Differences in the Composition of Leachate from Active and Non-Operational Municipal Waste Landfills in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Physicochemical Composition of Leachate
3. Results
4. Discussion
5. Conclusions
- The results of the study of the physicochemical properties of leachate showed high variability. Statistically significant differences occurred between the values for active and non-operational landfills for most of the analyzed parameters. The leachate from active landfills was characterized by higher EC and COD values and TKN, ON, AN, chlorides, TS and TDS concentration. The leachate from non-operational landfills contained more sulfates.
- The age of the landfill also influenced the variability of the results—the values characterizing older landfills (non-operational in Wrocław and active in Legnica) were more stable. The results of research on leachate from landfills put into operation at the turn of the 20th and 21st century (Bielawa, Jawor) showed greater variability, which sometimes blurred the differences resulting from the exploitation methods. This trend was visible, among others, in the case of COD, ON, TS, iron and manganese.
- The concentrations of heavy metals in the analyzed leachates were characterized by relatively low variability. The lack of significant differences between active and non-operational landfills could result from changes in the method of municipal waste disposal, due to which less waste containing these components is now sent to landfills.
- The conducted analyses showed the existence of significant differences between the surveyed active and closed landfills. These differences were especially visible in the cases of the following: EC, COD, TKN, ON, AN, TS, TDS, TSS, sulfates, chlorides, sodium, potassium, calcium, magnesium and nickel. No significant differences were found between the concentrations of other heavy metals (Cu, Zn, Cr, Pb, Cd) analyzed as part of the monitoring.
- EC, COD, ON, AN, chlorides and Ca appear to be particularly useful for monitoring purposes. These parameters are specified in the literature as characteristic of leachates, and in the conducted tests they also clearly showed differences between the tested landfills. They can complement monitoring due to their clear differentiation and widespread occurrence in landfill leachate.
Author Contributions
Funding
Conflicts of Interest
References
- Hussein, M.; Yoneda, K.; Zaki, Z.M.; Othman, N.A.; Amir, A. Leachate characterizations and pollution indices of active and closed unlined landfills in Malaysia. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100232. [Google Scholar] [CrossRef]
- EC Council Directive 1999/31/EC of the 26 April 1999 on the landfill of waste. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31999L0031 (accessed on 15 June 2020).
- Naveen, B.P.; Mahapatra, D.M.; Sitharam, T.G.; Sivapullaiah, P.V.; Ramachandra, T.V. Physico-chemical and biological characterization of urban municipal landfill leachate. Environ. Pollut. 2017, 220, 1–12. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.H.; Kjeldsen, P.; Bjerg, P.L.; Jensen, D.L.; Christensen, J.B.; Baun, A.; Albrechtsen, H.J.; Heron, G. Biogeochemistry of landfill leachate plumes. Appl. Geochem. 2001, 16, 659–718. [Google Scholar] [CrossRef]
- Fatta, D.; Papadopoulos, A.; Loizidou, M. A study on the landfill leachate and its impact on the groundwater quality of the greater area. Environ. Geochem. Health 1999, 21, 175–190. [Google Scholar] [CrossRef]
- Fitzke, B.; Blume, T.; Wienands, H.; Cambiella, Á. Hybrid processes for the treatment of leachate from landfills. In Economic Sustainability and Environmental Protection in Mediterranean Countries through Clean Manufacturing Methods; Coca-Prados, J., Gutiérrez-Cervelló, G., Eds.; Springer: Dordrecht, Germany, 2013; Chapter 6; p. 155. [Google Scholar] [CrossRef]
- Kheradmand, S.; Karimi-Jashni, A.; Sartaj, M. Treatment of municipal landfill leachate using a combined anaerobic digester and activated sludge system. Waste Manag. 2010, 30, 1025–1031. [Google Scholar] [CrossRef]
- Ahamad, A.; Raju, N.J.; Madhav, S.; Gossel, W.; Wycisk, P. Impact of non-engineered Bhalswa landfill on groundwater from Quaternary alluvium in Yamuna flood plain and potential human health risk, New Delhi, India. Quat. Int. 2019, 507, 352–369. [Google Scholar] [CrossRef]
- Singh, S.; Raju, N.J.; Gossel, W.; Wycisk, P. Assessment of pollution potential of leachate from the municipal solid waste disposal site and its impact on groundwater quality, Varanasi environs, India. Arabian J. Geosci. 2016, 9, 131. [Google Scholar] [CrossRef]
- Tałałaj, I.A.; Biedka, P.; Bartkowska, I. Treatment of landfill leachates with biological pretreatments and reverse osmosis. Environ. Chem. Lett. 2019, 17, 1177–1193. [Google Scholar] [CrossRef] [Green Version]
- Longe, E.O.; Balogun, M.R. Groundwater quality assessment near a municipal landfill, Lagos, Nigeria. Res. J. Appl. Sci. Eng. Technol. 2010, 2, 39–44. [Google Scholar]
- Bhatt, A.H.; Karanjekar, R.V.; Altouqi, S.; Sattler, M.L.; Hossain, M.D.S.; Chen, V.P. Estimating landfill leachate BOD and COD based on rainfall, ambient temperature, and waste composition: Exploration of a MARS statistical approach. Environ. Technol. Innov. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Raghab, S.M.; Abd El Meguid, A.M.; Hegazi, H.A. Treatment of leachate from municipal solid waste landfill. HBRC J. 2013, 9, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Grand-Ducal regulation of 24 February 2003 concerning the landfill of waste. 2013. Available online: http://eli.legilux.public.lu/eli/etat/leg/rgd/2003/02/24/n2/jo (in French) (accessed on 10 June 2020).
- Ministry of the Environment O do T and E. Diário da República no 90/2015. Series I of 2015-05-11; 2015. Available online: https://dre.pt/home/-/dre/67185043/details/maximized (accessed on 10 June 2020). (In Portuguese)
- Minister of the Environment and Water 20/2006. (IV. 5.). Decree of the Ministry of Environment and Water on Certain Rules and Conditions Related to Landfilling and the Landfill; Official publication: Budapest, Hungary, 2006. [Google Scholar]
- The Landfill Regulations (Northern Ireland). 2003. Available online: https://www.legislation.gov.uk/nisr/2003/496/contents/made (accessed on 20 May 2020).
- Regulation of the Minister of the Environment of 30 April 2013 on landfills. 2013, 1–10. Journal of Laws 2013, item 523, 14. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20130000523 (accessed on 30 August 2020). (In Polish)
- European Committee for Standardization ISO 10523:2008 Water Quality — Determination of pH; International Organization for Standardization: Brussels, Belgium, 2008.
- European Committee for Standardization ISO 7888:1985. Water Quality - Determination of Electrical Conductivity; International Organization for Standardization: Brussels, Belgium, 1993.
- European Committee for Standardization ISO 9280:1990. Water Quality — Determination of Sulfate — Gravimetric Method using Barium Chloride; International Organization for Standardization: Brussels, Belgium, 1990.
- European Committee for Standardization ISO 9297:1989 - Water Quality — Determination of Chloride — Silver Nitrate Titration with Chromate Indicator (Mohr’s method); International Organization for Standardization: Brussels, Belgium, 1989.
- European Committee for Standardization ISO 9964-1:1993 - Water Quality — Determination of Sodium and Potassium — Part 1: Determination of Sodium by Atomic Absorption Spectrometry; International Organization for Standardization: Brussels, Belgium, 1993.
- European Committee for Standardization ISO 9964-2:1993 - Water Quality — Determination of Sodium and Potassium — Part 2: Determination of Potassium by Atomic Absorption Spectrometry; International Organization for Standardization: Brussels, Belgium, 1993.
- European Committee for Standardization Iso 7980:1986 - Water Quality — Determination of Calcium and Magnesium — Atomic Absorption Spectrometric Method; International Organization for Standardization: Brussels, Belgium, 1986.
- European Committee for Standardization ISO 6332:1988. Water Quality - Determination of Iron -- Spectrometric Method using 1,10-Phenanthroline; International Organization for Standardization: Brussels, Belgium, 1998.
- European Committee for Standardization ISO 6333:1986 - Water Quality — Determination of Manganese — Formaldoxime Spectrometric Method; International Organization for Standardization: Brussels, Belgium, 1986.
- European Committee for Standardization ISO 15586:2003 - Water Quality — Determination of Trace Elements using Atomic Absorption Spectrometry with Graphite Furnace; International Organization for Standardization: Brussels, Belgium, 2003.
- European Committee for Standardization ISO 6060:1989. Water Quality — Determination of the Chemical Oxygen Demand; International Organization for Standardization: Brussels, Belgium, 1989.
- European Committee for Standardization ISO 5663:1984. Water Quality - Determination of Kjeldahl Nitrogen - Method after Mineralization with Selenium; International Organization for Standardization: Brussels, Belgium, 1984.
- European Committee for Standardization ISO 7150-1:1984 - Water Quality — Determination of Ammonium — Part 1_ Manual Spectrometric Method; International Organization for Standardization: Brussels, Belgium, 1984.
- European Committee for Standardization ISO 11923:1997. Water Quality — Determination of Suspended Solids by Filtration through Glass-Fibre Filters; International Organization for Standardization: Brussels, Belgium, 1997.
- Hajsardar, M.; Borghei, S.M.; Hassani, A.H.; Takdastan, A. Nitrogen removal from ammonium-rich pharmaceutical wastewater. A comparison between sequencing batch reactor (SBR) and sequencing batch biofilm reactor (SBBR). Environ. Prot. Eng. 2018, 44, 95–115. [Google Scholar] [CrossRef]
- Omer, H.N. Water quality parameters. In Water Quality - Science, Assessments and Policy; Summers, K., Ed.; IntechOpen: London, UK, 2020; Chapter 1. [Google Scholar] [CrossRef]
- Treister, R.; Nielsen, C.S.; Stubhaug, A.; Farrar, J.T.; Pud, D.; Sawilowsky, S.; Oaklander, A.L. Experimental comparison of parametric versus nonparametric analyses of data from the cold pressor test. J. Pain 2015, 16, 537–548. [Google Scholar] [CrossRef] [Green Version]
- Kühnast, C.; Neuhäuser, M. A note on the use of the non-parametric Wilcoxon-Mann-Whitney test in the analysis of medical studies. GMS Ger. Med. Sci. 2008, 6, Doc02. [Google Scholar]
- King, J.R.; Jackson, D.A. Variable selection in large environmental data sets using principal components analysis. Environ.: Off. J. Int. Environ. Soc. 1999, 10, 67–77. [Google Scholar] [CrossRef]
- Kumari, P.; Gupta, N.C.; Kaur, A.; Singh, K. Application of principal component analysis and correlation for assessing groundwater contamination in and around municipal solid waste landfill of ghazipur, delhi. J. Geol. Soc. India 2019, 94, 595–604. [Google Scholar] [CrossRef]
- de Engelmann, M.P.; dos Santos, V.H.J.M.; Barbieri, C.B.; Augustin, A.H.; Ketzer, J.M.M.; Rodrigues, L.F. Environmental monitoring of a landfill area through the application of carbon stable isotopes, chemical parameters and multivariate analysis. Waste Manag. 2018, 76, 591–605. [Google Scholar] [CrossRef]
- Grisa, A.M.C.; Paese, C.; Dotto, O.J.; Nicola, R.; Zeni, M. Chemometric analysis of an sanitary landfill leachate. J. Water Resour. Prot. 2012, 4, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Bennis, K.; Bahi, L. PCA and cluster analysis for criteria mapping in landfill siting. Int. J. Eng. Technol. 2014, 6, 2244–2260. [Google Scholar]
- Schober, P.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Peng, Y. Perspectives on technology for landfill leachate treatment. Arab. J. Chem. 2017, 10, 2567–2574. [Google Scholar] [CrossRef] [Green Version]
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and long-term composition of MSW landfill leachate: A review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- Nájera-Aguilar, H.A.; Gutiérrez-Hernández, R.F.; Bautista-Ramírez, J.; Martínez-Salinas, R.I.; Escobar-Castillejos, D.; Borraz-Garzón, R.; Rojas-Valencia, M.N.; Giácoman-Vallejos, G. Treatment of low biodegradability leachates in a serial system of aged refuse-filled bioreactors. Sustainability 2019, 11, 3193. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.A.; Jingsong, G.; Ping, L.Z.; Ya, P.Y.; Al-Rekabi, W.S. Review on landfill leachate treatments. Am. J. Appl. Sci. 2009, 6. [Google Scholar] [CrossRef]
- Jorstad, L.B.; Jankowski, J.; Acworth, R.I. Analysis of the distribution of inorganic constituents in a landfill leachate-contaminated aquifer Astrolabe Park, Sydney, Australia. Environ. Geol. 2004, 46, 263–272. [Google Scholar] [CrossRef]
- Demirbilek, D.; Öztüfekçi Önal, A.; Demir, V.; Uslu, G.; Arslanoglu-Isik, H. Characterization and pollution potential assessment of Tunceli, Turkey municipal solid waste open dumping site leachates. Environ. Monit. Assess. 2013, 185, 9435–9449. [Google Scholar] [CrossRef]
- Mohammad-pajooh, E.; Weichgrebe, D.; Cuff, G. Municipal landfill leachate characteristics and feasibility of retrofitting existing treatment systems with deammonification – A full scale survey. J. Environ. Manage. 2017, 187, 354–364. [Google Scholar] [CrossRef]
- Słomczyńska, B.; Słomczyński, T. Physico-chemical and toxicological characteristics of leachates from MSW landfills. Polish J. Environ. Stud. 2004, 13, 627–637. [Google Scholar]
- Wojciechowska, E.; Gajewska, M.; Obarska-Pempkowiak, H. Treatment of landfill leachate by constructed wetlands: Three case studies. Polish J. Environ. Stud. 2010, 19, 643–650. [Google Scholar]
- Tatsi, A.A.; Zouboulis, A.I.U. A field investigation of the quantity and quality of leachate from a municipal solid waste landfill in a Mediterranean climate (Thessaloniki, Greece). Adv. Environ. Res. 2002, 6, 207–219. [Google Scholar] [CrossRef]
- Przydatek, G. Multi-indicator analysis of the influence of old municipal landfill sites on the aquatic environment: Case study. Environ. Monit. Assess. 2019, 191, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.M.; Greice, R.; Marotta, D.S.; Campos, J.C. Land fi ll leachate treatment in Brazil – An overview. J. Environ. Manage. 2019, 232, 110–116. [Google Scholar] [CrossRef]
- Slack, R.J.; Gronow, J.R.; Voulvoulis, N. Household hazardous waste in municipal landfills: Contaminants in leachate. Sci. Total. Environ. 2005, 146, 501–509. [Google Scholar] [CrossRef]
- Alvarez-Vazquez, H.; Jefferson, B.; Judd, S.J. Membrane bioreactors vs conventional biological treatment of landfill leachate: A brief review. J. Chem. Technol. Biotechnol. 2004, 79, 1043–1049. [Google Scholar] [CrossRef]
- Tałałaj, I.A.; Biedka, P.; Walery, M.J.; Leszczyński, J. Monitoring of leachate quality at a selected municipal landfill site in Podlasie, Poland. J. Ecol. Eng. 2016, 17, 175–184. [Google Scholar] [CrossRef]
- Wdowczyk, A.; Szymańska-Pulikowska, A. Analysis of possibilities of water environment assessment in the area of the landfillment of municipal waste. Inży. Ekol. 2018, 19, 57–64. [Google Scholar] [CrossRef]
- Przydatek, G. Impact of small municipal solid waste landfill on groundwater quality. Environ. Monit. Assess. 2019, 191, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kulikowska, D.; Klimiuk, E. The effect of landfill age on municipal leachate composition. Bioresour. Technol. 2008, 99, 5981–5985. [Google Scholar] [CrossRef]
- Thomsen, N.I.; Milosevic, N.; Bjerg, P.L. Application of a contaminant mass balance method at an old landfill to assess the impact on water resources. Waste Manag. 2012, 32, 2406–2417. [Google Scholar] [CrossRef]

| Pollution Indicators | Unit | Non-Operational Landfills | |||
|---|---|---|---|---|---|
| Bielawa | Wrocław | ||||
| Range | Median | Range | Median | ||
| pH | - | 7.80–9.10 | 8.80 | 7.60–8.50 | 8.30 |
| EC | μS/cm | 3048–5075 | 3657 | 1915–2553 | 2358 |
| COD | mg O2/dm3 | 954–4270 | 2370 | 113–237 | 183 |
| TKN | mg N/dm3 | 32.20–294.50 | 131.20 | 0.18–5.34 | 1.81 |
| ON | mg Norg/dm3 | 14.60–182.80 | 80.50 | 0.16–5.34 | 1.54 |
| AN | mg NNH4/dm3 | 17.6–231.2 | 109.4 | 0.002–0.580 | 0.020 |
| TS | mg/dm3 | 2580–8745 | 3135 | 2225–2515 | 2280 |
| TDS | mg/dm3 | 2140–3050 | 2605 | 2185–2460 | 2240 |
| TSS | mg/dm3 | 105–5995 | 780 | 25–105 | 55 |
| Sulfates | mg SO4/dm3 | 139.1–1884 | 256.7 | 58–1457 | 1234 |
| Chlorides | mg Cl/dm3 | 5.5–765.0 | 636.0 | 0.82–125 | 104 |
| Sodium | mg Na/dm3 | 132.3–285.8 | 188.8 | 109–208 | 113 |
| Potassium | mg K/dm3 | 188.8–265.6 | 236.5 | 55–98 | 66 |
| Calcium | mg Ca/dm3 | 69.5–194.3 | 103.2 | 166–339 | 281 |
| Magnesium | mg Mg/dm3 | 61.3–133.4 | 74.2 | 51.4–68.2 | 60.8 |
| Iron | mg Fe/dm3 | 1.60–18.03 | 8.33 | 0.019–4.780 | 3.900 |
| Manganese | mg Mn/dm3 | 0.05–2.43 | 0.84 | 0.020–0.150 | 0.060 |
| Copper | mg Cu/dm3 | 0.021–4.716 | 0.352 | 0.020–6.670 | 3.210 |
| Zinc | mg Zn/dm3 | 0.263–1.572 | 0.693 | 0.123–1.515 | 0.338 |
| Chromium | mg Cr/dm3 | 0.0005–0.497 | 0.065 | 0.0005–1.429 | 0.017 |
| Lead | mg Pb/dm3 | 0.0005–0.179 | 0.066 | 0.0005–0.123 | 0.029 |
| Nickel | mg Ni/dm3 | 0.0005–0.052 | 0.032 | 0.0005–0.530 | 0.017 |
| Cadmium | mg Cd/dm3 | 0.0005–0.008 | 0.004 | 0.0005–0.013 | 0.004 |
| Pollution Indicators | Unit | Active Landfills | |||
|---|---|---|---|---|---|
| Legnica | Jawor | ||||
| Range | Median | Range | Median | ||
| pH | - | 8.0–8.9 | 8.7 | 7.4–8.6 | 8.2 |
| EC | μS/cm | 8417–11,370 | 10,411 | 5093–10,305 | 7427 |
| COD | mg O2/dm3 | 1585–3800 | 3490 | 843–4110 | 2880 |
| TKN | mg N/dm3 | 167.56–907.4 | 300.91 | 285.52–613.3 | 346.27 |
| ON | mg Norg/dm3 | 4.54–121.31 | 99.85 | 32.33–248.27 | 112.44 |
| AN | mg NNH4/dm3 | 66.07–786.09 | 460.91 | 148.57–460.91 | 256.08 |
| TS | mg/dm3 | 6210–8245 | 7780 | 4975–22,750 | 7775 |
| TDS | mg/dm3 | 6195–7830 | 7155 | 3470–5155 | 4630 |
| TSS | mg/dm3 | 15–1870 | 330 | 275–19,280 | 3280 |
| Sulfates | mg SO4/dm3 | 80.6–396.6 | 106.1 | 33.7–335.7 | 216.4 |
| Chlorides | mg Cl/dm3 | 21.95–2811 | 2323 | 11.5–1481 | 1074 |
| Sodium | mg Na/dm3 | 175.2–329.2 | 198.9 | 176.3–266.4 | 196.4 |
| Potassium | mg K/dm3 | 238.2–317.2 | 281 | 231.1–308.8 | 257.3 |
| Calcium | mg Ca/dm3 | 43.9–113.81 | 67.1 | 70.8–264.2 | 138.9 |
| Magnesium | mg Mg/dm3 | 70.2–133.82 | 78.1 | 77.5–233.45 | 85.3 |
| Iron | mg Fe/dm3 | 2.6–10.62 | 3.81 | 4.52–38.73 | 26.23 |
| Manganese | mg Mn/dm3 | 0.2–0.55 | 0.31 | 0.24–14.48 | 1.61 |
| Copper | mg Cu/dm3 | 0.07–4.03 | 1.44 | 0.142–4.07 | 0.954 |
| Zinc | mg Zn/dm3 | 0.145–2.03 | 0.56 | 0.232–4.82 | 0.991 |
| Chromium | mg Cr/dm3 | 0.023–0.58 | 0.3 | 0.025–0.59 | 0.157 |
| Lead | mg Pb/dm3 | 0.0005–0.26 | 0.04 | 0.0005–0.44 | 0.059 |
| Nickel | mg Ni/dm3 | 0.095–0.38 | 0.17 | 0.024–0.34 | 0.071 |
| Cadmium | mg Cd/dm3 | 0.0005–0.01 | 0.0005 | 0.002–0.07 | 0.007 |
| Variable | Sum of Ranks | U | Z | p | Z Corrected | p | |
|---|---|---|---|---|---|---|---|
| Closed | Active | ||||||
| pH | 206.5 | 199.5 | 94.5 | 0.138 | 0.89037 | 0.138 | 0.89019 |
| EC | 105.0 | 301.0 | 0.0 | −4.480 | 0.00001 | −4.480 | 0.00001 |
| COD | 145.0 | 261.0 | 40.0 | −2.642 | 0.00824 | −2.642 | 0.00824 |
| TKN | 111.0 | 295.0 | 6.0 | −4.204 | 0.00003 | −4.204 | 0.00003 |
| ON | 136.0 | 270.0 | 31.0 | −3.056 | 0.00225 | −3.056 | 0.00225 |
| AN | 113.0 | 293.0 | 8.0 | −4.112 | 0.00004 | −4.113 | 0.00004 |
| TS | 117.0 | 289.0 | 12.0 | −3.929 | 0.00009 | −3.929 | 0.00009 |
| TDS | 105.0 | 301.0 | 0.0 | −4.480 | 0.00001 | −4.481 | 0.00001 |
| TSS | 155.0 | 251.0 | 50.0 | −2.183 | 0.02907 | −2.183 | 0.02905 |
| Sulfates | 248.0 | 158.0 | 53.0 | 2.045 | 0.04089 | 2.045 | 0.04086 |
| Chlorides | 129.0 | 277.0 | 24.0 | −3.377 | 0.00073 | −3.378 | 0.00073 |
| Sodium | 137.0 | 269.0 | 32.0 | −3.010 | 0.00262 | −3.010 | 0.00262 |
| Potassium | 126.0 | 280.0 | 21.0 | −3.515 | 0.00044 | −3.515 | 0.00044 |
| Calcium | 253.0 | 153.0 | 48.0 | 2.274 | 0.02294 | 2.274 | 0.02294 |
| Magnesium | 137.5 | 268.5 | 32.5 | −2.987 | 0.00282 | −2.989 | 0.00280 |
| Iron | 164.0 | 242.0 | 59.0 | −1.769 | 0.07690 | −1.769 | 0.07690 |
| Manganese | 161.0 | 245.0 | 56.0 | −1.907 | 0.05654 | −1.907 | 0.05651 |
| Copper | 209.0 | 197.0 | 92.0 | 0.253 | 0.80049 | 0.253 | 0.80049 |
| Zinc | 198.0 | 208.0 | 93.0 | −0.207 | 0.83619 | −0.207 | 0.83619 |
| Chromium | 161.0 | 245.0 | 56.0 | −1.907 | 0.05654 | −1.907 | 0.05651 |
| Lead | 194.0 | 212.0 | 89.0 | −0.391 | 0.69613 | −0.400 | 0.68939 |
| Nickel | 126.0 | 280.0 | 21.0 | −3.515 | 0.00044 | −3.520 | 0.00043 |
| Cadmium | 185.5 | 220.5 | 80.5 | −0.781 | 0.43474 | −0.782 | 0.43405 |
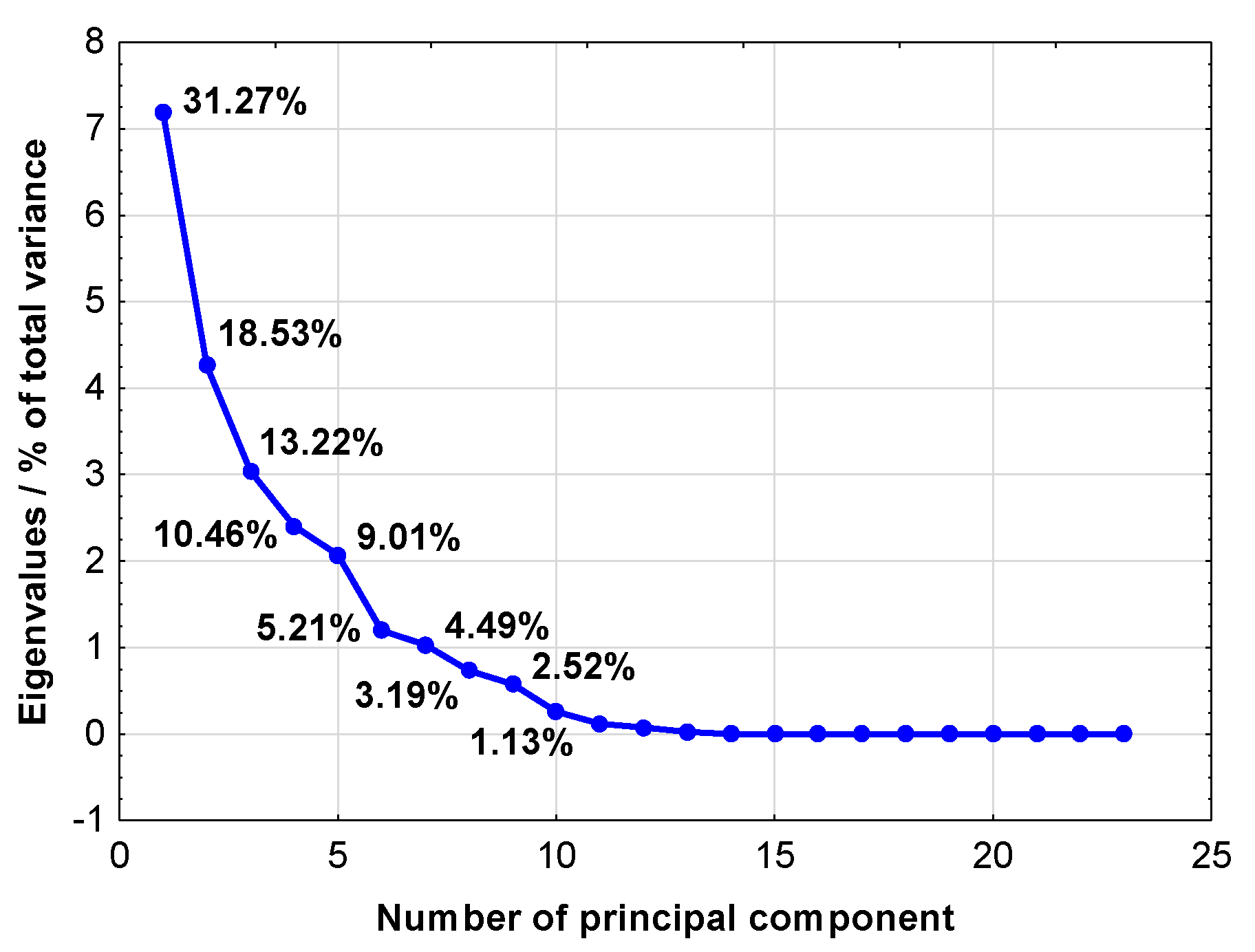 | Number of Principal Component | Cumulative Eigenvalue | Cumulative % of Total Variance | ||
| 1 | 7.193 | 31.273 | |||
| 2 | 11.456 | 49.807 | |||
| 3 | 14.496 | 63.028 | |||
| 4 | 16.902 | 73.485 | |||
| 5 | 18.973 | 82.491 | |||
| 6 | 20.171 | 87.701 | |||
| 7 | 21.204 | 92.193 | |||
| 8 | 21.938 | 95.384 | |||
| 9 | 22.519 | 97.907 | |||
| 10 | 22.777 | 99.033 | |||
| Factor loadings (correlations between variables and components) | |||||
| Variable | Component 1 | Component 2 | Component 3 | Component 4 | Component 5 |
| pH | 0.518 | −0.212 | 0.177 | −0.280 | −0.623 |
| EC | 0.796 | −0.347 | 0.236 | −0.100 | −0.053 |
| COD | −0.470 | −0.010 | 0.749 | 0.143 | 0.007 |
| TKN | −0.048 | −0.450 | −0.037 | −0.533 | −0.560 |
| ON | −0.710 | −0.108 | −0.077 | 0.161 | −0.545 |
| AN | 0.194 | −0.356 | 0.136 | −0.739 | −0.355 |
| TS | −0.421 | 0.483 | 0.005 | 0.315 | −0.603 |
| TDS | 0.719 | −0.150 | 0.627 | 0.034 | 0.058 |
| TSS | −0.563 | 0.460 | −0.181 | 0.272 | −0.530 |
| Sulfates | −0.449 | 0.394 | 0.497 | −0.354 | 0.249 |
| Chlorides | 0.714 | −0.318 | 0.115 | 0.460 | −0.176 |
| Sodium | 0.367 | −0.324 | −0.596 | −0.331 | 0.045 |
| Potassium | 0.273 | 0.095 | 0.661 | −0.062 | −0.192 |
| Calcium | −0.861 | −0.107 | −0.035 | −0.364 | 0.110 |
| Magnesium | −0.435 | 0.210 | 0.299 | −0.664 | 0.174 |
| Iron | −0.858 | −0.067 | −0.067 | −0.118 | 0.149 |
| Manganese | −0.675 | −0.642 | −0.057 | 0.210 | 0.040 |
| Copper | 0.516 | −0.144 | −0.653 | −0.073 | 0.178 |
| Zinc | −0.767 | −0.513 | 0.218 | 0.055 | 0.184 |
| Chromium | −0.039 | −0.720 | 0.404 | 0.396 | 0.008 |
| Lead | −0.346 | −0.785 | −0.342 | 0.003 | −0.095 |
| Nickel | 0.185 | −0.769 | 0.191 | 0.113 | 0.154 |
| Cadmium | −0.680 | −0.661 | 0.017 | 0.071 | −0.022 |
 | Number of Principal Component | Cumulative Eigenvalue | Cumulative % of Total Variance | ||
| 1 | 9.317 | 40.508 | |||
| 2 | 12.571 | 54.656 | |||
| 3 | 14.662 | 63.746 | |||
| 4 | 16.497 | 71.725 | |||
| 5 | 18.232 | 79.271 | |||
| 6 | 19.703 | 85.667 | |||
| 7 | 20.703 | 90.013 | |||
| 8 | 21.515 | 93.544 | |||
| 9 | 22.093 | 96.057 | |||
| 10 | 22.544 | 98.020 | |||
| Factor loadings (correlations between variables and components) | |||||
| Variable | Component 1 | Component 2 | Component 3 | Component 4 | Component 5 |
| pH | −0.565 | 0.042 | −0.608 | 0.040 | −0.465 |
| EC | −0.817 | −0.428 | 0.196 | −0.240 | −0.033 |
| COD | −0.849 | 0.392 | 0.109 | 0.081 | 0.130 |
| TKN | −0.876 | −0.182 | 0.171 | −0.299 | −0.182 |
| ON | −0.814 | −0.254 | −0.054 | −0.264 | −0.290 |
| AN | −0.722 | −0.180 | −0.193 | −0.351 | 0.057 |
| TS | −0.711 | −0.056 | 0.168 | 0.468 | −0.365 |
| TDS | −0.633 | −0.426 | 0.506 | −0.196 | 0.025 |
| TSS | −0.651 | 0.020 | 0.088 | 0.545 | −0.400 |
| Sulfates | 0.305 | 0.559 | 0.367 | −0.159 | −0.267 |
| Chlorides | −0.746 | −0.535 | −0.047 | 0.143 | 0.263 |
| Sodium | −0.553 | 0.083 | −0.467 | −0.055 | 0.383 |
| Potassium | −0.951 | −0.062 | 0.148 | −0.092 | 0.155 |
| Calcium | 0.857 | −0.035 | 0.188 | −0.111 | −0.360 |
| Magnesium | −0.611 | 0.467 | 0.454 | −0.371 | −0.047 |
| Iron | −0.628 | −0.053 | −0.315 | 0.388 | 0.257 |
| Manganese | −0.671 | 0.610 | −0.172 | −0.043 | 0.142 |
| Copper | 0.270 | −0.459 | −0.538 | −0.360 | −0.168 |
| Zinc | −0.294 | 0.693 | 0.068 | −0.107 | 0.323 |
| Chromium | 0.243 | 0.068 | 0.035 | 0.147 | 0.535 |
| Lead | −0.495 | 0.622 | −0.190 | 0.318 | −0.324 |
| Nickel | 0.232 | −0.300 | −0.068 | 0.215 | −0.031 |
| Cadmium | 0.025 | 0.474 | −0.476 | −0.513 | −0.181 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wdowczyk, A.; Szymańska-Pulikowska, A. Differences in the Composition of Leachate from Active and Non-Operational Municipal Waste Landfills in Poland. Water 2020, 12, 3129. https://doi.org/10.3390/w12113129
Wdowczyk A, Szymańska-Pulikowska A. Differences in the Composition of Leachate from Active and Non-Operational Municipal Waste Landfills in Poland. Water. 2020; 12(11):3129. https://doi.org/10.3390/w12113129
Chicago/Turabian StyleWdowczyk, Aleksandra, and Agata Szymańska-Pulikowska. 2020. "Differences in the Composition of Leachate from Active and Non-Operational Municipal Waste Landfills in Poland" Water 12, no. 11: 3129. https://doi.org/10.3390/w12113129





