Retrofractamide C Derived from Piper longum Alleviates Xylene-Induced Mouse Ear Edema and Inhibits Phosphorylation of ERK and NF-κB in LPS-Induced J774A.1
Abstract
:1. Introduction
2. Results and Discussion
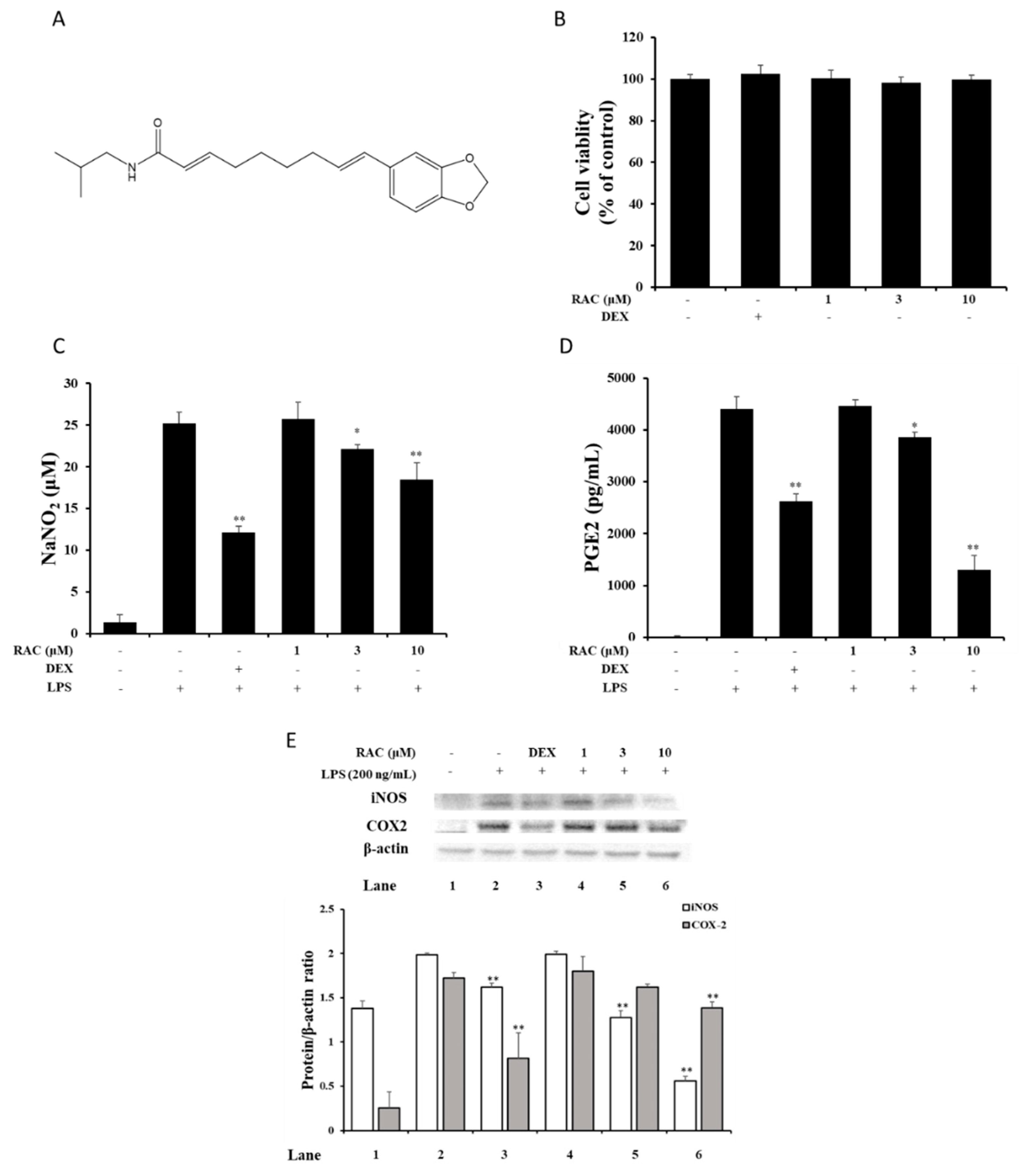
2.1. RAC Inhibits NO and PGE2 Production in LPS-Induced J774A.1 Cells
2.2. RAC Decreases iNOS and COX2 Expression in LPS-Induced J774A.1 Cells
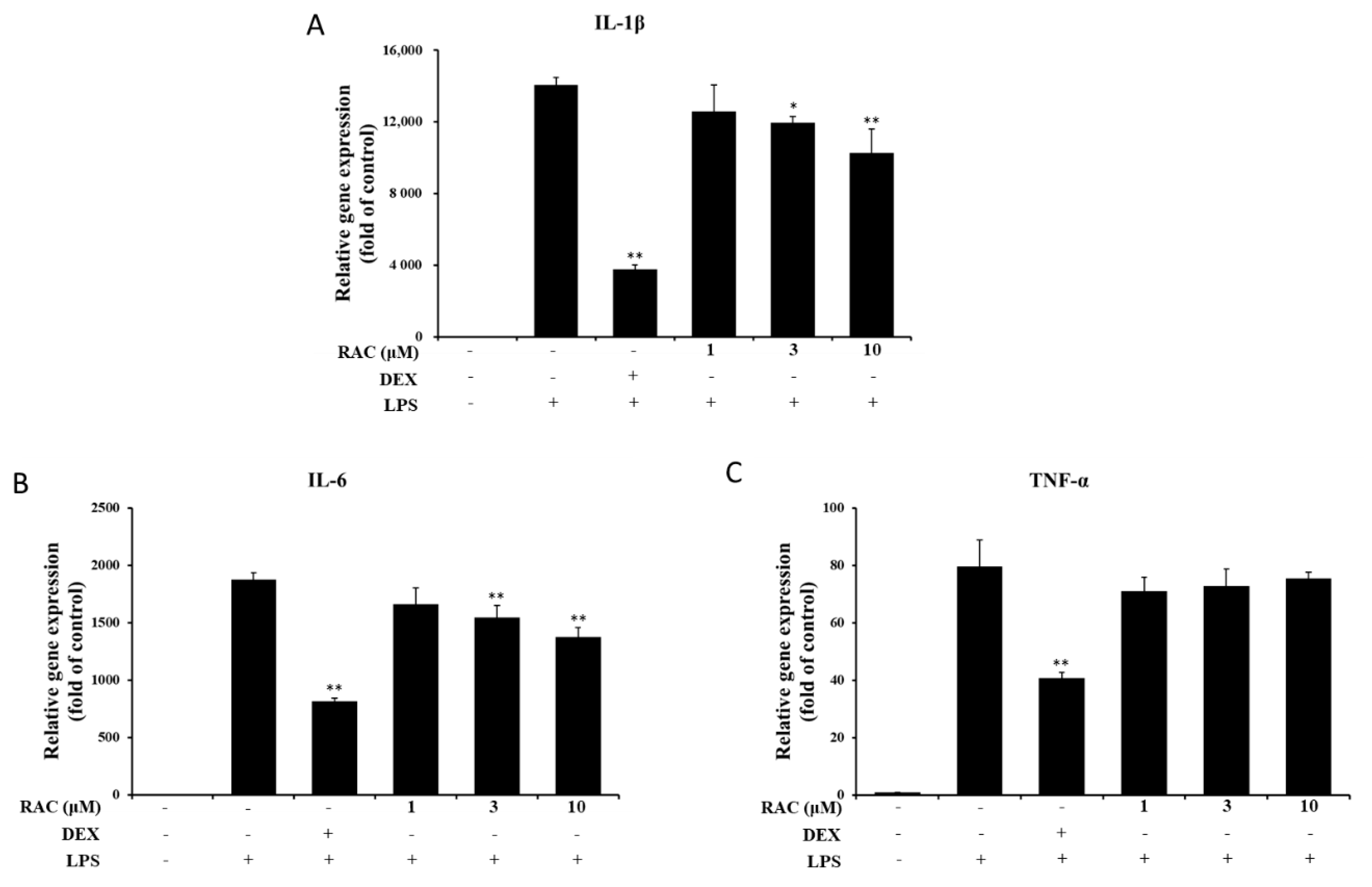
2.3. RAC Inhibits IL-1β and IL-6 But Not TNF-α Gene Expression in LPS-Induced J774A.1 Cells
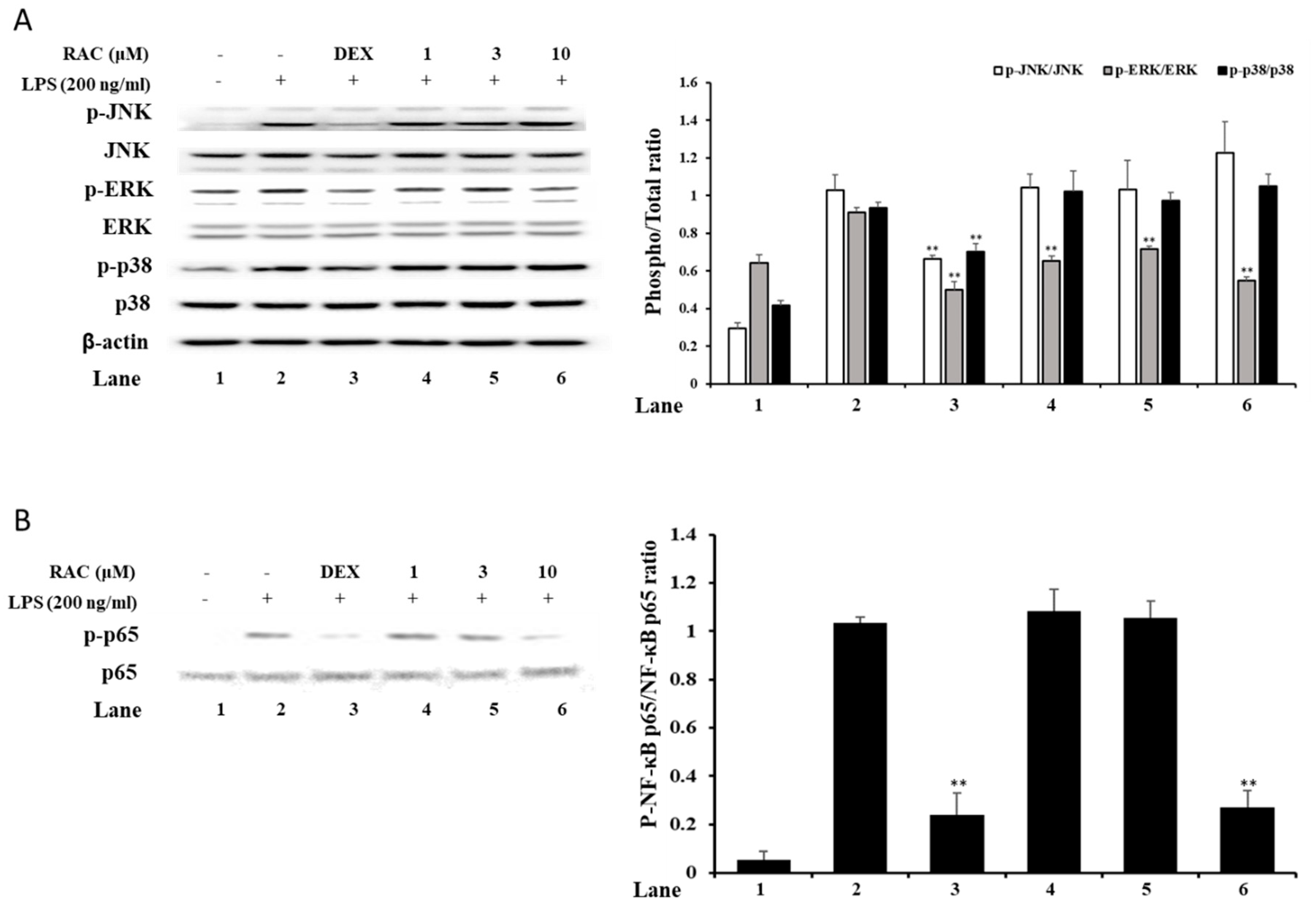
2.4. RAC Decreases Phosphorylated ERK and NF-κB p65 But Not JNK or p38 in LPS-Induced J774A.1 Cells
2.5. RAC Alleviates Xylene-Induced Mouse Ear Edema
3. Materials and Methods
3.1. Materials and Reagents
3.2. Isolation of Retrofractamide C
3.3. MTT Assay
3.4. NO Assay
3.5. ELISA
3.6. Quantitative Real-Time PCR
3.7. Immunoblot Analysis
3.8. Animals and Induction of a Xylene-Induced Ear Edema Model
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chovatiya, R.; Medzhitov, R. Stress, inflammation, and defense of homeostasis. Mol. Cell 2004, 54, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Higashiura, Y.; Shigetomi, H.; Kajihara, H. Pathogenesis of endometriosis: The role of initial infection and subsequent sterile inflammation. Mol. Med. Rep. 2014, 9, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Bergallo, M.; Galliano, I.; Montanari, P.; Calvi, C.; Rassu, M.; Dapra, V. Toll-like receptor 9 gene polymorphisms rs352140 confer susceptibility to graft-versus-host disease in allogenic hematopoietic stem cell transplantation. Minerva Biotecnol. 2019, 31, 49–53. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, S.; Niu, J.; Schmidt, C.; Sclabas, G.M.; Peng, B.; Uwagawa, T.; Li, Z.; Evans, D.B.; Abbruzzese, J.L.; Chiao, P.J. NF-κB and AP-1 connection: Mechanism of NF-κB-dependent regulation of AP-1 activity. Mol. Cell. Boil. 2004, 24, 7806–7819. [Google Scholar] [CrossRef] [Green Version]
- Khalaf, H.; Jass, J.; Olsson, P.E. Differential cytokine regulation by NF-κB and AP-1 in Jurkat T-cells. BMC Immunol. 2010, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, M.; Moochhala, S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 2004, 202, 145–156. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Djukanovic, R.; Wilson, S.J.; Kraft, M.; Jarjour, N.N.; Steel, M.; Chung, K.F.; Bao, W.; Fowler-Taylor, A.; Matthews, J.; Busse, W.W.; et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.B.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Yoon, O.B.; Beitz, A.J.; Lee, J.H. The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life Sci. 2002, 71, 191–204. [Google Scholar] [CrossRef]
- Cui, L.; Feng, L.; Zhang, Z.H.; Jia, X.B. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int. Immunopharmacol. 2014, 23, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.D.; Natanson, C. Anti-inflammatory therapies in sepsis and septic shock. Expert Opin. Inv. Drug. 2000, 9, 1651–1663. [Google Scholar] [CrossRef]
- Segura, M.; Vadeboncoeur, N.; Gottschalk, M. CD14-dependent and-independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clin. Exp. Immunol. 2002, 127, 243–254. [Google Scholar] [CrossRef]
- Wang, H.L.; Li, Y.X.; Niu, Y.T.; Zheng, J.; Wu, J.; Shi, G.J.; Ma, L.; Niu, Y.; Sun, T.; Yu, J.Q. Observing anti-inflammatory and anti-nociceptive activities of glycyrrhizin through regulating COX-2 and pro-inflammatory cytokines expressions in mice. Inflammation 2015, 38, 2269–2278. [Google Scholar] [CrossRef]
- Gunasekar, P.G.; Rogers, J.V.; Kabbur, M.B.; Garrett, C.M.; Brinkley, W.W.; McDougal, J.N. Molecular and histological responses in rat skin exposed to m-xylene. J. Biochem. Mol. Toxicol. 2003, 17, 92–94. [Google Scholar] [CrossRef]
- Rogers, J.V.; Gunasekar, P.G.; Garrett, C.M.; McDougal, J.N. Dermal exposure to m-xylene leads to increasing oxidative species and low molecular weight dna levels in rat skin. J. Biochem. Mol. Toxicol. 2001, 15, 228–230. [Google Scholar] [CrossRef]
- Ahaghotu, E.; Babu, R.J.; Chatterjee, A.; Singh, M. Effect of methyl substitution of benzene on the percutaneous absorption and skin irritation in hairless rats. Toxicol. Lett. 2005, 159, 261–271. [Google Scholar] [CrossRef]
- Ghoshal, S.; Prasad, B.K.; Lakshmi, V. Antiamoebic activity of Piper longum fruits against Entamoeba histolytica in vitro and in vivo. J. Ethnopharmacol. 1996, 50, 167–170. [Google Scholar] [CrossRef]
- Park, B.S.; Son, D.J.; Park, Y.H.; Kim, T.W.; Lee, S.E. Antiplatelet effects of acidamides isolated from the fruits of Piper longum L. Phytomedicine 2007, 14, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, K.S.; Oh, Y.N.; Lee, J.Y.; Son, B.Y.; Choi, W.; Lee, E.W.; Kwon, H.J.; Kim, B.W. Anti-oxidative and anti-inflammatory activities of seven medicinal herbs including Tetrapanax papyriferus and Piper longum Linne. Microbiol. Biotechnol. Lett. 2013, 41, 253–262. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lee, S.G.; Lee, H.K.; Kim, M.K.; Lee, S.H.; Lee, H.S. A piperidine amide extracted from Piper longum L. fruit shows activity against Aedes aegypti mosquito larvae. J. Agric. Food Chem. 2002, 50, 3765–3767. [Google Scholar] [CrossRef]
- Joy, B.; Sandhya, C.P.; Remitha, K.R. Comparison and bioevaluation of Piper longum fruit extracts. J. Chem. Pharm. Res. 2010, 2, 612–622. [Google Scholar]
- Khushbu, C.; Roshni, S.; Anar, P.; Carol, M.; Mayuree, P. Phytochemical and therapeutic potential of Piper longum Linn a review. Int. J. Res. Ayurveda Pharm. 2011, 2, 157–161. [Google Scholar]
- Lee, S.W.; Rho, M.C.; Park, H.R.; Choi, J.H.; Kang, J.Y.; Lee, J.W.; Kim, K.; Lee, H.S.; Kim, Y.K. Inhibition of diacylglycerol acyltransferase by alkamides isolated from the fruits of Piper longum and Piper nigrum. J. Agric. Food Chem. 2006, 54, 9759–9763. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, Y.K.; Kim, K.; Lee, H.S.; Choi, J.H.; Lee, W.S.; Jun, C.D.; Park, J.H.; Lee, J.M.; Rho, M.C. Alkamides from the fruits of Piper longum and Piper nigrum displaying potent cell adhesion inhibition. Bioorg. Med. Chem. Lett. 2008, 18, 4544–4546. [Google Scholar] [CrossRef]
- Okumura, Y.; Narukawa, M.; Iwasaki, Y.; Ishikawa, A.; Matsuda, H.; Yoshikawa, M.; Watanabe, T. Activation of TRPV1 and TRPA1 by black pepper components. Biosci. Biotech. Bioch. 2010, 74, 1068–1072. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardini, I.A.; FitzGerald, G.A. Selective inhibitors of cyclooxygenase-2: A growing class of anti-inflammatory drugs. Mol. Interv. 2001, 1, 30–38. [Google Scholar] [PubMed]
- Caiello, I.; Minnone, G.; Holzinger, D.; Vogl, T.; Prencipe, G.; Manzo, A.; Benedetti, F.D.; Strippoli, R. IL-6 amplifies TLR mediated cytokine and chemokine production: Implications for the pathogenesis of rheumatic inflammatory diseases. PLoS ONE 2014, 9, e107886. [Google Scholar] [CrossRef]
- Saferding, V.; Blüml, S. Innate immunity as the trigger of systemic autoimmune diseases. J. Autoimmun. 2020, 110, 102382. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, H.J.; Jang, H.J.; Lee, S.; Jung, K.; Lee, S.W.; Lee, S.J.; Rho, M.C. Portulaca oleracea extracts and their active compounds ameliorate inflammatory bowel diseases in vitro and in vivo by modulating TNF-α, IL-6 and IL-1β signalling. Food Res. Int. 2018, 106, 335–343. [Google Scholar] [CrossRef]
- Sommer, C.; Kress, M. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004, 361, 184–187. [Google Scholar] [CrossRef]
- Anderson, P.; Phillips, K.; Stoecklin, G.; Kedersha, N. Post-transcriptional regulation of proinflammatory proteins. J. Leukoc. Biol. 2004, 76, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Mino, T.; Takeuchi, O. Post-transcriptional regulation of cytokine mRNA controls the initiation and resolution of inflammation. Biotechnol. Genet. Eng. Rev. 2013, 29, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.J.; Bak, S.G.; Lim, H.J.; Lee, S.W.; Lee, S.; Ku, S.K.; Park, S.I.; Lee, S.J.; Rho, M.C. Acyclic Triterpenoid Isolated from Alpinia katsumadai Alleviates Formalin-Induced Chronic Mouse Paw Inflammation by Inhibiting the Phosphorylation of ERK and NF-κB. Molecules 2020, 25, 3345. [Google Scholar] [CrossRef]
- Park, J.Y.; Lim, M.S.; Kim, S.I.; Lee, H.J.; Kim, S.S.; Kwon, Y.S.; Chun, W. Quercetin-3-O-β-D-glucuronide suppresses lipopolysaccharide-induced JNK and ERK phosphorylation in LPS-challenged RAW264. 7 cells. Biomol. Ther. 2016, 24, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S.; et al. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264. 7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 2018, 41, 888–898. [Google Scholar] [PubMed]
- Uesugi, M.; Nakajima, K.; Tohyama, Y.; Kohsaka, S.; Kurihara, T. Nonparticipation of nuclear factor kappa B (NFκB) in the signaling cascade of c-Jun N-terminal kinase (JNK)-and p38 mitogen-activated protein kinase (p38MAPK)-dependent tumor necrosis factor alpha (TNFα) induction in lipopolysaccharide (LPS)-stimulated microglia. Brain Res. J. 2006, 1073, 48–59. [Google Scholar]
- Ashina, K.; Tsubosaka, Y.; Nakamura, T.; Omori, K.; Kobayashi, K.; Hori, M.; Ozaki, H.; Murata, T. Histamine induces vascular hyperpermeability by increasing blood flow and endothelial barrier disruption in vivo. PLoS ONE 2015, 10, e0132367. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A.; Kehrli, M.E., Jr.; Ackermann, M.R. Cell adhesion molecules, leukocyte trafficking, and strategies to reduce leukocyte infiltration. J. Vet. Intern. Med. 2001, 15, 516–529. [Google Scholar] [CrossRef]
- Back, Y.D.; Lee, H.S.; Ku, S.K. Effects of DHU001, a mixed herbal formula on acute inflammation in mice. Toxicol. Res. 2008, 24, 189–194. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.J.; Bak, S.G.; Park, E.J.; Ku, S.-K.; Lee, S.; Lee, S.W.; Lee, K.M.; Lee, S.-J.; Rho, M.-C. Retrofractamide C Derived from Piper longum Alleviates Xylene-Induced Mouse Ear Edema and Inhibits Phosphorylation of ERK and NF-κB in LPS-Induced J774A.1. Molecules 2020, 25, 4058. https://doi.org/10.3390/molecules25184058
Lim HJ, Bak SG, Park EJ, Ku S-K, Lee S, Lee SW, Lee KM, Lee S-J, Rho M-C. Retrofractamide C Derived from Piper longum Alleviates Xylene-Induced Mouse Ear Edema and Inhibits Phosphorylation of ERK and NF-κB in LPS-Induced J774A.1. Molecules. 2020; 25(18):4058. https://doi.org/10.3390/molecules25184058
Chicago/Turabian StyleLim, Hyung Jin, Seon Gyeong Bak, Eun Jae Park, Sae-Kwang Ku, Soyoung Lee, Seung Woong Lee, Kang Min Lee, Seung-Jae Lee, and Mun-Chual Rho. 2020. "Retrofractamide C Derived from Piper longum Alleviates Xylene-Induced Mouse Ear Edema and Inhibits Phosphorylation of ERK and NF-κB in LPS-Induced J774A.1" Molecules 25, no. 18: 4058. https://doi.org/10.3390/molecules25184058
APA StyleLim, H. J., Bak, S. G., Park, E. J., Ku, S.-K., Lee, S., Lee, S. W., Lee, K. M., Lee, S.-J., & Rho, M.-C. (2020). Retrofractamide C Derived from Piper longum Alleviates Xylene-Induced Mouse Ear Edema and Inhibits Phosphorylation of ERK and NF-κB in LPS-Induced J774A.1. Molecules, 25(18), 4058. https://doi.org/10.3390/molecules25184058




