke in Mice" International Journal of Molecular Sciences 21, no. 9: 3053. https://doi.org/10.3390/ijms21093053
APA StyleJain, S., Kim, E.-S., Kim, D., Burrows, D., De Felice, M., Kim, M., Baek, S.-H., Ali, A., Redgrave, J., Doeppner, T. R., Gardner, I., Bae, O.-N., & Majid, A. (2020). Comparative Cerebroprotective Potential of d- and l-Carnosine Following Ischemic Stroke in Mice. International Journal of Molecular Sciences, 21(9), 3053. https://doi.org/10.3390/ijms21093053







