Insights on the Extraction Performance of Alkanediols and Glycerol: Using Juglans regia L. Leaves as a Source of Bioactive Compounds
Abstract
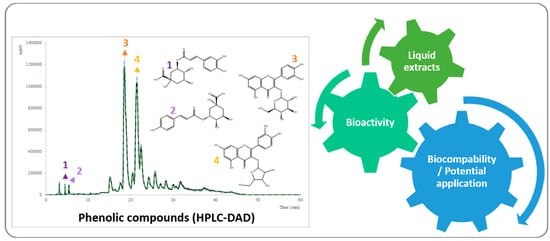
:1. Introduction
2. Results and Discussion
2.1. Extraction Yields
2.2. Cytotoxic and Anti-Inflammatory Activities
3. Materials and Methods
3.1. Standards and Reagents
3.2. Plant Material
3.3. Extraction Methodology
3.4. Chromatographic Analysis of the Main Phenolic Compounds
3.5. Cytotoxicity
3.6. Anti-Inflammatory Activity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rostagno, M.A.; Prado, J.M. Natural Product Extraction—Principles and Applications, 1st ed.; Rostagno, M.A., Prado, J.M., Eds.; The Royal Society of Green Chemistry: Cambridge, UK, 2013; ISBN 978-1-84973-606-0. [Google Scholar]
- Gawlik-Dziki, U.; Durak, A.; Pecio, Ł.; Kowalska, I. Nutraceutical Potential of Tinctures from Fruits, Green Husks, and Leaves of Juglans regia L. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.; Barros, L.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.; Dueñas, M. Leaves and decoction of Juglans regia L.: Different performances regarding bioactive compounds and in vitro antioxidant and antitumor effects. Ind. Crop. Prod. 2013, 51, 430–436. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.P.P.; Lobo, J.M.S. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Panth, N.; Paudel, K.R.; Karki, R. Phytochemical profile and biological activity of Juglans regia. J. Integr. Med. 2016, 14, 359–373. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans regia L.) Fruit and Tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. C. R. Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2018, 48, 962–982. [Google Scholar] [CrossRef] [Green Version]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of value-added components from food industry based and agro-forest biowastes by deep eutectic solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Baranauskaitė, J.; Jakštas, V.; Ivanauskas, L.; Kopustinskienė, D.M.; Drakšienė, G.; Masteikova, R.; Bernatonienė, J. Optimization of carvacrol, rosmarinic, oleanolic and ursolic acid extraction from oregano herbs (Origanum onites L., Origanum vulgare spp. hirtum and Origanum vulgare L.). Nat. Prod. Res. 2016, 30, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.D.L.; Da Silva, H.D.T.; Blanes, L.; Doble, P.A.; Tavares, M.F.M.; Dobb, P. Optimization of chemometric approaches for the extraction of isorhamnetin-3-O-rutinoside from Calendula officinalis L. J. Pharm. Biomed. Anal. 2016, 125, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Stojiljković, D.; Arsić, I.; Tadić, V. Extracts of wild apple fruit (Malus sylvestris (L.) Mill., Rosaceae) as a source of antioxidant substances for use in production of nutraceuticals and cosmeceuticals. Ind. Crop. Prod. 2016, 80, 165–176. [Google Scholar] [CrossRef]
- Manconi, M.; Marongiu, F.; Manca, M.L.; Caddeo, C.; Sarais, G.; Cencetti, C.; Pucci, L.; Longo, V.; Bacchetta, G.; Fadda, A.M. Nanoincorporation of bioactive compounds from red grape pomaces: In vitro and ex vivo evaluation of antioxidant activity. Int. J. Pharm. 2017, 523, 159–166. [Google Scholar] [CrossRef]
- Jimtaisong, A.; Krisdaphong, P. Antioxidant Activity of Pandanus amaryllifolius Leaf and Root Extract and its Application in Topical Emulsion. Trop. J. Pharm. Res. 2013, 12, 425–431. [Google Scholar] [CrossRef]
- Lahucky, R.; Nuernberg, K.; Kovac, L.; Bucko, O.; Nuernberg, G. Assessment of the antioxidant potential of selected plant extracts—In vitro and in vivo experiments on pork. Meat Sci. 2010, 85, 779–784. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Bujak, T.; Nizioł-Łukaszewska, Z.; Antosiewicz, B.; Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K. Stevia Rebaudiana Bert. Leaf Extracts as a Multifunctional Source of Natural Antioxidants. Molecules 2015, 20, 5468–5486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chulasiri, M.; Wanaswas, P.; Sriaum, D.; Nakamat, S.; Wongkrajang, Y.; Kongsaktrakoon, B.; Phornchirasilp, S.; Songchitsomboon, S.; Leelarungrayub, D. Utilizing hydroglycolic extract from myrobalan fruits to counteract reactive oxygen species. Int. J. Cosmet. Sci. 2011, 33, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Tubtimdee, C.; Shotipruk, A. Extraction of phenolics from Terminalia chebula Retz with water–ethanol and water–propylene glycol and sugaring-out concentration of extracts. Sep. Purif. Technol. 2011, 77, 339–346. [Google Scholar] [CrossRef]
- Lavaud, A.; Laguerre, M.; Birtic, S.; Fabiano Tixier, A.S.; Roller, M.; Chemat, F.; Bily, A.C. Eutectic Extraction Solvents, Extraction Methods by Eutectigenesis Using Said Solvents, and Extracts Derived from Said Extraction Methods. International Publication Number WO 2016/162703, 13 October 2016. [Google Scholar]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimisation of polyphenol extraction from Hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology. J. Appl. Res. Med. Aromat. Plants 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Apostolakis, A.; Grigorakis, S.; Makris, D.P. Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Sep. Purif. Technol. 2014, 128, 89–95. [Google Scholar] [CrossRef]
- Philippi, K.; Tsamandouras, N.; Grigorakis, S.; Makris, D.P. Ultrasound-Assisted Green Extraction of Eggplant Peel (Solanum melongena) Polyphenols Using Aqueous Mixtures of Glycerol and Ethanol: Optimisation and Kinetics. Environ. Process. 2016, 3, 369–386. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Aalim, H.; Limwachiranon, J.; Li, L.; Duan, Z.; Ren, G.; Luo, Z. Green recovery of phenolic compounds from rice byproduct (rice bran) using glycerol based on viscosity, conductivity and density. Int. J. Food Sci. Technol. 2018, 54, 1363–1371. [Google Scholar] [CrossRef]
- Kim, J.-M.; Chang, S.-M.; Kim, I.-H.; Kim, Y.E.; Hwang, J.-H.; Kim, K.-S.; Kim, W.-S. Design of optimal solvent for extraction of bio-active ingredients from mulberry leaves. Biochem. Eng. J. 2007, 37, 271–278. [Google Scholar] [CrossRef]
- European Commission Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products (2006/257/EC). Off. J. Eur. Union 2006, 1–528.
- Foti, C.; Bonamonte, D.; Cassano, N.; Conserva, A.; Vena, G.A. Allergic contact dermatitis to propyl gallate and pentylene glycol in an emollient cream. Australas. J. Dermatol. 2010, 51, 147–148. [Google Scholar] [CrossRef]
- Kerre, S. Allergic contact dermatitis to pentylene glycol in a cosmetic cream. Contact Dermat. 2008, 58, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.; Viflizzo, G.; Vecchio, F.; Parodi, A. Allergic contact dermatitis from pentilene glycol in an emolient cream, with possible co-sensitization to resveratrol. Contact Dermatitis 2003, 48, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jia, W.; Zhang, Y.; Tan, F.; Zhang, J. Synergistic effect of 1,4-cyclohexanediol and 1,2-hexanediol on percutaneous absorption and penetration of metronidazole. Int. J. Pharm. 2011, 415, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Verevkin, S.P. Determination of vapor pressures and enthalpies of vaporization of 1,2-alkanediols. Fluid Phase Equilibria 2004, 224, 23–29. [Google Scholar] [CrossRef]
- Mokbel, I.; Sawaya, T.; Zanota, M.-L.; Naccoul, R.A.; Jose, J.; De Bellefon, C. Vapor–Liquid Equilibria of Glycerol, 1,3-Propanediol, Glycerol + Water, and Glycerol + 1,3-Propanediol. J. Chem. Eng. Data 2012, 57, 284–289. [Google Scholar] [CrossRef]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef] [Green Version]
- Vieira, V.; Prieto, M.; Barros, L.; Coutinho, J.A.; Ferreira, I.C.; Ferreira, M.O.A.S. Enhanced extraction of phenolic compounds using choline chloride based deep eutectic solvents from Juglans regia L. Ind. Crop. Prod. 2018, 115, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Vieira, V.; Pereira, C.; Pires, T.C.; Calhelha, R.C.; Alves, M.J.; Ferreira, M.O.A.S.; Barros, L.; Ferreira, I.C. Phenolic profile, antioxidant and antibacterial properties of Juglans regia L. (walnut) leaves from the Northeast of Portugal. Ind. Crop. Prod. 2019, 134, 347–355. [Google Scholar] [CrossRef]
- Vieira, V.; Prieto, M.; Barros, L.; Coutinho, J.A.P.; Ferreira, M.O.A.S.; Ferreira, I.C. Optimization and comparison of maceration and microwave extraction systems for the production of phenolic compounds from Juglans regia L. for the valorization of walnut leaves. Ind. Crop. Prod. 2017, 107, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.-H.; Jiang, Z.-T.; Liu, T.; Li, R. Flavonoids in Juglans regia L. Leaves and Evaluation of In Vitro Antioxidant Activity via Intracellular and Chemical Methods. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Amaral, J.S.; Seabra, R.; Andrade, P.B.; Valentão, P.; Pereira, J.A.; Ferreres, F. Phenolic profile in the quality control of walnut (Juglans regia L.) leaves. Food Chem. 2004, 88, 373–379. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Lin, L.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Wan, R.; Wang, P.; Huo, W.; Dong, H.; Du, Q. Toxicity of imidazoles ionic liquid [C16mim]Cl to Hela cells. Ecotoxicol. Environ. Saf. 2018, 162, 408–414. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomasevic, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Hayyan, M.; Mbous, Y.P.; Looi, C.Y.; Wong, W.F.; Hayyan, A.; Salleh, M.Z.M.; Mohd-Ali, O. Natural deep eutectic solvents: Cytotoxic profile. SpringerPlus 2016, 5, 913. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, W.; Jorge, T.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; De Almeida, T.S.; Araújo, M.E.M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef]
- Abreu, R.; Ferreira, I.C.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rached, W.; Zeghada, F.Z.; Bennaceur, M.; Barros, L.; Calhelha, R.C.; Heleno, S.; Alves, M.; Carvalho, A.M.; Marouf, A.; Ferreira, I.C. Phytochemical analysis and assessment of antioxidant, antimicrobial, anti-inflammatory and cytotoxic properties of Tetraclinis articulata (Vahl) Masters leaves. Ind. Crop. Prod. 2018, 112, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.; Dueñas, M. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; De Souza, A.H.P.; Calhelha, R.C.; Barros, L.; Glamočlija, J.; Sokovic, M.; Peralta, R.M.; Bracht, A.; Ferreira, I.C. Bioactive formulations prepared from fruiting bodies and submerged culture mycelia of the Brazilian edible mushroom Pleurotus ostreatoroseus Singer. Food Funct. 2015, 6, 2155–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Plant Material | Origin | Solvent | Technique | Main Compounds | Bioactivities | References |
|---|---|---|---|---|---|---|
| Glycerol-based solvents | ||||||
| Artemisia arborescens L. Artemisia inculta Delile (aerial parts) | Greece | Glycerol + water (90%, w/v) | Stirring + heating | Total phenolic content Phenolic acids Flavonoids Others | Antioxidant Ferric reducing power DPPH | [27] |
| Hypericum perforatum L. (aerial parts) | Germany | Glycerol + water (10%, w/v) | Stirring + heating | Total phenolic content Phenolic acids Flavonols | Antioxidant Ferric reducing power | [26] |
| Olea europaea L. (leaves) | Greece | Glycerol + water (9.3%, w/v) | Stirring + heating | Total phenolic content Flavonoids Others | - | [28] |
| Origanum onites L. Origanum vulgare spp. hirtum Origanum vulgare L. (herbs) | Lithuania Turkey | Glycerol + ethanol (80–100%, v/v) Ethanol + water (30–96%, v/v) Methanol | UAE Heat-reflux Stirring Maceration Percolation | Rosmarinic acid Others | - | [16] |
| Oryza sativa L. (rice bran) | China | Glycerol + water (19.47%) | Shaking + heating | Total phenolic content Phenolic acids | - | [30] |
| Solanum melongena L. (peels) | Greece | Glycerol + water (90%, w/v) Ethanol + water (40%, v/v) | UAE | Total phenolic content Phenolic acids Flavonoids | Antioxidant Ferric reducing power DPPH | [29] |
| Propylene-glycol-based solvents | ||||||
| Calendula officinalis L. (flowers) | Brazil | Propylene glycol + ethanol + water (40% + 40% + 20%, v/v/v) | Shaking | Phenolic acids Flavonoids | - | [17] |
| Mellissa officinalis L. Origanum vulgaris L. Salvia officinalis L. (herbs) | Slovakia | Ethanol + propylene glycol | Commercial extracts | Total phenolic content | Antioxidant ABTS TBARS | [21] |
| Malus sylvestris (L.) Mill. (wild fruit) | Serbia | Ethanol + water (70%, v/v) Propylene glycol + water (45%, w/w) Propylene glycol + water (8%, w/w) Water | Maceration Percolation Soxhlet Ultrasonic | Total phenolic content Total flavonoids Total tannins | Antioxidant DPPH Ferric reducing power Inhibition of linoleic acid oxidation | [18] |
| Origanum onites L. Origanum vulgare spp. hirtum Origanum vulgare L. (herbs) | Lithuania Turkey | Propylene glycol + ethanol (70–90%, v/v) Ethanol + water (30–96%, v/v) Methanol | UAE Heat-reflux Stirring Maceration Percolation | Rosmarinic acidOthers | - | [16] |
| Pandanus amaryllifolius Roxb. (leaf and root) | Thailand | Propylene glycol Ethanol (95%) Propylene glycol + ethanol (1:4 and 1:1, v/v) | Maceration | Total phenolic content | Antioxidant DPPH Linoleic acid emulsion–thiocyanate method | [20] |
| Stevia rebaudiana Bert. (leaf) | Poland | Propylene glycol + water (4:1) Ethanol (96%) | Stirring | Total phenolic content Phenolic acids Flavonoids | Antioxidant DPPH ABTS Ferric reducing power Cytotoxicity CRL-2522 | [22] |
| Terminalia chebula Retz (dried fruits) | Thailand | Ethanol + water (76.4%, v/v) 1 Propylene glycol + water (36%, v/v) | Reflux | Total phenolic content Gallic acid Ellagic acid | Antioxidant ABTS | [24] |
| Terminalia cheubula Retz (fruits) | Thailand | Ethanol + water (30%, 50%, 70%, and 100%) Propylene glycol + water (30%, 50%, 70%, and 100%) | Maceration | Total phenolic content | Antioxidant DPPH H2O2 inhibition AAPH-induced haemolysis ABTS Photochemiluminescence | [23] |
| Vitis vinifera L. (pomace) | Italy | Propylene glycol + ethanol (1:1 and 1:3, v/v) | Stirring | Total phenolic content Gallic acid Flavonoids | Antioxidant DPPH AAPH-induced haemolysis | [19] |
| Ethylene glycol-based solvents | ||||||
| Morus alba L. (leaf) | Korea | Ethylene glycol + water (25%, 42%, and 58%) Acetone + water (47% and 57%) Acetone + methanol (27%) | Heat extraction | Total phenolic content | Antioxidant DPPH | [31] |
| Solvent | 3-O-Caffeoylquinic Acid | trans 3-p-Coumaroylquinic Acid | Quercetin 3-O-glucoside | Quercetin O-pentoside | Total HPLC |
|---|---|---|---|---|---|
| (mg/g Dry Plant) | (mg/g Dry Plant) | (mg/g Dry Plant) | (mg/g Dry Plant) | (mg/g Dry Plant) | |
| water | 5.16 ± 0.06b | 1.07 ± 0.03d | 4.3 ± 0.2h | 3.59 ± 0.07h | 14.1 ± 0.3g |
| ethanol | 4.52 ± 0.01d | 1.23 ± 0.02b | 11.7 ± 0.1c | 10.32 ± 0.06c | 27.8 ± 0.1b |
| 1,2-ethanediol | 5.79 ± 0.08a | 1.36 ± 0.02a | 12.6 ± 0.1b | 10.73 ± 0.08b | 30.5 ± 0.2a |
| 1,2-propanediol | 4.96 ± 0.06c | 1.14 ± 0.01c | 11.3 ± 0.2d | 9.8 ± 0.2d | 27.2 ± 0.4c |
| 1,3-propanediol | 5.30 ± 0.04b | 1.22 ± 0.04b | 11.1 ± 0.1d | 9.6 ± 0.07de | 27.3 ± 0.3c |
| 1,3-butanediol | 4.46 ± 0.01d | 1.03 ± 0.01d | 9.85 ± 0.2f | 8.6 ± 0.2f | 23.9 ± 0.3e |
| 1,2-pentanediol | 1.30 ± 0.02g | 0.300 ± 0.002g | 13.0 ± 0.3a | 11.45 ± 0.2a | 26.0 ± 0.5d |
| 1,5-pentanediol | 4.47 ± 0.07d | 1.22 ± 0.01b | 10.75 ± 0.2e | 9.5 ± 0.1e | 25.9 ± 0.4d |
| 1,2-hexanediol | 3.00 ± 0.05f | 0.8 ± 0.03f | 1.93 ± 0.04i | nd | 5.73 ± 0.09h |
| glycerol | 4.3 ± 0.1e | 0.94 ± 0.04e | 6.98 ± 0.2g | 6.1 ±0.1g | 18.3 ± 0.4f |
| Solvent | HeLa | PLP2 | ||
|---|---|---|---|---|
| Extract (µg/mL) | Solvent (%, v/v) | Extract (µg/mL) | Solvent (%, v/v) | |
| Water | >500 | >4 | >500 | >4 |
| Ethanol | 245 ± 14b | >4 | >500 | >4 |
| 1,2-ethanediol | 97 ± 10e | >4 | 142 ± 5b | >4 |
| 1,2-propanediol | 292 ± 24a | >4 | >500 | >4 |
| 1,3-propanediol | 216 ± 10c | >4 | >500 | >4 |
| 1,3-butanediol | 257 ± 12b | >4 | >500 | >4 |
| 1,2-pentanediol | 151 ± 12d | 0.63 ± 0.04a | 232 ± 13a | 0.89 ± 0.04a |
| 1,5-pentanediol | 212 ± 4c | 0.49 ± 0.02b | 141 ± 3b | 0.59 ± 0.02b |
| 1,2-hexanediol | 37 ± 2f | 0.36 ± 0.02c | 48 ± 5b | 0.285 ± 0.005c |
| Glycerol | 88 ± 4e | >4 | 143 ± 5b | >4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, V.; Calhelha, R.C.; Barros, L.; Coutinho, J.A.P.; C. F. R. Ferreira, I.; Ferreira, O. Insights on the Extraction Performance of Alkanediols and Glycerol: Using Juglans regia L. Leaves as a Source of Bioactive Compounds. Molecules 2020, 25, 2497. https://doi.org/10.3390/molecules25112497
Vieira V, Calhelha RC, Barros L, Coutinho JAP, C. F. R. Ferreira I, Ferreira O. Insights on the Extraction Performance of Alkanediols and Glycerol: Using Juglans regia L. Leaves as a Source of Bioactive Compounds. Molecules. 2020; 25(11):2497. https://doi.org/10.3390/molecules25112497
Chicago/Turabian StyleVieira, Vanessa, Ricardo C. Calhelha, Lillian Barros, João A. P. Coutinho, Isabel C. F. R. Ferreira, and Olga Ferreira. 2020. "Insights on the Extraction Performance of Alkanediols and Glycerol: Using Juglans regia L. Leaves as a Source of Bioactive Compounds" Molecules 25, no. 11: 2497. https://doi.org/10.3390/molecules25112497
APA StyleVieira, V., Calhelha, R. C., Barros, L., Coutinho, J. A. P., C. F. R. Ferreira, I., & Ferreira, O. (2020). Insights on the Extraction Performance of Alkanediols and Glycerol: Using Juglans regia L. Leaves as a Source of Bioactive Compounds. Molecules, 25(11), 2497. https://doi.org/10.3390/molecules25112497