Verification of a Novel Approach to Predicting Effects of Antibiotic Combinations: In Vitro Dynamic Model Study with Daptomycin and Gentamicin against Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. MICs of Daptomycin and Gentamicin Alone and in Combination
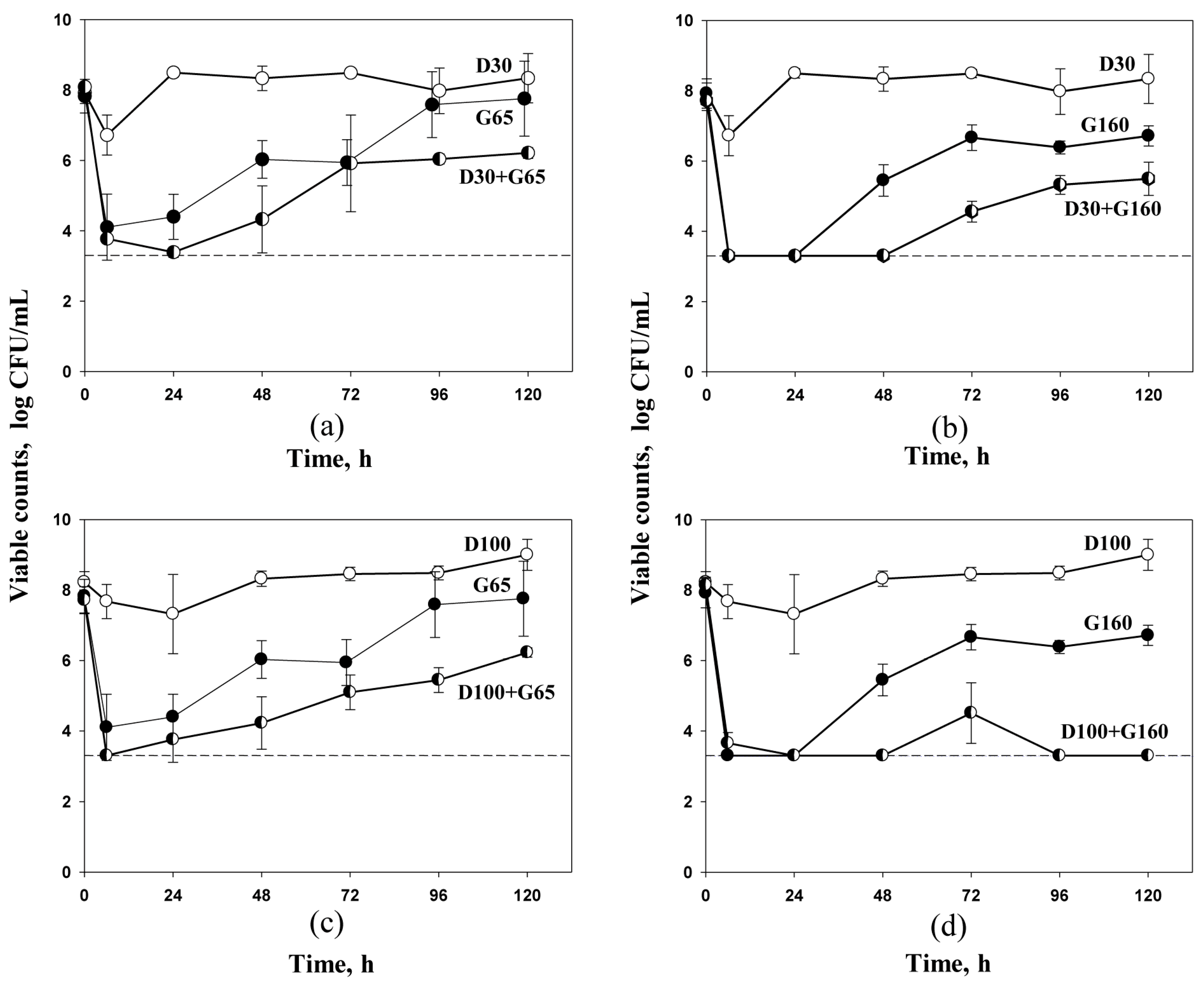
2.2. Antibiotic Pharmacodynamics with S. aureus
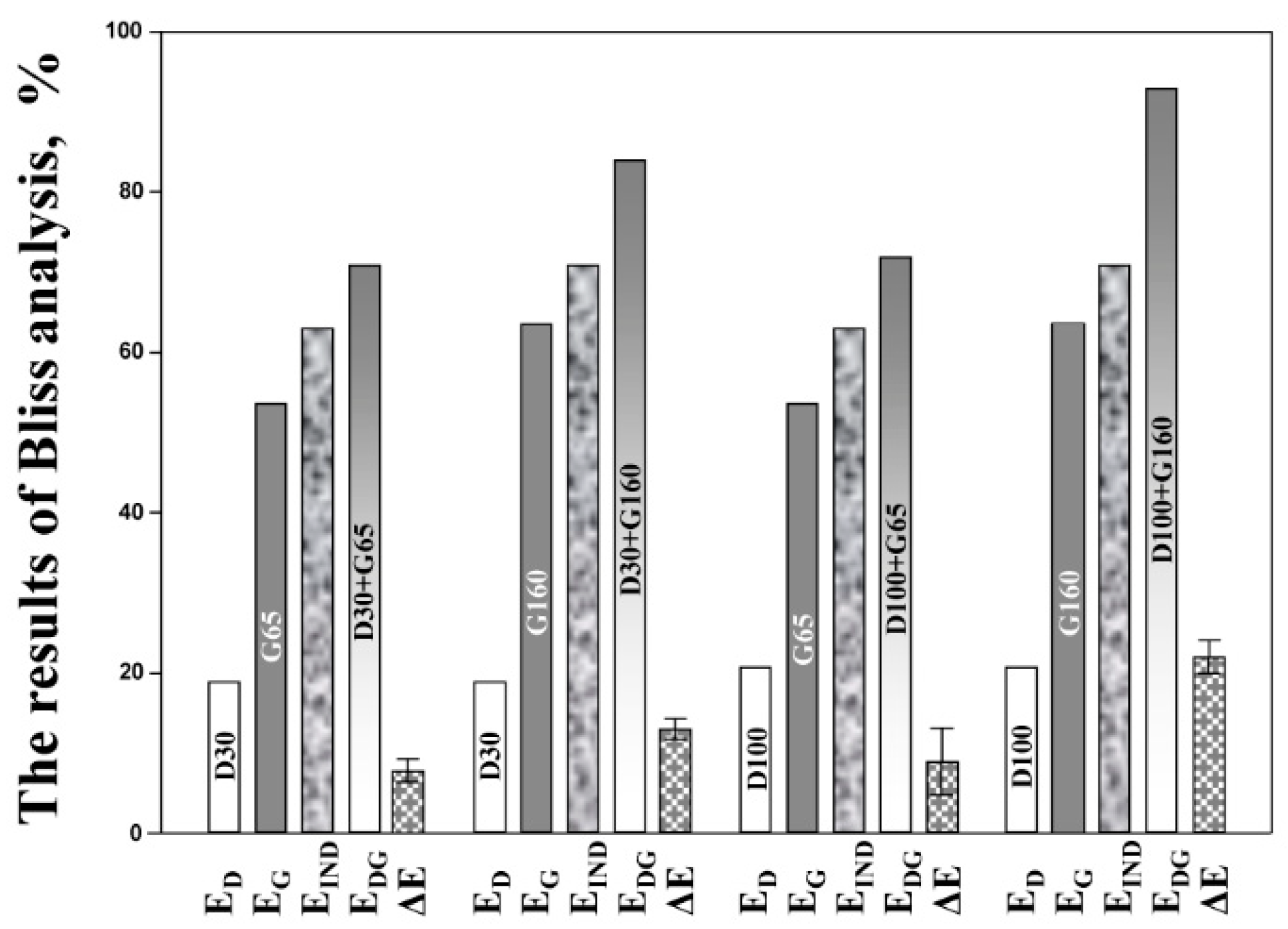
2.3. Pharmacodynamic Bliss Independence-Based Drug Interaction Analysis
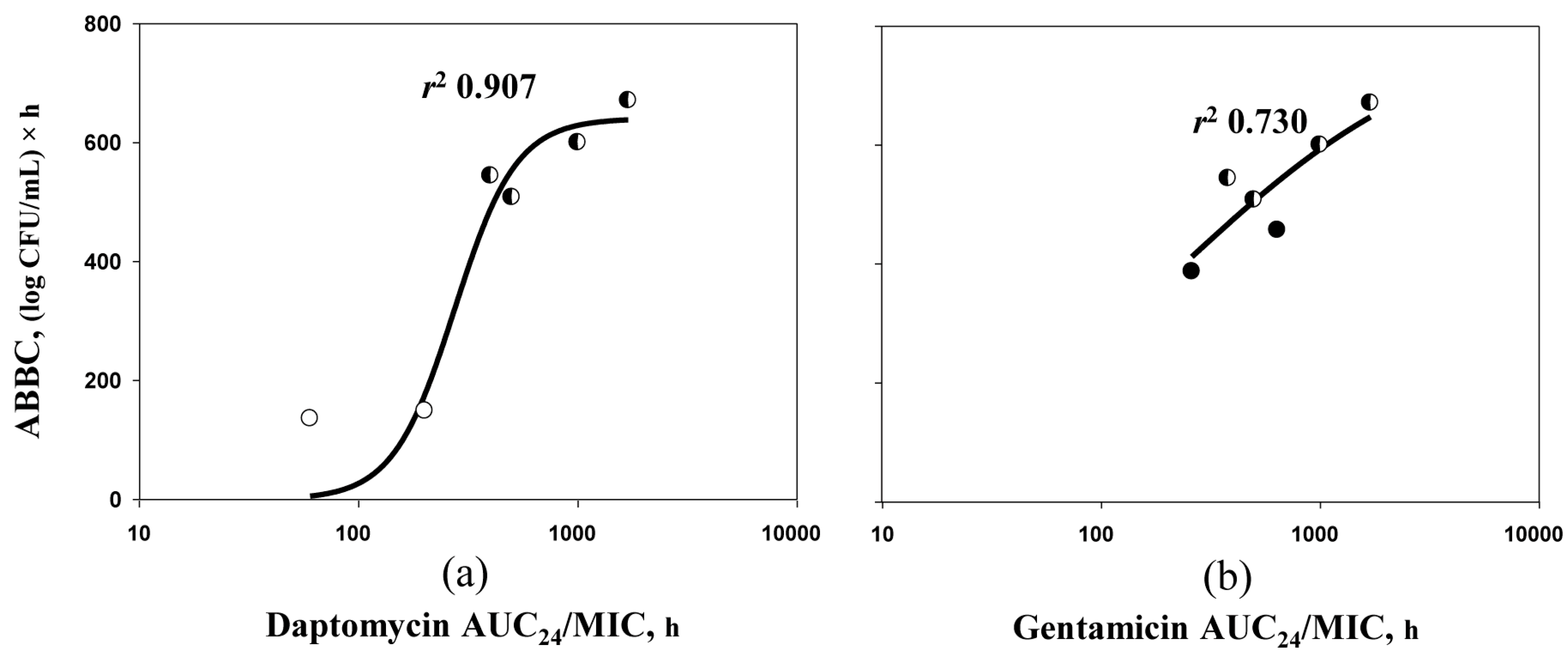
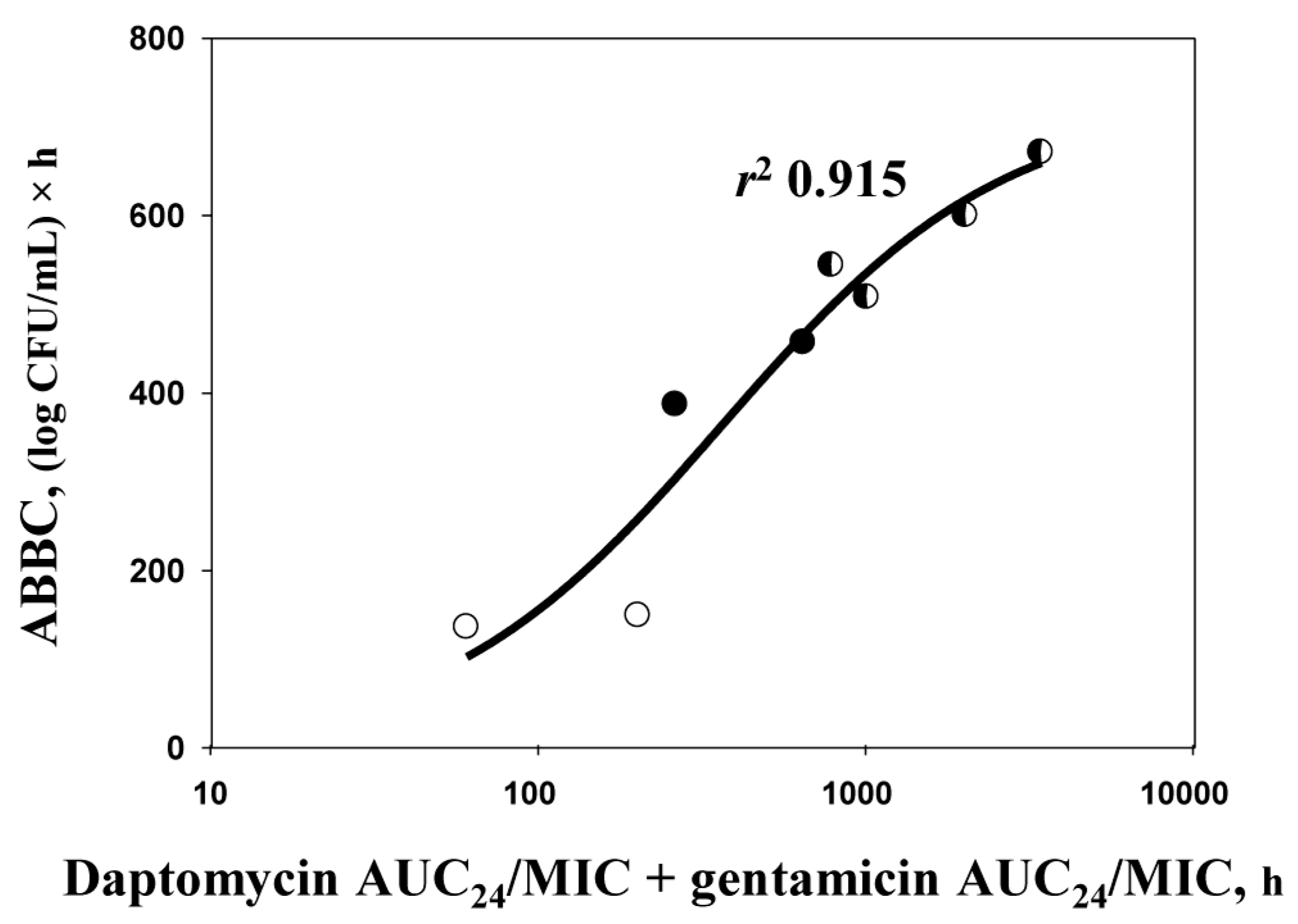
2.4. ABBC–AUC24/MIC Relationships
3. Discussion
4. Materials and Methods
4.1. Antimicrobial Agents and Bacterial Strain
4.2. Antibiotic Dosing Regimens and Simulated Pharmacokinetic Profiles
4.3. In Vitro Dynamic Model
4.4. Susceptibility Testing
4.5. Quantitation of the Antimicrobial Effect and Its Relationships with AUC24/MIC Ratios
4.6. Pharmacodynamic Bliss Independence-Based Drug Interaction Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mariani, P.G.; Sader, H.S.; Jones, R.N. Development of decreased susceptibility to daptomycin and vancomycin in a Staphylococcus aureus strain during prolonged therapy. J. Antimicrob. Chemother. 2006, 58, 481–483. [Google Scholar] [CrossRef]
- Skiest, D.J. Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J. Clin. Microbiol. 2006, 44, 655–656. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.W.; Murray, C.K.; Holmes, R.L.; Patterson, J.E.; Jorgensen, J.H. Diminished vancomycin and daptomycin susceptibility during prolonged bacteremia with methicillin-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2008, 60, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Riederer, K.; Chase, P.; Khatib, R. High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wang, M.C.; Huang, I.W.; Chen, F.J.; Lauderdale, T.L. Development of daptomycin non susceptibility with heterogeneous vancomycin-intermediate resistance and oxacillin susceptibility in methicillin resistant Staphylococcus aureus during high-dose daptomycin treatment. Antimicrob. Agents Chemother. 2010, 54, 4038–4040. [Google Scholar] [CrossRef] [Green Version]
- Rose, W.E.; Schulz, L.T.; Andes, D.; Striker, R.; Berti, A.D.; Hutson, P.R.; Shukla, S.K. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob. Agents Chemother. 2012, 56, 5296–5302. [Google Scholar] [CrossRef] [Green Version]
- Dortet, L.; Anguel, N.; Fortineau, N.; Richard, C.; Nordmann, P. In vivo acquired daptomycin resistance during treatment of methicillin-resistant Staphylococcus aureus endocarditis. Int. J. Infect. Dis. 2013, 17, e1076–e1077. [Google Scholar] [CrossRef] [Green Version]
- Sabat, A.J.; Tinelli, M.; Grundmann, H.; Akkerboom, V.; Monaco, M.; Del Grosso, M.; Errico, G.; Pantosti, A.; Friedrich, A.W. Daptomycin resistant Staphylococcus aureus clinical strain with novel non-synonymous mutations in the mprF and vraS genes: A new insight into daptomycin resistance. Front. Microbiol. 2018, 9, 2705–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, M.K.; Rezai, K.; Hayes, R.A.; Lolans, K.; Quinn, J.P.; Weinstein, R.A. Development of daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2005, 43, 5285–5287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vikram, H.R.; Havill, N.L.; Koeth, L.M.; Boyce, J.M. Clinical progression of methicillin-resistant Staphylococcus aureus vertebral osteomyelitis associated with reduced susceptibility to daptomycin. J. Clin. Microbiol. 2005, 43, 5384–5387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthy, M.H.; Olson, M.E.; Wickert, R.W.; Fey, P.D.; Jalali, Z. Daptomycin non-susceptible meticillin-resistant Staphylococcus aureus USA 300 isolate. J. Med. Microbiol. 2008, 57, 1036–1038. [Google Scholar] [CrossRef]
- Sakoulas, G.; Rose, W.; Rybak, M.J.; Pillai, S.; Alder, J.; Moellering, R.C., Jr.; Eliopoulos, G.M. Evaluation of endocarditis caused by methicillin-susceptible Staphylococcus aureus developing nonsusceptibility to daptomycin. J. Clin. Microbiol. 2008, 46, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Cunha, B.A.; Pherez, F.M. Daptomycin resistance and treatment failure following vancomycin for methicillin-resistant Staphylococcus aureus (MRSA) mitral valve acute bacterial endocarditis (ABE). Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 831–833. [Google Scholar] [CrossRef]
- Tenover, F.C.; Sinner, S.W.; Segal, R.E.; Huang, V.; Alexandre, S.S.; McGowan, J.E., Jr.; Weinstein, M.P. Characterisation of a Staphylococcus aureus strain with progressive loss of susceptibility to vancomycin and daptomycin during therapy. Int. J. Antimicrob. Agents 2009, 33, 564–568. [Google Scholar] [CrossRef] [Green Version]
- Van Hal, S.J.; Paterson, D.L.; Gosbell, I.B. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteraemia in a daptomycin-naïve patient—A review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 603–610. [Google Scholar] [CrossRef]
- Santimaleeworagun, W.; Changpradub, D.; Thunyaharn, S.; Hemapanpairoa, J. Optimizing the dosing regimens of daptomycin based on the susceptible dose-dependent breakpoint against vancomycin-resistant Enterococci infection. Antibiotics 2019, 8, 245. [Google Scholar] [CrossRef] [Green Version]
- Golikova, M.V.; Strukova, E.N.; Portnoy, Y.A.; Zinner, S.H.; Firsov, A.A. Predicting the antistaphylococcal effects of daptomycin–rifampicin combinations in an in vitro dynamic model. J. Antibiot. 2020, 73, 101–107. [Google Scholar] [CrossRef]
- Bollenbach, T. Antimicrobial interactions: Mechanisms and implications for drug discovery and resistance evolution. Curr. Opin. Microbiol. 2015, 27, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Aktas, G.; Derbentli, S. In vitro activity of daptomycin combinations with rifampicin, gentamicin, fosfomycin and fusidic acid against MRSA strains. J. Glob. Antimicrob. Resist. 2017, 10, 223–227. [Google Scholar] [CrossRef]
- Snydman, D.R.; McDermott, L.A.; Jacobus, N.V. Evaluation of in vitro interaction of daptomycin with gentamicin or beta-lactam antibiotics against Staphylococcus aureus and enterococci by FIC index and timed-kill curves. J. Chemother. 2005, 17, 614–621. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chen, P.Y.; Wang, J.T.; Chang, S.C. A study on combination of daptomycin with selected antimicrobial agents: In vitro synergistic effect of MIC value of 1 mg/L against MRSA strains. BMC Pharm. Toxicol. 2019, 20, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Credito, K.; Lin, G.; Appelbaum, P.C. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob. Agents Chemother. 2007, 51, 1504–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miro, J.M.; García-de-la-Maria, C.; Armero, Y.; Soy, D.; Moreno, A.; del Río, A.; Almela, M.; Sarasa, M.; Mestres, C.A.; Gatell, J.M.; et al. Addition of gentamicin or rifampin does not enhance the effectiveness of daptomycin in treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 4172–4177. [Google Scholar] [CrossRef] [Green Version]
- LaPlante, K.L.; Rybak, M.J. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 2004, 48, 4665–4672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, B.T.; Rybak, M.J. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 2005, 49, 2735–2745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, W.E.; Leonard, S.N.; Rybak, M.J. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 2008, 52, 3061–3067. [Google Scholar] [CrossRef] [Green Version]
- LaPlante, K.L.; Woodmansee, S. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant Staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob. Agents Chemother. 2009, 53, 3880–3886. [Google Scholar] [CrossRef] [Green Version]
- Firsov, A.A.; Smirnova, M.V.; Lubenko, I.Y.; Vostrov, S.N.; Portnoy, Y.A.; Zinner, S.H. Testing the mutant selection window hypothesis with Staphylococcus aureus exposed to daptomycin and vancomycin in an in vitro dynamic model. J. Antimicrob. Chemother. 2006, 58, 1185–1192. [Google Scholar] [CrossRef]
- Tam, V.H.; Kabbara, S.; Vo, G.; Schilling, A.N.; Coyle, E.A. Comparative pharmacodynamics of gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2626–2631. [Google Scholar] [CrossRef] [Green Version]
- Zinner, S.H.; Golikova, M.V.; Strukova, E.N.; Portnoy, Y.A.; Firsov, A.A. Predicting antibiotic combination effects on the selection of resistant Staphylococcus aureus: In vitro model studies with linezolid and gentamicin. Int. J. Antimicrob. Agents 2018, 52, 854–860. [Google Scholar] [CrossRef]
- Steenbergen, J.N.; Mohr, J.F.; Thorne, G.M. Effects of daptomycin in combination with other antimicrobial agents: A review of in vitro and animal model studies. J. Antimicrob. Chemother. 2009, 64, 1130–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadrah, K.; Strle, F. Antibiotic combinations with daptomycin for treatment of Staphylococcus aureus infections. Chemother. Res. Pract. 2011, 619321. [Google Scholar] [CrossRef] [PubMed]
- Dhand, A.; Sakoulas, G. Daptomycin in combination with other antibiotics for the treatment of complicated methicillin-resistant Staphylococcus aureus bacteremia. Clin. Ther. 2014, 36, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Humphries, R.; Ward, K.; Lewinski, M.A.; Yang, O.O. Combination therapy with daptomycin, linezolid, and rifampin as treatment option for MRSA meningitis and bacteremia. Diagn. Microbiol. Infect. Dis. 2011, 71, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Berti, A.D.; Hatch, J.B.; Maki, D.G. Relationship of in vitro synergy and treatment outcome with daptomycin plus rifampin in patients with invasive methicillin-resistant Staphylococcus aureus infections. Antimicrob. Agents Chemother. 2013, 57, 3450–3452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.-C.; Chen, C.-C.; Lu, Y.-C.; Lin, T.-P.; Chen, H.-J.; Su, B.-A.; Chao, C.-M.; Chuang, Y.-C.; Tang, H.-J. The potential role of sulbactam and cephalosporins plus daptomycin against daptomycin-nonsusceptible VISA and H-VISA isolates: An in vitro study. Antibiotics 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Cobussen, M.; Stassen, P.M.; Posthouwer, D.; van Tiel, F.H.; Savelkoul, P.H.M.; Havenith, T.; Haeseker, M.B. Improving peak concentrations of a single dose regimen of gentamicin in patients with sepsis in the emergency department. PLoS ONE 2019, 14, e0210012. [Google Scholar] [CrossRef] [Green Version]
- Demczar, D.J.; Nafziger, A.N.; Bertino Jr, J.S. Pharmacokinetics of gentamicin at traditional versus high doses: Implications for once-daily aminoglycoside dosing. Antimicrob. Agents Chemother. 1997, 41, 1115–1119. [Google Scholar] [CrossRef] [Green Version]
- Dvorchik, B.H.; Brazier, D.; DeBruin, M.F.; Arbeit, R.D. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 2003, 47, 1318–1323. [Google Scholar] [CrossRef] [Green Version]
- Firsov, A.A.; Shevchenko, A.A.; Vostrov, S.N.; Zinner, S.H. Inter- and intra-quinolone predictors of antimicrobial effect in an in vitro dynamic model: New insight into a widely used concept. Antimicrob. Agents Chemother. 1998, 42, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, P.C.; Barry, A.L.; Brown, S.D. Daptomycin susceptibility tests: Interpretive criteria, quality control, and effect of calcium on in vitro tests. Diagn. Microbiol. Infect. Dis. 2000, 38, 51–58. [Google Scholar] [CrossRef]
- Blaser, J.; Stone, B.B.; Zinner, S.H. Two compartment kinetic model with multiple artificial capillary units. J. Antimicrob. Chemother. 1985, 15, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Golikova, M.V.; Strukova, E.N.; Portnoy, Y.A.; Dovzhenko, S.A.; Kobrin, M.B.; Zinner, S.H.; Firsov, A.A. Predicting effects of antibiotic combinations using MICs determined at pharmacokinetically derived concentration ratios: In vitro model studies with linezolid- and rifampicin-exposed Staphylococcus aureus. J. Chemother. 2017, 29, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement M100-S25; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Eliopoulos, G.M.; Moellering Jr, R.C. Laboratory methods used to assess the activity of antimicrobial combinations. In Antibiotics in Laboratory Medicine; Lorian, V., Ed.; The Williams & Wilkins Co.: Baltimore, MD, USA, 1991; pp. 432–492. [Google Scholar]
- Firsov, A.A.; Saverino, D.; Ruble, M.; Gilbert, D.; Manzano, B.; Medeiros, A.A.; Zinner, S.H. Predictors of effect of ampicillin-sulbactam against TEM-1 beta-lactamase-producing Escherichia coli in an in vitro dynamic model: Enzyme activity versus MIC. Antimicrob. Agents Chemother. 1996, 40, 734–738. [Google Scholar] [CrossRef] [Green Version]
- Bliss, C.I. The toxicity of poisons applied jointly. Ann. Appl. Biol. 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharm. Rev. 1995, 47, 331–385. [Google Scholar]
- Vakil, V.; Trappe, W. Drug combinations: Mathematical modeling and networking methods. Pharmaceutics 2019, 11, 208. [Google Scholar] [CrossRef] [Green Version]
| Dosing Regimen | Daptomycin-to-Gentamicin AUC24 Ratio | MIC (mg/L) | FIC | |||
|---|---|---|---|---|---|---|
| Daptomycin | Gentamicin | FICD | FICG | ƩFIC | ||
| D30 | - | 0.5 | - | - | - | - |
| D100 | - | - | - | - | - | |
| G65 | - | - | 0.25 | - | - | - |
| G160 | - | - | - | - | - | |
| D100 + G65 | 1.5:1 | 0.25 | 0.17 | 0.5 | 0.68 | 1.18 |
| D100 + G160 | 1:1.5 | 0.06 | 0.09 | 0.12 | 0.36 | 0.48 |
| D30 + G65 | 1:2 | 0.06 | 0.13 | 0.12 | 0.52 | 0.64 |
| D30 + G160 | 1:5 | 0.03 | 0.16 | 0.06 | 0.64 | 0.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golikova, M.V.; Strukova, E.N.; Portnoy, Y.A.; Zinner, S.H.; Firsov, A.A. Verification of a Novel Approach to Predicting Effects of Antibiotic Combinations: In Vitro Dynamic Model Study with Daptomycin and Gentamicin against Staphylococcus aureus. Antibiotics 2020, 9, 538. https://doi.org/10.3390/antibiotics9090538
Golikova MV, Strukova EN, Portnoy YA, Zinner SH, Firsov AA. Verification of a Novel Approach to Predicting Effects of Antibiotic Combinations: In Vitro Dynamic Model Study with Daptomycin and Gentamicin against Staphylococcus aureus. Antibiotics. 2020; 9(9):538. https://doi.org/10.3390/antibiotics9090538
Chicago/Turabian StyleGolikova, Maria V., Elena N. Strukova, Yury A. Portnoy, Stephen H. Zinner, and Alexander A. Firsov. 2020. "Verification of a Novel Approach to Predicting Effects of Antibiotic Combinations: In Vitro Dynamic Model Study with Daptomycin and Gentamicin against Staphylococcus aureus" Antibiotics 9, no. 9: 538. https://doi.org/10.3390/antibiotics9090538
APA StyleGolikova, M. V., Strukova, E. N., Portnoy, Y. A., Zinner, S. H., & Firsov, A. A. (2020). Verification of a Novel Approach to Predicting Effects of Antibiotic Combinations: In Vitro Dynamic Model Study with Daptomycin and Gentamicin against Staphylococcus aureus. Antibiotics, 9(9), 538. https://doi.org/10.3390/antibiotics9090538





