Roles of Silk Fibroin on Characteristics of Hyaluronic Acid/Silk Fibroin Hydrogels for Tissue Engineering of Nucleus Pulposus
Abstract
1. Introduction
2. Materials and Methods
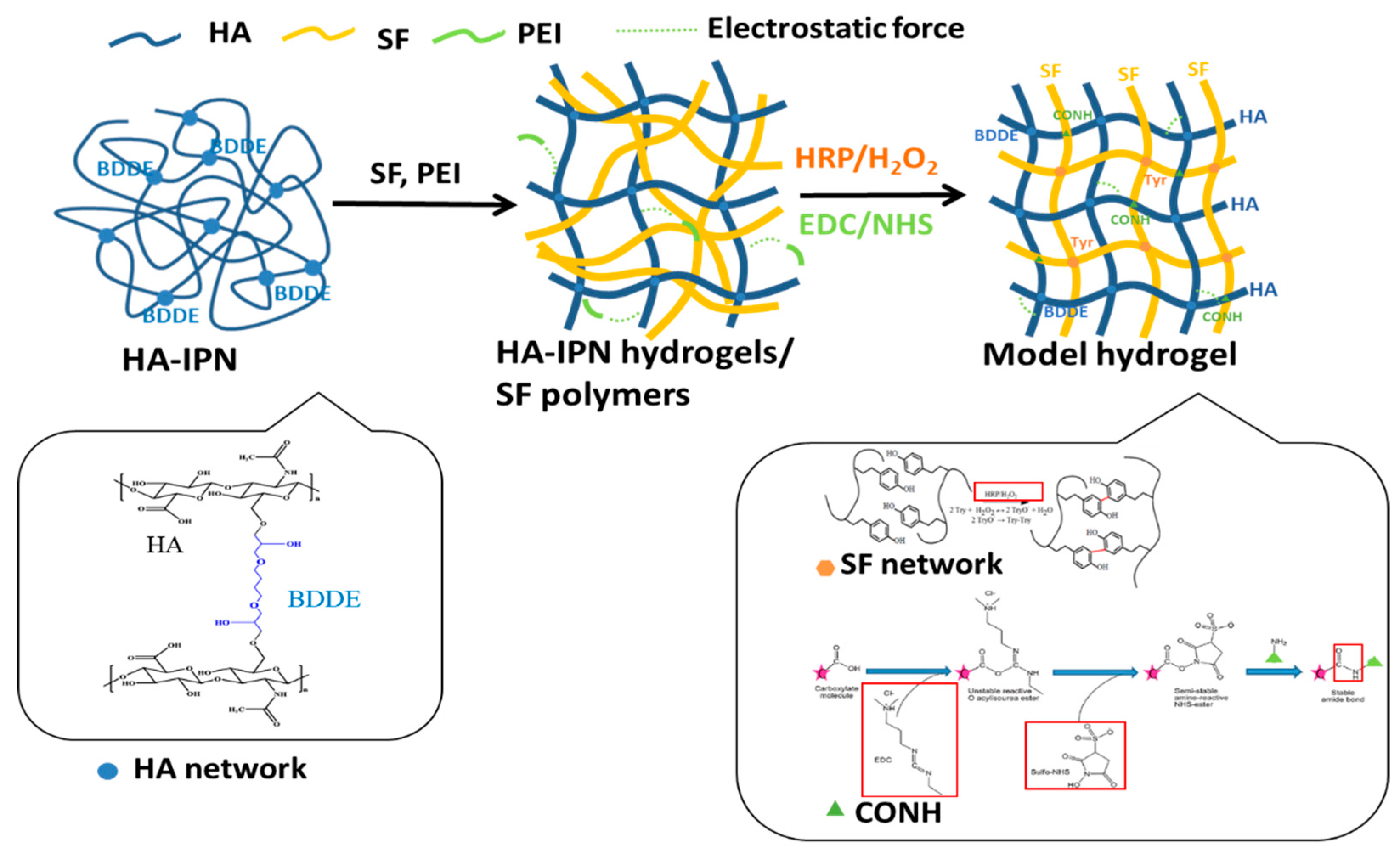
2.1. Fabrication of Interpenetrating Network HA-SF Hydrogels
2.2. Characterizations of HS-IPN Hydrogels
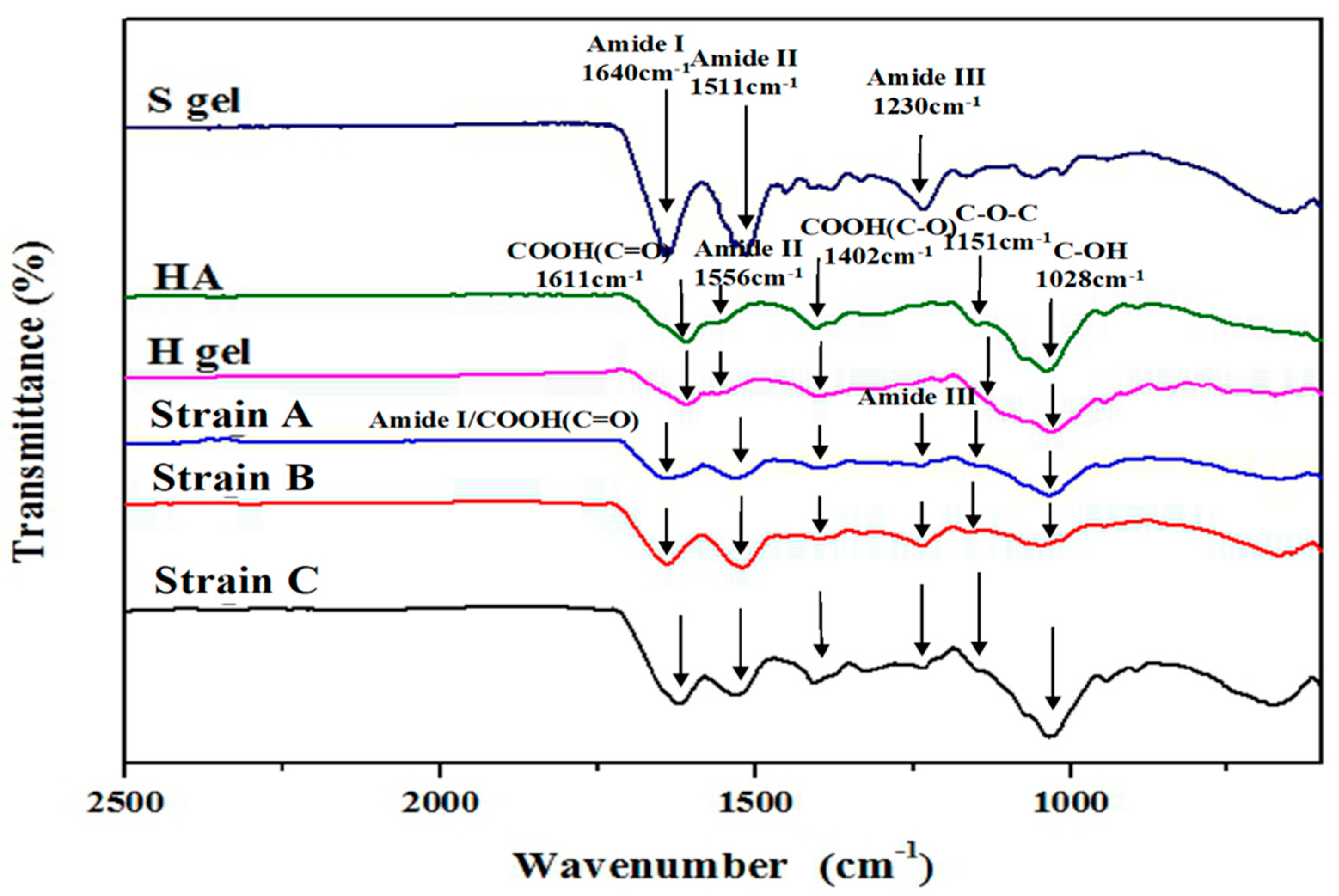
2.2.1. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectra of the Hydrogels
2.2.2. Swelling Ratios of H, S, Crosslinked H/PEI and the Model Hydrogels
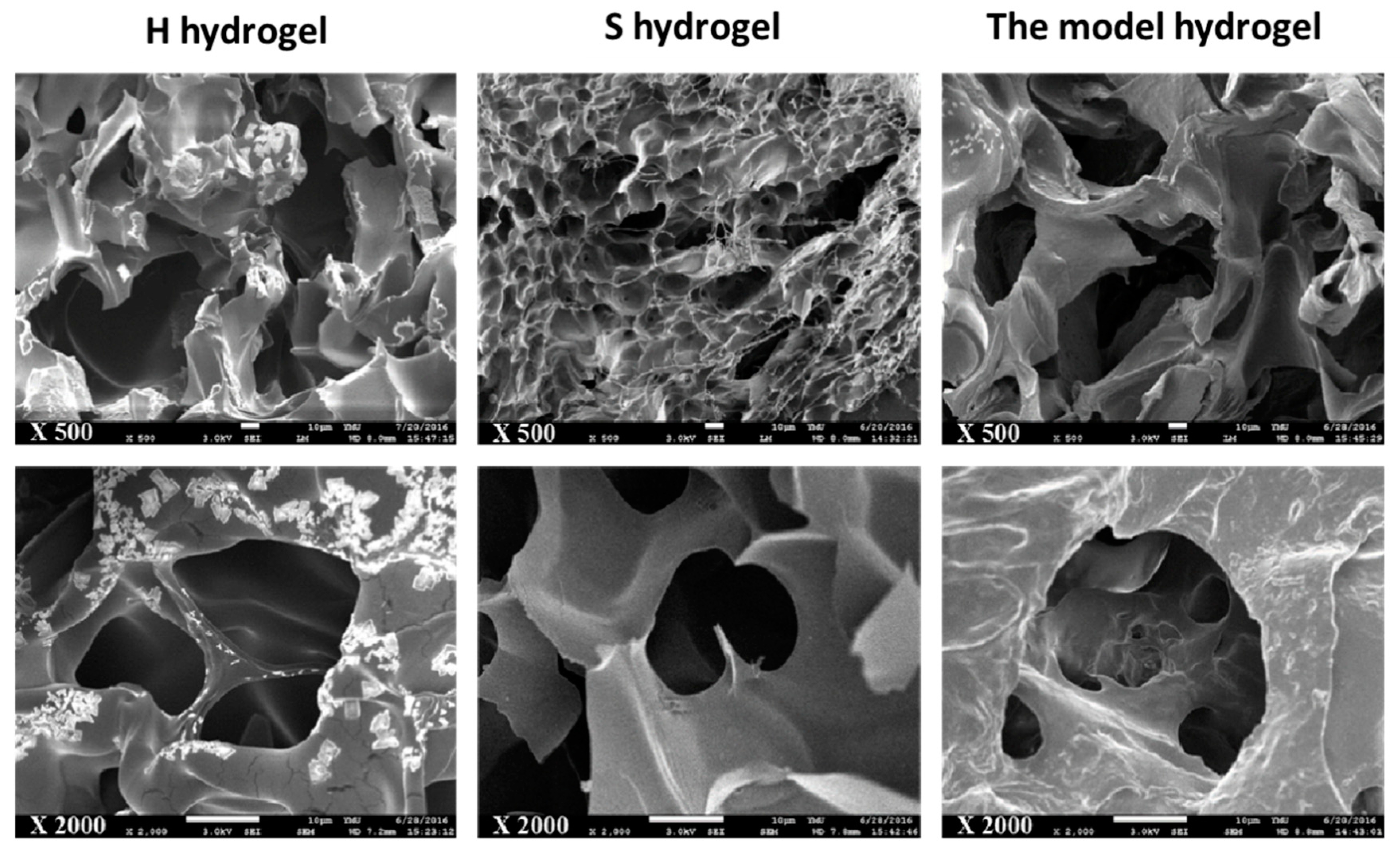
2.2.3. Morphology of HS-IPN Hydrogels
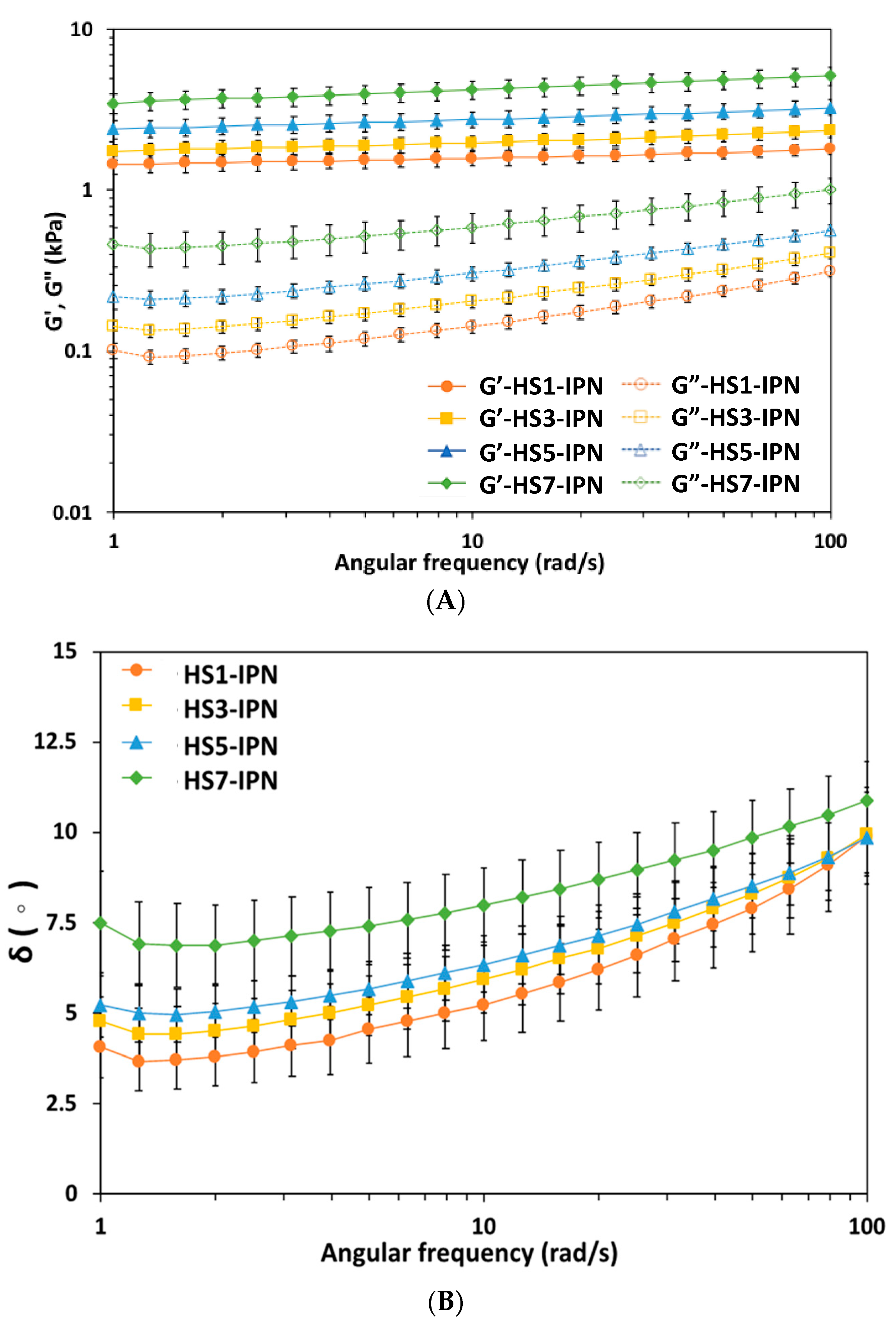
2.3. Rheological Studies of Vary Compositions of HS-IPN Hydrogels
Compressive Modules of the Model Hydrogels
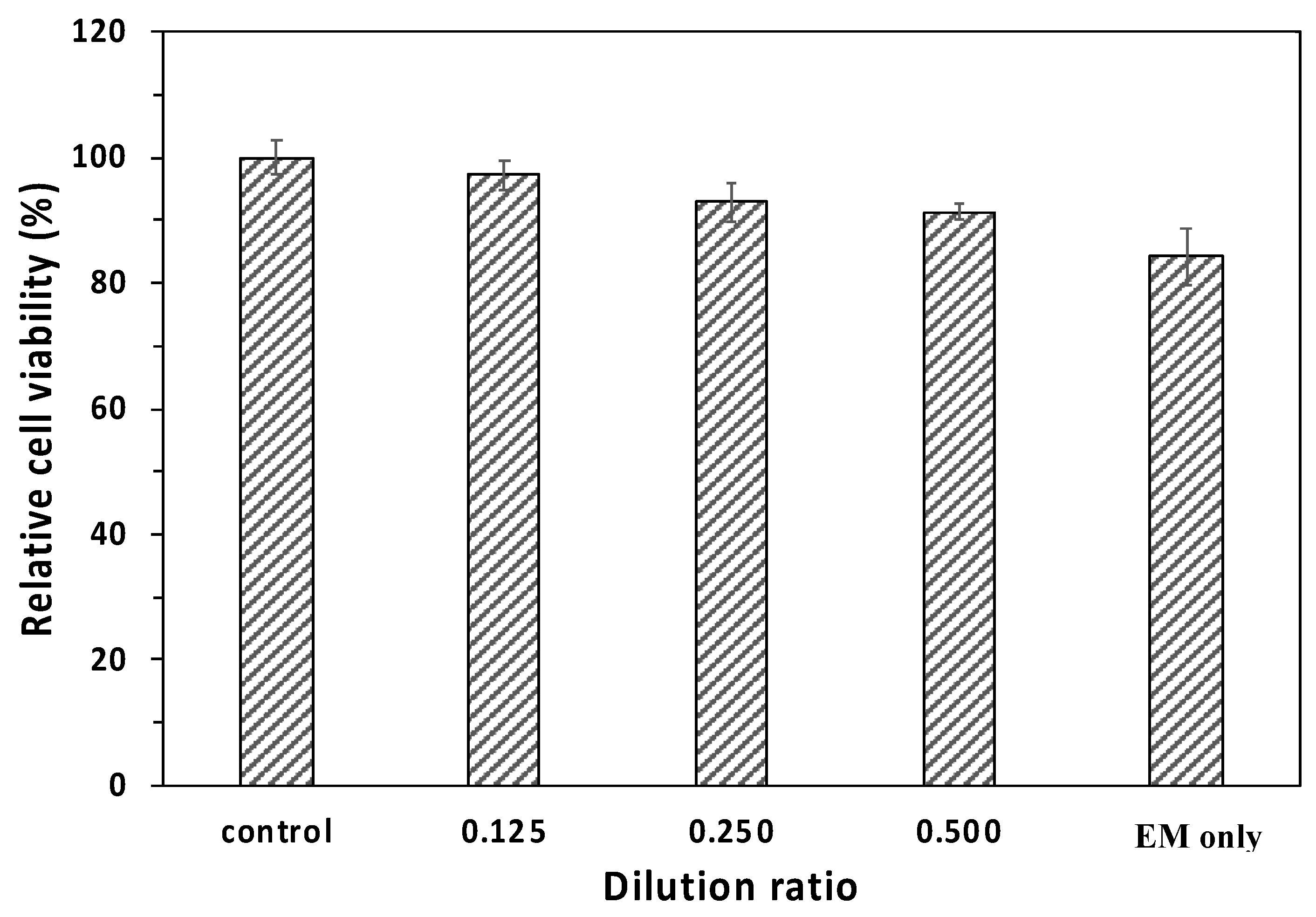
2.4. Cytotoxicity of the Model Hydrogels
2.5. Differentiations of Induced hBMSC to NP in the Model Hydrogels
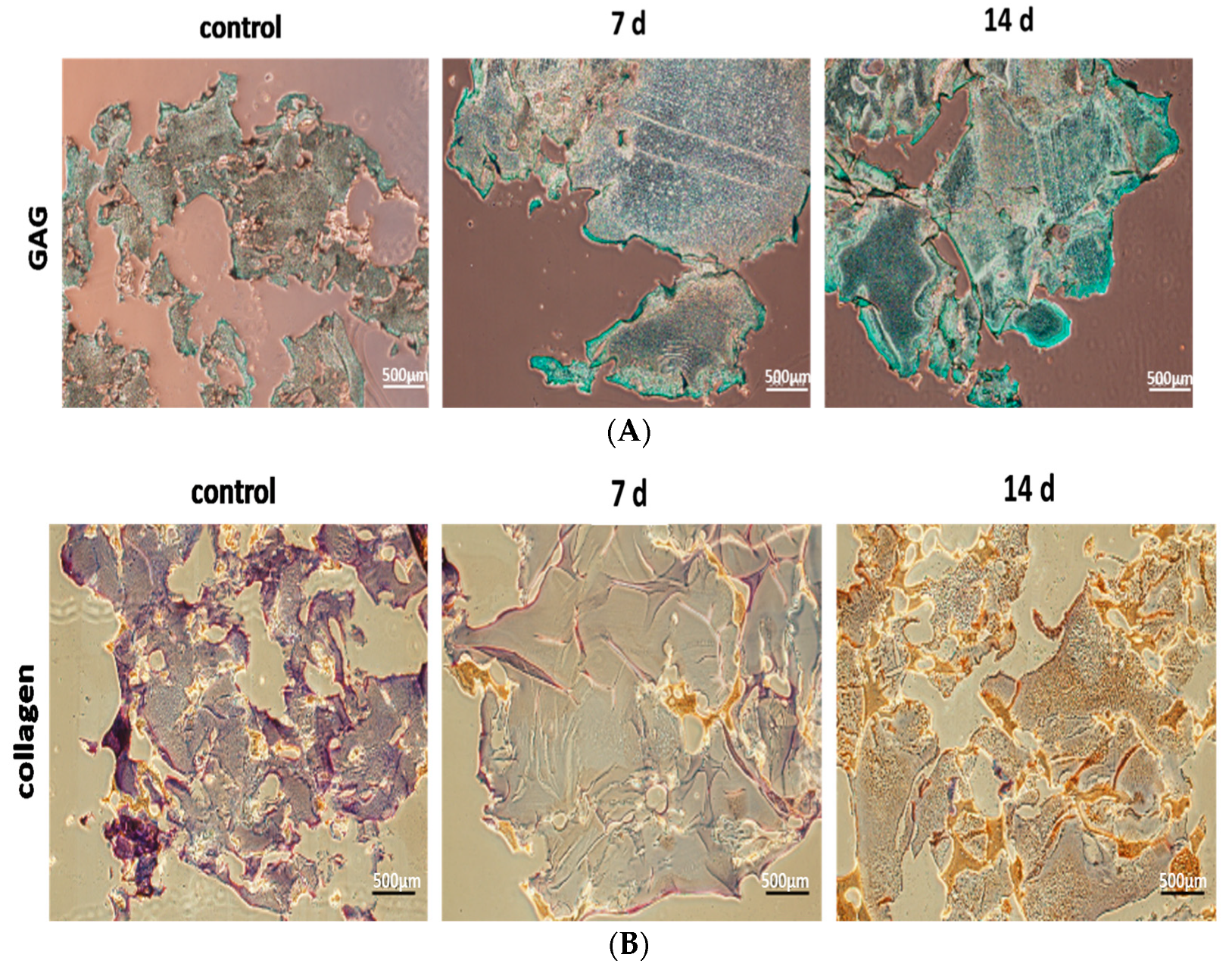
2.5.1. Immuno-Histochemical (IHC) Analysis of Specific Protein Expressions of the Differentiations of Induced hBMSC to NP
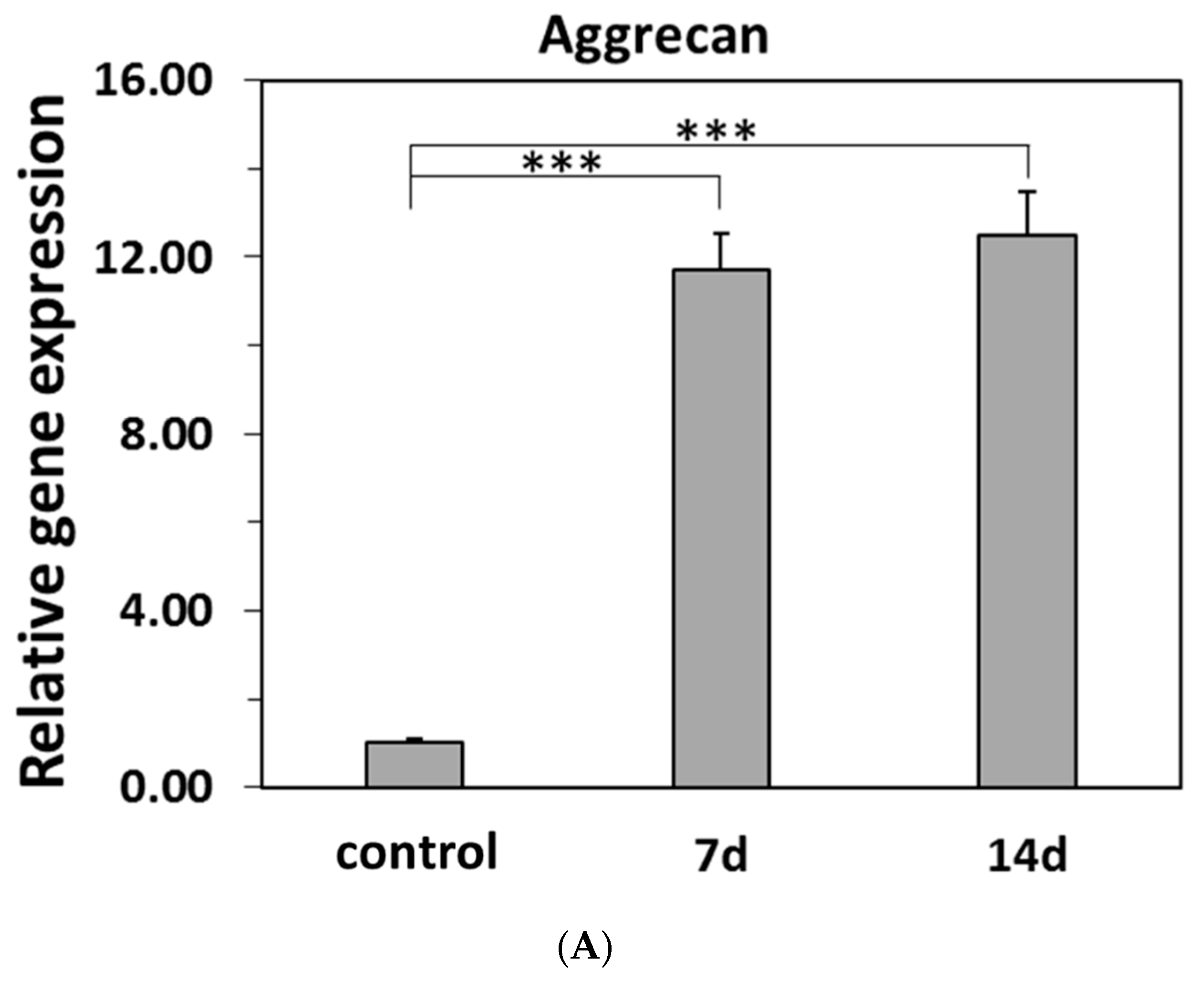
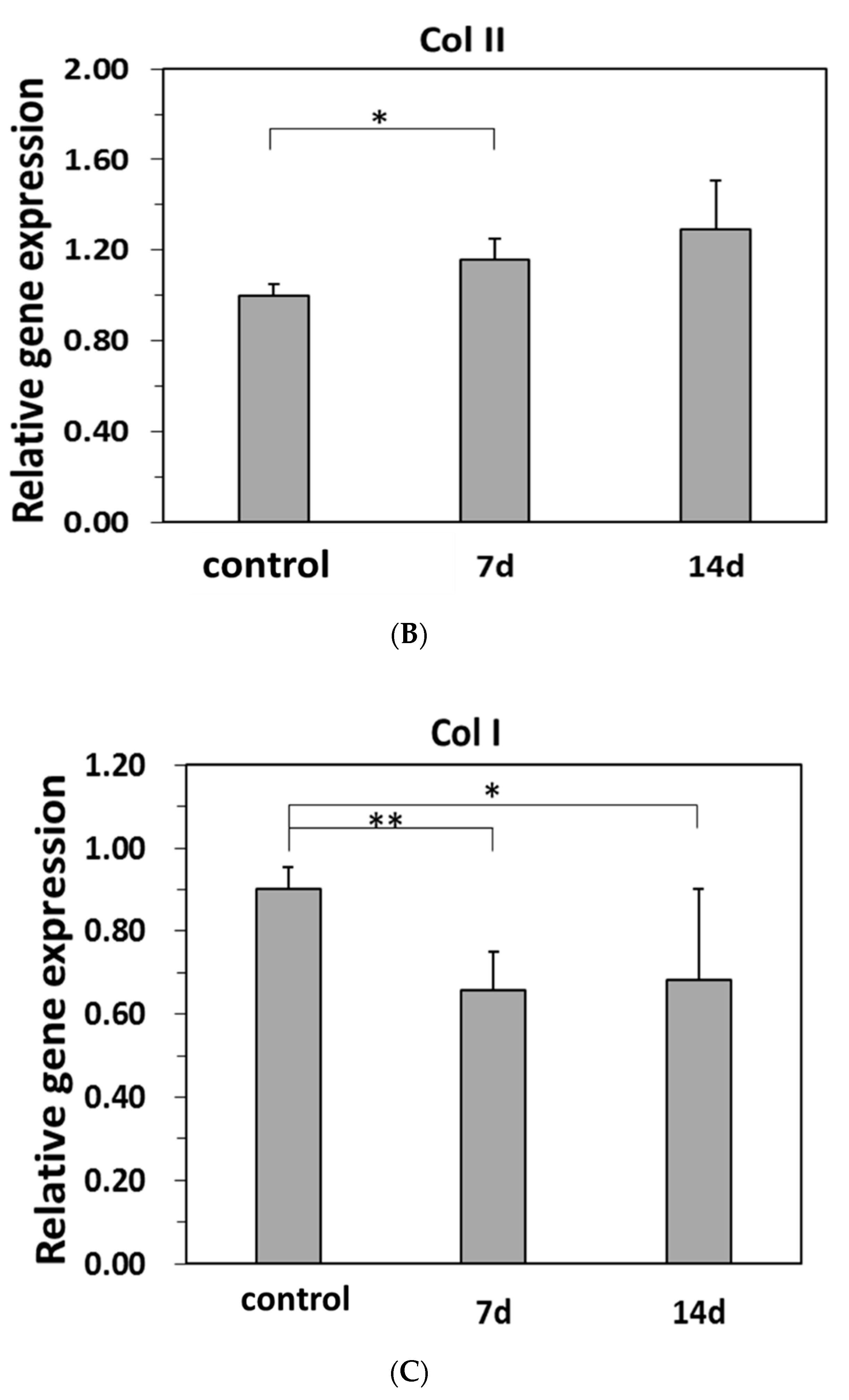
2.5.2. Real-Time PCR for Specific Gene Expressions of the Differentiations of Induced hBMSC to NP
2.6. Statistics
3. Results and Discussion
3.1. Fabricating Fluidity HA Hydrogels by Adjusting Parameters of HA, and Reactions Conditions of BDDE Crosslinking Reactions
Using HRP/H2O2 Reactions to Crosslink SF and Producing HS-IPN Hydrogels
3.2. Characterizations of HS-IPN Hydrogels
3.2.1. ATR-FTIR Spectra of HA, SF, and the Model Hydrogels Consisted of Varying Strains of SF
3.2.2. The Pore Structures of Scaffolds and Swelling Ratios for H, S and the Model Hydrogels
3.3. Varying Strains of SF Influenced the Rheological Properties of HS-IPN Hydrogels
Influence of the Weight Ratios of SF to HA in HS-IPN Hydrogels on Rheological Properties of Hydrogels
3.4. Confined Compressive Modules of the Model Hydrogels
3.5. Cytotoxicity Examinations for the Model Hydrogels
3.6. Differentiations of hBMSC to NP Cells in the Model Hydrogels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Nomenclature | Full name |
| HA | Hyaluronic acid |
| SF | Silk fibroin |
| H gel | HA hydrogel |
| S gel | SF hydrogel |
| HS1-IPN hydrogel | HA/SF hydrogel with weight ratios of HA to SF was 5:1 |
| HS3-IPN hydrogel | HA/SF hydrogel with weight ratios of HA to SF was 5:3 |
| HS5-IPN hydrogel | HA/SF hydrogel with weight ratios of HA to SF was 5:5 |
| HS7-IPN hydrogel (the model hydrogel) | HA/SF hydrogel with weight ratios of HA to SF was 5:7 |
References
- Higuchi, A.; Ling, Q.D.; Chang, Y.; Hsu, S.T.; Umezawa, A. Physical cues of biomaterials guide stem cell differentiation fate. Chem. Rev. 2013, 113, 3297–3328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, X.; Fan, D.; Zhu, C.; Deng, J.; Hui, J.; Ma, P. Synthesis and characterization of hyaluronic acid/human-like collagen hydrogels. Mater. Sci. Eng. C 2014, 43, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.P.J.; Cheng, B.; Roffler, S.R.; Lundy, D.J.; Yen, C.Y.T.; Chen, P.; Lai, J.J.; Pun, S.H.; Stayton, P.S.; Hsieh, P.C.H. Reloadable multidrug capturing delivery system for targeted ischemic disease treatment. Sci. Transl. Med. 2016, 8, 365ra160. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Ahrens, M.; Tsantrizos, A.; Donkersloot, P.; Martens, F.; Lauweryns, P.; Le Huec, J.C.; Moszko, S.; Fekete, Z.; Sherman, J.; Yuan, H.A.; et al. Nucleus replacement with the DASCOR disc arthroplasty device: Interim two-year efficacy and safety results from two prospective, non-randomized multicenter European studies. Spine 2009, 34, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Fussell, G.; Thomas, J.; Hsuan, A.; Lowman, A.; Karduna, A.; Vresilovic, E.; Marcolongo, M. Functional compressive mechanics of a PVA/PVP nucleus pulposus replacement. Biomaterials 2006, 27, 176–184. [Google Scholar] [CrossRef]
- Lanza, R.; Langer, R.; Vancati, J. Principles of Tissue Engineering, 3rd ed.; Academic Press: Burlington, VT, USA, 2007. [Google Scholar]
- Tsaryk, R.; Gloria, A.; Russo, T.; Anspach, L.; Santis, R.D.; Ghanaati, S.; Unger, R.E.; Ambrosio, L.; Kirkpatrick, C.J. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater. 2015, 20, 10–21. [Google Scholar] [CrossRef]
- Talebin, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, M.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-healing hydrogels: The next paradigm in tissue engineering. Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef]
- Yang, M.C.; Huang, Y.Y.; Wang, S.S.; Chou, N.K.; Chi, N.H.; Shieh, M.J.; Chang, Y.L.; Chung, T.W. The cardiomyogenic differentiation of rat mesenchymal stem cells on silk fibroin-polysaccharide cardiac patches in vitro. Biomaterials 2009, 30, 3757–3765. [Google Scholar] [CrossRef]
- Su, W.Y.; Chen, Y.C.; Lin, F.H. Injectable oxidized hyaluronic acid/adipic acid di-hydrazide hydrogel for nucleus pulposus regeneration. Acta Biomater. 2010, 6, 3044–3055. [Google Scholar] [CrossRef]
- Chen, Y.C.; Su, W.Y.; Yang, S.H.; Gefen, A.; Lin, F.H. In-situ forming hydrogels composed of oxidized high molecular weight hyaluronic acid and gelatin for nucleus pulposus regeneration. Acta Biomater. 2013, 9, 5181–5193. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Martin, J.T.; Elliott, D.M.; Smith, L.J.; Mauck, R.L. Phenotypic stability matrix elaboration and functional maturation of nucleus pulposus cells encapsulated in photo-crosslinkable hyaluronic acid hydrogels. Acta Biomater. 2015, 12, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Mehrali, M.; Bagherifard, S.; Akbari, M.; Thakur, A.; Mirani, B.; Mehrali, M.; Hasany, M.; Orive, G.; Das, P.; Dolatshahi-Prirouz, A. Bending electronics with human body: A pathway toward a cybernetic future. Adv. Sci. 2018, 5, 1700931. [Google Scholar] [CrossRef] [PubMed]
- Affas, S.; Schafer, F.-M.; Algarrahi, K.; Cristofaro, V.; Sullivan, P.; Yang, X.; Costa, K.; Sack, B.; Gharaee-Kermani, M.; Macoska, J.A.; et al. Augmentation cystoplasty of diseased porcine bladders with silk fibroin grafts. Tissue Eng. Part A 2019, 25, 855–866. [Google Scholar] [CrossRef]
- Chi, N.H.; Yang, M.C.; Chung, T.W.; Chen, J.Y.; Chou, N.K.; Wang, S.S. Cardiac repair achieved by bone marrow mesenchymal stem cells/silk fibroin/ hyaluronic acid patches in a rat of myocardial infarction model. Biomaterials 2012, 33, 5541–5550. [Google Scholar] [CrossRef]
- Yan, L.P.; Silva-Correia, J.; Ribeiro, V.P.; Miranda-Gonçalves, V.; Correia, C.; da Silva Morais, A.; Sousa, R.A.; Reis, R.M.; Oliveira, A.L.; Oliveira, J.M.; et al. Tumor growth suppression induced by biomimetic silk fibroin hydrogels. Sci. Rep. 2016, 6, 31037. [Google Scholar] [CrossRef]
- Gullbrand, S.E.; Schaer, T.P.; Agarwal, P.; Bendigo, J.R.; Dodge, G.R.; Chen, W.; Elliott, D.M.; Mauck, R.L.; Malhotra, N.R.; Smith, L.J. Translational of an injectable triple-interpenetrating-network hydrogels for intervertebral disc regeneration in a goat model. Acta Biomater. 2017, 60, 201–209. [Google Scholar] [CrossRef]
- Lin, H.A.; Gupta, M.S.; Varma, D.M.; Gilchrist, M.L.; Nicoll, S.B. Lower crosslinking density enhances functional nucleus pulposus-like matrix elaboration by human mesenchymal stem cells in carboxymethylcellulose hydrogels. J. Biomed. Mater. Res. Part A 2016, 104, 165–177. [Google Scholar] [CrossRef]
- Sakai, D.; Mochida, J.; Iwashina, T.; Hiyama, A.; Omi, H.; Imai, M.; Nakal, T.; Ando, K.; Hotta, T. Regenerative effects of transplanting mesenchymal stem cells embedded in aterlo-collagen to the degenerated intervertebral disc. Biomaterials 2006, 27, 335–345. [Google Scholar] [CrossRef]
- Francisco, A.T.; Mancino, R.J.; Bowle, R.D.; Brunger, J.M.; Tainter, D.M.; Chen, Y.T.; Richardson, W.R.; Guilak, F.; Setton, L.A. Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration. Biomaterials 2013, 34, 7381–7388. [Google Scholar] [CrossRef]
- Collin, E.C.; Grad, S.; Zeugolis, D.I.; Vinatier, C.S.; Clouet, J.R.; Guicheux, J.J.; Weiss, P.; Alini, M.; Pandit, A.S. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials 2011, 32, 2862–2870. [Google Scholar] [CrossRef]
- Yang, S.Q.; Wang, Q.S.; Tariq, Z.; You, R.C.; Li, X.F.; Li, M.Z.; Zhang, Q. Facile preparation of bioactive silk fibroin/hyaluronic acid hydrogels. Int. J. Biol. Macromol. 2018, 118, 775–782. [Google Scholar]
- Raia, N.R.; Partlow, B.P.; McGill, M.H.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kenne, L.; Gohil, S.; Nilsson, E.M.; Karlsson, A.; Ericsson, D.; Helander Kenne, A.; Nord, L.I. Modification and cross-linking parameters in hyaluronic acid hydrogels-definitions and analytical methods. Carbohydr. Polym. 2013, 91, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Tuin, A.; Zandstra, J.; Kluijtmans, S.G.; Bouwstra, J.B.; Harmsen, M.C.; Van Luyn, M.J. Hyaluronic acid-recombinant gelatin gels as a scaffold for soft tissue regeneration. Eur. Cell Mater. 2012, 24, 320–330. [Google Scholar] [CrossRef]
- Su, D.; Yao, M.; Liu, J.; Zhong, Y.; Chen, X.; Shao, Z. Enhancing mechanical properties of silk fibroin hydrogel through restricting the growth of β-sheet domains. ACS Appl. Mater. Interfaces 2017, 9, 17489–17498. [Google Scholar] [CrossRef]
- Iatridis, J.C.; Weidenbaum, M.; Setton, L.A.; Mow, V.C. Is the nucleus pulposus a solid or a fluid? mechanical behaviors of the nucleus pulposus of the human intervertebral disc. Spine 1996, 10, 1174–1184. [Google Scholar] [CrossRef]
- Lee, P.C.; Zan, B.S.; Chen, L.T.; Chung, T.W. Multi- functional PLGA-based Nanoparticles as a Controlled-Release Drug Delivery System for Antioxidant and Therapy. Int. J. Nanomed. 2019, 14, 1533–1549. [Google Scholar] [CrossRef]
- Choi, S.C.; Yoo, M.A.; Lee, S.Y.; Lee, H.J.; Son, D.H.; Jung, J.; Noh, I.; Kim, C.W. Modulation of biomechanical properties of hyaluronic acid hydrogels by crosslinking agents. J. Biomed. Mater. Res. A 2015, 103, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, E.M.; Hu, X.; Finley, V.; Kuo, C.K.; Kaplan, D.L. Effect of silk protein processing on drug delivery from silk films. Macromol. Biosci. 2013, 13, 311–320. [Google Scholar] [CrossRef]
- Partlow, B.P.; Hanna, C.W.; Rnjas-Kovacina, J.; Moreau, J.E.; Applegate, M.B.; Burke, K.A.; Marelli, B.; Mitropoulos, A.N.; Omenetto, F.G.; Kaplan, D.L. Highly tunable elastomeric silk biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.Q.; Qu, X.H.; Li, J.L.; Chen, L.; Tan, Y.F.; Li, J.; Li, B.; Liao, X.L. Synthesis and characterization of cell-laden double-network hydrogels based on silk fibroin and methacrylated hyaluronic acid. Eur. J. Polym. 2019, 118, 382–392. [Google Scholar] [CrossRef]
- Bian, L.; Zhai, D.Y.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. Enhanced MSC chondrogenesis following delivery of TGF-“β“ 3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011, 32, 6425–6434. [Google Scholar] [CrossRef] [PubMed]

| Materials | Viscoelastic Properties (0.01 Rad, 10 rad/s) | |||
|---|---|---|---|---|
| G′ (kPa) | G″ (kPa) | |G*| (kPa) | δ (°) | |
| Strain A | 4.09 ± 0.32 ** | 0.59 ± 0.16 | 4.13 ± 0.34 ** | 8.14 ± 1.66 |
| Strain B | 3.24 ± 0.16 | 0.50 ± 0.05 | 3.27 ± 0.16 | 8.69 ± 0.5 |
| Strain C | 3.40 ± 0.19 | 0.43 ± 0.07 | 3.43 ± 0.18 | 7.28 ± 1.19 |
| The Intensity of Blue Fluorescence of SF Hydrogels Consisted of Vary Strains of SF with Their Tyrosine Contents | ||
|---|---|---|
| Sample | OD Value | Tyrosine Content |
| Strain A | 0.80 ± 0.01 ** | 277 ± 11 ** |
| Strain B | 0.67 ± 0.01 | 255 ± 2 |
| Strain C | 0.67 ± 0.02 | 255 ± 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, T.-W.; Chen, W.-P.; Tai, P.-W.; Lo, H.-Y.; Wu, T.-Y. Roles of Silk Fibroin on Characteristics of Hyaluronic Acid/Silk Fibroin Hydrogels for Tissue Engineering of Nucleus Pulposus. Materials 2020, 13, 2750. https://doi.org/10.3390/ma13122750
Chung T-W, Chen W-P, Tai P-W, Lo H-Y, Wu T-Y. Roles of Silk Fibroin on Characteristics of Hyaluronic Acid/Silk Fibroin Hydrogels for Tissue Engineering of Nucleus Pulposus. Materials. 2020; 13(12):2750. https://doi.org/10.3390/ma13122750
Chicago/Turabian StyleChung, Tze-Wen, Weng-Pin Chen, Pei-Wen Tai, Hsin-Yu Lo, and Ting-Ya Wu. 2020. "Roles of Silk Fibroin on Characteristics of Hyaluronic Acid/Silk Fibroin Hydrogels for Tissue Engineering of Nucleus Pulposus" Materials 13, no. 12: 2750. https://doi.org/10.3390/ma13122750
APA StyleChung, T.-W., Chen, W.-P., Tai, P.-W., Lo, H.-Y., & Wu, T.-Y. (2020). Roles of Silk Fibroin on Characteristics of Hyaluronic Acid/Silk Fibroin Hydrogels for Tissue Engineering of Nucleus Pulposus. Materials, 13(12), 2750. https://doi.org/10.3390/ma13122750







