Spironolactone and XPB: An Old Drug with a New Molecular Target
Abstract
1. Introduction
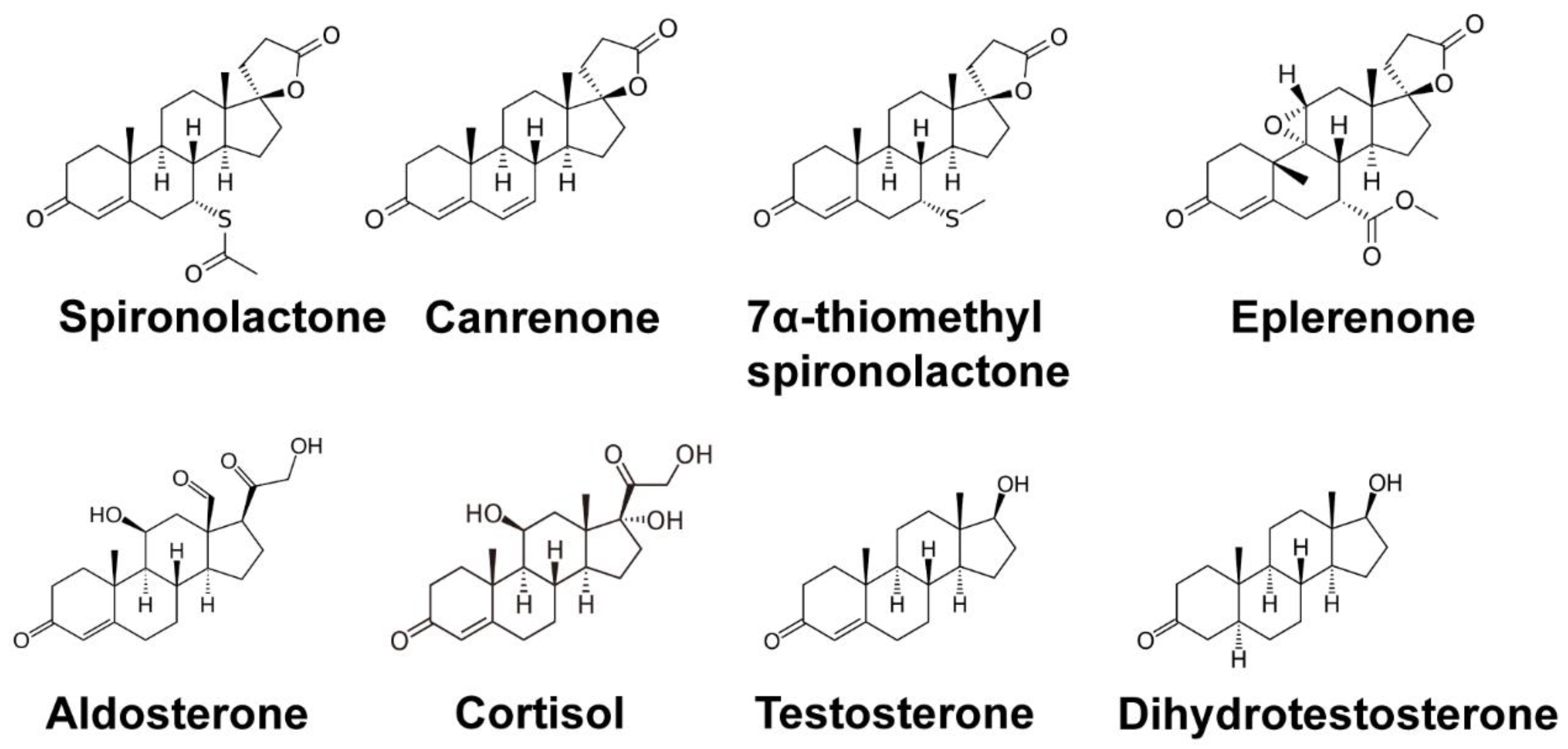
2. Clinical Uses and Canonical Targets of Spironolactone
2.1. SP as a Mineralocorticoid Receptor Antagonist
2.2. SP as an Androgen Receptor Antagonist
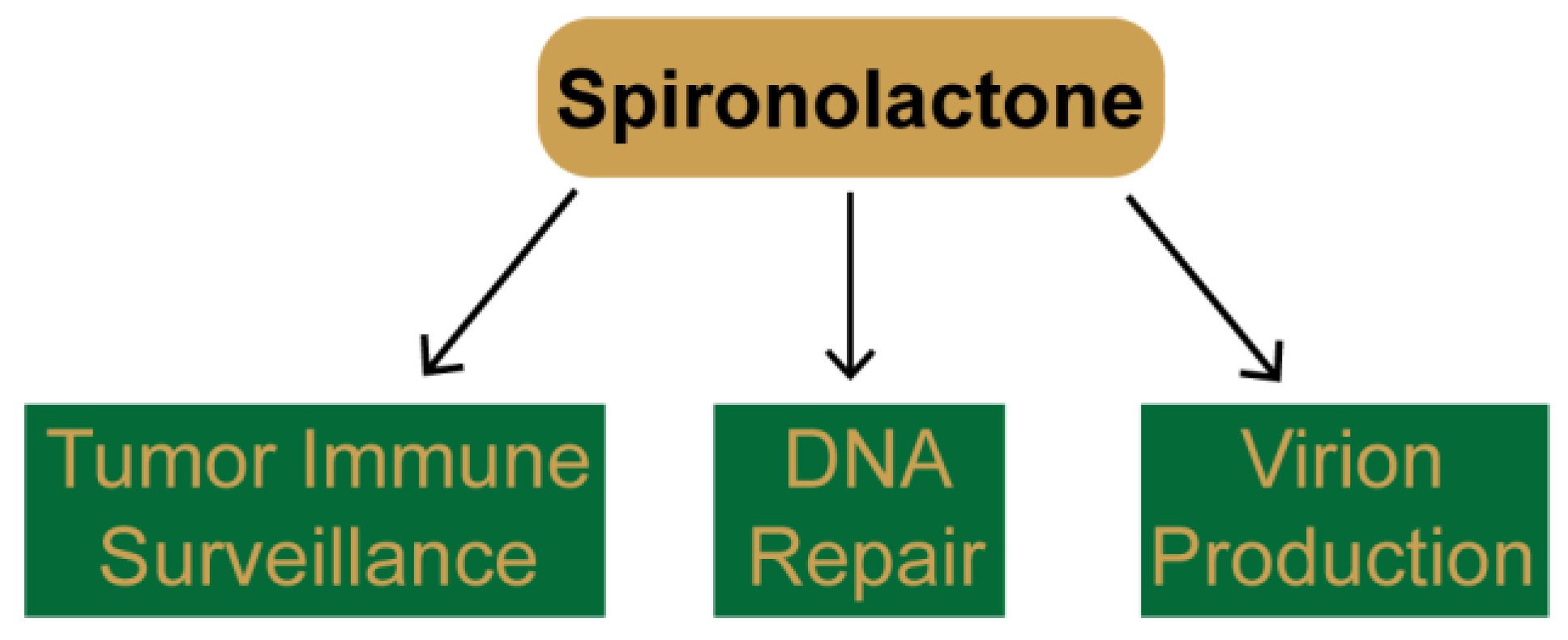
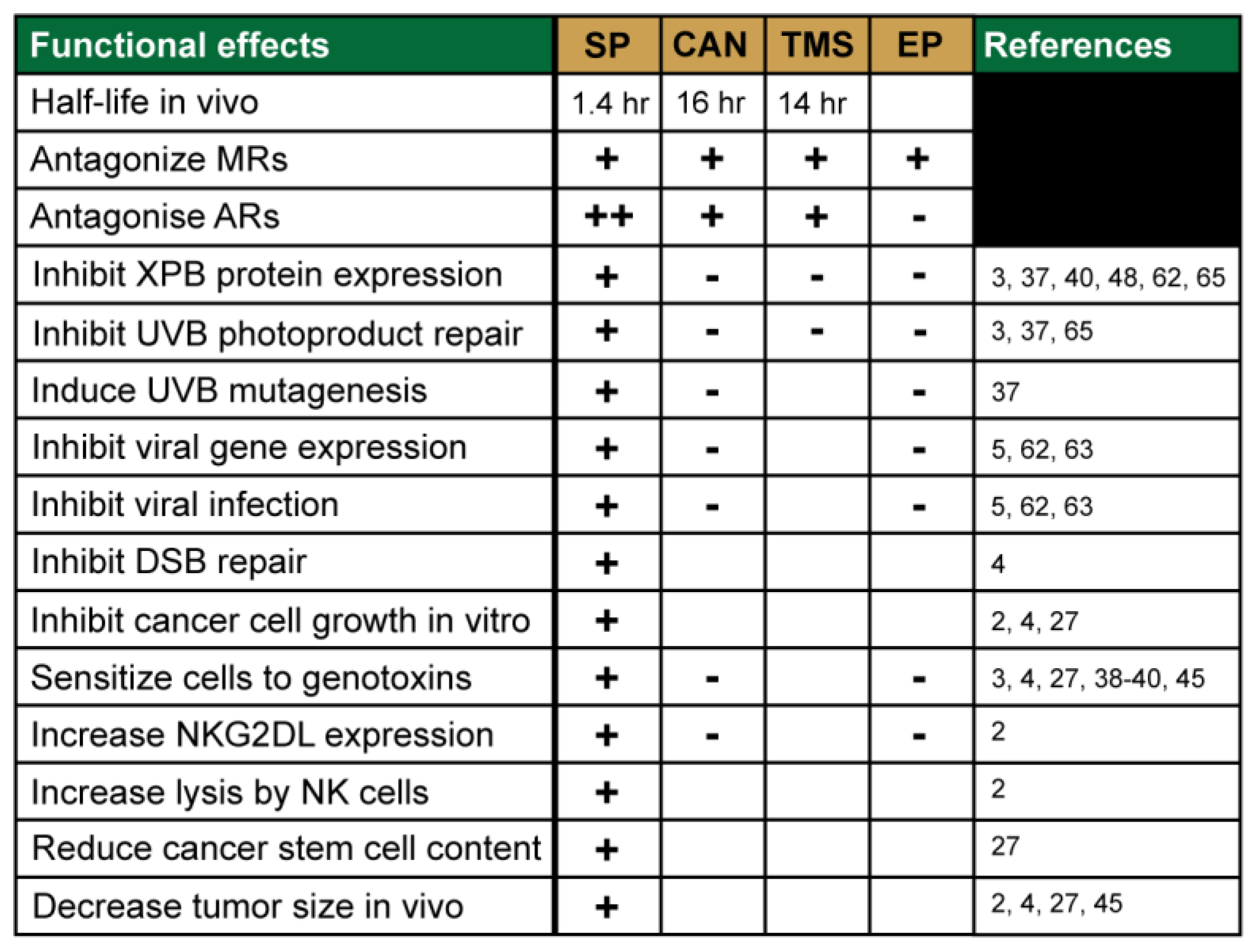
3. Identification of Novel Pharmacological Effects of Spironolactone
3.1. SP Promotes Tumor Cell Recognition by Natural Killer Cells
3.2. SP Inhibits DNA Repair and Sensitizes Cancer Cells to DNA Damaging Agents
3.3. SP Inhibits Viral Gene Transcription
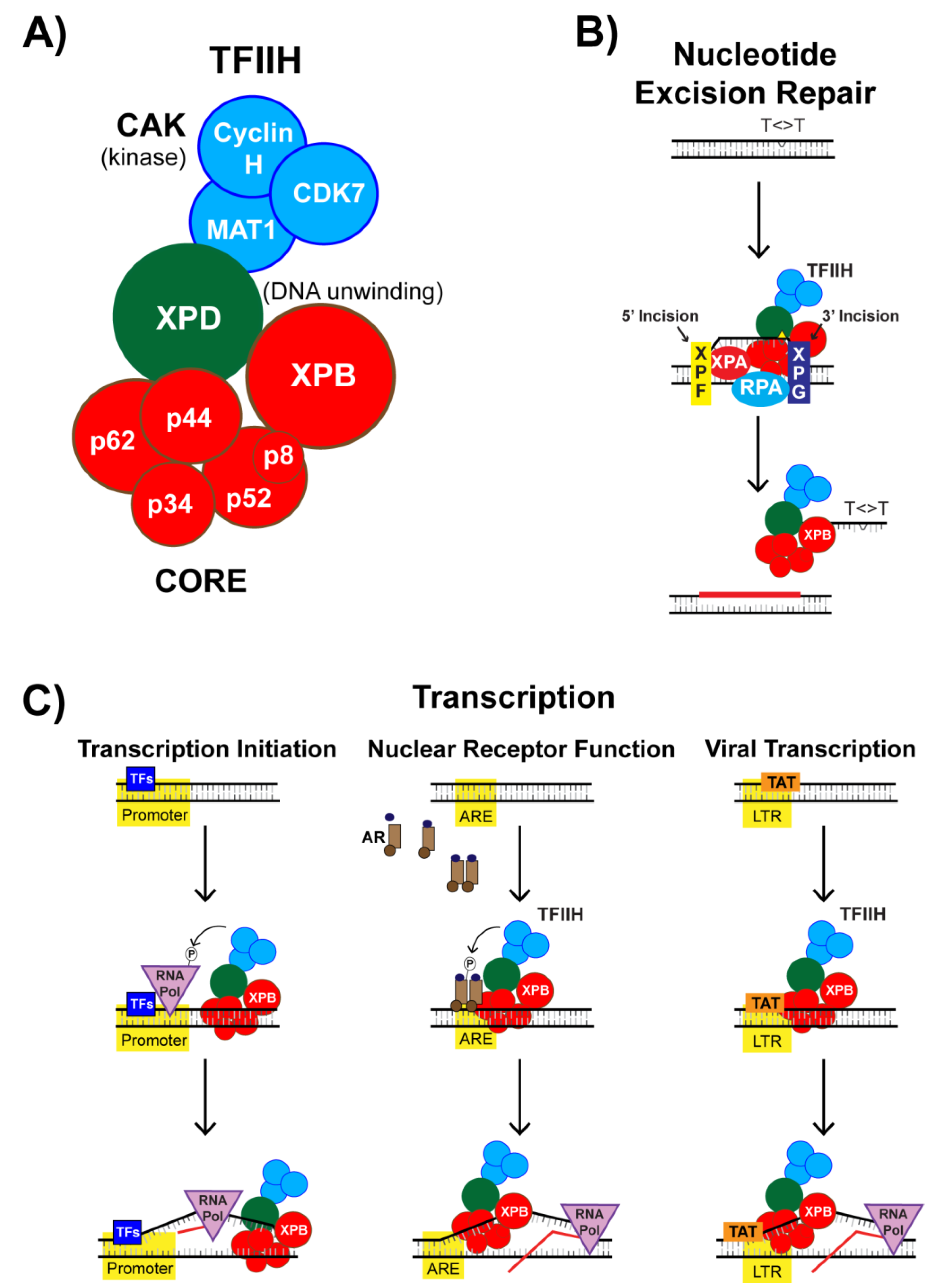
4. The XPB Protein as a Novel Target of Spironolactone
4.1. Identification of the DNA Repair Protein XPB as a Target of SP
4.2. SP Impacts TFIIH Function in Transcription Initiation
4.3. Nuclear Receptor-Dependent Transcription is Regulated by TFIIH and SP
4.4. SP Impacts TFIIH Function in Viral Transcription
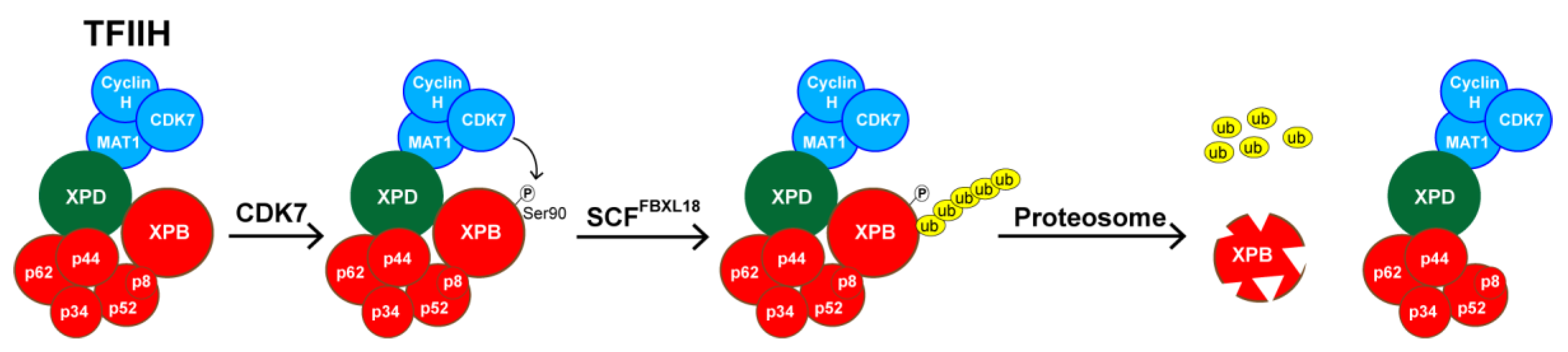
5. SP Promotes the Rapid Proteasomal Degradation of XPB
6. Implications for SP in the Skin and Potential Risks of Carcinogenesis
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, S.C.; Sung, B.; Prasad, S.; Webb, L.J.; Aggarwal, B.B. Cancer drug discovery by repurposing: Teaching new tricks to old dogs. Trends Pharmacol. Sci. 2013, 34, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.-H.; Vong, Q.P.; Lin, W.; Janke, L.; Chen, T.; Leung, W. Modulation of NKG2D ligand expression and metastasis in tumors by spironolactone via RXRγ activation. J. Exp. Med. 2013, 210, 2675–2692. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, S.; Ayadi, M.; Brino, L.; Egly, J.-M.; Larsen, A.K.; Coin, F. A Small Molecule Screen Identifies an Inhibitor of DNA Repair Inducing the Degradation of TFIIH and the Chemosensitization of Tumor Cells to Platinum. Chem. Biol. 2014, 21, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Shahar, O.D.; Kalousi, A.; Eini, L.; Fisher, B.; Weiss, A.; Darr, J.; Mazina, O.; Bramson, S.; Kupiec, M.; Eden, A.; et al. A high-throughput chemical screen with FDA approved drugs reveals that the antihypertensive drug Spironolactone impairs cancer cell survival by inhibiting homology directed repair. Nucleic Acids Res. 2014, 42, 5689–5701. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Thompson, J.; Swaminathan, S. Spironolactone blocks Epstein–Barr virus production by inhibiting EBV SM protein function. Proc. Natl. Acad. Sci. USA 2016, 113, 3609–3614. [Google Scholar] [CrossRef]
- Kagawa, C.M.; Cella, J.A.; Van Arman, C.G. Action of new steroids in blocking effects of aldosterone and desoxycorticosterone on salt. Science 1957, 126, 1015–1016. [Google Scholar] [CrossRef]
- Liddle, G.W. Sodium Diuresis Induced by Steroidal Antagonists of Aldosterone. Science 1957, 126, 1016–1018. [Google Scholar] [CrossRef]
- Cranston, W.; Juel-Jensen, B. The effects of spironolactone and chlorthalidone on arterial pressure. Lancet 1962, 279, 1161–1164. [Google Scholar] [CrossRef]
- Sturtevant, F.M. Antihypertensive Effects of an Aldosterone Antagonist. Science 1958, 127, 1393–1394. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Zannad, F.; McMurray, J.J.; Krum, H.; Van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef]
- Moore, K.; Aithal, G.P. Guidelines on the management of ascites in cirrhosis. Gut 2006, 55, vi1–vi12. [Google Scholar] [CrossRef] [PubMed]
- Dojki, F.K.; Bakris, G. Nonsteroidal mineralocorticoid antagonists in diabetic kidney disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.; Kim, H.; Bustamante, J. Drug-Induced Gynecomastia. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2012, 32, 1123–1140. [Google Scholar] [CrossRef]
- Bonne, C.; Raynaud, J. Mode of spironolactone anti-androgenic action: Inhibition of androstanolone binding to rat prostate androgen receptor. Mol. Cell. Endocrinol. 1974, 2, 59–67. [Google Scholar] [CrossRef]
- Corvol, P.; Michaud, A.; Menard, J.; Freifeld, M.; Mahoudeau, J. Antiandrogenic Effect of Spirolactones: Mechanism of Action. Endocrinology 1975, 97, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, D.; Sinclair, R. Use of spironolactone in dermatology. Skinmed 2011, 8, 328–332. [Google Scholar]
- Azarchi, S.; Bienenfeld, A.; Lo Sicco, K.; Marchbein, S.; Shapiro, J.; Nagler, A.R. Androgens in women: Hormone-modulating therapies for skin disease. J. Am. Acad. Dermatol. 2019, 80, 1509–1521. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Acne and sebaceous gland function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar] [CrossRef]
- Park, H.; Skopit, S. Safety Considerations and Monitoring in Patients Treated with Systemic Medications for Acne. Dermatol. Clin. 2016, 34, 185–193. [Google Scholar] [CrossRef]
- Sinclair, R.; Patel, M.; Dawson, T.; Yazdabadi, A.; Yip, L.; Perez, A.; Rufaut, N. Hair loss in women: Medical and cosmetic approaches to increase scalp hair fullness. Br. J. Dermatol. 2011, 165, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Fung, R.; Hellstern-Layefsky, M.; Tastenhoye, C.; Lega, I.C.; Steele, L. Differential Effects of Cyproterone Acetate vs Spironolactone on Serum High-Density Lipoprotein and Prolactin Concentrations in the Hormonal Treatment of Transgender Women. J. Sex. Med. 2016, 13, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Gardner, I.H.; Safer, J.D. Progress on the road to better medical care for transgender patients. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Menard, R.H.; Martin, H.F.; Stripp, B.; Gillette, J.R.; Bartter, F.C. Spironolactone and cytochrome P-450: Impairment of steroid hydroxylation in the adrenal cortex. Life Sci. 1974, 15, 1639–1648. [Google Scholar] [CrossRef]
- Menard, R.H.; Bartter, F.C.; Gillette, J.R. Spironolactone and cytochrome P-450: Impairment of steroid 21-hydroxylation in the adrenal cortex. Arch. Biochem. Biophys. 1976, 173, 395–402. [Google Scholar] [CrossRef]
- Sancar, A. Mechanisms of DNA Repair by Photolyase and Excision Nuclease (Nobel Lecture). Angew. Chem. Int. Ed. 2016, 55, 8502–8527. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.; Eini, L.; Nissim-Rafinia, M.; Viner, R.; Ezer, S.; Erez, K.; Aqaqe, N.; Hanania, R.; Milyavsky, M.; Meshorer, E.; et al. Spironolactone inhibits the growth of cancer stem cells by impairing DNA damage response. Oncogene 2019, 38, 3103–3118. [Google Scholar] [CrossRef]
- Oh, K.-S.; Khan, S.; Jaspers, N.G.J.; Raams, A.; Ueda, T.; Lehmann, A.; Friedmann, P.S.; Emmert, S.; Gratchev, A.; Lachlan, K.; et al. Phenotypic heterogeneity in the XPB DNA helicase gene (ERCC3): Xeroderma pigmentosum without and with Cockayne syndrome. Hum. Mutat. 2006, 27, 1092–1103. [Google Scholar] [CrossRef]
- Natale, V.; Raquer, H. Xeroderma pigmentosum-Cockayne syndrome complex. Orphanet J. Rare Dis. 2017, 12, 65. [Google Scholar] [CrossRef]
- Compe, E.; Egly, J.-M. Nucleotide Excision Repair and Transcriptional Regulation: TFIIH and Beyond. Annu. Rev. Biochem. 2016, 85, 265–290. [Google Scholar] [CrossRef]
- Compe, E.; Egly, J.-M. TFIIH: When transcription met DNA repair. Nat. Rev. Mol. Cell Biol. 2012, 13, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Rimel, J.K.; Taatjes, D.J. The essential and multifunctional TFIIH complex. Protein Sci. 2018, 27, 1018–1037. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Choi, J.-H.; Gaddameedhi, S.; Kemp, M.G.; Reardon, J.T.; Sancar, A. Nucleotide Excision Repair in Human Cells. J. Biol. Chem. 2013, 288, 20918–20926. [Google Scholar] [CrossRef]
- Kemp, M.G.; Reardon, J.T.; Lindsey-Boltz, L.; Sancar, A. Mechanism of Release and Fate of Excised Oligonucleotides during Nucleotide Excision Repair. J. Biol. Chem. 2012, 287, 22889–22899. [Google Scholar] [CrossRef] [PubMed]
- Oksenych, V.; De Jesus, B.B.; Zhovmer, A.; Egly, J.-M.; Coin, F. Molecular insights into the recruitment of TFIIH to sites of DNA damage. EMBO J. 2009, 28, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Marteijn, J.A.; Hoeijmakers, J.; Vermeulen, W. Check, Check …Triple Check: Multi-Step DNA Lesion Identification by Nucleotide Excision Repair. Mol. Cell 2015, 59, 885–886. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.G.; Krishnamurthy, S.; Kent, M.N.; Schumacher, D.L.; Sharma, P.; Excoffon, K.J.; Travers, J.B. Spironolactone Depletes the XPB Protein and Inhibits DNA Damage Responses in UVB-Irradiated Human Skin. J. Investig. Dermatol. 2019, 139, 448–454. [Google Scholar] [CrossRef]
- Hutcherson, R.J.; Kemp, M.G. ATR kinase inhibition sensitizes quiescent human cells to the lethal effects of cisplatin but increases mutagenesis. Mutat. Res. Mol. Mech. Mutagen. 2019, 111678, 111678. [Google Scholar] [CrossRef]
- Shaj, K.; Hutcherson, R.J.; Kemp, M.G. ATR Kinase Activity Limits Mutagenesis and Promotes the Clonogenic Survival of Quiescent Human Keratinocytes Exposed to UVB Radiation. Photochem. Photobiol. 2019, 96, 105–112. [Google Scholar] [CrossRef]
- Szalat, R.; Samur, M.K.; Fulciniti, M.; López, M.; Nanjappa, P.; Cleynen, A.; Wen, K.; Kumar, S.; Perini, T.; Calkins, A.S.; et al. Nucleotide excision repair is a potential therapeutic target in multiple myeloma. Leukemia 2017, 32, 111–119. [Google Scholar] [CrossRef]
- Zhang, M.; Du, W.; Acklin, S.M.; Jin, S.; Xia, F. SIRT2 protects peripheral neurons from cisplatin-induced injury by enhancing nucleotide excision repair. J. Clin. Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, L.; Cassel, T.; Helleday, T. The XPD subunit of TFIIH is required for transcription-associated but not DNA double-strand break-induced recombination in mammalian cells. Mutagenesis 2010, 25, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Moriel-Carretero, M.; Aguilera, A. A Postincision-Deficient TFIIH Causes Replication Fork Breakage and Uncovers Alternative Rad51- or Pol32-Mediated Restart Mechanisms. Mol. Cell 2010, 37, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Dungrawala, H.; Rose, K.L.; Bhat, K.; Mohni, K.N.; Glick, G.G.; Couch, F.B.; Cortez, D. The Replication Checkpoint Prevents Two Types of Fork Collapse without Regulating Replisome Stability. Mol. Cell 2015, 59, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Sanomachi, T.; Suzuki, S.; Togashi, K.; Sugai, A.; Seino, S.; Okada, M.; Yoshioka, T.; Kitanaka, C.; Yamamoto, M. Spironolactone, a Classic Potassium-Sparing Diuretic, Reduces Survivin Expression and Chemosensitizes Cancer Cells to Non-DNA-Damaging Anticancer Drugs. Cancers 2019, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Fishburn, J.; Tomko, E.; Galburt, E.; Hahn, S. Double-stranded DNA translocase activity of transcription factor TFIIH and the mechanism of RNA polymerase II open complex formation. Proc. Natl. Acad. Sci. USA 2015, 112, 3961–3966. [Google Scholar] [CrossRef]
- Schaeffer, L.; Roy, R.; Humbert, S.; Moncollin, V.; Vermeulen, W.; Hoeijmakers, J.; Chambon, P.; Egly, J. DNA repair helicase: A component of BTF2 (TFIIH) basic transcription factor. Science 1993, 260, 58–63. [Google Scholar] [CrossRef]
- Elinoff, J.M.; Chen, L.-Y.; Dougherty, E.J.; Awad, K.S.; Wang, S.; Biancotto, A.; Siddiqui, A.H.; Weir, N.A.; Cai, R.; Sun, J.; et al. Spironolactone-induced degradation of the TFIIH core complex XPB subunit suppresses NF-κB and AP-1 signalling. Cardiovasc. Res. 2018, 114, 65–76. [Google Scholar] [CrossRef]
- Rochette-Egly, C. Nuclear receptors: Integration of multiple signalling pathways through phosphorylation. Cell. Signal. 2003, 15, 355–366. [Google Scholar] [CrossRef]
- Chymkowitch, P.; Le May, N.; Charneau, P.; Compe, E.; Egly, J.-M. The phosphorylation of the androgen receptor by TFIIH directs the ubiquitin/proteasome process. EMBO J. 2010, 30, 468–479. [Google Scholar] [CrossRef]
- Gasser, S.; Orsulic, S.; Brown, E.J.; Raulet, D.H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005, 436, 1186–1190. [Google Scholar] [CrossRef]
- Arya, S.; Guo, C.; Josephs, S.; Wong-Staal, F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science 1985, 229, 69–73. [Google Scholar] [CrossRef]
- Sodroski, J.; Rosen, C.; Wong-Staal, F.; Salahuddin, S.; Popovic, M.; Arya, S.; Gallo, R.; Haseltine, W. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science 1985, 227, 171–173. [Google Scholar] [CrossRef]
- Berkhout, B.; Silverman, R.H.; Jeang, K.-T. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell 1989, 59, 273–282. [Google Scholar] [CrossRef]
- Das, A.T.; Harwig, A.; Berkhout, B. The HIV-1 Tat Protein Has a Versatile Role in Activating Viral Transcription. J. Virol. 2011, 85, 9506–9516. [Google Scholar] [CrossRef]
- Cujec, T.P.; Okamoto, H.; Fujinaga, K.; Meyer, J.; Chamberlin, H.; Morgan, D.O.; Peterlin, B.M. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genome Res. 1997, 11, 2645–2657. [Google Scholar] [CrossRef]
- García-Martínez, L.F.; Ivanov, D.; Gaynor, R.B. Association of Tat with Purified HIV-1 and HIV-2 Transcription Preinitiation Complexes. J. Biol. Chem. 1997, 272, 6951–6958. [Google Scholar] [CrossRef] [PubMed]
- Yoder, K.; Sarasin, A.; Kraemer, K.H.; McIlhatton, M.; Bushman, F.; Fishel, R. The DNA repair genes XPB and XPD defend cells from retroviral infection. Proc. Natl. Acad. Sci. USA 2006, 103, 4622–4627. [Google Scholar] [CrossRef] [PubMed]
- Yoder, K.; Roddick, W.; Hoellerbauer, P.; Fishel, R. XPB mediated retroviral cDNA degradation coincides with entry to the nucleus. Virology 2010, 410, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Espeseth, A.S.; Fishel, R.; Hazuda, D.; Huang, Q.; Xu, M.; Yoder, K.E.; Zhou, H. siRNA Screening of a Targeted Library of DNA Repair Factors in HIV Infection Reveals a Role for Base Excision Repair in HIV Integration. PLoS ONE 2011, 6, e17612. [Google Scholar] [CrossRef]
- Brass, A.L.; Dykxhoorn, D.M.; Benita, Y.; Yan, N.; Engelman, A.; Xavier, R.J.; Lieberman, J.; Elledge, S.J. Identification of Host Proteins Required for HIV Infection Through a Functional Genomic Screen. Science 2008, 319, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, B.; Morel, M.; Margottin-Goguet, F.; Ramirez, B.C. Specific Inhibition of HIV Infection by the Action of Spironolactone in T Cells. J. Virol. 2016, 90, 10972–10980. [Google Scholar] [CrossRef] [PubMed]
- Martella, C.; Tollenaere, A.I.; Waast, L.; Lacombe, B.; Groussaud, D.; Margottin-Goguet, F.; Ramirez, B.C.; Pique, C. HTLV-1 Transactivator Tax Exploits The Xpb Subunit Of Tfiih During Viral Transcription. J. Virol. 2020, 8, 02171-19. [Google Scholar] [CrossRef]
- Ueda, M.; Matsuura, K.; Kawai, H.; Wakasugi, M.; Matsunaga, T. Spironolactone-induced XPB degradation depends on CDK7 kinase and SCF FBXL18 E3 ligase. Genes Cells 2019, 24, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Han, S.; Kemp, M.G. Detection of the small oligonucleotide products of nucleotide excision repair in UVB-irradiated human skin. DNA Repair 2020, 86, 102766. [Google Scholar] [CrossRef]
- Riou, L.; Zeng, L.; Chevallier-Lagente, O.; Stary, A.; Nikaido, O.; Taïeb, A.; Weeda, G.; Mezzina, M.; Sarasin, A. The relative expression of mutated XPB genes results in xeroderma pigmentosum/Cockayne’s syndrome or trichothiodystrophy cellular phenotypes. Hum. Mol. Genet. 1999, 8, 1125–1133. [Google Scholar] [CrossRef]
- Salama, A.; Badran, M.; Elmowafy, M.; Soliman, G.M. Spironolactone-Loaded LeciPlexes as Potential Topical Delivery Systems for Female Acne: In Vitro Appraisal and Ex Vivo Skin Permeability Studies. Pharmaceutics 2019, 12, 25. [Google Scholar] [CrossRef]
- Ferreira-Nunes, R.; Ferreira, L.A.; Gratieri, T.; Cunha-Filho, M.; Gelfuso, G.M. Stability-indicating analytical method of quantifying spironolactone and canrenone in dermatological formulations and iontophoretic skin permeation experiments. Biomed. Chromatogr. 2019, 33, e4656. [Google Scholar] [CrossRef]
- Kelidari, H.; Saeedi, M.; Akbari, J.; Morteza-Semnani, K.; Gill, P.; Valizadeh, H.; Nokhodchi, A. Formulation optimization and in vitro skin penetration of spironolactone loaded solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 473–479. [Google Scholar] [CrossRef]
- Kelidari, H.R.; Saeedi, M.; Hajheidari, Z.; Akbari, J.; Morteza-Semnani, K.; Akhtari, J.; Valizadeh, H.; Asare-Addo, K.; Nokhodchi, A. Spironolactone loaded nanostructured lipid carrier gel for effective treatment of mild and moderate acne vulgaris: A randomized, double-blind, prospective trial. Colloids Surf. B Biointerfaces 2016, 146, 47–53. [Google Scholar] [CrossRef]
- Kelidari, H.R.; Saeedi, M.; Akbari, J.; Morteza-Semnani, K.; Valizadeh, H.; Maniruzzaman, M.; Farmoudeh, A.; Nokhodchi, A. Development and Optimisation of Spironolactone Nanoparticles for Enhanced Dissolution Rates and Stability. AAPS PharmSciTech 2016, 18, 1469–1474. [Google Scholar] [CrossRef]
- Afzali, B.M.; Yaghoobi, E.; Yaghoobi, R.; Bagherani, N.; Dabbagh, M.A. Comparison of the efficacy of 5% topical spironolactone gel and placebo in the treatment of mild and moderate acne vulgaris: A randomized controlled trial. J. Dermatol. Treat. 2010, 23, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Boix, J.; Sevilla, L.M.; Saez, Z.; Carceller, E.; Perez, P. Epidermal Mineralocorticoid Receptor Plays Beneficial and Adverse Effects in Skin and Mediates Glucocorticoid Responses. J. Investig. Dermatol 2016, 136, 2417–2426. [Google Scholar] [CrossRef]
- Boix, J.; Nguyen, V.T.; Farman, N.; Aractingi, S.; Pérez, P. Mineralocorticoid receptor blockade improves glucocorticoid-induced skin atrophy but partially ameliorates anti-inflammatory actions in an irritative model in human skin explants. Exp. Dermatol. 2017, 27, 185–187. [Google Scholar] [CrossRef]
- Boix, J.; Bigas, J.; Sevilla, L.M.; Iacobone, M.; Citton, M.; Torresan, F.; Caroccia, B.; Rossi, G.P.; Pérez, P. Primary aldosteronism patients show skin alterations and abnormal activation of glucocorticoid receptor in keratinocytes. Sci. Rep. 2017, 7, 15806. [Google Scholar] [CrossRef]
- Maubec, E.; Laouénan, C.; Deschamps, L.; Nguyen, V.T.; Scheer-Senyarich, I.; Wackenheim-Jacobs, A.-C.; Steff, M.; Duhamel, S.; Tubiana, S.; Brahimi, N.; et al. Topical Mineralocorticoid Receptor Blockade Limits Glucocorticoid-Induced Epidermal Atrophy in Human Skin. J. Investig. Dermatol. 2015, 135, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Farman, N.; Maubec, E.; Nassar, D.; Desposito, D.; Waeckel, L.; Aractingi, S.; Jaisser, F. Re-Epithelialization of Pathological Cutaneous Wounds Is Improved by Local Mineralocorticoid Receptor Antagonism. J. Investig. Dermatol. 2016, 136, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.; Is, M.; Sv, M.; L, W.; Am, T.; Tm, M. Faculty Opinions recommendation of Spironolactone use and risk of incident cancers: A retrospective, matched cohort study. Fac. Opin. Post Publ. Peer Rev. Biomed. Lit. 2017, 83, 653–663. [Google Scholar] [CrossRef]
- Gardiner, P.; Schrode, K.; Quinlan, D.; Martin, B.K.; Boreham, D.R.; Rogers, M.S.; Stubbs, K.; Smith, M.; Karim, A. Spironolactone Metabolism: Steady-State Serum Levels of the Sulfur-Containing Metabolites. J. Clin. Pharmacol. 1989, 29, 342–347. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabbard, R.D.; Hoopes, R.R.; Kemp, M.G. Spironolactone and XPB: An Old Drug with a New Molecular Target. Biomolecules 2020, 10, 756. https://doi.org/10.3390/biom10050756
Gabbard RD, Hoopes RR, Kemp MG. Spironolactone and XPB: An Old Drug with a New Molecular Target. Biomolecules. 2020; 10(5):756. https://doi.org/10.3390/biom10050756
Chicago/Turabian StyleGabbard, Ryan D., Robert R. Hoopes, and Michael G. Kemp. 2020. "Spironolactone and XPB: An Old Drug with a New Molecular Target" Biomolecules 10, no. 5: 756. https://doi.org/10.3390/biom10050756
APA StyleGabbard, R. D., Hoopes, R. R., & Kemp, M. G. (2020). Spironolactone and XPB: An Old Drug with a New Molecular Target. Biomolecules, 10(5), 756. https://doi.org/10.3390/biom10050756





