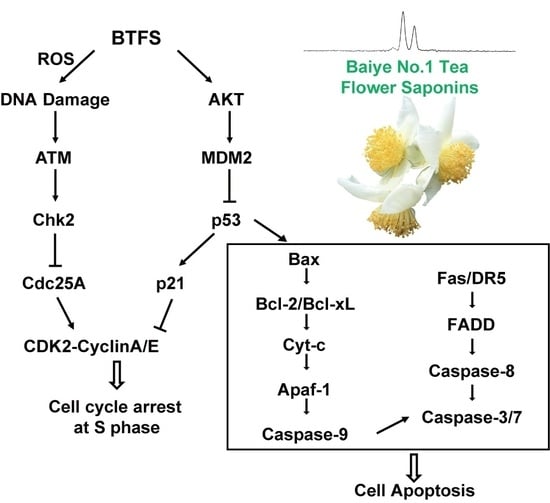
Standardized Saponin Extract from Baiye No.1 Tea (Camellia sinensis) Flowers Induced S Phase Cell Cycle Arrest and Apoptosis via AKT-MDM2-p53 Signaling Pathway in Ovarian Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Analysis and Identification of BTFS
2.2. BTFS Inhibits Ovarian Cancer Cell Proliferation
2.3. BTFS Induces Cell Cycle Arrest in the S Phase in A2780/CP70 Cells
2.4. The Effects of BTFS on Cell Cycle Regulatory Protein Expression
2.5. BTFS Activates Apoptosis in A2780/CP70 Cells
2.6. BTFS Mediates Apoptosis via Intrinsic and Extrinsic Apoptotic Pathways
2.7. BTFS Induces DNA Damage and Affects the Expression of Upstream Regulators AKT, MDM2, and P53
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction and Identification of Baiye No.1 Tea Flower Saponins (BTFS)
4.3. Cell Lines and Cell Culture
4.4. Cell Viability Assay
4.5. Colony Formation Assay
4.6. Cell Cycle Analysis by Flow Cytometry
4.7. Hoechse 33342 Staining
4.8. Evaluation of Mitochondrial Membrane Potential
4.9. Apoptosis Analysis by Flow Cytometry
4.10. Cellular Caspase Activity Assay
4.11. Detection of Intracellular ROS Production
4.12. Western Blotting
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA: Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA: Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Zhou, Q. Targeting Cyclin-Dependent Kinases in Ovarian Cancer. Cancer Investig. 2017, 35, 367–376. [Google Scholar] [CrossRef]
- D’Andrilli, G.; Kumar, C.; Scambia, G.; Giordano, A. Cell Cycle Genes in Ovarian Cancer. Clin. Cancer Res. 2004, 10, 8132. [Google Scholar] [CrossRef] [Green Version]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA: Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Zeng, J.Z.; Wong, A.S.T. Chemical Structures and Pharmacological Profiles of Ginseng Saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, D.; Ma, X.; Liu, Z.; Li, F.; Wu, D. Paris saponin VII suppressed the growth of human cervical cancer Hela cells. Eur. J. Med. Res. 2014, 19, 41. [Google Scholar] [CrossRef] [Green Version]
- Zong, J.; Wang, R.; Bao, G.; Ling, T.; Zhang, L.; Zhang, X.; Hou, R. Novel triterpenoid saponins from residual seed cake of Camellia oleifera Abel. show anti-proliferative activity against tumor cells. Fitoterapia 2015, 104, 7–13. [Google Scholar] [CrossRef]
- Xiao, X.; Zou, J.; Bui-Nguyen, T.M.; Bai, P.; Gao, L.; Liu, J.; Liu, S.; Xiao, J.; Chen, X.; Zhang, X.; et al. Paris saponin II of Rhizoma Paridis—a novel inducer of apoptosis in human ovarian cancer cells. Bioscience Trends 2012, 6, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.L.J.; He, K.; Pan, R.; Hu, Q.; Peng, B.; Liu, X. Apoptosisi induced by solanum lyratum on ovarian carcinoma cell SKOV3. World Sci. Technol. 2008, 60–63. [Google Scholar]
- Zhao, Y.Z.; Zhang, Y.Y.; Han, H.; Fan, R.P.; Hu, Y.; Zhong, L.; Kou, J.P.; Yu, B.Y. Advances in the antitumor activities and mechanisms of action of steroidal saponins. Chin. J. Nat. Med. 2018, 16, 732–748. [Google Scholar] [CrossRef]
- Koczurkiewicz, P.; Czyż, J.; Podolak, I.; Wójcik, K.; Galanty, A.; Janeczko, Z.; Michalik, M. Multidirectional effects of triterpene saponins on cancer cells—mini-review of in vitro studies. Acta Biochim. Pol. 2015, 62, 383–393. [Google Scholar] [CrossRef]
- Xu, X.H.; Li, T.; Fong, C.M.; Chen, X.; Chen, X.J.; Wang, Y.T.; Huang, M.Q.; Lu, J.J. Saponins from Chinese Medicines as Anticancer Agents. Molecules 2016, 21, 1326. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, N.; Morikawa, T.; Motai, C.; Ninomiya, K.; Okugawa, S.; Nishida, A.; Yoshikawa, M.; Muraoka, O. The Antiproliferative Effect of Chakasaponins I and II, Floratheasaponin A, and Epigallocatechin 3-O-Gallate Isolated from Camellia sinensis on Human Digestive Tract Carcinoma Cell Lines. Int. J. Mol. Sci. 2016, 17, 1979. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ren, N.; Rankin, G.O.; Li, B.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. Anti-proliferative effect and cell cycle arrest induced by saponins extracted from tea (Camellia sinensis) flower in human ovarian cancer cells. J. Funct. Foods 2017, 37, 310–321. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Yamamoto, K.; Kato, Y.; Nagatomo, A.; Matsuda, H. Floratheasaponins A-C, acylated oleanane-type triterpene oligoglycosides with anti-hyperlipidemic activities from flowers of the tea plant (Camellia sinensis). J. Nat. Prod. 2005, 68, 1360–1365. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Nakamura, S.; Kato, Y.; Matsuhira, K.; Matsuda, H. Medicinal flowers. XIV. New acylated oleanane-type triterpene oligoglycosides with antiallergic activity from flower buds of chinese tea plant (Camellia sinensis). Chem. Pharm. Bull. 2007, 55, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Shi, L.Z.; Pan, H.B.; Li, B.; Wu, Y.Y.; Tu, Y.Y. Identification of triterpenoid saponins in flowers of four Camellia Sinensis cultivars from Zhejiang province: Differences between cultivars, developmental stages, and tissues. Ind. Crop. Prod. 2017, 95, 140–147. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Shepherd, T.G.; Hoskin, D.W. Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol. Carcinog. 2017, 56, 75–93. [Google Scholar] [CrossRef]
- Jacobson, M.D. Reactive oxygen species and programmed cell death. Trends Biochem. Sci. 1996, 21, 83–86. [Google Scholar] [CrossRef]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. gammaH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Abedini, M.R.; Muller, E.J.; Bergeron, R.; Gray, D.A.; Tsang, B.K. Akt promotes chemoresistance in human ovarian cancer cells by modulating cisplatin-induced, p53-dependent ubiquitination of FLICE-like inhibitory protein. Oncogene 2010, 29, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Mahalanobish, S.; Saha, S.; Ghosh, S.; Sil, P.C. Natural products: An upcoming therapeutic approach to cancer. Food Chem. Toxicol. 2019, 128, 240–255. [Google Scholar] [CrossRef]
- Cao, S.; Norris, A.; Miller, J.S.; Ratovoson, F.; Razafitsalama, J.; Andriantsiferana, R.; Rasamison, V.E.; TenDyke, K.; Suh, T.; Kingston, D.G. Cytotoxic triterpenoid saponins of Albizia gummifera from the Madagascar rain forest. J. Nat. Prod. 2007, 70, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhao, L.; Zhang, Y.; Chen, W.; Liu, D.; Hou, H.; Ding, L.; Li, X. Ginsenoside 20(S)-Rg3 targets HIF-1α to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS ONE 2014, 9, 103887. [Google Scholar] [CrossRef]
- Nakata, H.; Kikuchi, Y.; Tode, T.; Hirata, J.; Kita, T.; Ishii, K.; Kudoh, K.; Nagata, I.; Shinomiya, N. Inhibitory effects of ginsenoside Rh2 on tumor growth in nude mice bearing human ovarian cancer cells. Jpn. J. Cancer Res. Gann 1998, 89, 733–740. [Google Scholar] [CrossRef]
- Fu, H.Z.; Wan, K.H.; Yan, Q.W.; Zhou, G.P.; Feng, T.T.; Dai, M.; Zhong, R.J. Cytotoxic triterpenoid saponins from the defatted seeds of Camellia oleifera Abel. J. Asian Nat. Prod. Res. 2018, 20, 412–422. [Google Scholar] [CrossRef]
- Jia, L.Y.; Wu, X.J.; Gao, Y.; Rankin, G.O.; Pigliacampi, A.; Bucur, H.; Li, B.; Tu, Y.Y.; Chen, Y.C. Inhibitory Effects of Total Triterpenoid Saponins Isolated from the Seeds of the Tea Plant (Camellia sinensis) on Human Ovarian Cancer Cells. Molecules 2017, 22, 1649. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.; Kim, E.; Gao, Y.; Rankin, G.O.; Li, B.; Chen, Y.C. Theaflavin-3, 3′-digallate induces apoptosis and G2 cell cycle arrest through the Akt/MDM2/p53 pathway in cisplatin-resistant ovarian cancer A2780/CP70 cells. Int. J. Oncol. 2016, 48, 2657–2665. [Google Scholar] [CrossRef] [Green Version]
- Blagosklonny, M.V. Cell immortality and hallmarks of cancer. Cell Cycle 2003, 2, 296–299. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.; Chen, Y.; Wen, L.; Yang, L.; Cui, G. Expression of connexin 32 and connexin 43 in acute myeloid leukemia and their roles in proliferation. Oncol. Lett. 2012, 4, 1003–1007. [Google Scholar] [CrossRef] [Green Version]
- Tin, M.M.; Cho, C.H.; Chan, K.; James, A.E.; Ko, J.K. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis 2007, 28, 1347–1355. [Google Scholar] [CrossRef]
- Qian, Y.; Han, Q.H.; Wang, L.C.; Guo, Q.; Wang, X.D.; Tu, P.F.; Zeng, K.W.; Liang, H. Total saponins of Albiziae Cortex show anti-hepatoma carcinoma effects by inducing S phase arrest and mitochondrial apoptosis pathway activation. J. Ethnopharmacol. 2018, 221, 20–29. [Google Scholar] [CrossRef]
- Jang, H.J.; Han, I.H.; Kim, Y.J.; Yamabe, N.; Lee, D.; Hwang, G.S.; Oh, M.; Choi, K.C.; Kim, S.N.; Ham, J.; et al. Anticarcinogenic effects of products of heat-processed ginsenoside Re, a major constituent of ginseng berry, on human gastric cancer cells. J. Agric. Food Chem. 2014, 62, 2830–2836. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.K.; Shah, M.A. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef]
- Desdouets, C.; Sobczak-Thépot, J.; Murphy, M.; Bréchot, C. Cyclin A: Function and expression during cell proliferation. Prog. Cell Cycle Res. 1995, 1, 115–123. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- You, L.; Yang, C.; Du, Y.; Liu, Y.; Chen, G.; Sai, N.; Dong, X.; Yin, X.; Ni, J. Matrine Exerts Hepatotoxic Effects via the ROS-Dependent Mitochondrial Apoptosis Pathway and Inhibition of Nrf2-Mediated Antioxidant Response. Oxid. Med. Cell Longev. 2019, 2019, 1045345. [Google Scholar] [CrossRef] [Green Version]
- Sur, S.; Agrawal, D.K. Phosphatases and kinases regulating CDC25 activity in the cell cycle: Clinical implications of CDC25 overexpression and potential treatment strategies. Mol. Cell. Biochem. 2016, 416, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Dozier, C.; Mazzolini, L.; Cénac, C.; Froment, C.; Burlet-Schiltz, O.; Besson, A.; Manenti, S. CyclinD-CDK4/6 complexes phosphorylate CDC25A and regulate its stability. Oncogene 2017, 36, 3781–3788. [Google Scholar] [CrossRef]
- Fulda, S. Targeting apoptosis for anticancer therapy. Semin. Cancer Biol. 2015, 31, 84–88. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Steven, A.J.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. CCS 2010, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.T.; Chen, S.Y.; Yang, A.G. Cancer gene therapy targeting cellular apoptosis machinery. Cancer Treat. Rev. 2012, 38, 868–876. [Google Scholar] [CrossRef] [Green Version]
- Lavrik, I.; Golks, A.; Krammer, P.H. Death receptor signaling. J. Cell Sci. 2005, 118, 265–267. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.C.; Borutaite, V. Regulation of apoptosis by the redox state of cytochrome c. Biochim. Biophys. Acta 2008, 1777, 877–881. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, R.; Kheirollahi, A.; Davoodi, J. Apaf-1: Regulation and function in cell death. Biochimie 2017, 135, 111–125. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Liu, Z.P. Natural products as anti-invasive and anti-metastatic agents. Curr. Med. Chem. 2011, 18, 808–829. [Google Scholar] [CrossRef]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Verrax, J.; Pedrosa, R.C.; Beck, R.; Dejeans, N.; Taper, H.; Calderon, P.B. In situ modulation of oxidative stress: A novel and efficient strategy to kill cancer cells. Curr. Med. Chem. 2009, 16, 1821–1830. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, Y.; Fu, C.; Xu, X.; Zhou, J.; Wang, S.; Kong, L.; Li, Z.; Guo, Q.; Wei, L. LZ-106, a novel analog of enoxacin, inducing apoptosis via activation of ROS-dependent DNA damage response in NSCLCs. Free Radic. Biol. Med. 2016, 95, 155–168. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, L.; Shi, S.; Wang, Y.; Ni, X.; Xiao, F.; Wang, S.; Li, P.; Ding, K. Oligosaccharide G19 inhibits U-87 MG human glioma cells growth in vitro and in vivo by targeting epidermal growth factor (EGF) and activating p53/p21 signaling. Glycobiology 2014, 24, 748–765. [Google Scholar] [CrossRef]
- Guachalla, L.M.; Rudolph, K.L. ROS induced DNA damage and checkpoint responses: Influences on aging? Cell Cycle 2010, 9, 4058–4060. [Google Scholar] [CrossRef] [Green Version]
- Maréchal, A.; Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, Y.; Chen, J.; Dong, X.; Gao, Z.; Zhang, H.; Wu, X.; Liu, Z.; Wu, Y. Aberrant Buildup of All-Trans-Retinal Dimer, a Nonpyridinium Bisretinoid Lipofuscin Fluorophore, Contributes to the Degeneration of the Retinal Pigment Epithelium. Investig. Ophthalmol Vis. Sci. 2017, 58, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Puente, X.S.; Jares, P.; Campo, E. Chronic lymphocytic leukemia and mantle cell lymphoma: Crossroads of genetic and microenvironment interactions. Blood 2018, 131, 2283–2296. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Wang, B.; Yue, M.; Cheng, Y. Pathway Analysis Based on Attractor and Cross Talk in Colon Cancer. Dis. Markers 2016, 2016, 2619828. [Google Scholar] [CrossRef] [Green Version]
- Urso, L.; Calabrese, F.; Favaretto, A.; Conte, P.; Pasello, G. Critical review about MDM2 in cancer: Possible role in malignant mesothelioma and implications for treatment. Crit. Rev. Oncol. Hematol. 2016, 97, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Shieh, S.Y.; Ikeda, M.; Taya, Y.; Prives, C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 1997, 91, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, D.; Xing, Z.; Liang, S.; Han, H.; Shi, H.; Zhang, Y.; Yang, Y.; Li, Q. N-Isopropylacrylamide-modified polyethylenimine-mediated p53 gene delivery to prevent the proliferation of cancer cells. Colloids Surf. B Biointerfaces 2015, 129, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, J.; Wang, C.Z.; Searle, J.; He, T.C.; Yuan, C.S.; Du, W. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 2011, 301, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Park, E.K.; Lee, E.J.; Lee, S.H.; Koo, K.H.; Sung, J.Y.; Hwang, E.H.; Park, J.H.; Kim, C.W.; Jeong, K.C.; Park, B.K.; et al. Induction of apoptosis by the ginsenoside Rh2 by internalization of lipid rafts and caveolae and inactivation of Akt. Br. J. Pharm. 2010, 160, 1212–1223. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds BTFS are not available from the authors. |







| Peak | Retention Time (min) | [M − H]− | MS2 | Formula | Peak Identity |
|---|---|---|---|---|---|
| 1 | 53.40 | 1245.59 | 1083, 1065, 951, 915, 753, 709, 611 | C60H94O27 | Unknown [20] |
| 2 | 54.45 | 1229.59 | 1083, 1067, 1049, 789, 611 | C60H94O26 | Floratheasaponin D [19,20] |
| 3 | 55.57 | 1215.58 | 1083, 1035, 951, 933, 789, 611 | C59H92O26 | Floratheasaponin A [18,20] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Y.; Chen, L.; Ren, N.; Li, B.; Wu, Y.; Rankin, G.O.; Rojanasakul, Y.; Wang, Y.; Chen, Y.C. Standardized Saponin Extract from Baiye No.1 Tea (Camellia sinensis) Flowers Induced S Phase Cell Cycle Arrest and Apoptosis via AKT-MDM2-p53 Signaling Pathway in Ovarian Cancer Cells. Molecules 2020, 25, 3515. https://doi.org/10.3390/molecules25153515
Tu Y, Chen L, Ren N, Li B, Wu Y, Rankin GO, Rojanasakul Y, Wang Y, Chen YC. Standardized Saponin Extract from Baiye No.1 Tea (Camellia sinensis) Flowers Induced S Phase Cell Cycle Arrest and Apoptosis via AKT-MDM2-p53 Signaling Pathway in Ovarian Cancer Cells. Molecules. 2020; 25(15):3515. https://doi.org/10.3390/molecules25153515
Chicago/Turabian StyleTu, Youying, Lianfu Chen, Ning Ren, Bo Li, Yuanyuan Wu, Gary O. Rankin, Yon Rojanasakul, Yaomin Wang, and Yi Charlie Chen. 2020. "Standardized Saponin Extract from Baiye No.1 Tea (Camellia sinensis) Flowers Induced S Phase Cell Cycle Arrest and Apoptosis via AKT-MDM2-p53 Signaling Pathway in Ovarian Cancer Cells" Molecules 25, no. 15: 3515. https://doi.org/10.3390/molecules25153515
APA StyleTu, Y., Chen, L., Ren, N., Li, B., Wu, Y., Rankin, G. O., Rojanasakul, Y., Wang, Y., & Chen, Y. C. (2020). Standardized Saponin Extract from Baiye No.1 Tea (Camellia sinensis) Flowers Induced S Phase Cell Cycle Arrest and Apoptosis via AKT-MDM2-p53 Signaling Pathway in Ovarian Cancer Cells. Molecules, 25(15), 3515. https://doi.org/10.3390/molecules25153515