Industrial Applications of Ionic Liquids
Abstract
:1. Introduction
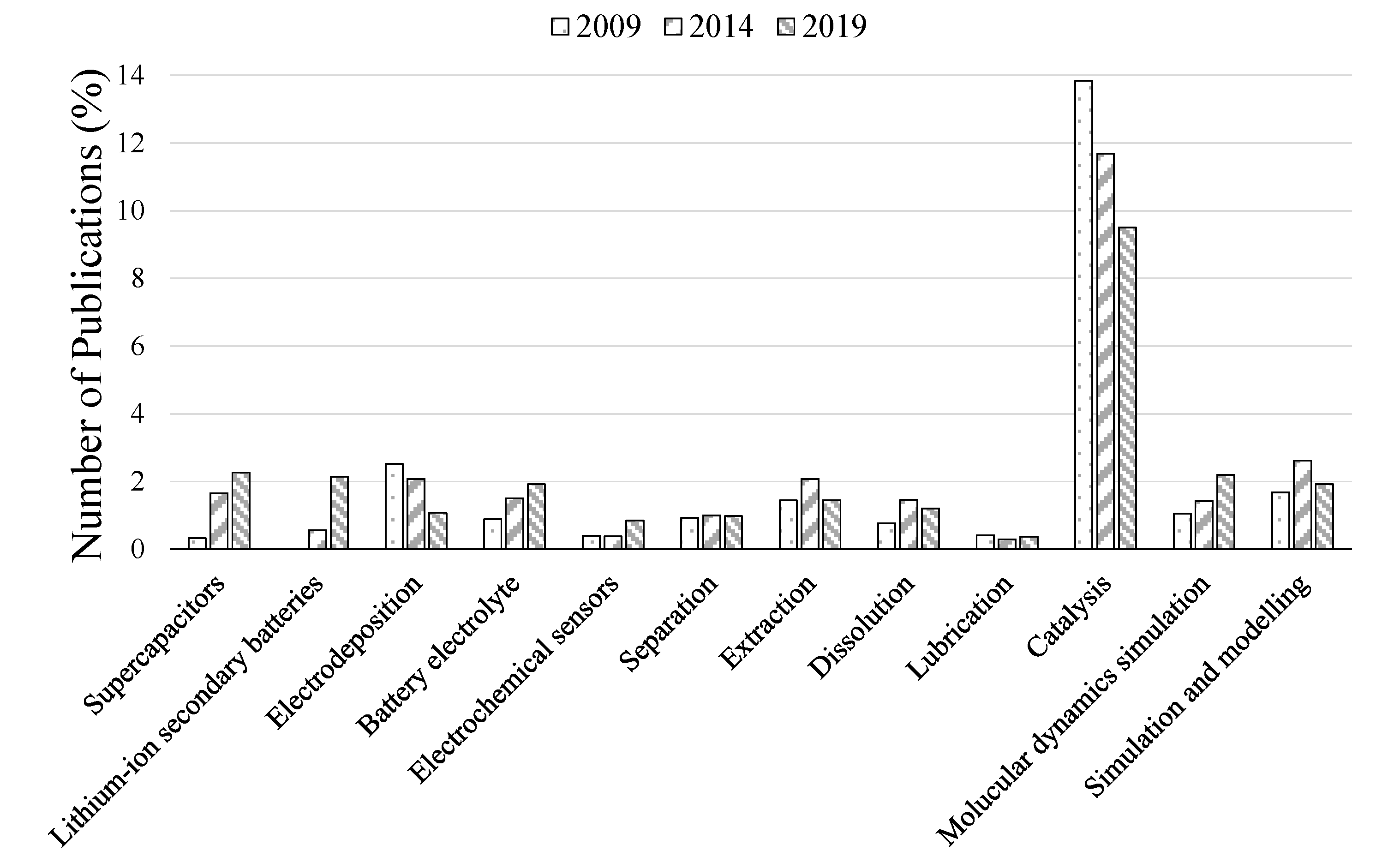
2. Growing Interest from the Academic and Industrial Communities
3. Industrial Applications of ILs
3.1. Commercial Scale Processes
3.1.1. Electrochemical Applications
3.1.2. Alkylation
3.1.3. Capture
3.1.4. Hydrogenation
3.1.5. Performance Additives
3.1.6. Dissolution
3.1.7. Operating Fluids
3.1.8. Analytical Uses
3.2. Pilot Scale Processes
3.2.1. Electrochemical Applications
3.2.2. Demethylation
3.2.3. Dimerisation
3.2.4. Chlorination
3.2.5. Hydrosilylation
3.2.6. Hydroformylation
3.2.7. Fluorination
3.2.8. Water-Gas Shift
3.2.9. Extraction
3.2.10. Separation
3.2.11. Dissolution
3.2.12. Operating Fluids
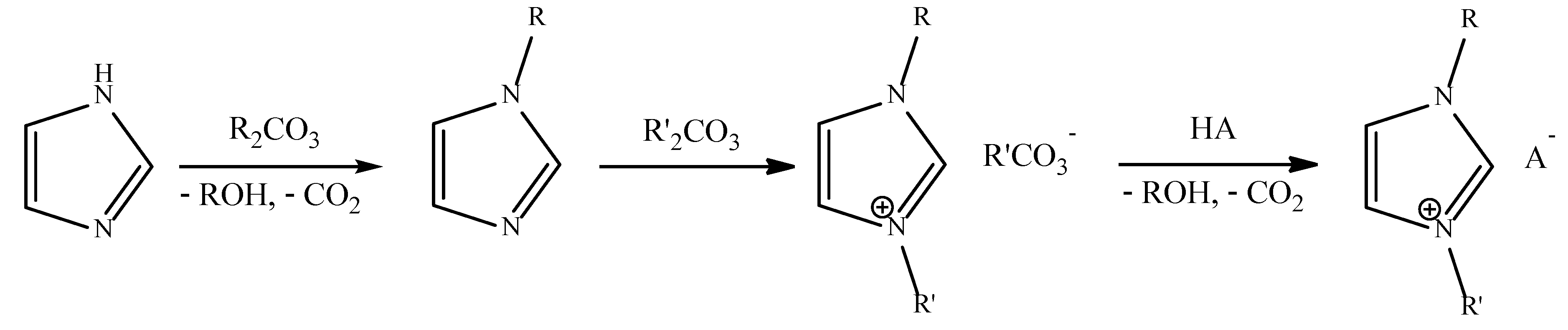
4. Industrial Synthesis of Ionic Liquids
5. Outlook
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walden, P. Ueber die Molekulargrösse und elektrische Leitfähigkeit einiger geschmolzenen. Bull. L’Académie Impériale Des Sci. St.-Pétersbourg 1914, 8, 405–422. [Google Scholar]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Kar, M.; Plechkova, N.V.; Seddon, K.R.; Pringle, J.M.; MacFarlane, D.R. Ionic Liquids-Further Progress on the Fundamental Issues. Aust. J. Chem. 2019, 72, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Paul, T. Anastas & John Charles Warner. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Cevasco, G.; Chiappe, C. Are ionic liquids a proper solution to current environmental challenges? Green Chem. 2014, 16, 2375–2385. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids in Green Chemistry. Green Chem. 2011, 13, 225. [Google Scholar] [CrossRef]
- Clark, J.H.; Tavener, S.J. Alternative solvents: Shades of green. Org. Process Res. Dev. 2007, 11, 149–155. [Google Scholar] [CrossRef]
- Thuy Pham, T.P.; Cho, C.W.; Yun, Y.S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef]
- e Silva, F.A.; Coutinho, J.A.P.; Ventura, S.P.M. Aquatic Toxicology of Ionic Liquids (ILs). In Encyclopedia of Ionic Liquids; Springer: Singapore, 2019; pp. 1–18. [Google Scholar] [CrossRef]
- Jordan, A.; Gathergood, N. Biodegradation of ionic liquids-a critical review. Chem. Soc. Rev. 2015, 44, 8200–8237. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Ionic liquids are not always green: Hydrolysis of 1-butyl-3- methylimidazolium hexafluorophosphate. Green Chem. 2003, 5, 361–363. [Google Scholar] [CrossRef]
- Rooney, D.; Jacquemin, J. Safer Solvents and Processes. Ionic Liquids. In Handbook of Solvents: Second Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 2, pp. 679–706. [Google Scholar] [CrossRef]
- Nancarrow, P.; Mohammed, H. Ionic Liquids in Space Technology - Current and Future Trends. Chembioeng Rev. 2017, 4, 106–119. [Google Scholar] [CrossRef]
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Lopes, J.N.C.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Substances Restricted under REACH (Last Updated 10 August 2020). Available online: https://echa.europa.eu/substances-restricted-under-reach (accessed on 1 September 2020).
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef]
- Kazakov, A.; Magee, J.W.; Chirico, R.D.; Paulechka, E.; Diky, V.; Muzny, C.D.; Kroenlein, K.; Frenkel, M. NIST Standard Reference Database 147: NIST Ionic Liquids Database—(ILThermo), Version 2.0, National Institute of Standards and Technology, Gaithersburg MD, 20899. Available online: https://ilthermo.boulder.nist.gov (accessed on 28 September 2020).
- Dong, Q.; Muzny, C.D.; Kazakov, A.; Diky, V.; Magee, J.W.; Widegren, J.A.; Chirico, R.D.; Marsh, K.N.; Frenkel, M. ILThermo: A free-access web database for thermodynamic properties of ionic liquids. J. Chem. Eng. Data 2007, 52, 1151–1159. [Google Scholar] [CrossRef]
- Tzschucke, C.C.; Markert, C.; Bannwarth, W.; Roller, S.; Hebel, A.; Haag, R. Modern Separation Techniques for the Efficient Workup in Organic Synthesis. Angew. Chem. Int. Ed. 2002, 41, 3964–4000. [Google Scholar] [CrossRef]
- Lee, C.W. Diels-Alder reactions in chloroaluminate ionic liquids: Acceleration and selectivity enhancement. Tetrahedron Lett. 1999, 40, 2461–2464. [Google Scholar] [CrossRef]
- Uerdingen, M.; Treber, C.; Balser, M.; Schmitt, G.; Werner, C. Corrosion behaviour of ionic liquids. In Green Chemistry; Royal Society of Chemistry: London, UK, 2005; Volume 7, pp. 321–325. [Google Scholar] [CrossRef]
- Shiflett, M.B. (Ed.) Commercial Applications of Ionic Liquids; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Hurley, F.H.; WIer, T.P. Electrodeposition of Metals from Fused Quaternary Ammonium Salts. J. Electrochem. Soc. 1951, 98, 203. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium Chloroaluminate Melts: A New Class of Room-Temperature Ionic Liquids for Electrochemistry, Spectroscopy, and Synthesis. Inorg. Chem. 1982, 21, 1263–1264. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Zaworotko, M.J. Air and Water STable 1-Ethyl-3-Methylimidazolium Based Ionic Liquids. J. Chem. Soc. Commun. 1992, 965–967. [Google Scholar] [CrossRef]
- Bonhôte, P.; Dias, A.-P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef]
- Morton, M.D.; Hamer, C.K. Ionic liquids—The beginning of the end or the end of the beginning?—A look at the life of ionic liquids through patent claims. Sep. Purif. Technol. 2018, 196, 3–9. [Google Scholar] [CrossRef]
- Howard, K.A.; Mitchell, H.L.; Waghorne, R.H. Liquid Salt Extraction of Aromatics from Process Feed Streams. U.S. Patent 4359596A, 16 November 1982. [Google Scholar]
- Holbrey, J.D.; Plechkova, N.V.; Seddon, K.R. Recalling COIL. In Green Chemistry; Royal Society of Chemistry: London, UK, 2006; Volume 8, pp. 411–414. [Google Scholar] [CrossRef]
- Phillips, G.W.; Falling, S.N.; Kingsport, T.; Godleski, S.A.; Monnier, J.R. Continuous Process for the Manufacture of 2,5-dihydrofurans from γ, δ-epoxybutenes. U.S. Patent 5315019A, 24 May 1994. [Google Scholar]
- García-Verdugo, E.; Altava, B.; Burguete, M.I.; Lozano, P.; Luis, S.V. Ionic liquids and continuous flow processes: A good marriage to design sustainable processes. Green Chem. 2015, 17, 2693–2713. [Google Scholar] [CrossRef] [Green Version]
- Solvay. Press Release: Solvay Successfully Completes the Acquisition of Cytec and Launches Integration Plans; Solvay: Brussels, Belgium, 2015. [Google Scholar]
- Freemantle, M. BASF’S Smart Ionic Liquid. Chem. Eng. News Arch. 2003, 81, 9. [Google Scholar] [CrossRef]
- Volland, M.; Verena, S.; Maase, M.; Flores, M. Method for Separation of Acids from Chemical Reaction Mixtures by Means of Ionic Fluids. WO2003062171A2, 31 July 2003. [Google Scholar]
- Seddon, K.R. Ionic liquids: A taste of the future. Nat. Mater. 2003, 2, 363–365. [Google Scholar] [CrossRef]
- Abbott, A.P.; Davies, D.L.; Capper, G.; Rasheed, R.K.; Tambyrajah, V. Ionic Liquids and Their Use. WO2002026381A2, 4 April 2002. [Google Scholar]
- Abbott, A.P.; Davies, D.L.; Capper, G.; Rasheed, R.K.; Tambyrajah, V. Ionic Liquids and Their Use as Solvents. WO2002026701A2, 4 April 2002. [Google Scholar]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Archer, J.; John, C. Electrodeposition of chromium black from ionic liquids. Trans. Inst. Met. Finish. 2004, 82, 14–17. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K. Ionic liquid analogues formed from hydrated metal salts. Chem. A Eur. J. 2004, 10, 3769–3774. [Google Scholar] [CrossRef]
- Professor Andrew P Abbott—University of Leicester. Available online: https://www2.le.ac.uk/departments/chemistry/people/academic-staff/andrew_p_abbott (accessed on 10 June 2020).
- Runge, W. Supplement to the Treatise. Technology Entrepreneurship: A Treatise on Entrepreneurs and Entrepreneurship for and in Technology Ventures; KIT Scientific Publishing: Karlsrue, Germany, 2014. [Google Scholar]
- NOHMs Technologies Inc. Available online: http://www.nohms.com/technology/ (accessed on 10 June 2020).
- Moganty, S.S.; Lee, J. Hybrid Ionic Liquid Electrolytes. US20160164137A1, 9 June 2016. [Google Scholar]
- Zyga, L.; Metal-Air Battery Could Store 11 Times More Energy than Lithium-Ion. Phys.Org. 2009. Available online: https://phys.org/news/2009-11-metal-air-battery-energy-lithium-ion.html (accessed on 10 June 2020).
- Tullo, A.H. Batteries that breathe air. CEn Glob. Enterp. 2017, 95, 21–22. [Google Scholar] [CrossRef]
- Friesen, C.A.; Krishnan, R.; Tang, T.; Wolfe, D. Metal-Air Cell with Tuned Hydrophobicity. WO2011159391A1, 22 December 2011. [Google Scholar]
- Friesen, C.A.; Wolfe, D.; Johnson, P.B. Metal-Air Cell with Ion Exchange Material. WO2012174558A1, 20 December 2012. [Google Scholar]
- Vagt, U. Ionic Liquids Unique Materials with Multiple Prospects. CHEManager Eur. 2008, 11, 8. [Google Scholar]
- Kalb, R.S. Toward Industrialization of Ionic Liquids. In Commercial Applications of Ionic Liquids; Shiflett, M.B., Ed.; Springer: Cham, Switzerland, 2020; pp. 261–282. [Google Scholar] [CrossRef]
- Tullo, A.H. The time is now for ionic liquids. Chem. Eng. News 2020, 98, 24. [Google Scholar]
- McCoy, M. Chevron embraces ionic liquids. Chem. Eng. News 2016, 94, 16. [Google Scholar]
- Timken, H.K.; Luo, H.; Chang, B.-K.; Carter, E.; Cole, M. ISOALKYTM Technology: Next-Generation Alkylate Gasoline Manufacturing Process Technology Using Ionic Liquid Catalyst. In Commercial Applications of Ionic Liquids; Shiflett, M.B., Ed.; Springer: Cham, Switzerland, 2020; pp. 33–47. [Google Scholar] [CrossRef]
- Seddon, K. QUILL rewrites the future of industrial solvents. Green Chem. 1999, 1, G58. [Google Scholar] [CrossRef]
- Timken, H.K.C.; Elomari, S.; Trumbull, S.; Cleverdon, R. Integrated Alkylation Process Using Ionic Liquid Catalysts. WO2006073749A2, 13 July 2006. [Google Scholar]
- Elomari, S.; Trumbull, S.; Timken, H.K.C.; Cleverdon, R. Alkylation Process Using Chloroaluminate Ionic Liquid Catalysts. WO2006068983A2, 29 June 2006. [Google Scholar]
- Martins, S.C.; Nafis, D.A.; Bhattacharyya, A. Alkylation Process Using Phosphonium-Based Ionic Liquids. US9156028B2, 13 October 2013. [Google Scholar]
- Liu, Y.; Hu, R.; Xu, C.; Su, H. Alkylation of isobutene with 2-butene using composite ionic liquid catalysts. Appl. Catal. A Gen. 2008, 346, 189–193. [Google Scholar] [CrossRef]
- Huang, C.P.; Liu, Z.C.; Xu, C.M.; Chen, B.H.; Liu, Y.F. Effects of additives on the properties of chloroaluminate ionic liquids catalyst for alkylation of isobutane and butene. Appl. Catal. A Gen. 2004, 277, 41–43. [Google Scholar] [CrossRef]
- Chung, W.; Zhang, R.; Song, D. Safe and sustainable alkylation: Performance and update on composite ionic liquid alkylation technology. Hydrocarb. Process. 2020. [Google Scholar]
- Well Resources. Ionikylation. Available online: https://www.wellresources.ca/ionikylation (accessed on 12 June 2020).
- Freemantle, M. Ionic liquids make splash in industry. Chem. Eng. News 2005, 83, 33–38. [Google Scholar] [CrossRef]
- Tempel, D.J.; Henderson, P.B.; Brzozowski, J.R. Reactive Liquid Based Gas Storage and Delivery Systems. US7172646B2, 6 February 2007. [Google Scholar]
- Abai, M.; Atkins, M.P.; Hassan, A.; Holbrey, J.D.; Kuah, Y.; Nockemann, P.; Oliferenko, A.A.; Plechkova, N.V.; Rafeen, S.; Rahman, A.A.; et al. An ionic liquid process for mercury removal from natural gas. Dalt. Trans. 2015, 44, 8617–8624. [Google Scholar] [CrossRef] [Green Version]
- Boada, R.; Cibin, G.; Coleman, F.; Diaz-Moreno, S.; Gianolio, D.; Hardacre, C.; Hayama, S.; Holbrey, J.D.; Ramli, R.; Seddon, K.R.; et al. Mercury capture on a supported chlorocuprate(ii) ionic liquid adsorbent studied using: Operando synchrotron X-ray absorption spectroscopy. Dalt. Trans. 2016, 45, 18946–18953. [Google Scholar] [CrossRef] [Green Version]
- Rogers, R.D.; Holbrey, J.; Rodriguez, H. Process for Removing Metals from Hydrocarbons. WO2010116165A2, 14 October 2010. [Google Scholar]
- Abai, M.; Atkins, M.P.; Cheun, K.Y.; Holbrey, J.; Nockemann, P.; Seddon, K.; Srinivasan, G.; Zou, Y. Process for Removing Metals from Hydrocarbons. WO2012046057A2, 12 April 2012. [Google Scholar]
- Cheun, K.Y.; Holbrey, J.D.; Atkins, M.P. Process for Removing Heavy Metals from Hydrocarbons. WO2016139280A1, 9 September 2016. [Google Scholar]
- Werner, S.; Szesni, N.; Kaiser, M.; Haumann, M.; Wasserscheid, P. A scalable preparation method for SILP and SCILL ionic liquid thin-film materials. Chem. Eng. Technol. 2012, 35, 1962–1967. [Google Scholar] [CrossRef]
- Clariant Specialty Chemicals. Available online: https://www.clariant.com/en/Corporate (accessed on 28 August 2020).
- Szesni, N.; Fischer, R.; Hagemeyer, A.; Grossmann, F.; Hou, H.C.; Boyer, J.; Sun, M.; Urbancic, M.; Lugmair, C.; Lowe, D.M. Catalyst Composition for Selective Hydrogenation with Improved Characteristics. WO2013057244A1, 25 April 2013. [Google Scholar]
- Barth, T.; Korth, W.; Jess, A. Selectivity-Enhancing Effect of a SCILL Catalyst in Butadiene Hydrogenation. Chem. Eng. Technol. 2017, 40, 395–404. [Google Scholar] [CrossRef]
- 3MTM Antistatic Additives. Ionic Liquids and Solids; 3M: Saint Paul, MI, USA, 2016. [Google Scholar]
- BASF. Basionics—High-Performance Antistatic Additives Brochure; BASF: Ludwigshafen, Germany, 2016. [Google Scholar]
- Evonik Industries. Press Release. New Tego Products Increase Conductivity and Electrostatic Charge Dissipation of Paints and Coatings. 2009. Available online: https://corporate.evonik.com/en/new-tego-products-increase-conductivity-and-electrostatic-charge-dissipation-of-paints-and-coatings-101049.html (accessed on 19 August 2020).
- Koei Chemical Co., L. Ionic Liquids-Type Antistatic Agents for Resins. Available online: https://www.koeichem.com/en/en_product/ion/antistatic.html (accessed on 22 August 2020).
- Hoff, A.; Jost, C.; Prodi-Schwab, A.; Weyershausen, B.; Schmidt, F.G. Ionic Liquids: New designer compounds for more efficient chemistry. Elem. Degussa Sci. Newsl. 2004, 9, 10–15. [Google Scholar]
- Weyershausen, B.; Lehmann, K. Industrial application of ionic liquids as performance additives. Green Chem. 2005, 7, 15–19. [Google Scholar] [CrossRef]
- Iolitec. Ionic Liquids Technologies. Available online: https://iolitec.de/ (accessed on 23 July 2020).
- CORDIS European Commission. Final Report Summary—IOLICAP (Novel IOnic LΙquid and Supported Ionic Liquid Solvents for Reversible CAPture of CO2). Available online: https://cordis.europa.eu/project/id/283077/reporting (accessed on 17 August 2020).
- Natural Fiber Welding, Inc. Available online: https://naturalfiberwelding.com/ (accessed on 25 August 2020).
- DeLong, H.C.; Trulove, P.C.; Haverhals, L.M.; Reichert, W.M. Natural Fiber Welding. US8202379B1, 19 June 2012. [Google Scholar]
- Haverhals, L.M.; Reichert, W.M.; De Long, H.C.; Trulove, P.C. Natural fiber welding. Macromol. Mater. Eng. 2010, 295, 425–430. [Google Scholar] [CrossRef]
- Haverhals, L.M.; Durkin, D.P.; Trulove, P.C. Natural Fiber Welding. In Commercial Applications of Ionic Liquids; Shiflett, M.B., Ed.; Springer: Cham, Switzerland, 2020; pp. 211–226. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Oster, K.; Tedstone, A.; Greer, A.; Budgen, N.; Garforth, A.; Hardacre, C. Dehydrochlorination of PVC in multi-layered blisterpacks using ionic liquids. Green Chem. 2020, 22, 5132–5142. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Liu, Y.; Zhang, X.; Zhang, S. Degradation of poly(ethylene terephthalate) using ionic liquids. Green Chem. 2009, 11, 1568–1575. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, Y.; Lu, X.; Zhang, S. First-row transition metal-containing ionic liquids as highly active catalysts for the glycolysis of poly(ethylene terephthalate) (PET). ACS Sustain. Chem. Eng. 2015, 3, 340–348. [Google Scholar] [CrossRef]
- Ioniqa. Ioniqa Takes First 10 Kiloton PET Upcycling Factory into Operation. 2019. Available online: https://ioniqa.com/ioniqa-takes-first-10-kiloton-pet-upcycling-factory-into-operation/ (accessed on 3 June 2020).
- Timonen, J.; Hooghoudt, T.; Vilaplana Artigas, M.; Philipse, A.; Sanchez, C.G.; Ribot, J.C.; Philippi, V.; De Groot, R. Magnetic Fluid. WO2014142661A2, 18 September 2014. [Google Scholar]
- Hooghoudt, T.; Philippi, V.; Vilaplana Artigas, M. Polymer Degradation. WO2016105200A1, 30 June 2016. [Google Scholar]
- Van Berkum, S.; Philippi, V.; Vilaplana Artigas, M.; De Groot, R.; Hooghoudt, T. Improved Reusable Capture Complex. WO2016105198A1, 30 June 2016. [Google Scholar]
- Ioniqa. Available online: https://ioniqa.com/ (accessed on 29 August 2020).
- Oster, K.; Goodrich, P.; Jacquemin, J.; Hardacre, C.; Ribeiro, A.P.C.; Elsinawi, A. A new insight into pure and water-saturated quaternary phosphonium-based carboxylate ionic liquids: Density, heat capacity, ionic conductivity, thermogravimetric analysis, thermal conductivity and viscosity. J. Chem. 2018, 121, 97–111. [Google Scholar] [CrossRef]
- Oster, K.; Hardacre, C.; Jacquemin, J.; Ribeiro, A.P.C.; Elsinawi, A. Ionic liquid-based nanofluids (ionanofluids) for thermal applications: An experimental thermophysical characterization. In Pure and Applied Chemistry; De Gruyter: Berlin, Germany, 2019; Volume 91, pp. 1309–1340. [Google Scholar] [CrossRef]
- Mettop GmbH. Safe & Effective Cooling. ILTEC Technology. Available online: https://mettop.com/en/products/8 (accessed on 18 August 2020).
- Proionic. Ionic Liquid Applications—Intrinsically Safe High-Temperature Cooling. Available online: https://www.proionic.com/ionic-liquids/applications-high-temperature-cooling.php (accessed on 18 August 2020).
- Mettop GmbH. ILTEC Technology. Ionic Liquid Cooling Technology-Description and Application. Available online: https://mettop.com/api/cdn/uploads/1526032491_k7timr64.pdf (accessed on 19 August 2020).
- Mettop GmbH. ILTEC Technology. A New Formula for Furnace Safety. 2020. Available online: https://mettop.com/api/cdn/uploads/1586336515_becqeuwu.pdf (accessed on 19 August 2020).
- Hanel, M.; Filzwieser, A.; Krassnig, H.-J.; Degel, R. ILTEC—Highlighting Potentials and Eliminating Concerns Regarding the Safe and Water-Free Cooling Technology. Bhm Berg Hüttenmännische Mon. 2019, 164, 281–286. [Google Scholar] [CrossRef]
- Klüber Lubrication. Ionic Liquids—Innovative Lightning Conductor in e-Mobility. Available online: https://www.klueber.com/uk/en/company/newsroom/news/ionic-liquids-innovative-lightning-conductor-in-e-mobility/ (accessed on 19 August 2020).
- Bosch. Conductive Lubricants will Protect the Electric Motors of the Future. Available online: https://www.bosch-presse.de/pressportal/de/en/conductive-lubricants-will-protect-the-electric-motors-of-the-future-42628.html (accessed on 19 August 2020).
- Adler, R.; Siebert, G. Method and Devices for Compressing a Gaseous Medium. US20070258828A1, 8 November 2007. [Google Scholar]
- Kompf, M. Mobility under high pressure. In Reports on Science and Technology; Linde: Pullach, Germany, 2006; pp. 24–29. [Google Scholar]
- Mayer, M. From prototype to serial production. Manufacturing hydrogen fuelling stations. In Proceedings of the Presented at Eco-Mobility 2014, Austrian Association for Advanced Propulsion Systems A3PS, Vienna, Austria, 8 October 2014. [Google Scholar]
- BOC. Kittybrewster, Aberdeen: Europe’s Largest Hydrogen Vehicle Refuelling Station Case Study; BOC: Guildford, UK, 2019. [Google Scholar]
- Linde. The Driving Force. Managing Hydrogen Projects with Linde; Brochure tcm17-233488; Linde: Pullach, Germany, 2012. [Google Scholar]
- Supelco Ionic Liquid GC Column Literature & Applications. Available online: https://www.sigmaaldrich.com/analytical-chromatography/gas-chromatography/columns/ionic-liquid-literature.html (accessed on 17 August 2020).
- Anderson, J.L.; Armstrong, D.W. Immobilized ionic liquids as high-selectivity/high-temperature/high- stability gas chromatography stationary phases. Anal. Chem. 2005, 77, 6453–6462. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.; Earle, M.J.; Gilea, M.A.; Plechkova, N.V.; Seddon, K.R. Ionic Liquid–Liquid Chromatography: A Novel Separation Method. In Commercial Applications of Ionic Liquids; Shiflett, M.B., Ed.; Springer: Cham, Switzerland, 2020; pp. 167–189. [Google Scholar] [CrossRef]
- Earle, M.; Seddon, K.; Self, R.; Brown, L. Ionic Liquid Separations. WO2013121218A1, 22 August 2013. [Google Scholar]
- Earle, M.; Seddon, K. Ionic Liquid Separations. WO2013121219A1, 22 August 2013. [Google Scholar]
- Earle, M.; Gilea, M. Lentinan Extraction Process from Mushrooms Using Ionic Liquid. WO2013140185A1, 26 September 2013. [Google Scholar]
- Earle, M.; Seddon, K. Ionic Liquid Separations. WO2013121220A1, 22 August 2013. [Google Scholar]
- Brown, L.; Earle, M.J.; Gîlea, M.A.; Plechkova, N.V.; Seddon, K.R. Ionic Liquid-Liquid Separations Using Countercurrent Chromatography: A New General-Purpose Separation Methodology. Aust. J. Chem. 2017, 70, 923–932. [Google Scholar] [CrossRef]
- Brown, L.; Earle, M.J.; Gîlea, M.A.; Plechkova, N.V.; Seddon, K.R. Ionic Liquid–Liquid Chromatography: A New General Purpose Separation Methodology. Top. Curr. Chem. 2017, 375, 1–41. [Google Scholar] [CrossRef] [Green Version]
- AECS-QuikPrep Ltd. Available online: http://www.quattroprep.com/ (accessed on 1 September 2020).
- Hitachi High-Tech Corporation. Hitachi Ionic liquid HILEM© for Easy Sample PreparationHitachi Ionic liquid HILEM© for Easy Sample Preparation. Available online: https://www.hitachi-hightech.com/global/expo/science/panel/ (accessed on 16 August 2020).
- Hitachi High-Tech Corporation. Various EM Imaging by Using Ionic Liquid (IL) Easy Preparation Method. Available online: https://www.hitachi-hightech.com/global/expo/science/panel/ (accessed on 16 August 2020).
- Joubert, L.-M.; McDonald, K. SEM Visualization of Biological Samples using Hitachi Ionic Liquid HILEM® IL 1000: A Comparative Study. Microsc. Microanal. 2016, 22, 1170–1171. [Google Scholar] [CrossRef] [Green Version]
- IoLiTec. Electrolytes for Metal Deposition. Available online: https://iolitec.de/node/652 (accessed on 10 June 2020).
- O’Meara, M.; Alemany, A.; Maase, M.; Vagt, U.; Malkowsky, I. Deposition of aluminum using ionic liquids: BASF process improves adhesion and coating density. Met. Finish. 2009, 107, 38–39. [Google Scholar] [CrossRef]
- Alemany, A.; Malkowsky, I.; Vagt, U.; Maase, M.; O’Meara, M. Recent developments in the field of aluminum deposition using ionic liquids. Plat. Surf. Finish. 2010, 97, 34–37. [Google Scholar]
- Freydina, E.; Abbott, J.G.; Lund, A.C.; Hilty, R.D.; Ruan, S.; Reese, J.; Chan, L.J.; Wright, J.A.; Curran, J.A. Nanostructured Aluminum Alloys for Improved Hardness. US10590558B2, 17 March 2020. [Google Scholar]
- CORDIS European Commission. Final Report Summary—SCAIL-UP (Scaling- up of the Aluminium Plating Process from Ionic Liquids). 2017. Available online: https://cordis.europa.eu/project/id/608698/reporting (accessed on 25 August 2020).
- Teramoto, D.; Yokoyama, R.; Kagawa, H.; Sada, T.; Ogata, N. A Novel Ionic Liquid-Polymer Electrolyte for the Advanced Lithium Ion Polymer Battery. In Molten Salts and Ionic Liquids: Never the Twain? John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 367–388. [Google Scholar] [CrossRef]
- ZapGo Ltd. Carbon-IonTM: A new, safer and faster charging category of rechargeable energy storage devices. In Brochure; ZapGo: Oxford, UK, 2016; pp. 1–16. [Google Scholar]
- Schmid, C.R.; Beck, C.A.; Cronin, J.S.; Staszak, M.A. Demethylation of 4-methoxyphenylbutyric acid using molten pyridinium hydrochloride on multikilogram scale. Org. Process Res. Dev. 2004, 8, 670–673. [Google Scholar] [CrossRef]
- Forestière, A.; Olivier-Bourbigou, H.; Saussine, L. Oligomerization of Monoolefins by Homogeneous Catalysts. Oil Gas Sci. Technol. 2009, 64, 649–667. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Freemantle, M. An Introduction to Ionic Liquids; RSC Publishing: Cambridge, UK, 2009. [Google Scholar]
- Stegmann, V.; Massonne, K. Method for Producing Haloalkanes from Alcohols. WO2005026089A2, 24 March 2005. [Google Scholar]
- Geldbach, T.J.; Zhao, D.; Castillo, N.C.; Laurenczy, G.; Weyershausen, B.; Dyson, P.J. Biphasic hydrosilylation in ionic liquids: A process set for industrial implementation. J. Am. Chem. Soc. 2006, 128, 9773–9780. [Google Scholar] [CrossRef]
- Weyershausen, B.; Hell, K.; Hesse, U. Industrial application of ionic liquids as process aid. Green Chem. 2005, 7, 283–287. [Google Scholar] [CrossRef]
- Welton, T.; Wasserscheid, P. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Franke, R.; Hahn, H. A catalyst that goes to its limits. Evonik Ind. 2015, 2, 18–23. [Google Scholar]
- Kohlpaintner, C.W.; Fischer, R.W.; Cornils, B. Aqueous biphasic catalysis: Ruhrchemie/Rhône-Poulenc oxo process. Appl. Catal. A Gen. 2001, 221, 219–225. [Google Scholar] [CrossRef]
- Magna, L.; Harry, S.; Proriol, D.; Saussine, L.; Olivier-Bourbigou, H. Hydroformylation of 1-hexene with a cobalt catalyst in ionic liquids: A new efficient approach for generation and recycling of the catalyst. Oil Gas Sci. Technol. 2007, 62, 775–780. [Google Scholar] [CrossRef]
- Hillebrand, G.; Hirschauer, A.; Commereuc, D.; Olivier-Bourbigou, H.; Saussine, L. Process for Hydroformylation Using a Catalyst Based on Cobalt and/or Rhodium Employed in a Two-Phase Medium. US20010039363A1, 8 November 2001. [Google Scholar]
- House of Commons Environmental Audit Committee. UK Progress on Reducing F-Gas Emissions; Fifth Report of Session 2017–19; House of Commons Environmental Audit Committee: London, UK, 2018. [Google Scholar]
- Bonnet, P. Catalysis in Nonaqueous Ionic Liquids. In Multiphase Homogeneous Catalysis; Cornils, B., Herrmann, W.A., Horváth, I.T., Leitner, W., Mecking, S., Olivier-Bourbigou, H., Vogt, D., Eds.; Wiley: Hoboken, NJ, USA, 2005; pp. 535–542. [Google Scholar] [CrossRef]
- Bonnet, P.; Lacroix, É.; Schirmann, J.-P. Ion Liquids Derived from Lewis Acid Based on Titanium, Niobium, Tantalum, or Antimony, and Uses Thereof. WO2001081353A1, 1 November 2001. [Google Scholar]
- Haumann, M. Continuous Catalytic Processes with Supported Ionic Liquid Phase (SILP) Materials. In Commercial Applications of Ionic Liquids; Shiflett, M.B., Ed.; Springer: Cham, Switzerland, 2020; pp. 49–67. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Fila, D.; Gajda, B.; Gęga, J.; Hubicki, Z. Rare Earth Elements—Separation Methods Yesterday and Today. In Applications of Ion Exchange Materials in the Environment; Inamuddin Ahamed, M.I., Asiri, A.M., Eds.; Springer: Cham, Switzerland, 2019; pp. 161–185. [Google Scholar]
- Nockemann, P.; Ritesh, R. Separation of Rare Earth Metals. WO2018109483A1, 21 June 2018. [Google Scholar]
- Nockemann, P.; Brolly, D.; Bradley, E.; McCourt, E. Countercurrent Rare Earth Separation Process. WO2019239151A1, 19 December 2019. [Google Scholar]
- Nockemann, P.; Brolly, D.; Bradley, E.; McCourt, E. Enhanced Separation of Rare Earth Metals. WO2019239150A1, 19 December 2019. [Google Scholar]
- SEREN AG. Seren Opens REM Magnet Recycling Demonstration Plant. 2018. Available online: https://www.seren-ag.com/rem-magnet-recycling/ (accessed on 15 June 2020).
- Arlt, W.; Seiler, M.; Jork, C.; Schneider, T. Ionic Liquids as Selective Additives for the Separation of Close-Boiling or Azeotropic Mixtures. WO2002074718A2, 26 September 2002. [Google Scholar]
- Jork, C.; Seiler, M.; Beste, Y.A.; Arlt, W. Influence of ionic liquids on the phase behavior of aqueous azeotropic systems. J. Chem. Eng. Data 2004, 49, 852–857. [Google Scholar] [CrossRef]
- Beste, Y.A.; Schoenmakers, H.; Arlt, W.; Seiler, M.; Jork, C. Recycling of Ionic Liquids Produced in Extractive Distillation. WO2005016484A1, 24 February 2005. [Google Scholar]
- Beste, Y.; Eggersmann, M.; Schoenmakers, H. Extraktivdestillation mit ionischen flüssigkeiten. Chem. Ing. Tech. 2005, 77, 1800–1808. [Google Scholar] [CrossRef]
- Hembre, R.T.; Barnicki, S.D.; Sink, C.W.; Hardacre, C. Quaternary Phosphinates with Co-Solvents for Extracting c1 to c4 Carboxylic Acids from Aqueous Streams. WO2016100769A1, 23 June 2016. [Google Scholar]
- Hembre, R.T.; Barnicki, S.D.; Sink, C.W.; Hardacre, C.; Liu, J.; Qi, F. Quaternary Carboxylate Compositions for Extracting C1 to C4 Carboxylic Acids from Aqueous Streams. US9573078B2, 21 February 2017. [Google Scholar]
- Liu, J.; Wang, S.; Hardacre, C.; Rooney, D.W.; Hembre, R.T.; Barnicki, S.D.; Sink, C.W. Quaternary Arylcarboxylate Compositions for Extracting C1 to C4 Carboxylic Acids from Aqueous Streams. WO2017105476, 22 June 2017. [Google Scholar]
- Dorn, S.; Wendler, F.; Meister, F.; Heinze, T. Interactions of ionic liquids with Polysaccharides—7: Thermal stability of cellulose in ionic liquids and N-methylmorpholine-N-oxide. Macromol. Mater. Eng. 2008, 293, 907–913. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Rogers, R.D.; Holbrey, J.D. Dissolution And Processing Of Cellulose Using Ionic Liquids. WO2003029329A2, 10 April 2003. [Google Scholar]
- BASF Intermediates. Processing Cellulose with Ionic Liquids. Available online: http://www.intermediates.basf.com/chemicals/web/en/function/conversions:/publish/content/news-and-publications/brochures/download/BASF_Processing_Cellulose.pdf (accessed on 22 August 2020).
- Clough, M.T.; Geyer, K.; Hunt, P.A.; Son, S.; Vagt, U.; Welton, T. Ionic liquids: Not always innocent solvents for cellulose. Green Chem. 2015, 17, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Ioncell. Available online: https://ioncell.fi/ (accessed on 22 August 2020).
- Michud, A.; Tanttu, M.; Asaadi, S.; Ma, Y.; Netti, E.; Kääriainen, P.; Persson, A.; Berntsson, A.; Hummel, M.; Sixta, H. Ioncell-F: Ionic liquid-based cellulosic textile fibers as an alternative to viscose and Lyocell. Text. Res. J. 2016, 86, 543–552. [Google Scholar] [CrossRef]
- Metsä Fibre. Shaping the New Sustainable Textile Fibre Future. 2019. Available online: https://www.metsafibre.com/en/media/Stories/Pages/Shaping-the-new-textile-fibre-future.aspx (accessed on 23 August 2020).
- Grete Project. Green Chemicals and Technologies for the Wood-to-Textile Value Chain. Available online: https://www.greteproject.eu/ (accessed on 23 August 2020).
- Imperial Innovations. ionoSolv. Ethanol Production Using Cheap Pretreatment to Retrofit 2G to 1G Existing Processes. Available online: https://www.imperial.tech/media/uploads/files/ionoSolv_6555_WhitePaper_Imperial_Innovations.pdf (accessed on 23 August 2020).
- George, A.; Brandt, A.; Tran, K.; Zahari, S.M.S.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015, 17, 1728–1734. [Google Scholar] [CrossRef] [Green Version]
- Abouelela, A.R.; Gschwend, F.V.; Malaret, F.; Hallett, J.P. Commercial Aspects of Biomass Deconstruction with Ionic Liquids. In Commercial Applications of Ionic Liquids; Shiflett, M.B., Ed.; Springer: Cham, Switzerland, 2020; pp. 87–127. [Google Scholar] [CrossRef]
- Chen, L.; Sharifzadeh, M.; Mac Dowell, N.; Welton, T.; Shah, N.; Hallett, J.P. Inexpensive ionic liquids: [HSO4]-based solvent production at bulk scale. Green Chem. 2014, 16, 3098–3106. [Google Scholar] [CrossRef] [Green Version]
- Lixea. Sustainable Solutions. Available online: https://www.lixea.co/#home (accessed on 23 August 2020).
- Brandt, A.; Hallett, J.; Leak, D.J.; Murphy, R.J.; Welton, T. Treatment. WO2012080702A2, 21 June 2012. [Google Scholar]
- Hallett, J.P.; Fennell, P.; Gschwend, F.; Brandt-Talbot, A.; Kelsall, G. Process For The Extraction of Metal Pollutants From Treated Cellulosic Biomass. WO2017085516A1, 26 May 2017. [Google Scholar]
- Filtration + Separation. BioFlex Turns Wood into Green Bioproducts. 2020. Available online: https://www.filtsep.com/chemicals/features/bioflex-turns-wood-into-green-bioproducts-10/ (accessed on 25 August 2020).
- Bartlett, D.H.; Farooq, A. Chitin, Cholera, and Competence. Science 2005, 310, 1775–1777. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, X.; Sun, N.; Rogers, R.D. Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem. 2010, 12, 968. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in Functional Chitin Materials: A Review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Rogers, R.D. Are Ionic Liquids Enabling Technology? Startup to Scale-Up to Find Out. In Commercial Applications of Ionic Liquids; Shiflett, M.B., Ed.; Springer: Cham, Switzerland, 2020; pp. 69–85. [Google Scholar] [CrossRef]
- Mari Signum, Mid Atlantic, LLC. Available online: https://www.marisignum.com/ (accessed on 22 August 2020).
- Drahl, C. Shell Game. ScienceNews 2019. Available online: https://secure.dashdigital.com/sciencenews/june_22__2019/MobilePagedArticle.action?articleId=1496488#articleId1496488 (accessed on 22 August 2020).
- Seiler, M.; Schneider, M.-C. Ionic liquids: Cooling and heating with heat. Evonik Ind. 2011, 3, 12–19. [Google Scholar]
- Kühn, A.; Seiler, M.; Radspieler, M.; Kotenko, O. Ionic liquids as new absorbents for absorption chillers and heat pumps. In Thermally Driven Heat Pumps for Heating And Cooling; Kühn, A., Ed.; Universitätsverlag der TU Berlin: Berlin, Germany, 2013; pp. 215–220. [Google Scholar]
- Chubu Electric Power Co., lnc. Press Release. Developed a New Technology for Adopting Ionic Liquid in the Humidity Control Agent for the Liquid Based Humidity Controlling Air Conditioning Unit. 2018. Available online: https://www.chuden.co.jp/english/corporate/ecor_releases/erel_pressreleases/3269300_18939.html (accessed on 18 August 2020).
- BASF Intermediates. Basionics Ionic Liquids—Solutions for Your Success. Product Catalogue. Available online: http://www.intermediates.basf.com/chemicals/brochures/intermediates (accessed on 23 July 2020).
- Solvay. Cytec Acquisition Successfully Completed. 2015. Available online: https://www.solvay.com/en/press-release/solvay-successfully-completes-acquisition-cytec-and-launches-integration-plans (accessed on 22 July 2020).
- Bradaric, C.J.; Downard, A.; Kennedy, C.; Robertson, A.J.; Zhou, Y. Industrial preparation of phosphonium ionic liquids. Green Chem. 2003, 5, 143–152. [Google Scholar] [CrossRef]
- Solvionic. Private Correspondence; Solvionic: Toulouse, France, 2020. [Google Scholar]
- Runge, W. Technology Entrepreneurship: A Treatise on Entrepreneurs and Entrepreneurship for and in Technology Ventures; KIT Scientific Publishing: Karlsrue, Germany, 2013; Volume 2. [Google Scholar] [CrossRef]
- Walker, A. Solvents. WO2009034329A1, 19 March 2009. [Google Scholar]
- Runge, W. Supplement to the Treatise. Technology Entrepreneurship: A Treatise on Entrepreneurs and Entrepreneurship for and in Technology Ventures. Solvent Innovation GmbH; KIT Scientific Publishing: Karlsrue, Germany, 2014. [Google Scholar]
- Kalb, R. Method For Producing Ionic Liquids, Ionic Solids Or Mixtures Thereof. WO2005021484A2, 10 March 2005. [Google Scholar]
- Kalb, R. Method For Producing Quaternary Carbonates. WO2008052860A1, 8 May 2008. [Google Scholar]
- Kalb, R. Method for Reacting 1,3-heteroaromatic 2-carboxylates with Water. WO2008052863A2, 8 May 2008. [Google Scholar]
- Kalb, R.S.; Stepurko, E.N.; Emel’yanenko, V.N.; Verevkin, S.P. Carbonate based ionic liquid synthesis (CBILS®): Thermodynamic analysis. Phys. Chem. Chem. Phys. 2016, 18, 31904–31913. [Google Scholar] [CrossRef]
- Kalb, R.S.; Damm, M.; Verevkin, S.P. Carbonate Based Ionic Liquid Synthesis (CBILS®): Development of the continuous flow method for preparation of ultra-pure ionic liquids. React. Chem. Eng. 2017, 2, 432–436. [Google Scholar] [CrossRef]
- Solvionic. Cleaner Solvents for Sustainable Chemistry. Available online: https://en.solvionic.com/ (accessed on 23 July 2020).
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Ionic Liquid Webshop—Proionic. Available online: https://www.proionic.com/ionic-liquids/webshop.php (accessed on 11 August 2020).
- Sayah, S.; Ghamouss, F.; Santos-Peña, J.; Tran-Van, F.; Lemordant, D. The Intriguing Properties of 1-Ethyl-3-methylimidazolium bis(fluorosulfonyl)imide Ionic Liquid. J. Solut. Chem. 2019, 48, 992–1008. [Google Scholar] [CrossRef]
- Khan, M.I.; Zaini, D.; Shariff, A.M.; Moniruzzaman, M. Framework for Ecotoxicological Risk Assessment of Ionic Liquids. In Procedia Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 148, pp. 1141–1148. [Google Scholar] [CrossRef] [Green Version]
- Abramenko, N.; Kustov, L.; Metelytsia, L.; Kovalishyn, V.; Tetko, I.; Peijnenburg, W. A review of recent advances towards the development of QSAR models for toxicity assessment of ionic liquids. J. Hazard. Mater. 2020, 384, 121429. [Google Scholar] [CrossRef]
- Das, R.N.; Roy, K. Advances in QSPR/QSTR models of ionic liquids for the design of greener solvents of the future. Mol. Divers. 2013, 17, 151–196. [Google Scholar] [CrossRef]
- Rooney, D.; Jacquemin, J.; Gardas, R. Thermophysical properties of ionic liquids. Top. Curr. Chem. 2009, 290, 185–212. [Google Scholar] [CrossRef]
- Coutinho, J.A.P.; Carvalho, P.J.; Oliveira, N.M.C. Predictive methods for the estimation of thermophysical properties of ionic liquids. RSC Adv. 2012, 2, 7322–7346. [Google Scholar] [CrossRef]
- Ab Manan, N.; Hardacre, C.; Jacquemin, J.; Rooney, D.W.; Youngs, T.G.A. Evaluation of gas solubility prediction in ionic liquids using COSMOthermX. J. Chem. Eng. Data 2009, 54, 2005–2022. [Google Scholar] [CrossRef]
- Ji, X.; Held, C.; Sadowski, G. Modeling imidazolium-based ionic liquids with ePC-SAFT. Fluid Phase Equilib. 2012, 335, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.S.; Lin, S.T. Screening of ionic liquids for CO2 capture using the COSMO-SAC model. Chem. Eng. Sci. 2015, 121, 157–168. [Google Scholar] [CrossRef]
- Lee, B.S.; Lin, S.T. Prediction and screening of solubility of pharmaceuticals in single- and mixed-ionic liquids using COSMO-SAC model. Aiche J. 2017, 63, 3096–3104. [Google Scholar] [CrossRef]
- Patel, N.K.; Joshipura, M.H. Generalized PSRK model for prediction of liquid density of ionic liquids. In Procedia Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 51, pp. 386–394. [Google Scholar] [CrossRef] [Green Version]
- Jouyban, A.; Mirheydari, S.N.; Barzegar-Jalali, M.; Shekaari, H.; Acree, W.E. Comprehensive models for density prediction of ionic liquid + molecular solvent mixtures at different temperatures. Phys. Chem. Liq. 2020, 58, 309–324. [Google Scholar] [CrossRef]
- Gardas, R.L.; Coutinho, J.A.P. A group contribution method for viscosity estimation of ionic liquids. Fluid Phase Equilib. 2008, 266, 195–201. [Google Scholar] [CrossRef]
- Ge, R.; Hardacre, C.; Jacquemin, J.; Nancarrow, P.; Rooney, D.W. Heat capacities of ionic liquids as a function of temperature at 0.1 MPa. Measurement and prediction. J. Chem. Eng. Data 2008, 53, 2148–2153. [Google Scholar] [CrossRef]
- Valderrama, J.O.; Martinez, G.; Rojas, R.E. Predictive model for the heat capacity of ionic liquids using the mass connectivity index. Acta 2011, 513, 83–87. [Google Scholar] [CrossRef]
- Mirkhani, S.A.; Gharagheizi, F. Predictive quantitative structure-property relationship model for the estimation of ionic liquid viscosity. Ind. Eng. Chem. Res. 2012, 51, 2470–2477. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Ilani-Kashkouli, P.; Mohammadi, A.H.; Ramjugernath, D.; Richon, D. Development of a group contribution method for determination of viscosity of ionic liquids at atmospheric pressure. Chem. Eng. Sci. 2012, 80, 326–333. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Ilani-Kashkouli, P.; Mohammadi, A.H. Computation of normal melting temperature of ionic liquids using a group contribution method. Fluid Phase Equilib. 2012, 329, 1–7. [Google Scholar] [CrossRef]
- Lazzús, J.A. A group contribution method to predict the melting point of ionic liquids. Fluid Phase Equilib. 2012, 313, 1–6. [Google Scholar] [CrossRef]
- Paduszyński, K.; Domańska, U. A new group contribution method for prediction of density of pure ionic liquids over a wide range of temperature and pressure. Ind. Eng. Chem. Res. 2012, 51, 591–604. [Google Scholar] [CrossRef]
- Zhao, N.; Jacquemin, J.; Oozeerally, R.; Degirmenci, V. New method for the estimation of viscosity of pure and mixtures of ionic liquids based on the UNIFAC–VISCO model. J. Chem. Eng. Data 2016, 61, 2160–2169. [Google Scholar] [CrossRef] [Green Version]
- Oster, K.; Jacquemin, J.; Hardacre, C.; Ribeiro, A.P.C.; Elsinawi, A. Further development of the predictive models for physical properties of pure ionic liquids: Thermal conductivity and heat capacity. J. Chem. 2018, 118, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Soares, L.H.; Guirardello, R.; Rolemberg, M.P. A simple group contribution model to predict thermal conductivity of pure ionic liquids. Chem. Eng. Trans. 2019, 74, 1195–1200. [Google Scholar] [CrossRef]
- Jacquemin, J.; Nancarrow, P.; Rooney, D.W.; Gomes, M.F.C.; Husson, P.; Majer, V.; Pádua, A.A.H.; Hardacre, C. Prediction of ionic liquid properties. II. Volumetric properties as a function of temperature and pressure. J. Chem. Eng. Data 2008, 53, 2133–2143. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Hemmati-Sarapardeh, A. Toward a predictive model for estimating viscosity of ternary mixtures containing ionic liquids. J. Mol. Liq. 2014, 200, 340–348. [Google Scholar] [CrossRef]
- Trohalaki, S.; Pachter, R.; Drake, G.W.; Hawkins, T. Quantitative structure-property relationships for melting points and densities of ionic liquids. Energy Fuels 2005, 19, 279–284. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Mirkhani, S.A.; Keshavarz, M.H.; Farahani, N.; Tumba, K. A molecular-based model for prediction of liquid viscosity of pure organic compounds: A quantitative structure property relationship (QSPR) approach. J. Taiwan Inst. Chem. Eng. 2013, 44, 359–364. [Google Scholar] [CrossRef]
- Valderrama, J.O.; Zarricueta, K. A simple and generalized model for predicting the density of ionic liquids. Fluid Phase Equilib. 2009, 275, 145–151. [Google Scholar] [CrossRef]
- Preiss, U.; Bulut, S.; Krossing, I. In silico prediction of the melting points of ionic liquids from thermodynamic considerations: A case study on 67 salts with a melting point range of 337 °C. J. Phys. Chem. B 2010, 114, 11133–11140. [Google Scholar] [CrossRef]
- Mehrkesh, A.; Karunanithi, A.T. Predicting Melting point and Viscosity of Ionic Liquids Using New Quantum Chemistry Descriptors. Fluid Phase Equilib. 2016, 427, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Canongia Lopes, J.N.; Pádua, A.A.H.; Shimizu, K. Molecular force field for ionic liquids IV: Trialkylimidazolium and alkoxycarbonyl-imidazolium cations; alkylsulfonate and alkylsulfate anions. J. Phys. Chem. B 2008, 112, 5039–5046. [Google Scholar] [CrossRef]
- Chaban, V.V.; Voroshylova, I.V.; Kalugin, O.N. A new force field model for the simulation of transport properties of imidazolium-based ionic liquids. In Physical Chemistry Chemical Physics; Royal Society of Chemistry: London, UK, 2011; Volume 13, pp. 7910–7920. [Google Scholar] [CrossRef]
- Batista, M.L.S.; Coutinho, J.A.P.; Gomes, J.R.B. Prediction of Ionic Liquids Properties through Molecular Dynamics Simulations. Curr. Phys. Chem. 2014, 4, 151–172. [Google Scholar] [CrossRef]
- Shen, C.; Li, C.X.; Li, X.M.; Lu, Y.Z.; Muhammad, Y. Estimation of densities of ionic liquids using Patel-Teja equation of state and critical properties determined from group contribution method. Chem. Eng. Sci. 2011, 66, 2690–2698. [Google Scholar] [CrossRef]
- Chorązewski, M.; Postnikov, E.B.; Jasiok, B.; Nedyalkov, Y.V.; Jacquemin, J. A Fluctuation Equation of State for Prediction of High-Pressure Densities of Ionic Liquids. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckner, W.; Mao, C.M.; Pfaendtner, J. Statistical models are able to predict ionic liquid viscosity across a wide range of chemical functionalities and experimental conditions. Mol. Syst. Des. Eng. 2018, 3, 253–263. [Google Scholar] [CrossRef]
- Zhao, N.; Oozeerally, R.; Degirmenci, V.; Wagner, Z.; Bendová, M.; Jacquemin, J. New Method Based on the UNIFAC–VISCO Model for the Estimation of Ionic Liquids Viscosity Using the Experimental Data Recommended by Mathematical Gnostics. J. Chem. Eng. Data 2016, 61, 3908–3921. [Google Scholar] [CrossRef] [Green Version]
- Chirico, R.D.; Frenkel, M.; Magee, J.W.; Diky, V.; Muzny, C.D.; Kazakov, A.F.; Kroenlein, K.; Abdulagatov, I.; Hardin, G.R.; Acree, W.E.; et al. Improvement of quality in publication of experimental thermophysical property data: Challenges, assessment tools, global implementation, and online support. J. Chem. Eng. Data 2013, 58, 2699–2716. [Google Scholar] [CrossRef] [Green Version]
- Seddon, K.R.; Stark, A.; Torres, M.J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Clare, B.R.; Bayley, P.M.; Best, A.S.; Forsyth, M.; MacFarlane, D.R. Purification or contamination? The effect of sorbents on ionic liquids. Chem. Commun. 2008, 2689. [Google Scholar] [CrossRef]
- Schubert, T.J.S. Commercial Production of Ionic Liquids. In Commercial Applications of Ionic Liquids; Shiflett, M.B., Ed.; Springer: Cham, Switzerland, 2020; pp. 191–208. [Google Scholar] [CrossRef]
- Welton, T. Solvents and sustainable chemistry. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Schubert, T.J.S. Current and future ionic liquid markets. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2017; Volume 1250, pp. 35–65. [Google Scholar] [CrossRef]
- Anton, A.M.; Frenzel, F.; Yuan, J.; Tress, M.; Kremer, F. Hydrogen bonding and charge transport in a protic polymerized ionic liquid. Soft Matter 2020, 16, 6091–6101. [Google Scholar] [CrossRef]
- Xiao, W.; Yang, Q.; Zhu, S. Comparing ion transport in ionic liquids and polymerized ionic liquids. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Mao, X.; Brown, P.; Červinka, C.; Hazell, G.; Li, H.; Ren, Y.; Chen, D.; Atkin, R.; Eastoe, J.; Grillo, I.; et al. Self-assembled nanostructures in ionic liquids facilitate charge storage at electrified interfaces. Nat. Mater. 2019, 18, 1350–1357. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Yoshio, M.; Seki, A. Ion-conductive nanostructured polymer films formed by photopolymerization of lyotropic columnar liquid-crystalline monomers, composed of a zwitterionic compound and a protic ionic liquid. Crystals 2020, 10, 276. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yan, G.; Wang, J.; Zhou, J.; Bie, L.; Jia, Y.; Meng, F. Self-assembly, phase behaviour and ion-conducting property of pyridinium-based ionic liquid-crystalline oligomers. Liq. Cryst. 2020, 1–14. [Google Scholar] [CrossRef]
- Taylor, R.; McCrellis, C.; McStay, C.; Jacquemin, J.; Hardacre, C.; Mercy, M.; Bell, R.G.; de Leeuw, N.H. CO2 Capture in Wet and Dry Superbase Ionic Liquids. J. Solut. Chem. 2015, 44, 511–527. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.F.R.; McClung, M.; McReynolds, C.; Daly, H.; Greer, A.J.; Jacquemin, J.; Hardacre, C. Understanding the Competitive Gas Absorption of CO2 and SO2 in Superbase Ionic Liquids. Ind. Eng. Chem. Res. 2018, 57, 17033–17042. [Google Scholar] [CrossRef] [Green Version]
- Greer, A.J.; Taylor, S.F.R.; Daly, H.; Quesne, M.; Catlow, C.R.A.; Jacquemin, J.; Hardacre, C. Investigating the Effect of NO on the Capture of CO2 Using Superbase Ionic Liquids for Flue Gas Applications. ACS Sustain. Chem. Eng. 2019, 7, 3567–3574. [Google Scholar] [CrossRef]
- Wang, C.; Luo, X.; Luo, H.; Jiang, D.E.; Li, H.; Dai, S. Tuning the basicity of ionic liquids for equimolar CO2 capture. Angew. Chem. Int. Ed. 2011, 50, 4918–4922. [Google Scholar] [CrossRef]
- Hollingsworth, N.; Taylor, S.F.R.; Galante, M.T.; Jacquemin, J.; Longo, C.; Holt, K.B.; de Leeuw, N.H.; Hardacre, C. Reduction of Carbon Dioxide to Formate at Low Overpotential using a Superbase Ionic Liquid. Angew. Chem. 2015, 127, 14370–14374. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.; Hollingsworth, N.; Galante, M.T.; Jacquemin, J.; Longo, C.; Holt, K.B.; de Leeuw, N.H.; Hardacre, C. CO2 Capture and Electrochemical Conversion using Super Basic [P66614][124-Triz]. Faraday Discuss. 2015, 183, 389–400. [Google Scholar]
- Chen, Y.; Mu, T. Conversion of CO2 to value-added products mediated by ionic liquids. Green Chem. 2019, 21, 2544–2574. [Google Scholar] [CrossRef]
- Luo, Q.; Pentzer, E. Encapsulation of Ionic Liquids for Tailored Applications. ACS Appl. Mater. Interfaces 2020, 12, 5169–5176. [Google Scholar] [CrossRef]
- Jessop, P.G.; Heldebrant, D.J.; Li, X.; Eckert, C.A.; Liotta, C.L. Reversible nonpolar-to-polar solvent. Nature 2005, 436, 1102. [Google Scholar] [CrossRef]
- Jessop, P.G.; Phan, L.; Carrier, A.; Robinson, S.; Dürr, C.J.; Harjani, J.R. A solvent having switchable hydrophilicity. Green Chem. 2010, 12, 809. [Google Scholar] [CrossRef]
- Giri, N.; Del Pópolo, M.G.; Melaugh, G.; Greenaway, R.L.; Rätzke, K.; Koschine, T.; Pison, L.; Gomes, M.F.C.; Cooper, A.I.; James, S.L. Liquids with permanent porosity. Nature 2015, 527, 216–220. [Google Scholar] [CrossRef]
- Porous Liquid Technologies. Available online: http://www.porousliquidtechnologies.com/ (accessed on 6 September 2020).
- Ma, L.; Haynes, C.J.E.; Grommet, A.B.; Walczak, A.; Parkins, C.C.; Doherty, C.M.; Longley, L.; Tron, A.; Stefankiewicz, A.R.; Bennett, T.D.; et al. Coordination cages as permanently porous ionic liquids. Nat. Chem. 2020, 12, 270–275. [Google Scholar] [CrossRef]
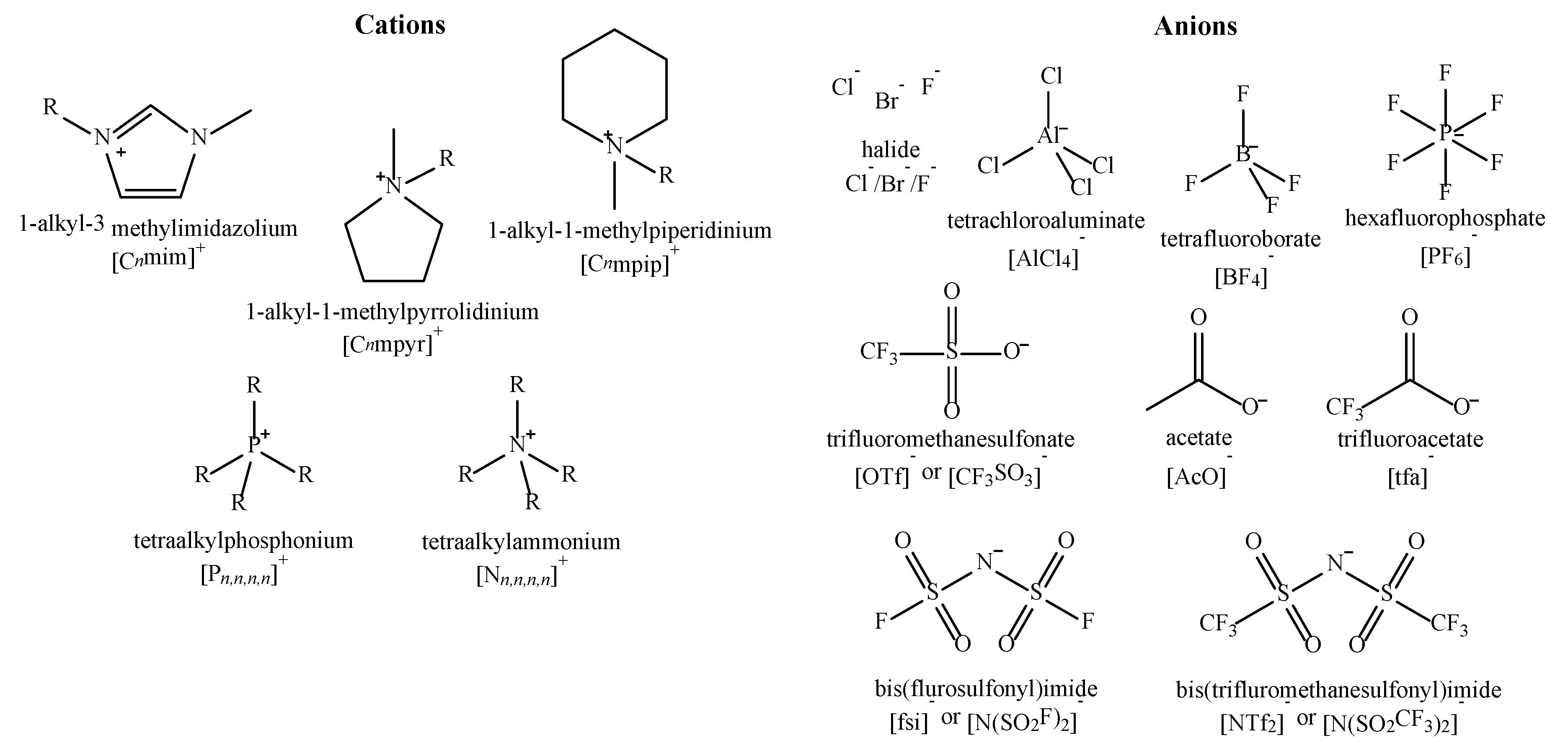
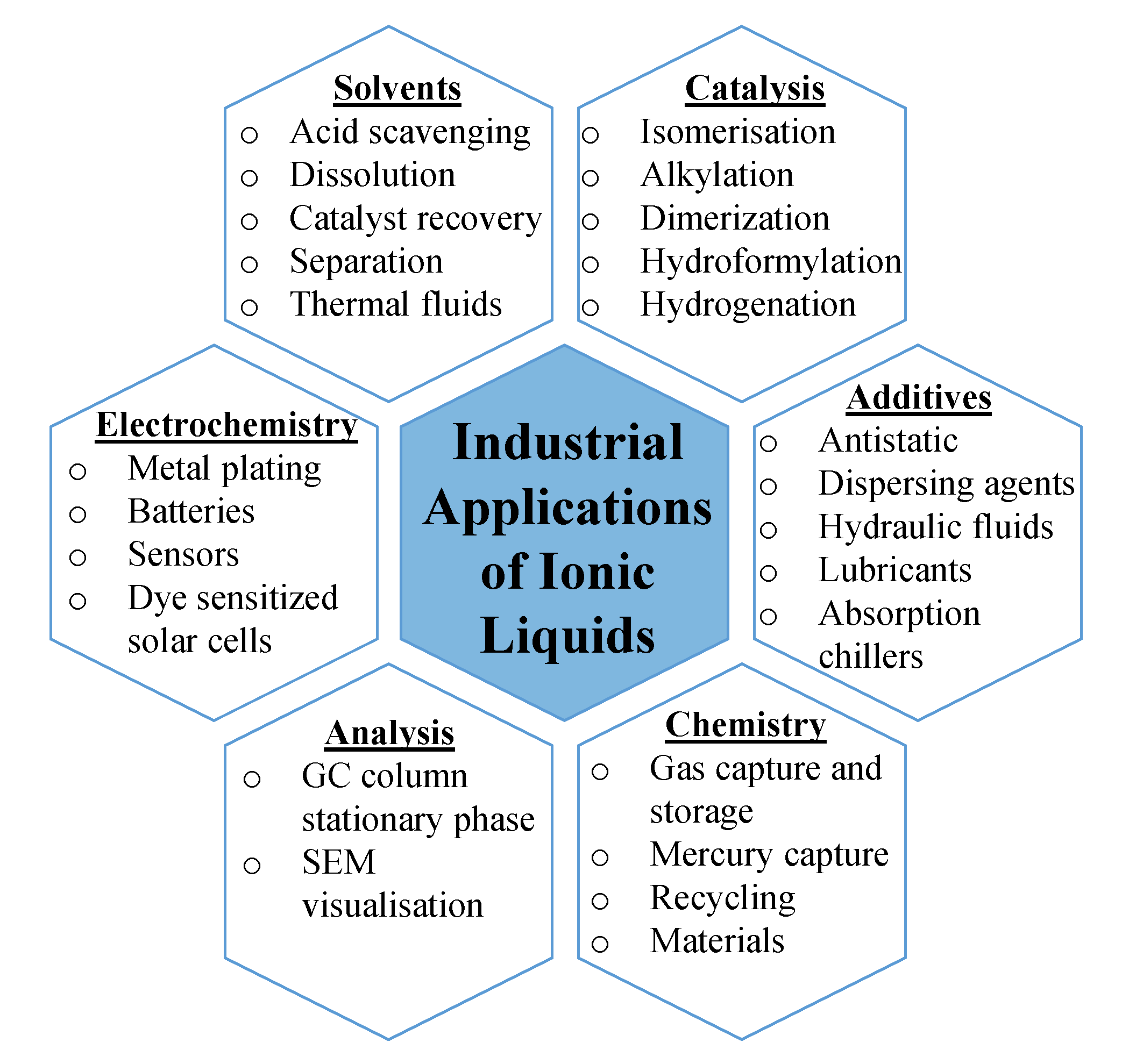
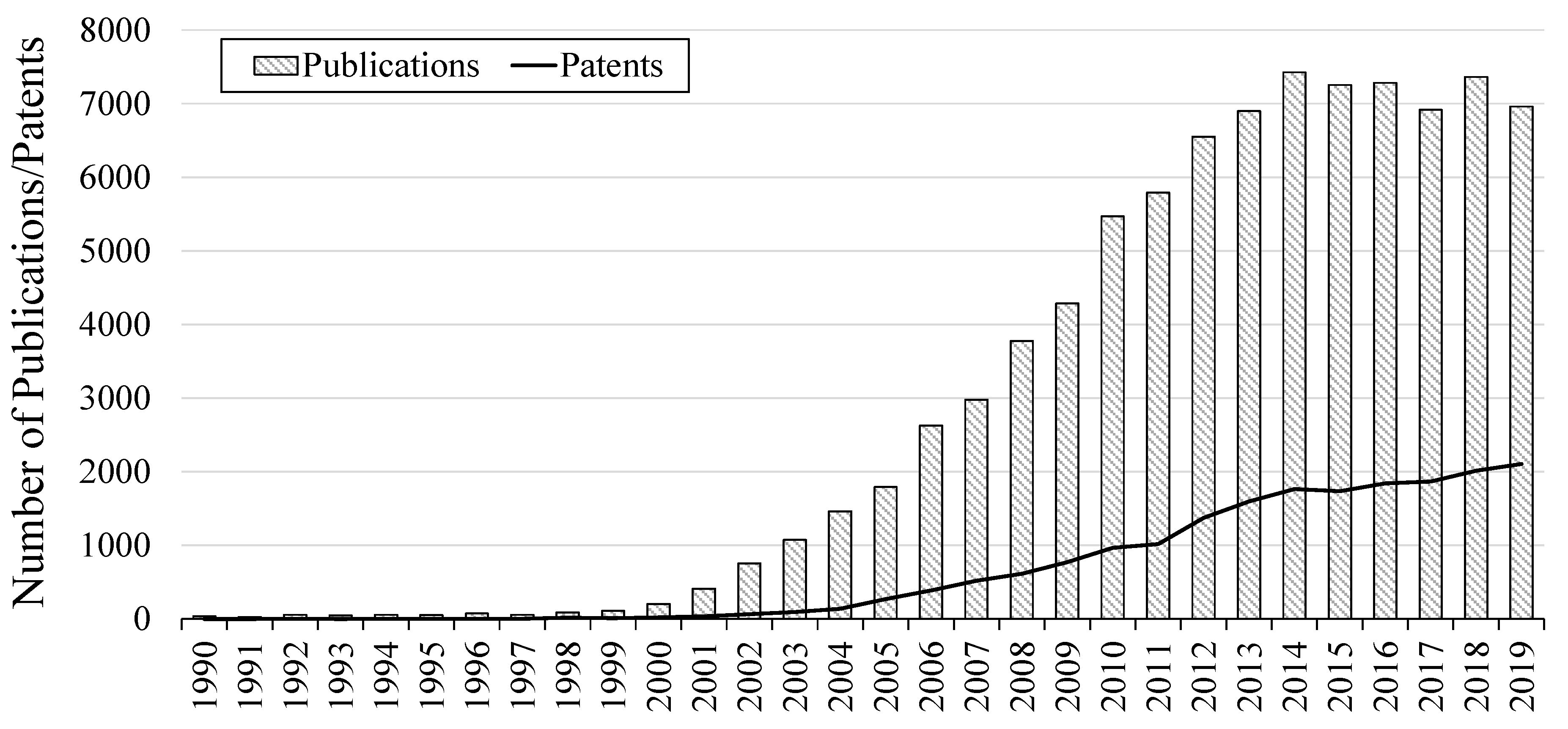
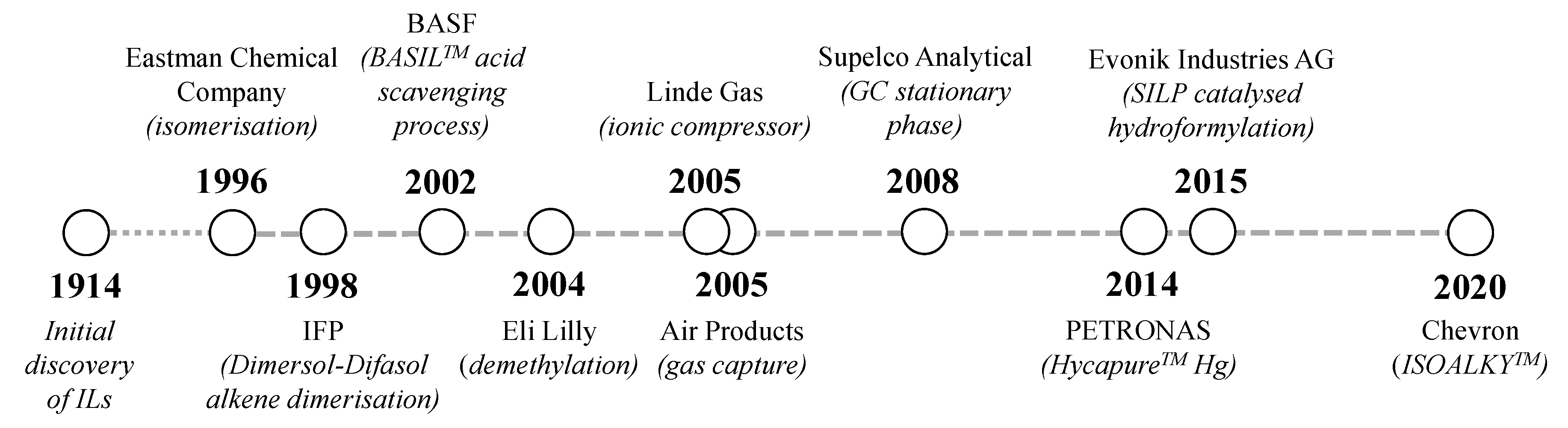
| Property | Organic Solvents | Ionic Liquids |
|---|---|---|
| Number of solvents | >1000 | >106 |
| Applicability in a given process | Single function | Multifunction |
| Cost | Generally cheap | 2 to 100 times more expensive than organic solvents |
| Recyclability/Toxicity | Green imperative—survey of toxicity of organic solvents is controlled by REACH | Economic imperative—toxicity and biodegradability are often not well known |
| Vapour pressure | Measurable and generally well-known—several organic solvents have vapour pressure > limit used in the classification of volatile organic compounds (VOCs) | For aprotic ILs: negligible vapour pressure under normal conditions |
| Flammability | Usually flammable | Usually non-flammable, but some ILs are used as propellants |
| Tuneability | Limited range of solvents available | Virtually unlimited range means “designer solvents” |
| Chirality | Rare | Common and tuneable |
| Catalytic ability | Rare | Common and tuneable |
| Viscosity/mPa·s | 0.2–100 | 20–97,000 |
| Density/g·cm−3 | 0.6–1.7 | 0.8–3.3 |
| Refractive Index | 1.3–1.6 | 1.3–2.2 |
| Electrical conductivity/mS·cm−1 | Usually insulator | Up to 120 |
| Thermal conductivity/W·m−1·K−1 | 0.1–0.6 | 0.1–0.3 |
| Company | Product lines | Scale |
|---|---|---|
| Iolitec | Ammonium, imidazolium, phosphonium, piperidinium, pyridinium, pyrrolidinium, sulfonium ILs | Portfolio of >250 ILs [83] Multi-kg scale >1 tonne by 2020 [55] |
| Proionic | Imidazolium, pyrrolidinium ILs | >1 tonne |
| Scionix | Ammonium ILs [41,42,46] | Ten 200 kg batches of IL and one IL made on a tonne scale [46] |
| Solvionic | Ammonium, imidazolium, phosphonium, piperidinium, pyrrolidinium ILs | >1.5 tonnes/month by 2021 >50 tonnes/month by 2023 [189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. https://doi.org/10.3390/molecules25215207
Greer AJ, Jacquemin J, Hardacre C. Industrial Applications of Ionic Liquids. Molecules. 2020; 25(21):5207. https://doi.org/10.3390/molecules25215207
Chicago/Turabian StyleGreer, Adam J., Johan Jacquemin, and Christopher Hardacre. 2020. "Industrial Applications of Ionic Liquids" Molecules 25, no. 21: 5207. https://doi.org/10.3390/molecules25215207
APA StyleGreer, A. J., Jacquemin, J., & Hardacre, C. (2020). Industrial Applications of Ionic Liquids. Molecules, 25(21), 5207. https://doi.org/10.3390/molecules25215207







