Kinetic and Equilibrium Studies of Doxorubicin Adsorption onto Carbon Nanotubes
Abstract
:1. Introduction
2. Results and Discussion
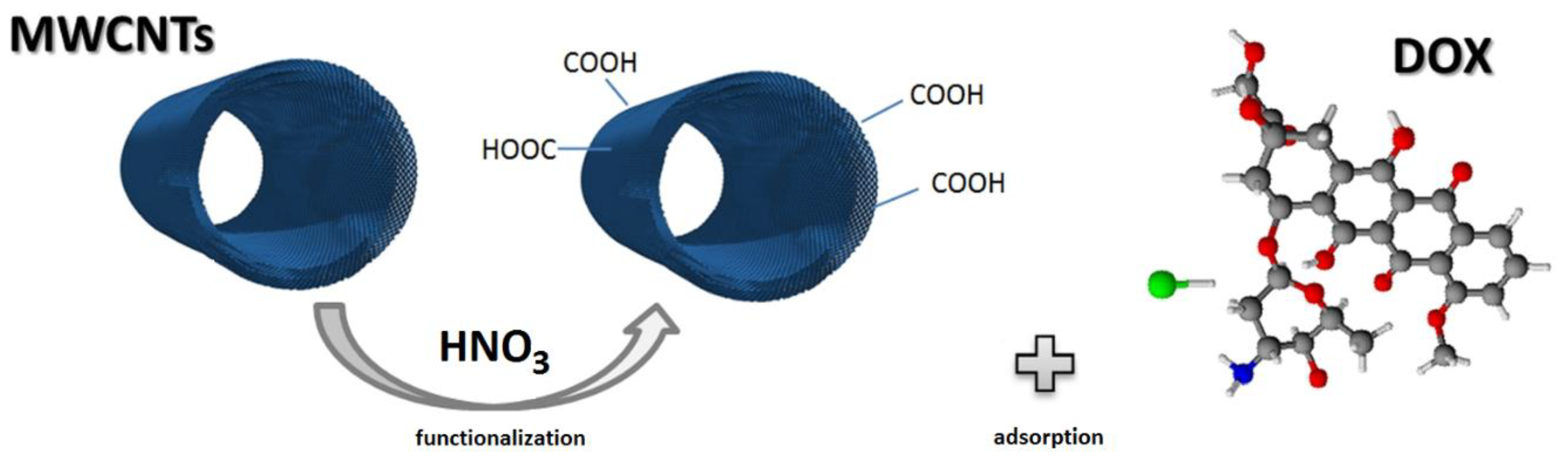
2.1. Qualitative and Quantitative Determination of Surface Functional Groups on Modified Multiwall Carbon Nanotubes
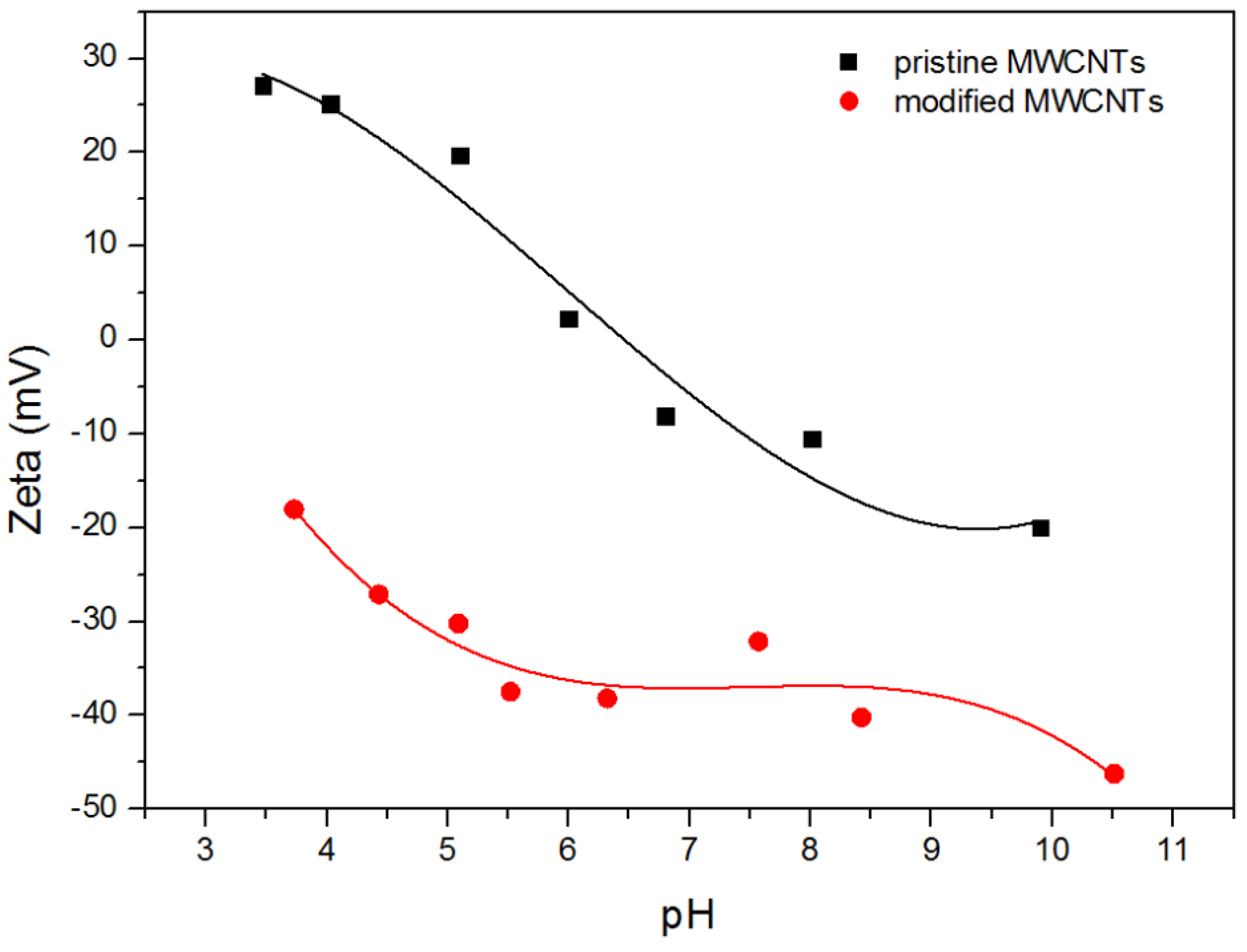
2.1.1. Zeta Potential
2.1.2. Energy-Dispersive X-ray Spectroscopy
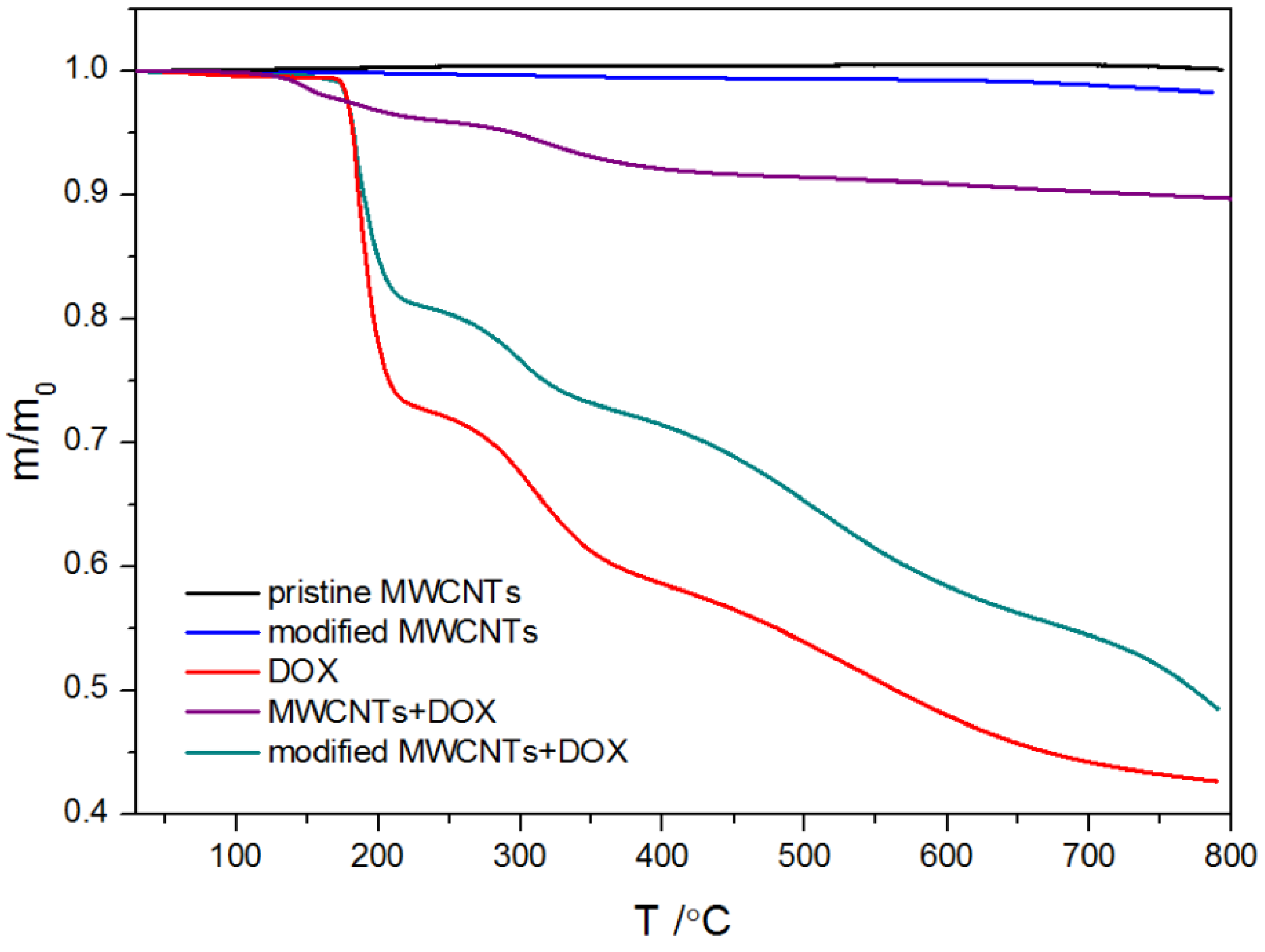
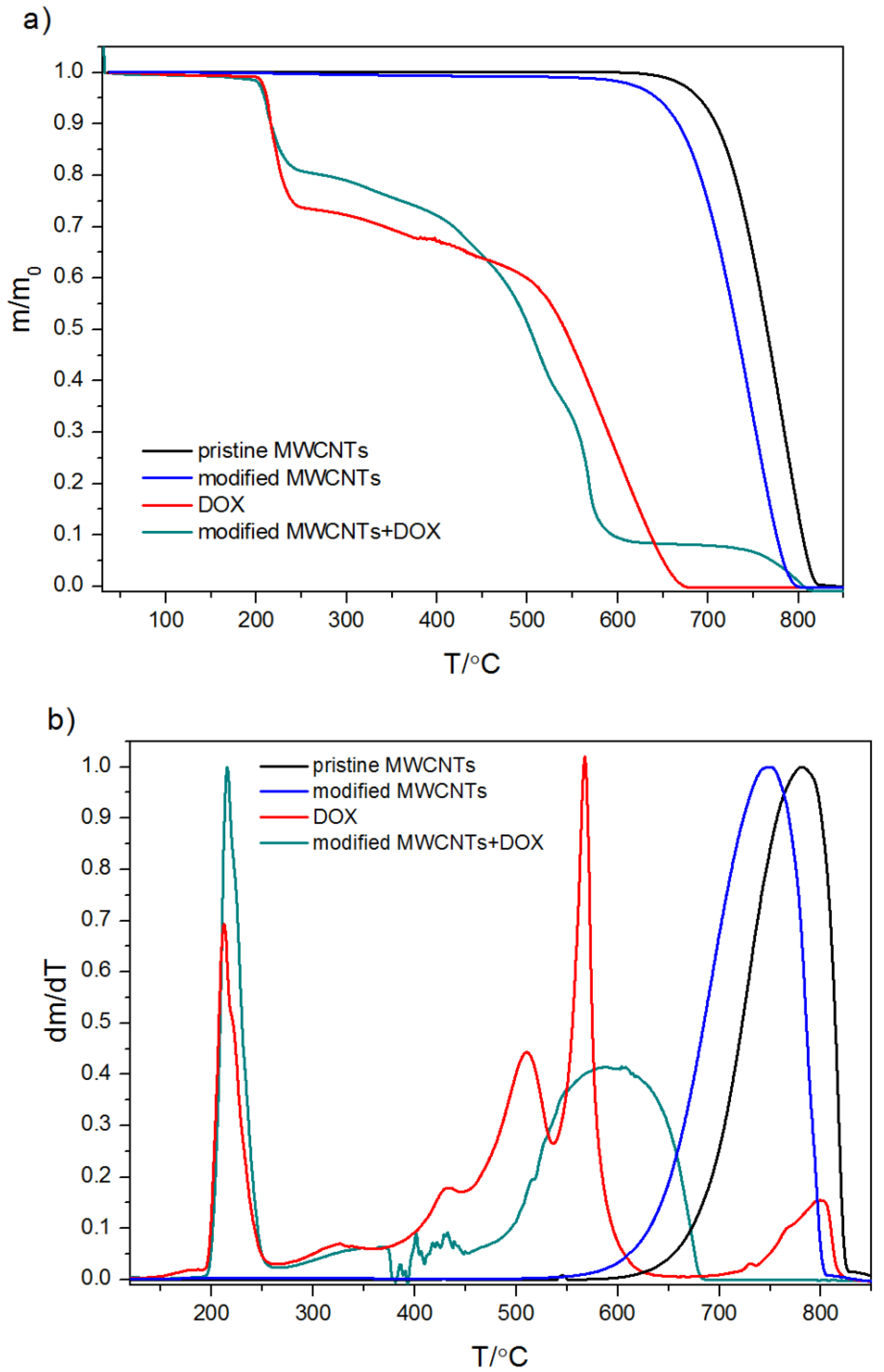
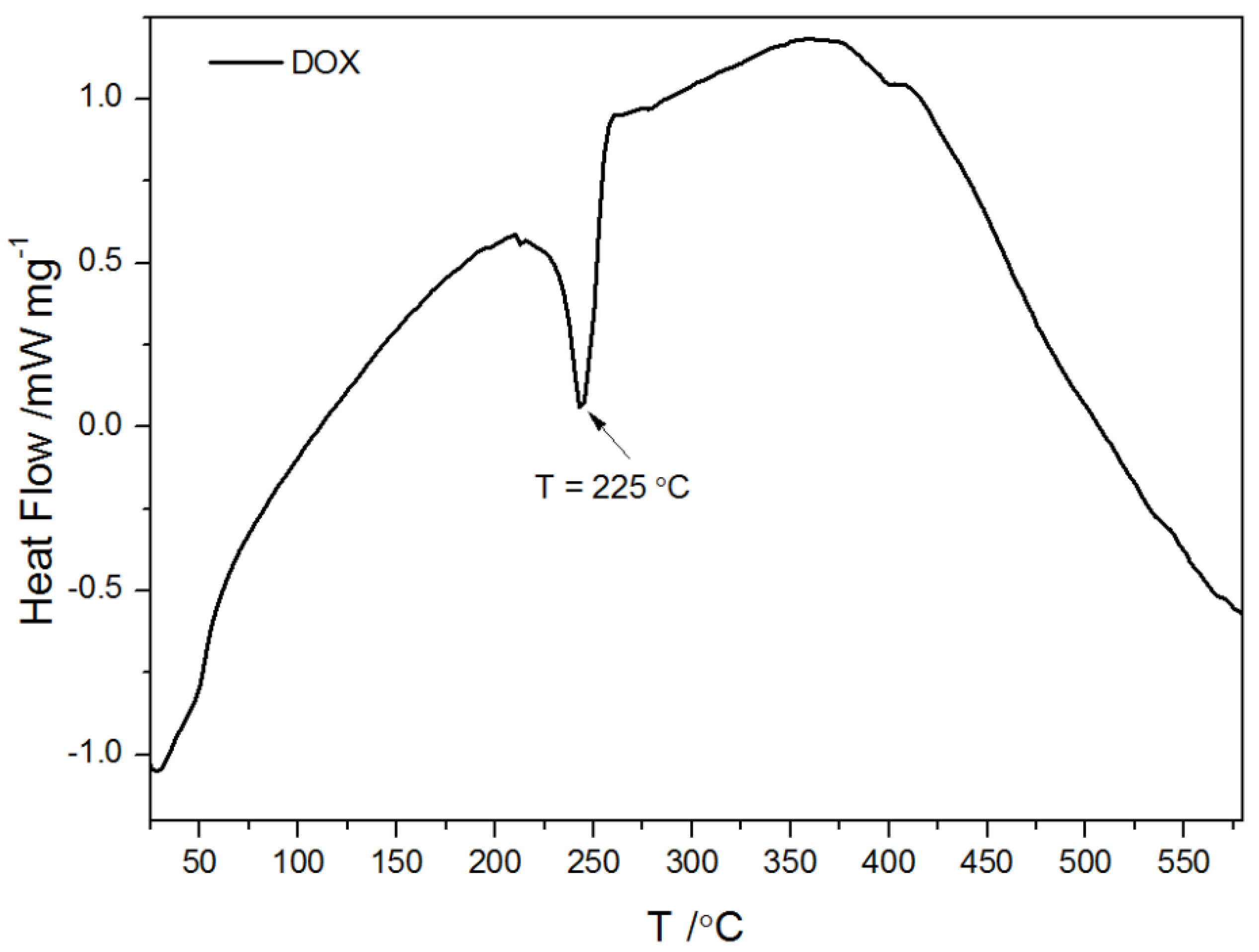
2.1.3. Thermogravimetric Analysis and Differential Scanning Calorimetry
2.2. Adsorption of DOX on Carbon Nanotubes
2.2.1. Computer Simulation for Adsorption of DOX on CNTs
2.2.2. Adsorption Isotherm of DOX Adsorption on Pristine MWCNTs and Modified MWCNTs
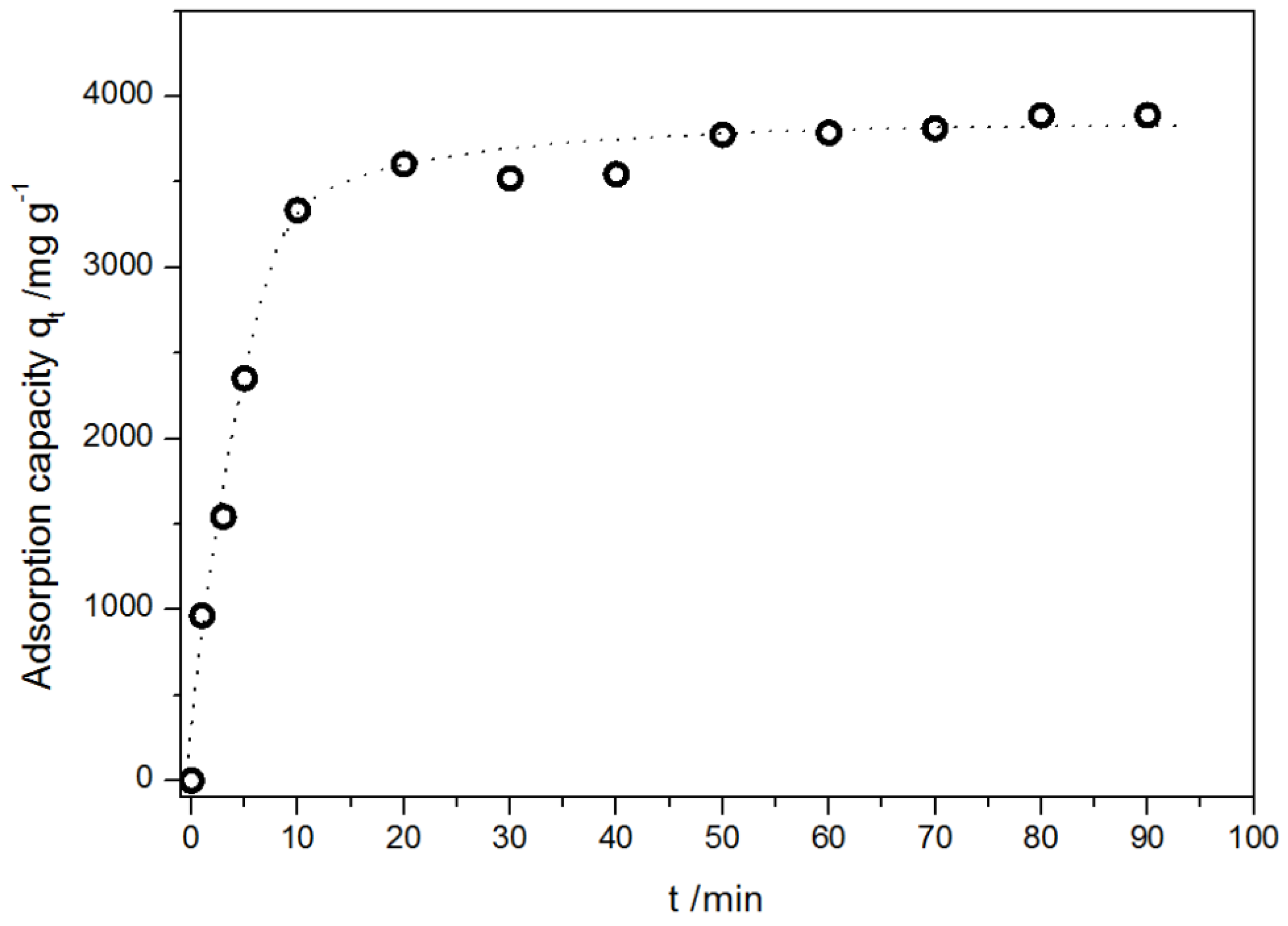
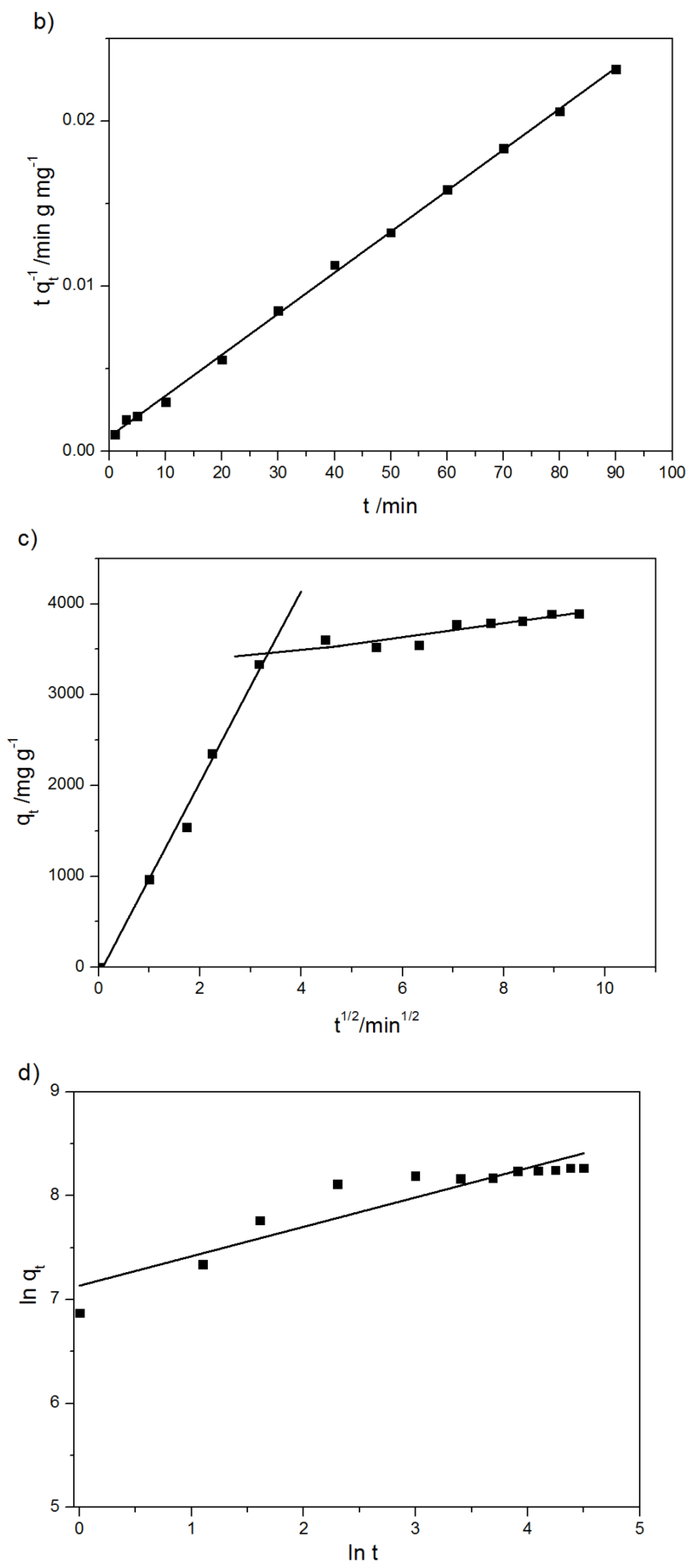
2.2.3. Kinetics of Adsorption
2.2.4. Mechanism of Adsorption
3. Experimental
3.1. Materials
Functionalization of MWCNTs
3.2. Methods
3.2.1. Thermal Analysis
3.2.2. UV–VIS Spectroscopy
3.2.3. Zeta Potential
3.2.4. Adsorption of DOX on Functionalized MWCNTs
3.2.5. Determination of Adsorption Kinetics
3.2.6. Molecular Dynamics Simulations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singal, P.K.; Iliskovic, N. Doxorubicin-Induced Cardiomyopathy. N. Engl. J. Med. 1998, 339, 900–905. [Google Scholar] [CrossRef]
- Singal, P.; Li, T.; Kumar, D.; Danelisen, I.; Iliskovic, N. Adriamycin-induced heart failure: Mechanisms and modulation. Mol. Cell. Biochem. 2000, 207, 77–86. [Google Scholar] [CrossRef]
- Abdullah, C.S.; Alam, S.; Aishwarya, R.; Miriyala, S.; Bhuiyan, M.A.N.; Panchatcharam, M.; Pattillo, C.B.; Orr, A.W.; Sadoshima, J.; Hill, J.A.; et al. Doxorubicin-induced cardiomyopathy associated with inhibition of autophagic degradation process and defects in mitochondrial respiration. Sci. Rep. 2019, 9, 1–20. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.V.N.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological overviews from bench to bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef]
- Ewer, M.S.; Ewer, S.M. Cardiotoxicity of anticancer treatments: What the cardiologist needs to know. Nat. Rev. Cardiol. 2010, 7, 564–575. [Google Scholar] [CrossRef]
- Scheede-Bergdahl, C.; Jagoe, R.T. After the chemotherapy: Potential mechanisms for chemotherapy-induced delayed skeletal muscle dysfunction in survivors of acute lymphoblastic leukaemia in childhood. Front. Pharmacol. 2013, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Suh, S.; Jo, A.; Traore, M.A.; Zhan, Y.; Coutermarsh-Ott, S.; Ringel-Scaia, V.M.; Allen, I.C.; Davis, R.M.; Behkam, B. Nanoscale Bacteria-Enabled Autonomous Drug Delivery System (NanoBEADS) Enhances Intratumoral Transport of Nanomedicine. Adv. Sci. 2018, 6, 1801309. [Google Scholar] [CrossRef] [Green Version]
- Zgurskaya, H.I.; Weeks, J.W.; Ntreh, A.T.; Nickels, L.M.; Ewolloscheck, D. Mechanism of coupling drug transport reactions located in two different membranes. Front. Microbiol. 2015, 6, 100. [Google Scholar] [CrossRef] [Green Version]
- Heimann, D.M.; Rosenberg, S.A. Continuous Intravenous Administration of Live Genetically Modified Salmonella Typhimurium in Patients with Metastatic Melanoma. J. Immunother. 2003, 26, 179–180. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Bahreman, A.; Daudey, G.; Bussmann, J.; Olsthoorn, R.C.L.; Kros, A. Drug delivery via cell membrane fusion using lipopeptide modified liposome. ACS Cent. Sci. 2016, 28, 621–630. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Ahmad, R.R.H.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [Green Version]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. ChemBiomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, A.; Yadav, T.; Sharma, S.; Nayak, A.; Kumari, A.; Mishra, N. Polymers for drug delivery. J. Biosci. Med. 2016, 4, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Chaniotakis, N.A.; Thermos, K.; Kalomiraki, M. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2015, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lala, R.; Thorat, A.; Gargote, C. Current trends in β-cyclodextrin based drug delivery systems. Int. J. Res. Ayurveda Pharm. 2011, 2, 1520–1526. [Google Scholar]
- Orozco, J.; Campuzano, S.; Kagan, D.; Zhou, M.; Gao, W.; Wang, J. Dynamic Isolation and Unloading of Target Proteins by Aptamer-Modified Microtransporters. Anal. Chem. 2011, 83, 7962–7969. [Google Scholar] [CrossRef] [Green Version]
- Paxton, W.F.; Sundararajan, S.; Mallouk, T.E.; Sen, A. Chemical locomotion. Angew. Chem. Int. Ed. 2006, 45, 5420–5429. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Balasubramanian, S.; Kagan, D.; Manesh, K.M.; Campuzano, S.; Wang, J. Motion-based DNA detection using catalytic nanomotors. Nat. Commun. 2010, 1, 36. [Google Scholar] [CrossRef]
- Sánchez, S.; Ananth, A.N.; Fomin, V.M.; Viehrig, M.; Schmidt, O.G. Superfast Motion of Catalytic Microjet Engines at Physiological Temperature. J. Am. Chem. Soc. 2011, 133, 14860–14863. [Google Scholar] [CrossRef]
- Fischer, T.; Agarwal, A.; Hess, H. A smart dust biosensor powered by kinesin motors. Nat. Nanotechnol. 2009, 4, 162–166. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.; Mia, M.; Rahman, M.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fam, D.; Palaniappan, A.; Tok, A.; Liedberg, B.; Moochhala, S. A review on technological aspects influencing commercialization of carbon nanotube sensors. Sens. Actuators B Chem. 2011, 157, 1–7. [Google Scholar] [CrossRef]
- Harrison, B.S.; Atala, A. Carbon nanotube applications for tissue engineering. Biomaterials 2007, 28, 344–353. [Google Scholar] [CrossRef]
- Fisher, C.; Han, Z.; Levchenko, I.; Ostrikov, K. Control of dense carbon nanotube arrays via hierarchical multilayer catalyst. Appl. Phys. Lett. 2011, 99, 143104. [Google Scholar] [CrossRef]
- Gruner, G. Carbon nanotube transistors for biosensing applications. Anal. Bioanal. Chem. 2005, 384, 322–335. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon Nanotubes: Applications in Pharmacy and Medicine. BioMed. Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.-W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A Review of Carbon Nanotube Toxicity and Assessment of Potential Occupational and Environmental Health Risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar] [CrossRef]
- Lacerda, L.; Bianco, A.; Prato, M.; Kostarelos, K. Carbon nanotubes as nanomedicines: From toxicology to pharmacology. Adv. Drug Deliv. Rev. 2006, 58, 1460–1470. [Google Scholar] [CrossRef]
- Shim, M.; Kam, N.W.S.; Chen, R.J.; Li, Y.; Dai, H. Functionalization of Carbon Nanotubes for Biocompatibility and Biomolecular Recognition. Nano Lett. 2002, 2, 285–288. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef]
- Wu, W.; Wieckowski, S.; Pastorin, G.; Benincasa, M.; Klumpp, C.; Briand, J.-P.; Gennaro, R.; Prato, M.; Bianco, A. Targeted Delivery of Amphotericin B to Cells by Using Functionalized Carbon Nanotubes. Cheminform 2006, 37, 6358–6362. [Google Scholar] [CrossRef]
- Zhou, F.; Xing, D.; Ou, Z.; Wu, B.; Resasco, D.E.; Chen, W.R. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J. Biomed. Opt. 2009, 14, 021009. [Google Scholar] [CrossRef]
- Gannon, C.J.; Cherukuri, P.; Yakobson, B.I.; Cognet, L.; Kanzius, J.S.; Kittrell, C.; Weisman, R.B.; Pasquali, M.; Schmidt, H.K.; Smalley, R.E.; et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer 2007, 110, 2654–2665. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, D.; Mays, J.W.; Zhang, X.P.; Bratcher, M.S.; Zhang, X.P. Carbon Nanotubes with Covalently Linked Porphyrin Antennae: Photoinduced Electron Transfer. J. Am. Chem. Soc. 2005, 127, 6916–6917. [Google Scholar] [CrossRef] [Green Version]
- Panczyk, T.; Wolski, P.; Lajtar, L. Coadsorption of Doxorubicin and Selected Dyes on Carbon Nanotubes. Theoretical Investigation of Potential Application as a pH-Controlled Drug Delivery System. Langmuir 2016, 32, 4719–4728. [Google Scholar] [CrossRef]
- Rungnim, C.; Rungrotmongkol, T.; Poo-Arporn, R.P. pH-controlled doxorubicin anticancer loading and release from carbon nanotube noncovalently modified by chitosan: MD simulations. J. Mol. Graph. Model. 2016, 70, 70–76. [Google Scholar] [CrossRef]
- Hao, G.; Xu, Z.P.; Li, L. Manipulating extracellular tumour pH: An effective target for cancer therapy. RSC Adv. 2018, 8, 22182–22192. [Google Scholar] [CrossRef] [Green Version]
- Gómez, S.; Rendtorff, N.M.; Aglietti, E.F.; Sakka, Y.; Suarez, G. Intensity of sulfonitric treatment on multiwall carbon nanotubes. Chem. Phys. Lett. 2017, 689, 135–141. [Google Scholar] [CrossRef]
- Wang, Y.; Iqbal, A.Z.; Mitra, S. Rapidly Functionalized, Water-Dispersed Carbon Nanotubes at High Concentration. J. Am. Chem. Soc. 2006, 128, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mitra, S. Fast Microwave-Assisted Purification, Functionalization and Dispersion of Multi-Walled Carbon Nanotubes. J. Nanosci. Nanotechnol. 2008, 8, 5770–5775. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Brown, J.M.; Chen, R.; Ke, P.C.; Lai, X.; Mitra, S.; Witzmann, F.A. Comparison of Nanotube-Protein Corona Composition in Cell Culture Media. Small 2013, 9, 2171–2181. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.-T.; Wang, Y.; Liu, Y.; Wang, H. Adsorption and desorption of doxorubicin on oxidized carbon nanotubes. Colloids Surf. B Biointerfaces 2012, 97, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Farahani, B.V.; Behbahani, G.R.; Javadi, N. Functionalized Multi Walled Carbon Nanotubes as a Carrier for Doxorubicin: Drug Adsorption Study and Statistical Optimization of Drug Loading by Factorial Design Methodology. J. Braz. Chem. Soc. 2015, 27, 694–705. [Google Scholar] [CrossRef]
- Vanyoreka, L.; Meszarosa, R.; Barany, S. Surface and electrosurface characterization of surface-oxidized multi-walled N-doped carbon nanotubes. Colloid Surf. A 2014, 448, 140–146. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intraparticle diffusion processes during acid dye adsorption on chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef]
- Geng, X.; Jing, J.; Cen, Y.; Datta, R.; Liang, J. In Situ Synthesis and characterization of polyethyleneimine-modified carbon nanotubes supported PtRuelectrocatalyst for methanol oxidation. J. Nanomater. 2015, 2015, 296589. [Google Scholar] [CrossRef] [Green Version]
- Pu, Y.; Yang, X.; Zheng, H.; Wang, D.; Su, Y.; He, J. Adsorption and desorption of thallium(I) on multiwalled carbon nanotubes. Chem. Eng. J. 2013, 219, 403–410. [Google Scholar] [CrossRef]
- Li, W.; Xiao, L.; Qin, C. Synthesis and relevant electrochemical properties of 2-hydroxypropyltrimethyl ammonium chloride chitosan-grafted multiwalled carbon nanotubes. J. Mater. Sci. 2010, 45, 5915–5922. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Aksu, Z. Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions onto Chlorella vulgaris. Process. Biochem. 2002, 38, 89–99. [Google Scholar] [CrossRef]
- Langmiur, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; Zhu, Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Song, L.; Mashayekhi, H.; Chefetz, B.; Xing, B. Adsorption and desorption of phenanthrene on carbon nanotubes in simulated gastrointestinal fluids. Environ. Sci. Technol. 2011, 45, 6018–6024. [Google Scholar] [CrossRef]
- Malek, S.K.; Gabris, M.A.; Jume, B.H.; Baradaran, R.; Aziz1, M.; Karim, K.J.B.A.; Nodeh, H.R. Adsorption and in vitro release study of curcumin form polyethyleneglycol functionalized multi walled carbon nanotube: Kinetic and isotherm study. Daru 2019, 27, 9–20. [Google Scholar] [CrossRef]
- Temkin, M.; Pyzhev, V. Kinetics of the synthesis of ammonia on promoted iron catalysts. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Nwabue, F.I.; Itumoh, E.J. Adsorption isotherm and kinetic modeling of a novel procedure for physical modification of silica gel using aqueous solutions of 4,4′-(1,2-ethanediyldinitrilo)bis-(2-pentanone) for preconcentration of Ni(II) ion. Sep. Sci. Technol. 2019, 55, 2919–2932. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Sadik, Z.; Mahdi, D.K.; Alshrefi, S.M.; Al-Sammarraie, A.M.; Alamgir, F.M.; Singh, P.M.; Aljeboree, A.M. Preparation, structure and adsorption properties of synthesized multiwall carbon nanotubes for highly effective removal of maxilon blue dye. Korean J. Chem. Eng. 2015, 32, 2456–2462. [Google Scholar] [CrossRef]
- Shahbeig, H.; Bagheri, N.; Ghorbanian, S.A.; Hallajisani, A.; Poorkarimi, S. A new adsorption isotherm model of aqueous solutions on granular activated carbon. World J. Model. Simul. 2013, 9, 243–254. [Google Scholar]
- Khan, T.A.; Chaudhry, S.A.; Ali, I. Equilibrium uptake, isotherm and kinetic studies of cd (II) adsorption onto iron oxide activated red mud from aqueous solution. J. Mol. Liq. 2015, 202, 165–175. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, X.; Han, M.; Ma, W. Adsorption kinetic character of cooper ions onto a modified chitosan transparent thin membrane from aqueous solution. J. Hazard. Mater. 2010, 182, 408–415. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, L.; Lu, Q.; Fei, Z.; Dyson, P.J. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials 2009, 30, 6041–6047. [Google Scholar] [CrossRef]
- Enayatpour, B.; Rajabi, M.; Moradi, O.; Asdolehzade, N.; Nayak, A.; Agarwal, S.; Gupta, V.K. Adsorption kinetics of lysozyme on multi-walled carbon nanotubes and amino functionalized multi-walled carbon nanotubes from aqueous solution. J. Mol. Liq. 2018, 254, 93–97. [Google Scholar] [CrossRef]
- Yoo, H.S.; Lee, K.H.; Oh, J.E.; Park, T.G. In Vitro of nanoparticles based on doxorubicin-PLGA conjugates. J. Control Release 2000, 68, 419–431. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, M.; Neelesh, J.; Keerti, J.N.K. Biomedical applications of carbon nanotubes: A critical review. Curr. Drug Deliv. 2016, 13, 796–817. [Google Scholar] [CrossRef] [PubMed]
- Dettlaff-Weglikowska, U.; Benoit, J.-M.; Chiu, P.-W.; Graupner, R.; Lebedkin, S.; Roth, S. Chemical functionalization of single walled carbon nanotubes. Curr. Appl. Phys. 2002, 2, 497–501. [Google Scholar] [CrossRef]
- Sanson, C.; Schatz, C.; Le Meins, J.-F.; Soum, A.; Thévenot, J.; Garanger, E.; Lecommandoux, S. A simple method to achieve high doxorubicin loading in biodegradable polymersomes. J. Control. Release 2010, 147, 428–435. [Google Scholar] [CrossRef]
- Berendsen, H.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.; Fraaije, J. LINCS: A linear constraint solver for molecular simulations. J. Comput Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Minoia, A. YASC. Available online: http://chembytes.wikidot.com/buildcstruct (accessed on 15 October 2020).
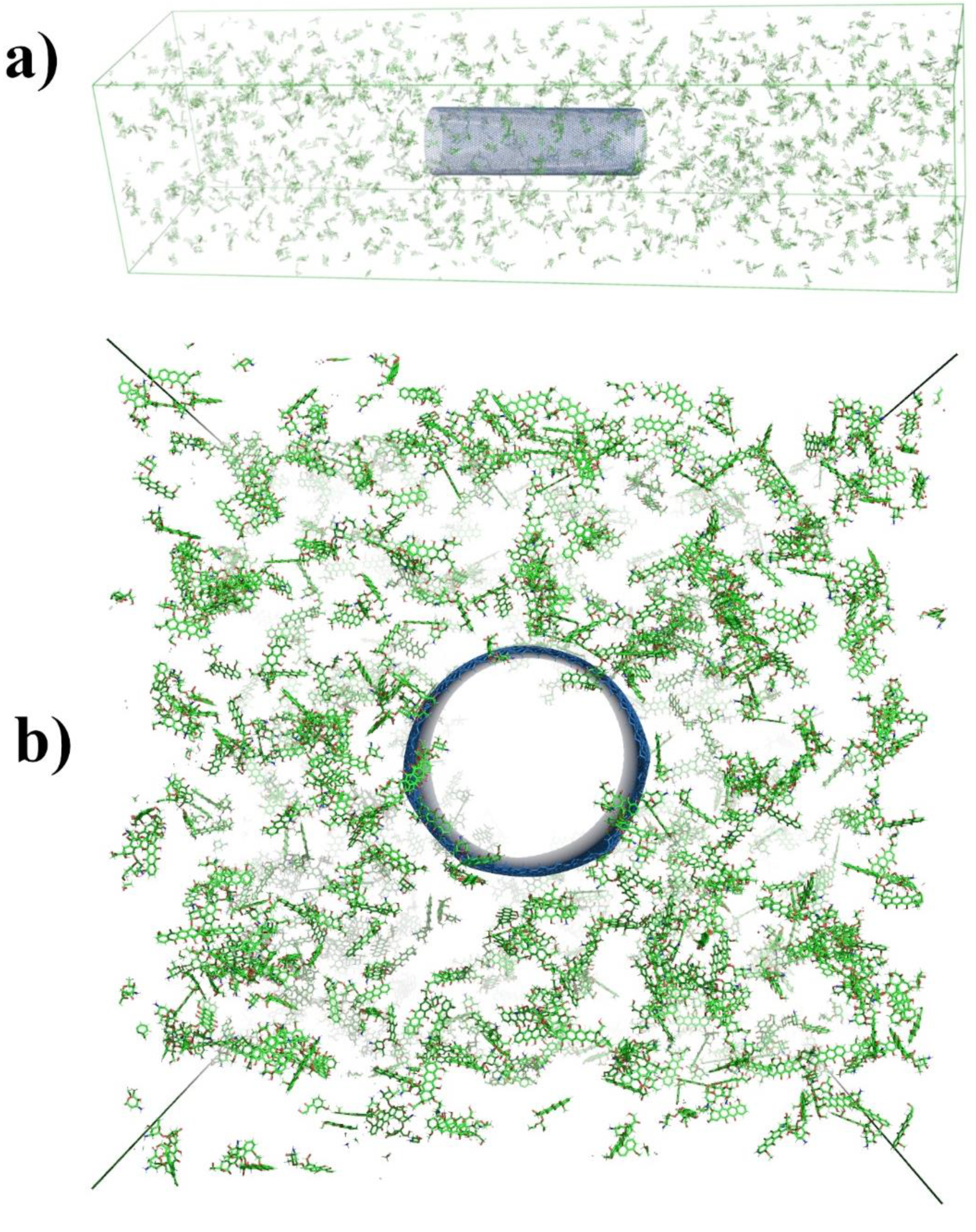

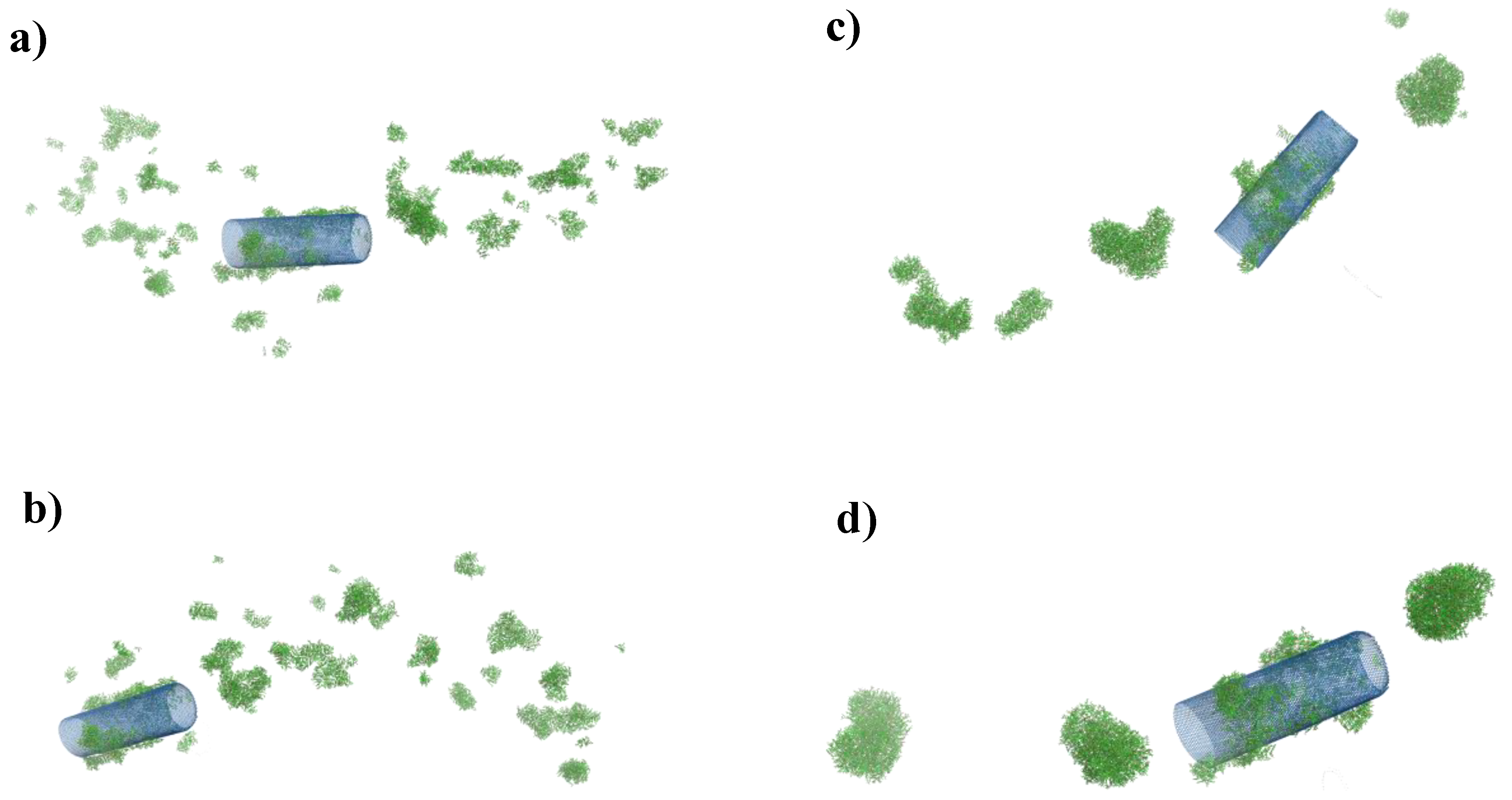
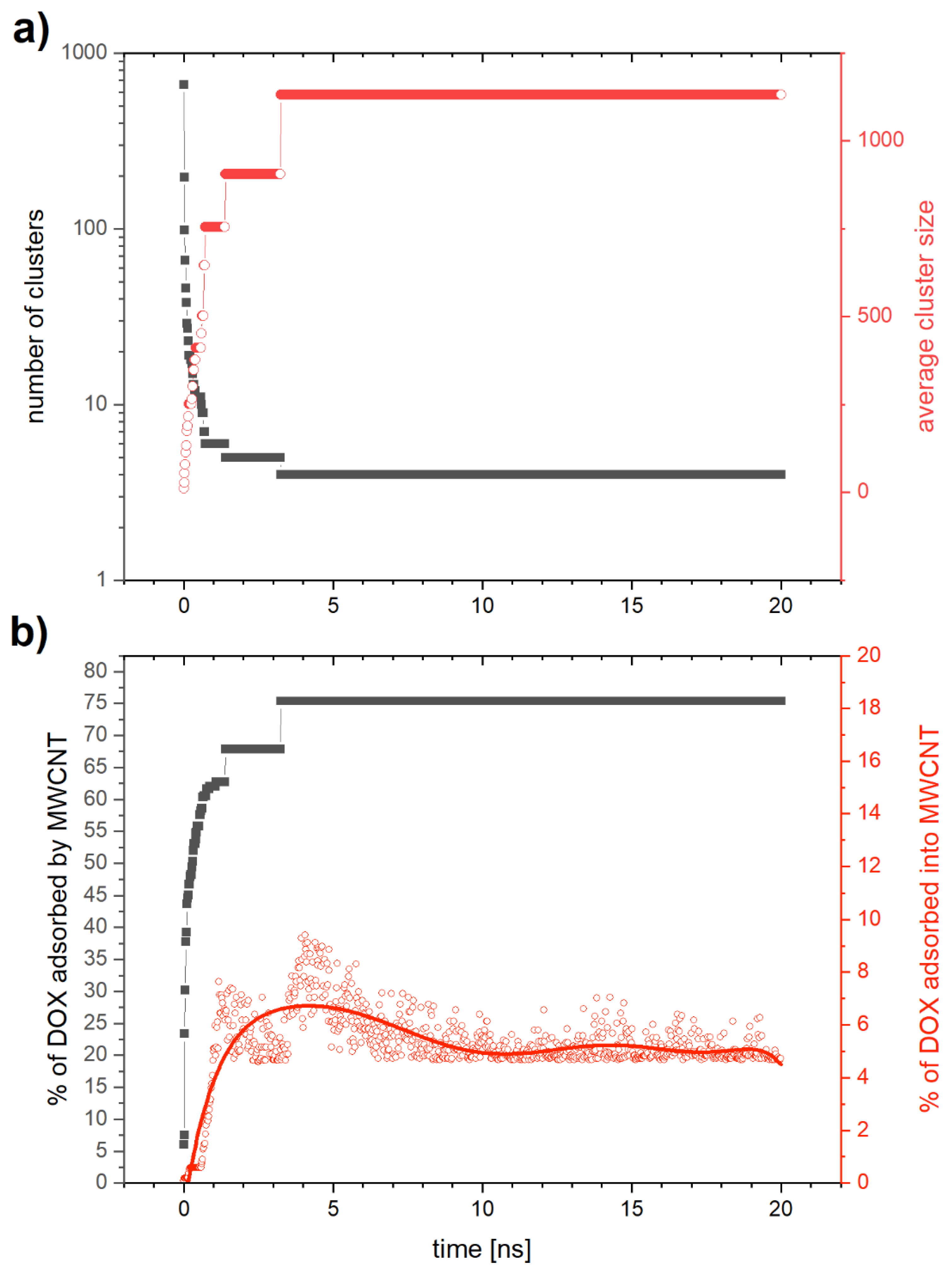
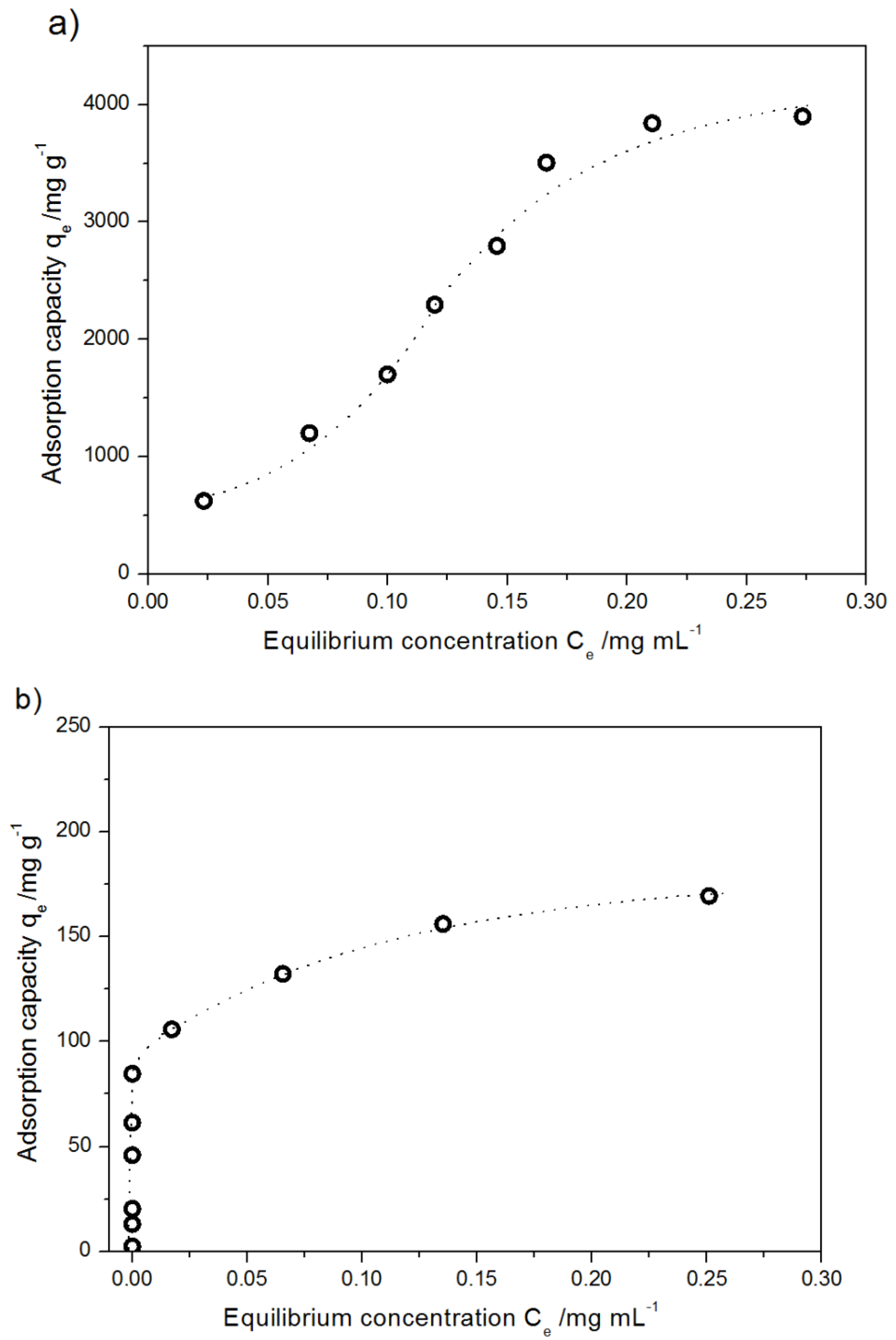


| Material | Carbon (wt %) | Nitrogen (wt %) | Oxygen (wt %) | Chlorine (wt %) |
|---|---|---|---|---|
| MWCNTs | 97.84 | 1.22 | 0.94 | 0.00 |
| modified MWCNTs | 95.75 | 1.46 | 2.79 | 0.00 |
| DOX-MWCNTs | 74.28 | 3.36 | 17.42 | 3.52 |
| DOX | 64.10 | 3.85 | 25.84 | 6.21 |
| Freundlich | Langmuir | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1/n | kF | R2 | qmax mg g−1 | KL mL g−1 | R2 | b kJ mol−1 | kt mL g−1 | R2 | |
| Pristine MWCNTs | 0.1725 ± 0.009 | 64.99 ± 6.10 | 0.9918 | 185.2 ± 20.4 | 0.0492 ± 0.004 | 0.9985 | 0.104 ± 0.009 | 4.70 ± 0.04 | 0.9891 |
| Modified MWCNTs | 0.8234 ± 0.063 | 42.61 ± 8.53 | 0.9658 | - | - | - | 0.00165 ± 0.00053 | 0.0464 ± 0.009 | 0.8750 |
| Kinetic Model | Parameters | Values |
|---|---|---|
| Pseudo-first order | qe/mg g−1 | 2290 ± 173 |
| k1/min−1 | 0.0110 ± 0.0009 | |
| R2 | 0.915 | |
| Pseudo-second order | qe/mg g−1 | 4029 ± 241 |
| k2/g mg−1 min−1 | 6.92 × 10−5 ± 0.16 × 10−5 | |
| R2 | 0.999 | |
| Intra-particle diffusion | k1/mg g−1 min −1/2 | 1058.6 ± 62.9 |
| C1/mg g−1 | 82.59 ± 2.4 | |
| R2 | 0.991 | |
| k2/mg g−1 min −1/2 | 77.12 ± 1.7 | |
| C2/mg g−1 | 317.0 ± 21.3 | |
| R2 | 0.903 | |
| Fractional power | v | 0.283 ± 0.09 |
| KFP | 1264.4 ± 74.4 | |
| R2 | 0.859 | |
| Elovich model | α/mg g−1 min −1 | 4136.2 ± 167.7 |
| β/g mg−1 | 0.001537 ± 0.00001 | |
| R2 | 0.904 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudoba, D.; Łudzik, K.; Jażdżewska, M.; Wołoszczuk, S. Kinetic and Equilibrium Studies of Doxorubicin Adsorption onto Carbon Nanotubes. Int. J. Mol. Sci. 2020, 21, 8230. https://doi.org/10.3390/ijms21218230
Chudoba D, Łudzik K, Jażdżewska M, Wołoszczuk S. Kinetic and Equilibrium Studies of Doxorubicin Adsorption onto Carbon Nanotubes. International Journal of Molecular Sciences. 2020; 21(21):8230. https://doi.org/10.3390/ijms21218230
Chicago/Turabian StyleChudoba, Dorota, Katarzyna Łudzik, Monika Jażdżewska, and Sebastian Wołoszczuk. 2020. "Kinetic and Equilibrium Studies of Doxorubicin Adsorption onto Carbon Nanotubes" International Journal of Molecular Sciences 21, no. 21: 8230. https://doi.org/10.3390/ijms21218230






