Liraglutide Increases Serum Levels of MicroRNA-27b, -130a and -210 in Patients with Type 2 Diabetes Mellitus: A Novel Epigenetic Effect
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Biochemical Analyses
4.2. Extraction of miRNAs and Stem-Loop Real-Time Polymerase Chain Reaction
5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cianflone, D.; Rizvi, A.A.; Rizzo, M. Microvascular and macrovascular effects of liraglutide. Int. J. Cardiol. 2019, 286, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.M.; Wan, T.F.; Wang, Y.; Yang, Q.W. Cardiovascular outcomes of liraglutide in patients with type 2 diabetes: A systematic review and meta-analysis. Medicine 2019, 98, e17860. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.A.; Patti, A.M.; Giglio, R.V.; Nikolic, D.; Amato, A.; Al-Busaidi, N.; Al-Rasadi, K.; Soresi, M.; Banach, M.; Montalto, G.; et al. Liraglutide improves carotid intima-media thickness in patients with type 2 diabetes and non-alcoholic fatty liver disease: An 8-month prospective pilot study. Expert Opin. Biol. Ther. 2015, 15, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Sachinidis, A.; Nikolic, D.; Stoian, A.P.; Papanas, N.; Tarar, O.; Rizvi, A.A.; Rizzo, M. Cardiovascular outcomes trials with incretin-based medications: A critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors. Metabolism 2020, 111, 154343. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [Green Version]
- Simonson, B.; Das, S. MicroRNA Therapeutics: The Next Magic Bullet? Mini Rev. Med. Chem. 2015, 15, 467–474. [Google Scholar] [CrossRef]
- Romaine, S.P.; Tomaszewski, M.; Condorelli, G.; Samani, N.J. MicroRNAs in cardiovascular disease: An introduction for clinicians. Heart 2015, 101, 921–928. [Google Scholar] [CrossRef]
- Brennan, E.; Wang, B.; McClelland, A.; Mohan, M.; Marai, M.; Beuscart, O.; Derouiche, S.; Gray, S.; Pickering, R.; Tikellis, C.; et al. Protective Effect of let-7 miRNA Family in Regulating Inflammation in Diabetes-Associated Atherosclerosis. Diabetes 2017, 66, 2266–2277. [Google Scholar] [CrossRef] [Green Version]
- Regazzi, R. MicroRNAs as therapeutic targets for the treatment of diabetes mellitus and its complications. Expert Opin. Ther. Targets 2018, 22, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.L. Elucidating the contributory role of microRNA to cardiovascular diseases (a review). Vascul. Pharmacol. 2019, 114, 31–48. [Google Scholar] [CrossRef]
- Shang, J.; Li, J.; Keller, M.P.; Hohmeier, H.E.; Wang, Y.; Feng, Y.; Zhou, H.H.; Shen, X.; Rabaglia, M.; Soni, M.; et al. Induction of miR-132 and miR-212 Expression by Glucagon-Like Peptide 1 (GLP-1) in Rodent and Human Pancreatic beta-Cells. Mol. Endocrinol. 2015, 29, 1243–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Li, Q.; Wang, W.; Guo, L.; Guo, C.; Sun, Y.; Zhang, J. GLP-1 contributes to increases in PGC-1alpha expression by downregulating miR-23a to reduce apoptosis. Biochem. Biophys. Res. Commun. 2015, 466, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Capuani, B.; Pacifici, F.; Della-Morte, D.; Lauro, D. Glucagon Like Peptide 1 and MicroRNA in Metabolic Diseases: Focusing on GLP1 Action on miRNAs. Front. Endocrinol. (Lausanne) 2018, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cao, H.; Zhuang, J.; Wan, J.; Guan, M.; Yu, B.; Li, X.; Zhang, W. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin. Chim. Acta 2011, 412, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Bostjancic, E.; Zidar, N.; Glavac, D. MicroRNA microarray expression profiling in human myocardial infarction. Dis. Markers 2009, 27, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.S.; Volsi, G.L.; Pitruzzella, A.; Fiore, V.; Mangiafico, M.; Vanella, L.; Parenti, R.; Rizzo, M.; Volti, G.L. Circulating miR-130a, miR-27b, and miR-210 in Patients With Peripheral Artery Disease and Their Potential Relationship With Oxidative Stress. Angiology 2016, 67, 945–950. [Google Scholar] [CrossRef]
- Sun, L.; Trajkovski, M. MiR-27 orchestrates the transcriptional regulation of brown adipogenesis. Metabolism: Clin. Exp. 2014, 63, 272–282. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 968–976. [Google Scholar] [CrossRef]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.I.; Katsura, A.; Mihira, H.; Horie, M.; Saito, A.; Miyazono, K. Regulation of TGF-beta-mediated endothelial-mesenchymal transition by microRNA-27. J. Biochem. 2017, 161, 417–420. [Google Scholar] [CrossRef] [Green Version]
- Qun, L.; Wenda, X.; Weihong, S.; Jianyang, M.; Wei, C.; Fangzhou, L.; Zhenyao, X.; Pingjin, G. miRNA-27b modulates endothelial cell angiogenesis by directly targeting Naa15 in atherogenesis. Atherosclerosis 2016, 254, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Li, D.; Yang, J.; Xie, J.; Yu, F.; Ma, Y.; Zhu, X.; Zhao, J.; Lv, Z. MicroRNA-130a Targets MAP3K12 to Modulate Diabetic Endothelial Progenitor Cell Function. Cell. Physiol. Biochem. 2015, 36, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhu, M.; Mao, X.; Long, M.; Du, X.; Wu, Y.; Abudureyimu, K.; Zhang, C.; Wang, Y.; Tao, Y.; et al. MicroRNA-130a expression is decreased in Xinjiang Uygur patients with type 2 diabetes mellitus. Am. J. Transl. Res. 2015, 7, 1984–1991. [Google Scholar] [PubMed]
- Jiang, Y.R.; Du, J.Y.; Wang, D.D.; Yang, X. miRNA-130a improves cardiac function by down-regulating TNF-alpha expression in a rat model of heart failure. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8454–8461. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.Y.; Liu, P.; Zhou, H.T.; Sun, W.X.; Song, J.; Shu, J.; Cui, G.J.; Yang, Z.J.; Jia, E.Z. Circular RNAs promote TRPM3 expression by inhibiting hsa-miR-130a-3p in coronary artery disease patients. Oncotarget 2017, 8, 60280–60290. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.W.; Chen, Z.H.; Ding, X.Q.; Liu, J.Y.; Ge, P.C.; An, F.H.; Li, L.H.; Wang, L.S.; Ma, W.Z.; Yang, Z.J.; et al. Predictive Effects of Circulating miR-221, miR-130a and miR-155 for Coronary Heart Disease: A Multi-Ethnic Study in China. Cell. Physiol. Biochem. 2017, 42, 808–823. [Google Scholar] [CrossRef]
- Devlin, C.; Greco, S.; Martelli, F.; Ivan, M. miR-210: More than a silent player in hypoxia. IUBMB Life 2011, 63, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Song, X.; Sun, W.; Wang, Y.; Liu, B. Effect of Hypoxia-Induced MicroRNA-210 Expression on Cardiovascular Disease and the Underlying Mechanism. Oxid. Med. Cell. Longev. 2019, 2019, 4727283. [Google Scholar] [CrossRef]
- Li, J.; Su, L.; Gong, Y.Y.; Ding, M.L.; Hong, S.B.; Yu, S.; Xiao, H.P. Downregulation of miR-139-5p contributes to the antiapoptotic effect of liraglutide on the diabetic rat pancreas and INS-1 cells by targeting IRS1. PLoS ONE 2017, 12, e0173576. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M. A glucagon-like peptide-1 analog, liraglutide, ameliorates endothelial dysfunction through miRNAs to inhibit apoptosis in rats. PeerJ 2019, 7, e6567. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Chen, J.; Hu, L.; Liang, M.; Wang, X.; Feng, S.; Shen, J.; Luan, X. Liraglutide regulates the viability of pancreatic alpha-cells and pancreatic beta-cells through cAMP-PKA signal pathway. Life Sci. 2018, 195, 87–94. [Google Scholar] [CrossRef]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic Complications of Diabetes Mellitus: A Mini Review. Curr. Diabetes Rev. 2017, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Corrado, E.; Rizzo, M.; Coppola, G.; Muratori, I.; Carella, M.; Novo, S. Endothelial dysfunction and carotid lesions are strong predictors of clinical events in patients with early stages of atherosclerosis: A 24-month follow-up study. Coron. Artery Dis. 2008, 19, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020, 63, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauck, M.; Rizzo, M.; Johnson, A.; Bosch-Traberg, H.; Madsen, J.; Cariou, B. Once-Daily Liraglutide Versus Lixisenatide as Add-on to Metformin in Type 2 Diabetes: A 26-Week Randomized Controlled Clinical Trial. Diabetes Care 2016, 39, 1501–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Martinez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Diaz-Lopez, A.; Drevon, C.A.; et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patti, A.M.; Al-Rasadi, K.; Giglio, R.V.; Nikolic, D.; Mannina, C.; Castellino, G.; Chianetta, R.; Banach, M.; Cicero, A.F.G.; Lippi, G.; et al. Natural approaches in metabolic syndrome management. Arch. Med. Sci. 2018, 14, 422–441. [Google Scholar] [CrossRef]
- Toth, P.P.; Patti, A.M.; Nikolic, D.; Giglio, R.V.; Castellino, G.; Biancucci, T.; Geraci, F.; David, S.; Montalto, G.; Rizvi, A.; et al. Bergamot Reduces Plasma Lipids, Atherogenic Small Dense LDL, and Subclinical Atherosclerosis in Subjects with Moderate Hypercholesterolemia: A 6 Months Prospective Study. Front. Pharmacol. 2015, 6, 299. [Google Scholar] [CrossRef] [Green Version]
- Andrikou, E.; Tsioufis, C.; Andrikou, I.; Leontsinis, I.; Tousoulis, D.; Papanas, N. GLP-1 receptor agonists and cardiovascular outcome trials: An update. Hell. J. Cardiol. 2019, 60, 347–351. [Google Scholar] [CrossRef]
- Rizzo, M.; Nikolic, D.; Banach, M.; Patti, A.M.; Montalto, G.; Rizvi, A.A. Incretin-based therapies, glucometabolic health and endovascular inflammation. Curr. Pharm. Des. 2014, 20, 4953–4960. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, M.; Rizvi, A.A.; Spinas, G.A.; Rini, G.B.; Berneis, K. Glucose lowering and anti-atherogenic effects of incretin-based therapies: GLP-1 analogues and DPP-4-inhibitors. Expert Opin. Investig. Drugs 2009, 18, 1495–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, M.; Abate, N.; Chandalia, M.; Rizvi, A.A.; Giglio, R.V.; Nikolic, D.; Marino Gammazza, A.; Barbagallo, I.; Isenovic, E.R.; Banach, M.; et al. Liraglutide reduces oxidative stress and restores heme oxygenase-1 and ghrelin levels in patients with type 2 diabetes: A prospective pilot study. J. Clin. Endocrinol. Metab. 2015, 100, 603–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Li, C.; Yang, C.; Li, B.; Wei, J.; Lin, Y.; Ye, P.; Hu, G.; Li, J. Liraglutide reduces hepatic glucolipotoxicityinduced liver cell apoptosis through NRF2 signaling in Zucker diabetic fatty rats. Mol. Med. Rep. 2018, 17, 8316–8324. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Fang, Q.H.; Cui, Y.T.; Shen, Q.L.; Liu, Q.; Wang, P.H.; Yu, D.M.; Li, C.J. Liraglutide prevents beta-cell apoptosis via inactivation of NOX2 and its related signaling pathway. J. Diabetes Complicat. 2019, 33, 267–277. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, X.; Teng, X. Liraglutide attenuates myocardial ischemia/reperfusion injury possibly through reducing cardiomyocytes apoptosis and oxidation in rats. Zhonghua Xin Xue Guan Bing Za Zhi 2015, 43, 259–263. [Google Scholar]
- Rizzo, M.; Chandalia, M.; Patti, A.M.; di Bartolo, V.; Rizvi, A.A.; Montalto, G.; Abate, N. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-Month prospective pilot study. Cardiovasc. Diabetol. 2014, 13, 49. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, M.; Rizvi, A.A.; Patti, A.M.; Nikolic, D.; Giglio, R.V.; Castellino, G.; Li Volti, G.; Caprio, M.; Montalto, G.; Provenzano, V.; et al. Liraglutide improves metabolic parameters and carotid intima-media thickness in diabetic patients with the metabolic syndrome: An 18-month prospective study. Cardiovasc. Diabetol. 2016, 15, 162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.Y.; Gong, Y.L.; Li, C.J.; Qi, Q.; Zhang, Q.M.; Yu, D.M. Circulating MiRNA biomarkers serve as a fingerprint for diabetic atherosclerosis. Am. J. Transl. Res. 2016, 8, 2650–2658. [Google Scholar]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef]
- Kang, T.; Lu, W.; Xu, W.; Anderson, L.; Bacanamwo, M.; Thompson, W.; Chen, Y.E.; Liu, D. MicroRNA-27 (miR-27) targets prohibitin and impairs adipocyte differentiation and mitochondrial function in human adipose-derived stem cells. J. Biol. Chem. 2013, 288, 34394–34402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, S.A.; Kalluri, R. Angiogenesis is controlled by miR-27b associated with endothelial tip cells. Blood 2012, 119, 2439–2440. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.D.; Shen, Y.; Wei, X.; Zhang, F.Q.; Liu, Y.Y.; Ma, L. Inhibitory effect of microRNA-27b on interleukin 17 (IL-17)-induced monocyte chemoattractant protein-1 (MCP1) expression. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, H.; Li, H.; Gao, Z.; Song, K. Atrial overexpression of microRNA-27b attenuates angiotensin II-induced atrial fibrosis and fibrillation by targeting ALK5. Hum. Cell 2018, 31, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Vickers, K.C.; Shoucri, B.M.; Levin, M.G.; Wu, H.; Pearson, D.S.; Osei-Hwedieh, D.; Collins, F.S.; Remaley, A.T.; Sethupathy, P. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology 2013, 57, 533–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Zhang, X.; Zhou, Z.; Sun, B.; Gu, W.; Liu, J.; Zhang, H. Liraglutide inhibits the proliferation and promotes the apoptosis of MCF-7 human breast cancer cells through downregulation of microRNA-27a expression. Mol. Med. Rep. 2018, 17, 5202–5212. [Google Scholar] [CrossRef] [Green Version]
- Ofori, J.K.; Salunkhe, V.A.; Bagge, A.; Vishnu, N.; Nagao, M.; Mulder, H.; Wollheim, C.B.; Eliasson, L.; Esguerra, J.L. Elevated miR-130a/miR130b/miR-152 expression reduces intracellular ATP levels in the pancreatic beta cell. Sci. Rep. 2017, 7, 44986. [Google Scholar] [CrossRef] [Green Version]
- Perri, R.; Nares, S.; Zhang, S.; Barros, S.P.; Offenbacher, S. MicroRNA modulation in obesity and periodontitis. J. Dent. Res. 2012, 91, 33–38. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiang, L.; Chen, M.; Xiang, C. MicroRNA130a inhibits the proliferation, migration and invasive ability of hepatocellular carcinoma cells by downregulating Rhokinase 2. Mol. Med. Rep. 2018, 18, 3077–3084. [Google Scholar] [CrossRef]
- Mantovani, A.; Targher, G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: Spotlight on nonalcoholic fatty liver disease. Ann. Transl. Med. 2017, 5, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha Thi, H.T.; Kim, H.Y.; Kim, Y.M.; Hong, S. MicroRNA-130a modulates a radiosensitivity of rectal cancer by targeting SOX4. Neoplasia 2019, 21, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Peeters, P.J.; Bazelier, M.T.; Leufkens, H.G.; de Vries, F.; de Bruin, M.L. The risk of colorectal cancer in patients with type 2 diabetes: Associations with treatment stage and obesity. Diabetes Care 2015, 38, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jung, Y.D.; Choi, Y.S.; Lee, Y.M. Targeting of RUNX3 by miR-130a and miR-495 cooperatively increases cell proliferation and tumor angiogenesis in gastric cancer cells. Oncotarget 2015, 6, 33269–33278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, K.S.; Chan, E.W.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Diabetes Increases Risk of Gastric Cancer After Helicobacter pylori Eradication: A Territory-Wide Study With Propensity Score Analysis. Diabetes Care 2019, 42, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Fasanaro, P.; D’Alessandra, Y.; di Stefano, V.; Melchionna, R.; Romani, S.; Pompilio, G.; Capogrossi, M.C.; Martelli, F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J. Biol. Chem. 2008, 283, 15878–15883. [Google Scholar] [CrossRef] [Green Version]
- Zaccagnini, G.; Maimone, B.; di Stefano, V.; Fasanaro, P.; Greco, S.; Perfetti, A.; Capogrossi, M.C.; Gaetano, C.; Martelli, F. Hypoxia-induced miR-210 modulates tissue response to acute peripheral ischemia. Antioxid. Redox Signal. 2014, 21, 1177–1188. [Google Scholar] [CrossRef] [Green Version]
- Eken, S.M.; Jin, H.; Chernogubova, E.; Li, Y.; Simon, N.; Sun, C.; Korzunowicz, G.; Busch, A.; Backlund, A.; Osterholm, C.; et al. MicroRNA-210 Enhances Fibrous Cap Stability in Advanced Atherosclerotic Lesions. Circ. Res. 2017, 120, 633–644. [Google Scholar] [CrossRef]
- Diao, H.; Liu, B.; Shi, Y.; Song, C.; Guo, Z.; Liu, N.; Song, X.; Lu, Y.; Lin, X.; Li, Z. MicroRNA-210 alleviates oxidative stress-associated cardiomyocyte apoptosis by regulating BNIP3. Biosci. Biotechnol. Biochem. 2017, 81, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, M.; Nikolic, D.; Patti, A.M.; Mannina, C.; Montalto, G.; McAdams, B.S.; Rizvi, A.A.; Cosentino, F. GLP-1 receptor agonists and reduction of cardiometabolic risk: Potential underlying mechanisms. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Manning, P.; Katare, R. Cardiovascular microRNAs: As modulators and diagnostic biomarkers of diabetic heart disease. Cardiovasc. Diabetol. 2014, 13, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athyros, V.G.; Katsiki, N.; Karagiannis, A. Is Targeting microRNAs the Philosopher’s Stone for Vascular Disease? Curr. Vasc. Pharmacol. 2016, 14, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Katsiki, N.; Karagiannis, A. Editorial: microRNAs: Potential Targets for the Treatment of Cardiovascular Disease. Curr. Vasc. Pharmacol. 2015, 13, 366–367. [Google Scholar] [CrossRef]
- Hu, S.; Huang, M.; Li, Z.; Jia, F.; Ghosh, Z.; Lijkwan, M.A.; Fasanaro, P.; Sun, N.; Wang, X.; Martelli, F.; et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 2010, 122, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Zhang, Z.; Wang, L. Small interference RNA against PTP-1B reduces hypoxia/reoxygenation induced apoptosis of rat cardiomyocytes. Apoptosis 2008, 13, 383–393. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Katsiki, N.; Behnam, B.; Iranpanah, H.; Sahebkar, A. MicroRNAs and type 2 diabetes mellitus: Molecular mechanisms and the effect of antidiabetic drug treatment. Metabolism 2018, 87, 48–55. [Google Scholar] [CrossRef]
- Li, J.; Fu, L.Z.; Liu, L.; Xie, F.; Dai, R.C. Glucagon-Like Peptide-1 (GLP-1) Receptor Agonist Liraglutide Alters Bone Marrow Exosome-Mediated miRNA Signal Pathways in Ovariectomized Rats with Type 2 Diabetes. Med. Sci. Monit. 2017, 23, 5410–5419. [Google Scholar] [CrossRef] [Green Version]
- Quintanilha, B.J.; Reis, B.Z.; Duarte, G.B.S.; Cozzolino, S.M.F.; Rogero, M.M. Nutrimiromics: Role of microRNAs and Nutrition in Modulating Inflammation and Chronic Diseases. Nutrients 2017, 9, 1168. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; de Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef] [Green Version]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO: Body Mass Index—BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 19 August 2020).
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Nagele, U.; Hagele, E.O.; Sauer, G.; Wiedemann, E.; Lehmann, P.; Wahlefeld, A.W.; Gruber, W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J. Clin. Chem. Clin. Biochem. 1984, 22, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Warnick, G.R.; Nguyen, T.; Albers, A.A. Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin. Chem. 1985, 31, 217–222. [Google Scholar] [CrossRef] [PubMed]
| Variable | |
|---|---|
| Age (years), mean ± sd | 64.6 ± 8.4 |
| Women, n (%) | 9 (36) |
| Smoking habit, n (%) | 10 (40) |
| Family history of cardiovascular diseases, n (%) | 14 (56) |
| Diabetes duration (years), mean ± sd | 9.6 ± 7.1 |
| Systolic blood pressure (mmHg), mean ± sd | 128.2 ± 19.3 |
| Diastolic blood pressure (mmHg), mean ± sd | 79.4 ± 7.7 |
| Heart rate, bpm | 78 ± 9 |
| Hypertension, n (%) | 18 (72) |
| Dyslipidaemia, n (%) | 19 (76) |
| Obesity, n (%) | 16 (64) |
| Antihypertensive Therapies | |
| Beta-blockers, n (%) | 12 (48) |
| Angiotensin-converting enzyme inhibitors, n (%) | 7 (28) |
| Calcium entry blockers, n (%) | 7 (28) |
| Diuretics, n (%) | 8 (32) |
| Lipid-lowering Drugs | |
| Statins, n (%) | 13 (52) |
| Omega-3 fatty acids, n (%) | 7 (28) |
| Fibrates, n (%) | 0 (0) |
| Aspirin use, n (%) | 7 (28) |
| Baseline | After 4 Months | p-Value | |
|---|---|---|---|
| Weight (kg), mean ± sd | 78.2 ± 12.4 | 75 ± 10.9 | 0.0609 * |
| BMI (kg/m2), mean ± sd | 29.2 ± 4.3 | 28.3 ± 3.5 | 0.0645 * |
| Waist circumference (cm), mean ± sd | 105.7 ± 10.7 | 103.2 ± 10.8 | 0.0894 * |
| Fasting glucose (mmol/L), median (IQR) | 8.4 (3.4) | 6.8 (2.4) | 0.0052 † |
| HbA1c (%), median (IQR) | 8.3 (0.6) | 6.2 (1.5) | 0.0009 † |
| Total cholesterol (mmol/L), mean ± sd | 5.0 ± 1.0 | 4.0 ± 0.7 | 0.0007 * |
| Triglycerides (mmol/L), mean ± sd | 1.9 ± 1.0 | 1.5 ± 0.8 | 0.0478 * |
| HDL-C (mmol/L), mean ± sd | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.7659 * |
| LDL-C (mmol/L), mean ± sd | 2.9 ± 1.2 | 2.2 ± 0.6 | 0.0150 * |
| Fold Change | p-Value * | |
|---|---|---|
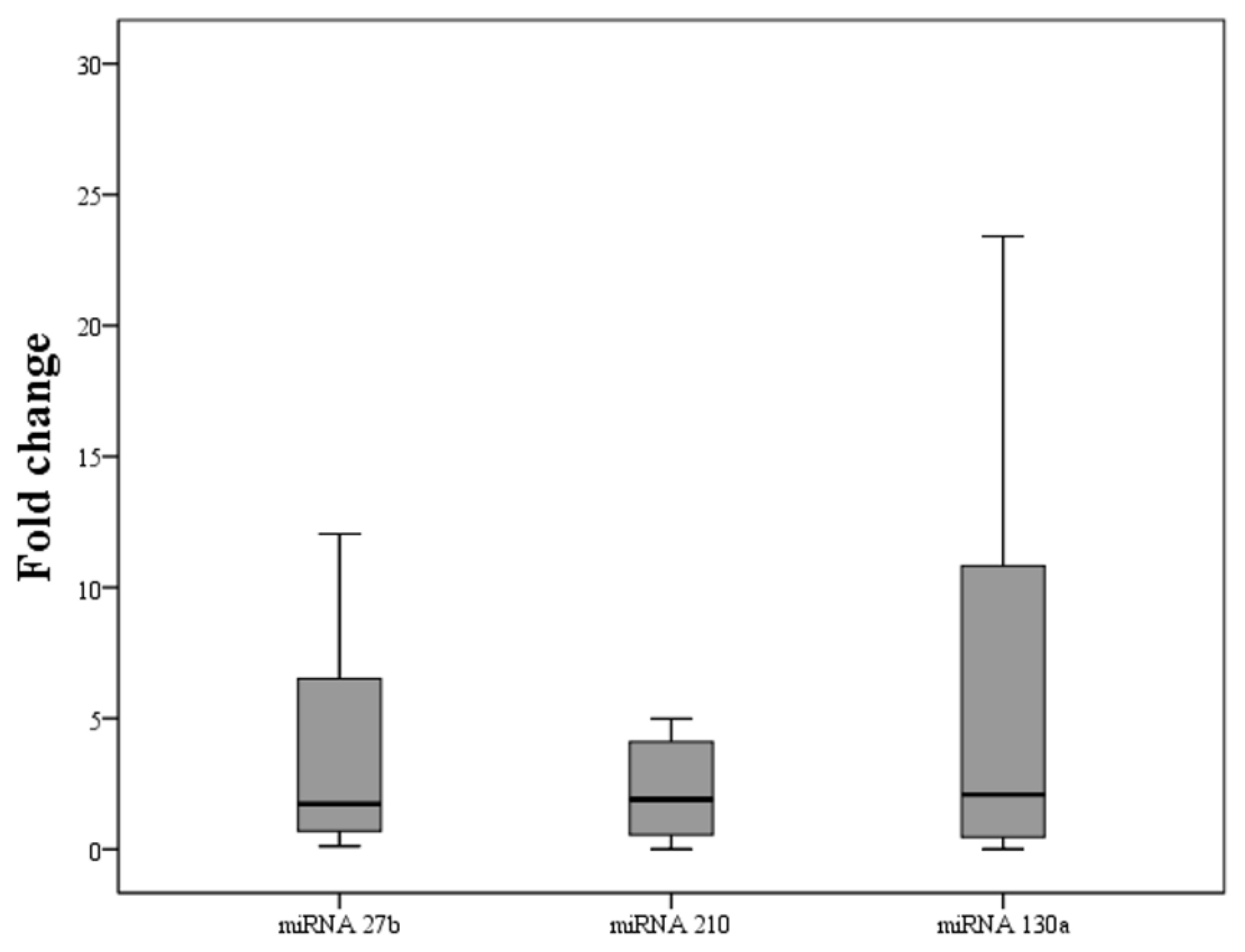
| miRNA-27b | 1.73 (7.12) | 0.0401 |
| miRNA-130a | 1.91 (3.64) | 0.0401 |
| miRNA-210 | 2.09 (11.0) | 0.0486 |
| miRNA-27b | miRNA-130a | miRNA-210 | ||||
|---|---|---|---|---|---|---|
| Correlation Coefficient (r2) | p-Value | Correlation Coefficient (r2) | p-Value | Correlation Coefficient (r2) | p-Value | |
| Weight | 0.187 | 0.4433 | 0.069 | 0.7805 | 0.062 | 0.7998 |
| BMI | 0.179 | 0.4633 | 0.066 | 0.7889 | 0.072 | 0.7670 |
| Waist circumference | 0.332 | 0.1643 | 0.244 | 0.3146 | 0.165 | 0.5009 |
| Fasting glucose | 0.072 | 0.7699 | 0.169 | 0.4881 | 0.008 | 0.9744 |
| HbA1c | −0.172 | 0.4813 | −0.199 | 0.4133 | −0.373 | 0.1156 |
| Total cholesterol | 0.260 | 0.2830 | 0.104 | 0.6731 | 0.221 | 0.3629 |
| Triglycerides | 0.098 | 0.6885 | 0.317 | 0.1855 | 0.001 | 0.9971 |
| HDL cholesterol | −0.198 | 0.4159 | −0.322 | 0.1887 | −0.333 | 0.1641 |
| LDL cholesterol | 0.256 | 0.2898 | 0.220 | 0.3648 | 0.408 | 0.0828 |
| miRNA ID | Reverse-Transcribed Primers (48-bp Stem-Loop + 6-bp miRNA-Specific Sequence) | Forward | Reverse (Common Sequence) |
|---|---|---|---|
| Hsa-miRNA-27b | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGCAGAA | ATGCTTCACAGTGGCT | GTGCGGTCGTGGAGTC |
| Hsa-miRNA-130a | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACATGCCC | ATGCCAGTGCAATGTT | GTGCGGTCGTGGAGTC |
| Hsa-miRNA-210 | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTCAGCC | ATGCCTGTGCGTGTGA | GTGCGGTCGTGGAGTC |
| Common Reverse Primers | GTGCGTGTCGTGGAGTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giglio, R.V.; Nikolic, D.; Volti, G.L.; Stoian, A.P.; Banerjee, Y.; Magan-Fernandez, A.; Castellino, G.; Patti, A.M.; Chianetta, R.; Castracani, C.C.; et al. Liraglutide Increases Serum Levels of MicroRNA-27b, -130a and -210 in Patients with Type 2 Diabetes Mellitus: A Novel Epigenetic Effect. Metabolites 2020, 10, 391. https://doi.org/10.3390/metabo10100391
Giglio RV, Nikolic D, Volti GL, Stoian AP, Banerjee Y, Magan-Fernandez A, Castellino G, Patti AM, Chianetta R, Castracani CC, et al. Liraglutide Increases Serum Levels of MicroRNA-27b, -130a and -210 in Patients with Type 2 Diabetes Mellitus: A Novel Epigenetic Effect. Metabolites. 2020; 10(10):391. https://doi.org/10.3390/metabo10100391
Chicago/Turabian StyleGiglio, Rosaria Vincenza, Dragana Nikolic, Giovanni Li Volti, Anca Pantea Stoian, Yajnavalka Banerjee, Antonio Magan-Fernandez, Giuseppa Castellino, Angelo Maria Patti, Roberta Chianetta, Carlo Castruccio Castracani, and et al. 2020. "Liraglutide Increases Serum Levels of MicroRNA-27b, -130a and -210 in Patients with Type 2 Diabetes Mellitus: A Novel Epigenetic Effect" Metabolites 10, no. 10: 391. https://doi.org/10.3390/metabo10100391
APA StyleGiglio, R. V., Nikolic, D., Volti, G. L., Stoian, A. P., Banerjee, Y., Magan-Fernandez, A., Castellino, G., Patti, A. M., Chianetta, R., Castracani, C. C., Montalto, G., Rizvi, A. A., Sesti, G., & Rizzo, M. (2020). Liraglutide Increases Serum Levels of MicroRNA-27b, -130a and -210 in Patients with Type 2 Diabetes Mellitus: A Novel Epigenetic Effect. Metabolites, 10(10), 391. https://doi.org/10.3390/metabo10100391









