Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks
Abstract
:1. Introduction
1.1. Chemical Characteristics of Tetracycline Antibiotics
1.2. Characteristics of Sulfonamide Antibiotics
2. Presence of TCs and SAs in Manures and Slurries
3. Presence of TCs and SAs in Soils
4. Dynamics and Fate of Antibiotics in Soils
4.1. Adsorption/Desorption
4.1.1. Influence of Edaphic Variables and Adsorption/Desorption Mechanisms
Tetracycline Antibiotics
Sulfonamide Antibiotics
4.2. Degradation
4.2.1. Hydrolysis
4.2.2. Photodegradation
- (a)
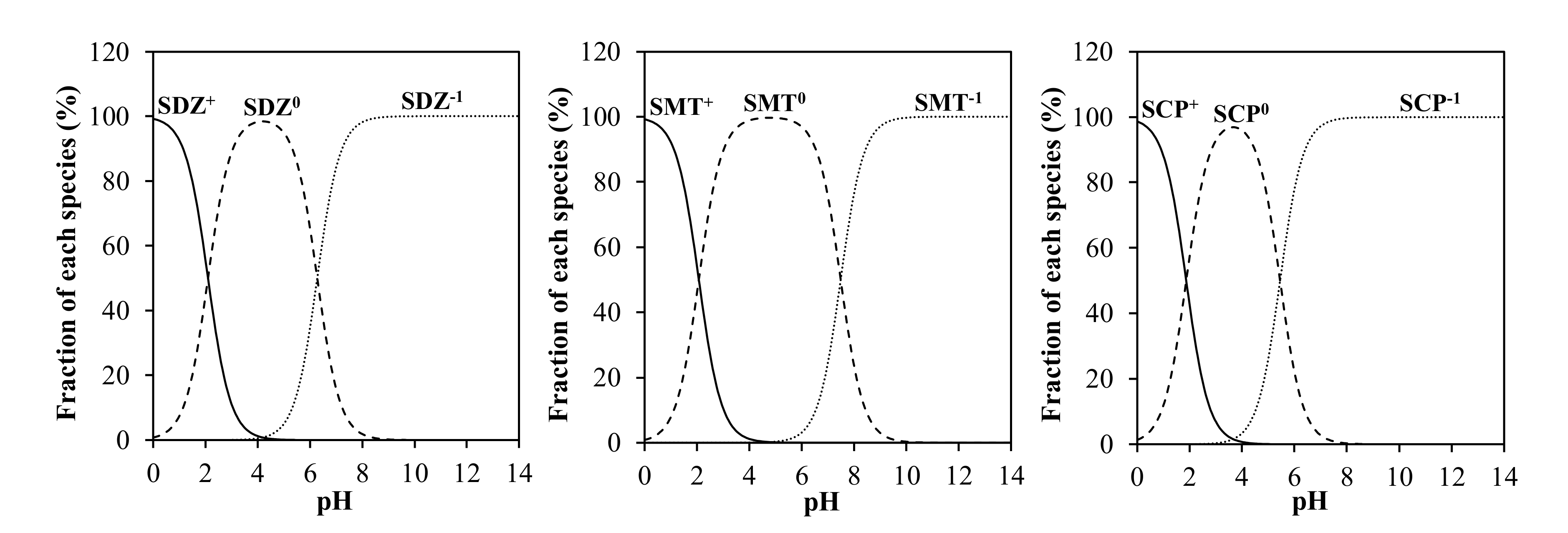
- TCs are amphoteric molecules, with three acid dissociation constants (pKa), therefore, depending on the pH of the medium, they can be found in cationic, zwitterionic, and/or anionic form. For example, at pH 4.0, 17% of TC species are in the cationic form, while at pH 7.2 the cationic TC species only represent 0.01%, with percentage distribution being very similar for OTC and CTC. At the same time, increasing the pH from 4.0 to 7.2 causes a progressive decrease in the zwitterionic forms and a significant increase in the negatively charged forms. Furthermore, different researchers indicated that the degree of absorption of light radiation of the anionic species of TCs overlaps more with the spectrum of simulated sunlight, compared to the neutral and cationic species [142,143,144]. Therefore, an increase in pH is expected to favor direct photolysis of CTs.
- (b)
- An increase in pH gives rise to an increase in the concentration of OH− ions, which react with the hydroxyl radicals (HO·) generated by the presence of the TCs molecules in the solution, producing highly reactive oxygen species (O·−), according to the following equation proposed by Liu et al. [147]:
- (c)
- During the photolysis of TCs, H2O2 is also formed, which can also lead to the formation of HO·radicals as a consequence of the photolysis of the peroxide bond (-O-O-) [141]:
- (a)
- On the one hand, as in the case of TCs, SAs are amphoteric molecules, presenting two acid dissociation constants (pKa), therefore, depending on the pH of the medium, one or other species of these compounds (cationic, neutral, and/or anionic) will dominate. In this sense, Boreen et al. [157] pointed out that the different species of SAs have different reactivity to light, indicating that the quantum yield (moles of a compound that are transformed per mole of photons that are absorbed by the compound) is 17, 3, and 8 times higher for the anionic species of SMT, SDZ, and SCP, respectively, than for the neutral species. Baeza and Knappe [158] also indicate that photolysis is higher for anionic species in relation to the neutral molecules. In this sense, at pH 4, 98%, 99%, and 96% of the species of SDZ, SMT, and SCP, respectively, are in neutral form. However, as the pH increases, the proportion of neutral species decreases, at the same time as the proportion of anionic species increases. Thus, at pH 7.2, the anionic species represent 89%, 34%, and 98% for SDZ, SMT, and SCP, respectively, thus justifying an increase in direct photolysis with increasing pH.
- (b)
- On the other hand, an increase in pH also leads to an increase in indirect photolysis, favoring the oxidation of these antibiotics due to the generation of highly reactive free radicals [159]. This is due to the increase in the concentration of OH− ions available to react with hydroxyl radicals (OH·), which are generated by the presence of SAs molecules in the solution, thus increasing the generation of reactive species O·− [147].
4.2.3. Biodegradation
5. Environmental Risks Associated to the Presence of Tetracycline and Sulfonamide Antibiotics
5.1. Transport of Antibiotics and Presence in Waterbodies
5.2. Influence on Soil Organisms
5.3. Entry of Antibiotics in the Food Chain Through Crops
Author Contributions
Funding
Conflicts of Interest
References
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Liu, W. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.P.; Bu, D.P.; Carriqque-Mas, J.; Fèvre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S.; et al. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Med. Hyg. 2016, 110, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Ozumchelouei, E.J.; Hamidian, A.H.; Zhang, Y.; Yang, M. Physicochemical properties of antibiotics: A review with an emphasis on detection in the aquatic environment. Water. Environ. Res. 2020, 92, 177–188. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- OIE. OIE Annual Report on Antimicrobial Agents Intended for Use in Animals: Better Understanding of the Global Situation. Third Report; OIE: Paris, France, 2018. [Google Scholar]
- EMA (ESVAC). Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2017; EMA: Amsterdam, Netherlands, 2019. [Google Scholar]
- WHO. Critically Important Antimicrobials for Human Medicine, 6th Revision; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Chen, M.; Yi, Q.; Hong, J.; Lin, K.; Yuan, D. Simultaneous determination of 32 antibiotics and 12 pesticides in sediment using ultrasonic-assisted extraction and high performance liquid chromatography-tandem mass spectrometry. Anal. Methods 2015, 7, 1896–1905. [Google Scholar] [CrossRef]
- Babić, S.; Horvat, A.J.M.; Mutavdžić-Pavlović, D.; Kaštelan-Macan, M. Determination of pKa values of active pharmaceutical ingredients. Trends Anal. Chem. 2007, 26, 1043–1061. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (Vas) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Pikkemaat, M.G.; Yassin, H.; van-der-Fels-Klerx, H.J.; Berendsen, B.J.A. Antibiotic Residues and Resistance in the Environment; RIKILT Wageningen UR: Wageningen, The Netherlands, 2016. [Google Scholar] [CrossRef] [Green Version]
- Kuppusamy, S.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M.; Yoon, Y.-E.; Lee, Y.B. Veterinary antibiotics (VAs) contamination as a global agro-ecological issue: A critical view. Agric. Ecosyst. Environ. 2018, 257, 47–59. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghirardini, A.; Grillini, V.; Verlicchi, P. A review of the occurrence of selected micropollutants and microorganisms in different raw and treated manure—Environmental risk due to antibiotics after application to soil. Sci. Total Environ. 2020, 707. [Google Scholar] [CrossRef] [PubMed]
- Wohde, M.; Berkner, S.; Junker, T.; Konradi, S.; Schwarz, L.; Düring, R.-A. Occurrence and transformation of veterinary pharmaceuticals and biocides in manure: A literature review. Environ. Sci. Eur. 2016, 28, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Qiang, Z.; Ben, W.; Chen, M. Residual veterinary antibiotics in swine manure from concentrated animal feeding operations in Shandong Province, China. Chemosphere 2011, 84, 695–700. [Google Scholar] [CrossRef]
- Widyasari-Mehta, A.; Hartung, S.; Kreuzig, R. From the application of antibiotics to antibiotic residues in liquid manures and digestates: A screening study in one European center of conventional pig husbandry. J. Environ. Manag. 2016, 177, 129–137. [Google Scholar] [CrossRef]
- Martínez-Carballo, E.; González-Barreiro, C.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007, 148, 570–579. [Google Scholar] [CrossRef]
- Spielmeyer, A. Occurrence and fate of antibiotics in manure during manure treatments: A short review. Sustain. Chem. Pharm. 2018, 9, 76–86. [Google Scholar] [CrossRef]
- Boxall, A.B.A. Fate of Veterinary Medicines Applied to Soils. In Pharmaceuticals in the Environment; Kümmerer, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.-F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J. Environ. Qual. 2009, 38, 1086–1108. [Google Scholar] [CrossRef] [Green Version]
- Ezzariai, A.; Hafidi, M.; Khadra, A.; Aemig, Q.; Fels, L.E.; Barret, M.; Merlina, G.; Patureau, D.; Pinelli, E. Human and veterinary antibiotics during composting of sludge or manure: Global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 2018, 359, 465–481. [Google Scholar] [CrossRef]
- Xie, W.-Y.; Shen, Q.; Zhao, F.J. Antibiotics and antibiotic resistance from animal manures to soil: A review. Eur. J. Soil Sci. 2018, 69, 181–195. [Google Scholar] [CrossRef] [Green Version]
- Elmund, G.K.; Morrison, S.M.; Grant, D.W.; Nevins, M.P. Role of excreted chlortetracycline in modifying the decomposition process in feedlot waste. Bull. Environ. Contam. Toxicol. 1971, 6, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Winckler, C.; Grafe, A. Use of veterinary drugs in intensive animal production. Evidence for persistence of tetracycline in pig slurry. J. Soils Sediments 2001, 1, 66–70. [Google Scholar] [CrossRef]
- Ince, B.; Coban, H.; Turker, G.; Ertekin, E.; Ince, O. Effect of oxytetracycline on biogas production and active microbial populations during batch anaerobic digestion of cow manure. Bioprocess Biosyst. Eng. 2013, 36, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Lamshöft, M.; Sukul, P.; Zühlke, S.; Spiteller, M. Metabolism of 14C-labelled and non-labelled sulfadiazine after administration to pigs. Anal. Bioanal. Chem. 2007, 388, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhao, T.; Liu, Q.; He, J.; He, D.; Wu, G.; Li, Y.; Jiang, C.; Xu, Z. Residual veterinary antibiotics in pig excreta after oral administration of sulfonamides. Environ. Geochem. Health 2016, 38, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Duffee, N.E.; Bevill, R.F.; Thurmon, J.C.; Luther, H.G.; Nelson, D.E.; Hacker, F.E. Pharmacokinetics of sulfamethazine in male, female and castrated male swine. J. Vet. Pharmacol. Ther. 1984, 7, 203–211. [Google Scholar] [CrossRef]
- Karcı, A.; Balcıoğlu, I.A. Investigation of the tetracycline, sulfonamide, and fluoroquinolone antimicrobial compounds in animal manure and agricultural soils in Turkey. Sci. Total Environ. 2009, 407, 4652–4664. [Google Scholar] [CrossRef]
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. Clean-Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- De-Liguoro, M.; Cibin, V.; Capolongo, F.; Halling-Sørensen, B.; Montesissa, C. Use of oxytetracycline and tylosin in intensive calf farming: Evaluation of transfer to manure and soil. Chemosphere 2003, 52, 203–212. [Google Scholar] [CrossRef]
- Hamscher, G.; Sczesny, S.; Höper, H.; Nau, H. Determination of Persistent Tetracycline Residues in Soil Fertilized with Liquid Manure by High-Performance Liquid Chromatography with Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Tasho, R.P.; Cho, J.Y. Veterinary antibiotics in animal waste, its distribution in soil and uptake by plants: A review. Sci. Total Environ. 2016, 563–564, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, J.; Wang, J.; Ma, Z.; Han, P.; Luan, Y.; Lu, A. Occurrence of antibiotics in soils and manures from greehouse vegetable production bases of Beijing, China and an associated risk assessment. Sci. Total Environ. 2015, 521–522, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef]
- Huang, X.; Liu, C.; Li, K.; Liu, F.; Liao, D.; Liu, L.; Zhu, G.; Liao, J. Occurrence and distribution of veterinary antibiotics and tetracycline resistance genes in farmland soils around swine feedlots in Fujian Province, China. Environ. Sci. Pollut. Res. Int. 2013, 20, 9066–9074. [Google Scholar] [CrossRef]
- Hou, J.; Wan, W.; Mao, D.; Wang, C.; Mu, Q.; Qin, S.; Luo, Y. Occurrence and distribution of sulfonamides, tetracyclines, quinolones, macrolides, and nitrofurans in livestock manure and amended soils of Northern China. Environ. Sci. Pollut. Res. 2015, 22, 4545–4554. [Google Scholar] [CrossRef]
- Li, Y.-W.; Wu, X.-L.; Mo, C.-H.; Tai, Y.-P.; Huang, X.-P.; Xiang, L. Investigation of sulfonamide, tetracycline, and quinolone antibiotics in vegetable farmland soil in the Pearl River Delta Area, Southern China. J. Agric. Food Chem. 2011, 59, 7268–7276. [Google Scholar] [CrossRef]
- Wei, R.; He, T.; Zhang, S.; Zhu, L.; Shang, B.; Li, Z.; Wang, R. Occurrence of seventeen veterinary antibiotics and resistant bacterias in manure-fertilized vegetable farm soil in four provinces of China. Chemosphere 2019, 215, 234–240. [Google Scholar] [CrossRef]
- Xiang, L.; Wu, X.-L.; Jiang, Y.-N.; Yan, Q.-Y.; Li, Y.-W.; Huang, X.-P.; Cai, Q.-Y.; Mo, C.-H. Occurrence and risk assessment of tetracycline antibiotics in soil from organic vegetable farms in a subtropical city, south China. Environ. Sci. Pollut. Res. 2016, 23, 13984–13995. [Google Scholar] [CrossRef]
- An, J.; Chen, H.; Wei, S.; Gu, J. Antibiotic contamination in animal manure, soil, and sewage sludge in Shenyang, northeast China. Environ. Earth Sci. 2015, 74, 5077–5086. [Google Scholar] [CrossRef]
- Sun, J.; Zeng, Q.; Tsang, D.C.W.; Zhu, L.Z.; Li, X.D. Antibiotics in the agricultural soils from the Yangtze River Delta, China. Chemosphere 2017, 189, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.M.; Halling-Sørensen, B.; Ingerslev, F.; Hansen, S.H. Simultaneous extraction of tetracycline, macrolide and sulfonamide antibiotics from agricultural soils using pressurized liquid extraction, followed by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2004, 1038, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Pawelzick, H.T.; Höper, H.; Nau, H.; Hamscher, G. A survey of the occurrence of various tetracyclines and sulfamethazine in sandy soils in northwestern Germany fertilized with liquid manure. In Proceedings of the SETAC Euro 14th Annual Meeting, Prague, Czech Republic, 18–22 April 2004. [Google Scholar]
- Awad, Y.M.; Kim, S.-C.; El-Azeem, S.A.M.; Kim, K.-H.; Kim, K.-R.; Kim, K.; Jeon, C.; Lee, S.S.; Ok, Y.S. Veterinary antibiotics contamination in water, sediment, and soil near a swine manure composting facility. Environ. Earth Sci. 2014, 71, 1433–1440. [Google Scholar] [CrossRef]
- Ok, Y.S.; Kim, S.-C.; Kim, K.-R.; Lee, S.S.; Moon, D.H.; Lim, K.J.; Sung, J.-K.; Hur, S.-O.; Yang, J.E. Monitoring of selected veterinary antibiotics in environmental compartments near a composting facility in Gangwon Province, Korea. Environ. Monit. Assess. 2011, 174, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Occurrence of veterinary antibiotics and progesterone in broiler manure and agricultural soil in Malaysia. Sci. Total Environ. 2014, 488–489, 261–267. [Google Scholar] [CrossRef]
- Andreu, V.; Vazquez-Roig, P.; Blasco, C.; Picó, Y. Determination of tetracycline residues in soil by pressurized liquid extraction and liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2009, 394, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- García-Galán, M.J.; Díaz-Cruz, S.; Barceló, D. Multiresidue trace analysis of sulfonamide antibiotics and their metabolites in soils and sewage sludge by pressurized liquid extraction followed by liquid chromatography-electrospray-quadrupole linear ion trap mass spectrometry. J. Chromatogr. A 2013, 1275, 32–40. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Álvarez-Esmorís, C.; Paradelo-Núñez, R.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. Occurrence of tetracyclines and sulfonamides in manures, agricultural soils and crops from different áreas in Galicia (NW Spain). J. Clean. Prod. 2018, 197, 491–500. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Fogg, L.A.; Baird, D.J.; Lewis, C.; Telfer, T.C.; Kolpin, D.; Gravell, A.; Pemberton, E.; Boucard, T. Targeted Monitoring Study for Veterinary Medicines in the UK Environment; Environmental Agency: Bristol, TN, USA, 2005. [Google Scholar]
- Linn, D.M.; Carski, T.H.; Brusseau, M.L.; Chang, F.H. Sorption and Degradation of Pesticides and Organic Chemicals in Soil; SSSA: Madison, WI, USA, 1993. [Google Scholar]
- Kümmerer, K. Resistance in the environment. J. Antimicrob. Chemother. 2004, 54, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Calvet, R. Adsorption of organic chemicals in soils. Environ. Health Perspect. 1989, 83, 145–177. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S. Pharmaceutical antibiotic compounds in soils—A review. J. Plant Nutr. Soil Sci. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, Q.; Wan, Y.; Yu, Q.; Xie, X. Effects of soil/solution ratios and cation types on adsorption and desorption of tetracycline in soils. Soil Sci. Soc. Am. J. 2010, 74, 1553–1561. [Google Scholar] [CrossRef] [Green Version]
- Sassman, S.A.; Lee, L.S. Sorption of three tetracyclines by several soils: Assessing the role of pH and cation exchange. Environ. Sci. Technol. 2005, 39, 7452–7459. [Google Scholar] [CrossRef] [PubMed]
- Rabølle, M.; Spiild, H. Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere 2000, 40, 715–722. [Google Scholar] [CrossRef]
- Kong, W.; Li, C.; Dolhi, J.M.; Li, S.; He, J.; Qiao, M. Characteristics of oxytetracycline sorption and potential bioavailability in soils with various physical-chemical properties. Chemosphere 2012, 87, 542–548. [Google Scholar] [CrossRef]
- Gong, W.; Liu, X.; He, H.; Wang, L.; Dai, G. Quantitatively modeling soil-water distribution coefficients of three antibiotics using soil physicochemical properties. Chemosphere 2012, 89, 825–831. [Google Scholar] [CrossRef]
- Laak, T.L.T.; Gebbink, W.A.; Tolls, J. Estimation of soil coefficients of veterinary pharmaceuticals from soil properties. Environ. Toxicol. Chem. 2006, 25, 933–941. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Q.; Deng, X.; Nan, Z.; Liang, X.; Wen, H.; Huang, K.; Wu, Y. Single and competitive sorption of sulfadiazine and chlortetracycline on loess soil from Northwest China. Environ. Pollut. 2020, 263, 114650. [Google Scholar] [CrossRef]
- Leal, R.M.P.; Alleoni, L.R.F.; Tornisielo, V.L.; Regitano, J.B. Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils. Chemosphere 2013, 92, 979–985. [Google Scholar] [CrossRef] [Green Version]
- Sukul, P.; Lamshöft, M.; Zühlke, S.; Spiteller, M. Sorption and desorption of sulfadiazine in soil and soil-manure systems. Chemosphere 2008, 73, 1344–1350. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sarmah, A.K.; Manley-Harris, M. Soprtion of selected veterinary antibiotics onto dairy farming soils of contrasting nature. Sci. Total Environ. 2014, 472, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.P.; Rath, S.; Fostier, A.H. Sorption of sulfachloropyridazine in Brazilian soils. J. Braz. Chem. Soc. 2017, 28, 158–167. [Google Scholar] [CrossRef]
- Álvarez-Esmorís, C.; Conde-Cid, M.; Ferreira-Coelho, G.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Adsorption/desorption of sulfamethoxypyridazine and enrofloxacin in agricultural soils. Sci. Total Environ. 2020, 706, 136015. [Google Scholar] [CrossRef] [PubMed]
- Thiele-Bruhn, S.; Aust, M.O. Effects of pig slurry on the sorption of sulfonamide antibiotics in soil. Arch. Environ. Contam. Toxicol. 2004, 47, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wegst-Uhrich, S.R.; Navarro, D.A.G.; Zimmerman, L.; Aga, D.S. Assessing antibiotic sorption in soil: A literature review and new case studies on sulfonamides and macrolides. Chem. Cent. J. 2014, 8, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Tolls, J. Sorption of veterinary pharmaceuticals in soils: A review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Sun, K.; Zhao, Y.; Lin, C. Sorption of tetracycline to sediments and soils: Assessing the roles of pH, the presence of cadmium and properties of sediments and soils. Front. Environ. Sci. Eng. 2010, 4, 421–429. [Google Scholar] [CrossRef]
- Teixidó, M.; Granados, M.; Prat, M.D.; Beltrán, J.L. Sorption of tetracyclines onto natural soils: Data analysis and prediction. Environ. Sci. Pollut. Res. 2012, 19, 3087–3095. [Google Scholar] [CrossRef]
- Wan, Y.; Bao, Y.; Zhou, Q. Simultaneous adsorption and desorption of cadmium and tetracycline on cinnamon soil. Chemosphere 2010, 80, 807–812. [Google Scholar] [CrossRef]
- Li, Y.; Pan, T.; Miao, D.; Chen, Z.; Tao, Y. Sorption-Desorption of Typical Tetracyclines on Different Soils: Environment Hazards Analysis with Partition Coefficients and Hysteresis Index. Environ. Eng. Sci. 2015, 32, 865–871. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Experimental data and model prediction of tetracycline adsorption and desorption in agricultural soils. Environ. Res. 2019, 177, 108607. [Google Scholar] [CrossRef] [PubMed]
- Goetesch, H.E.; Mylon, S.E.; Butler, S.; Zilles, J.L.; Nguyen, T.H. Oxytetracycline interactions at the soil-water interface: Effects of environmental surfaces on natural transformation and growth inhibition of Azotobacter vinelandii. Environ. Toxicol. Chem. 2012, 31, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, S.; Han, M.; Cho, J. Sorption characteristics of oxytetracycline, amoxicillin, and sulfathiazole in two different soil types. Geoderma 2012, 185–186, 97–101. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Estimation of adsorption/desorption Freundlich’s affinity coefficients for oxytetracycline and chlortetracycline from soil properties: Experimental data and pedotransfer functions. Ecotoxicol. Environ. Safe. 2020, 196, 110584. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, Y.; Shen, G.; Zhang, H.; Yuan, Z.; Zhang, W. Adsorption/desorption behavior and mechanisms of sulfadiazine and sulfamethoxazole in agricultural soil systems. Soil Till. Res. 2019, 186, 233–241. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, Y.; Hu, S.; Zhang, H.; Yuan, Z.; Zhang, W. Adsorption and degradation of sulfadiazine and sulfamethoxazole in an agricultural soil system under an anaerobic condition: Kinetics and environmental risks. Chemosphere 2018, 194, 266–274. [Google Scholar] [CrossRef]
- Lertpaitoonpan, S.; Ong, S.K.; Moorman, T.B. Effect of organic carbon and pH on soil sorption sulfamethazine. Chemosphere 2009, 76, 558–564. [Google Scholar] [CrossRef]
- Pavlović, D.M.; Ćurković, L.; Blažek, D.; Župan, J. The sorption of sulfamethazine on soil samples: Isotherms and error analysis. Sci. Total Environ. 2014, 497–498, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, P.; Sarmah, A.K. Assessing the sorption and leaching behaviour of three sulfonamides in pasture soils through batch and column studies. Sci. Total Environ. 2014, 493, 535–543. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Blackwell, P.; Cavallo, R.; Kay, P.; Tolls, J. The sorption and transport of a sulphonamide antibiotic in soil systems. Toxicol. Lett. 2002, 131, 19–28. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Nóvoa-Muñoz, J.C.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Pedotransfer functions to estimate the adsorption and desorption of sulfadiazine in agricultural soils. Sci. Total Environ. 2019, 691, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Fostier, A.H.; Pereira, L.A.; Dionisio, A.C.; Ferreira, F.O.; Doretto, K.M.; Peruchi, L.M.; Viera, A.; Neto, O.F.O.; Bosco, S.M.D.; et al. Sorption behaviors of antimicrobial and antiparasitic veterinary drugs on subtropical soils. Chemosphere 2019, 214, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, Y.; Jing, M.; Chen, J. Sorption and transport of sulfonamides in soils amended with wheat straw-derived biochar: Effects of water pH, coexistence copper ion, and dissolved organic matter. J. Soils Sediments 2017, 17, 771–779. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Nóvoa-Muñoz, J.C.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Experimental data and modeling for sulfachloropyridazine and sulfamethazine adsorption/desorption on agricultural acid soils. Micropor. Mesopor. Mat. 2019, 288, 109601. [Google Scholar] [CrossRef]
- Kodešová, R.; Grabic, R.; Kočárek, M.; Klement, A.; Golovko, O.; Fér, M.; Nikodema, A.; Jakšík, O. Pharmaceuticals’ sorptions relative to properties of thirteen different soils. Sci. Total Environ. 2015, 511, 435–443. [Google Scholar] [CrossRef]
- Kočárek, M.; Kodešova, R.; Vondráčková, L.; Golovko, O.; Fér, M.; Klement, A.; Nikodem, A.; Jakšík, O.; Grabic, R. Simultaneous sorption of four ionizable pharmaceuticals in different horizons of three soil types. Environ. Pollut. 2016, 218, 563–573. [Google Scholar] [CrossRef]
- Maszkowska, J.; Białk-Bielińska, A.; Mioduszewska, K.; Wagil, M.; Kumirska, J.; Stepnowski, P. Sorption of sulfisoxazole onto soil-an insight into different influencing factors. Environ. Sci. Pollut. Res. 2015, 22, 12182–12189. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, Q.; Wang, Y.; Yu, Q.; Xie, X. Adsorption and desorption of three tetracycline antibiotics in cinnamon soils of China. China Environ. Sci. 2010, 30, 1383–1388. [Google Scholar]
- Bao, Y.; Zhou, Q.; Bao, Y.; Liu, Y.; Xie, X. Adsorption and desorption of three tetracycline antibiotics in petroleum-contaminated soils. China Environ. Sci. 2012, 32, 1257–1262. [Google Scholar]
- Chen, Z.; Li, G.; Sum, L.; Li, Y. Tetracyclines sorption in the presence of cadmium on river sediments: The effects of sorption mechanism and complex properties. Water, Air, Soil Pollut. 2016, 227–283. [Google Scholar] [CrossRef]
- Avisar, D.; Primor, O.; Gozlan, I.; Mamane, H. Sorption of sulfonamides and tetracyclines to montmorillonite clay. Water Air Soil Pollut. 2010, 209, 439–450. [Google Scholar] [CrossRef]
- Álvarez-Esmorís, C.; Conde-Cid, M.; Fernández-Calviño, D.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Adsorption-desorption of doxycycline in agricultural soils: Batch and stirred flow-chamber experiments. Environ. Res. 2020, 186, 109565. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calviño, D.; Bermúdez-Couso, A.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Kinetics of tetracycline, oxytetracycline, and chlortetracyclineadsorption and desorption on two acid soils. Environ. Sci. Pollut. Res. 2015, 22, 425–433. [Google Scholar] [CrossRef]
- Pils, J.R.; Laird, D.A. Sorption of tetracycline and chlortetracycline on K- and Ca-saturated soil clays, humic substances, and clay-humic complexes. Environ. Sci. Technol. 2007, 41, 1928–1933. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Adsorption/desorption and transport of sulfadiazine, sulfachloropyridazine, and sulfamethazine, in acid agricultural soils. Chemosphere 2019, 234, 978–986. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Seibicke, T.; Schulten, H.R.; Leinweber, P. Sorption of sulfonamide pharmaceutical antibiotics on whole soils and particle-size fractions. J. Environ. Qual. 2004, 33, 1331–1342. [Google Scholar] [CrossRef]
- Carda-Broch, S.; Berthod, A. Countercurrent chromatography for the measurement of the hydrophobicity of sulfonamide amphoteric compounds. Chromatographia 2004, 59, 79–87. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, W.; Ma, Q.; Zhou, H. Interactive effects of sulfadiazine and Cu(II) on their sorption and desorption on two soils with different characteristics. Chemosphere 2015, 138, 701–707. [Google Scholar] [CrossRef]
- Doretto, K.M.; Rath, S. Sorption of sulfadiazine on Brazilian soils. Chemosphere 2013, 90, 2027–2034. [Google Scholar] [CrossRef] [Green Version]
- Pinna, M.V.; Castaldi, P.; Deiana, P.; Pusino, A.; Garau, G. Sorption behavior of sulfamethazine on unamended and manure-amended soils and short-term impact on soil microbial community. Ecotoxicol. Environ. Saf. 2012, 84, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, E.; Laird, D.A.; Koskinen, W.C.; Dowdy, R.H. Atrazine desorption from smectites. Soil Sci. Soc. Am. J. 1994, 58, 1632–1638. [Google Scholar] [CrossRef]
- Doretto, K.M.; Peruchi, L.M.; Rath, S. Sorption and desorption of sulfadimethoxine, sulfaquinoxaline and sulfamethazine antimicrobials in Brazilian soils. Sci. Total Environ. 2014, 476–477, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Munira, S.; Farenhorst, A. Sorption and desorption of glyphosate, MCPA and tetracycline and their mixtures in soil as influenced by phosphate. J. Environ. Sci. Health B 2017, 52, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Ding, H.; Bao, Y. Effects of temperature on the adsorption and desorption of tetracycline in soils. Adv. Mat. Res. 2013, 726–731, 344–347. [Google Scholar] [CrossRef]
- Jones, A.D.; Bruland, G.L.; Agrawal, S.G.; Vasudevan, D. Factors influencing the sorption of oxytetracycline to soils. Environ. Toxicol. Chem. 2005, 24, 761–770. [Google Scholar] [CrossRef]
- Yao, F.; Zhaojun, L.; Xiaoqing, H. Impacts of soil organic matter, iron-aluminium oxides and pH on adsorption-desorption behaviors of oxytetracycline. Res. J. Biotech. 2016, 11, 121–131. [Google Scholar]
- Zhu, F.; Jiao, S.J.; Shan, Z.J.; Kong, D.Y.; Ge, F.; Wang, N. Sorption and desorption behaviour of doxycycline on a Wushantu soil in Taihu Lake region, China. Fresenius Environ. Bull. 2015, 24, 3295–3299. [Google Scholar]
- Chu, B.; Goyne, K.W.; Anderson, S.H.; Lin, C.H.; Lerch, R.N. Sulfamethazine sorption to soil: Vegetative management, pH, and dissolved organic matter effects. J. Environ. Qual. 2013, 42, 794–805. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J. Coll. Interf. Sci. 2011, 361, 247–251. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology 2011, 20, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gu, X.; Gao, S.; Geng, J.; Wang, X. Adsorption of tetracycline (TC) onto montmorillonite: Cations and humic acid effects. Geoderma 2012, 183–184, 12–18. [Google Scholar] [CrossRef]
- Gu, C.; Karthikeyan, K.G.; Sibley, S.D.; Pedersen, J.A. Complexation of the antibiotic tetracycline with humic acid. Chemosphere 2007, 66, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Shi, X.; Mao, J.; Zhu, D. Tetracycline sorption to coal and soil humic acids: An examination of humic structural heterogeneity. Environ. Toxicol. Chem. 2010, 29, 1934–1942. [Google Scholar] [CrossRef]
- Sithole, B.; Guy, R.D. Models for tetracycline in aquatic environments—II. Interaction with humic substances. Water Air Soil Pollut. 1987, 32, 315–321. [Google Scholar] [CrossRef]
- Sithole, B.B.; Guy, R.D. Models for tetracycline in aquatic environments. I. Interaction with Bentonite Clay Systems. Water Air Soil Pollut. 1987, 32, 303–314. [Google Scholar] [CrossRef]
- Białk-Bielińska, A.; Maszkowska, J.; Mrozik, W.; Bielawska, A.; Kołodziejska, M.; Palavinskas, R.; Stepnowski, P.; Kumirska, J. Sulfadimethoxine and sulfaguanidine: Their sorption potential on natural soils. Chemosphere 2012, 86, 1059–1065. [Google Scholar] [CrossRef]
- Wang, N.; Guo, X.; Xu, J.; Hao, L.; Kong, D.; Gao, S. Sorption and transport of five sulfonamide antibiotics in agricultural soil and soil-manure systems. J. Environ. Sci. Health B 2015, 50, 23–33. [Google Scholar] [CrossRef]
- Chen, K.-L.; Liu, L.-C.; Chen, W.-R. Adsorption of sulfamethoxazole and sulfapyridine antibiotics in high organic content soils. Environ. Pollut. 2017, 231, 1163–1171. [Google Scholar] [CrossRef]
- Białk-Bielińska, A.; Stolte, S.; Matzke, M.; Fabiańska, A.; Maszkowska, J.; Kołodziejska, M.; Liberek, B.; Stepnowski, P.; Kumirska, J. Hydrolysis of sulphonamides in aqueous solutions. J. Hazard. Mater. 2012, 221–222, 264–274. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J. pH and temperature effects on the hydrolysis of three β-lactam antibiotics: Ampicillin, cefalotin and cefoxitin. Sci. Total Environ. 2014, 466–467, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B. Algal toxicity of antibacterial agents used in intensive farming. Chemosphere 2000, 40, 731–739. [Google Scholar] [CrossRef]
- Paesen, J.; Cypers, W.; Pauwels, K.; Roets, E.; Hoogmartens, J. Study of the stability of tylosin A in aqueous solutions. J. Pharm. Biomed. Anal. 1995, 13, 1153–1159. [Google Scholar] [CrossRef]
- Xuan, R.; Arisi, L.; Wang, Q.; Yates, S.R.; Biswas, K.C. Hydrolysis and photolysis of oxytetracycline in aqueous solution. J. Environ. Sci. Heal. B 2010, 45, 73–81. [Google Scholar] [CrossRef]
- Charuaud, L.; Jarde, E.; Jaffrezic, A.; Thomas, M.-F.; Bot, B.L. Veterinary pharmaceutical residues from natural water to tap water: Sales, occurrence and fate. J. Hazard. Mater. 2019, 361, 169–186. [Google Scholar] [CrossRef] [Green Version]
- Voigt, M.; Jaeger, M. On the photodegradation of azithromycin, erythromycin and tylosin and their transformation products—A kinetic study. Sustain. Chem. Pharm. 2017, 5, 131–140. [Google Scholar] [CrossRef]
- Batchu, S.R.; Panditi, V.R.; Gardinali, P.R. Photodegradation of sulfonamide antibiotics in simulated and natural sunlight: Implications for their environmental fate. J. Environ. Sci. Heal. B 2014, 49, 200–211. [Google Scholar] [CrossRef]
- Batchu, S.R.; Panditi, V.R.; O’Shea, K.E.; Gardinali, P.R. Photodegradation of antibiotics under simulated solar radiation: Implications for their environmental fate. Sci. Total Environ. 2014, 470–471, 299–310. [Google Scholar] [CrossRef]
- Timm, A.; Borowska, E.; Majewsky, M.; Merel, S.; Zwiener, C.; Bräse, S.; Horn, H. Photolysis of four β-lactam antibiotics under simulated environmental conditions: Degradation, transformation products and antibacterial activity. Sci. Total Environ. 2019, 651, 1605–1612. [Google Scholar] [CrossRef]
- Jin, X.; Xu, H.; Qiu, S.; Jia, M.; Wang, F.; Zhang, A.; Jiang, X. Direct photolysis of oxytetracycline: Influence of initial concentration, pH and temperature. J. Photochem. Photobiol. A 2017, 332, 224–231. [Google Scholar] [CrossRef]
- Niu, J.; Li, Y.; Wang, W. Light-source-dependent role of nitrate and humic acid in tetracycline photolysis: Kinetics and mechanism. Chemosphere 2013, 92, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Thiele-Bruhn, S.; Peters, D. Photodegradation of pharmaceuticals antibiotics on slurry and soil surfaces. Landbauforschung Völkenrode 2007, 1, 13–23. [Google Scholar]
- López-Peñalver, J.J.; Sánchez-Polo, M.; Gómez-Pacheco, C.V.; Rivera-Utrilla, J. Photodegradation of tetracyclines in aqueous solution by using UV and UV/H2O2 oxidation processes. J. Chem. Technol. Biotechnol. 2010, 85, 1325–1333. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, C.; Qu, J.; Yang, M. Photodegradation of tetracycline and formation of reactive oxygen species in aqueous tetracycline solution under simulated sunlight irradiation. J. Photochem. Photobiol. A 2008, 197, 81–87. [Google Scholar] [CrossRef]
- Jiao, S.; Zheng, S.; Yin, D.; Wang, L.; Chen, L. Aqueous oxytetracycline degradation and the toxicity change of degradation compounds in photoirradiation process. J. Environ. Sci. 2008, 20, 806–813. [Google Scholar] [CrossRef]
- Jiao, S.; Zheng, S.; Yin, D.; Wang, L.; Chen, L. Aqueous photolysis of tetracycline and toxicity of photolytic products to luminescent bacteria. Chemosphere 2008, 73, 377–382. [Google Scholar] [CrossRef]
- Werner, J.J.; Arnold, W.A.; McNeill, K. Water hardness as a phtochemical parameter: Tetracycline photolysis as a function of calcium concentration, magnesium concentration, and pH. Environ. Sci. Technol. 2006, 40, 7236–7241. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Díaz-Raviña, M.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E. Biotic and abiotic dissipation of tetracyclines using simulated sunlight and in the dark. Sci. Total Environ. 2018, 635, 1520–1529. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Duan, X.; Fu, Y.; Dionysiou, D.D. Photochemical Degradation of oxytetracycline: Influence of pH and role of carbonate radical. Chem. Eng. J. 2015, 276, 113–121. [Google Scholar] [CrossRef]
- Leal, J.F.; Esteves, V.I.; Santos, E.B.H. Use of sunlight to degrade oxytetracycline in marine aquaculture´s waters. Environ. Pollut. 2016, 213, 932–939. [Google Scholar] [CrossRef]
- Batista, A.P.S.; Pires, F.C.C.; Teixeira, A.C.S.C. Photochemical degradation of sulfadiazine, sulfamerazine and sulfamethazine: Relevance of concentration and heterocyclic aromatic groups to degradation kinetics. J. Photochem. Photobiol. A 2014, 286, 40–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhong, Z.; Guo, C.; Li, L.; He, Y.; Fan, W.; Chen, Y. Degradation of sulfonamides antibiotics in lake water and sediment. Environ. Sci. Pollut. Res. 2013, 20, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Díaz-Raviña, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E. Degradation of sulfadiazine, sulfachloropyrydazine and sulfamethazine in aqueous media. J. Environ. Manag. 2018, 228, 239–248. [Google Scholar] [CrossRef]
- Lian, J.; Qiang, Z.; Li, M.; Bolton, J.R.; Qu, J. UV photolysis kinetics of sulfonamides in aqueous solution based on optimized fluence quantification. Water Res. 2015, 75, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Zhang, J. Photodegradation of Sulfadiazine in Aqueous Solution and the Affeting Factors. J. Chem. 2016, 75, 8358960. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.Z.; Glady-Croué, J.; Croué, J.P. Photodegradation of sulfathiazole under simulated sunlight: Kinetics, photo-induced structural rearrangement, and antimicrobial activities of photoproducts. Water Res. 2017, 124, 576–583. [Google Scholar] [CrossRef]
- Periša, M.; Babić, S.; Škorić, I.; Frömel, T.; Knepper, T.P. Photodegradation of sulfonamides and their N4-acetylated metabolites in water by simulated sunlight irradiation: Kinetics and identification of photoproducts. Environ. Sci. Pollut. Res. 2013, 20, 8934–8946. [Google Scholar] [CrossRef]
- Song, Y.; Tian, J.; Gao, S.; Shao, P.; Qi, J.; Cui, F. Photodegradation of sulfonamides by g-C3N4 under visible light irradiation: Effectiveness, mechanism and pathways. Appl. Catal. B 2017, 210, 88–96. [Google Scholar] [CrossRef]
- Boreen, A.L.; Arnold, W.A.; Mcneill, K. Triplet-sensitized photodegradation of sulfa drugs containing six-membered heterocyclic groups: Identification of an SO2 extrusion photoproduct. Environ. Sci. Technol. 2005, 39, 3630–3638. [Google Scholar] [CrossRef]
- Baeza, C.; Knappe, D.R.U. Transformation kinetics of biochemically active compounds in low-pressure UV Photolysis and UV/H2O2 advanced oxidation processes. Water Res. 2011, 45, 4531–4543. [Google Scholar] [CrossRef]
- Nassar, R.; Trivella, A.; Mokh, S.; Al-Iskandarani, M.; Budzinski, H.; Mazellier, P. Photodegradation of sulfamethazine, sulfamethoxypiridazine, amitriptyline, and clomipramine drugs in aqueous media. J. Photochem. Photobiol. A 2017, 336, 176–182. [Google Scholar] [CrossRef]
- Babić, S.; Zrnćic, M.; Ljubas, D.; Ćurković, L.; Škorić, I. Photolytic and thin TiO2 film assisted photocatalytic degradation of sulfamethazine in aqueous solution. Environ. Sci. Pollut. Res. 2015, 22, 11372–11386. [Google Scholar] [CrossRef] [PubMed]
- Bahnmüller, S.; von Guten, U.; Canonica, S. Sunlight-induced transformation of sulfadiazine and sulfamethoxazole in surface waters and wastewater effluents. Water Res. 2014, 57, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Biošić, M.; Mitrevski, M.; Babić, S. Environmental behavior of sulfadiazine, sulfamethazine, and their metabolites. Environ. Sci. Pollut. Res. 2017, 24, 9802–9812. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, Y.; Zhou, X. Degradation of Bezafibrate with UV/H2O2 in Surface Water and Wastewater Treatment Plant Effluent. Clean-Soil Air Water 2012, 40, 239–245. [Google Scholar] [CrossRef]
- Sukul, P.; Lamshöft, M.; Zühlke, S.; Spiteller, M. Photolysis of 14C-sulfadiazine in water and manure. Chemosphere 2008, 71, 717–725. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Wang, Z.; Tao, T.; Wei, D.; Hu, C. Photolysis of chlortetracycline in aqueous solution: Kinetics, toxicity and products. J. Environ. Sci. 2012, 24, 254–260. [Google Scholar] [CrossRef]
- Baena-Nogueras, R.M.; González-Mazo, E.; Lara-Martín, P.A. Photolysis of antibiotics under simulated sunlight irradiation: Identification of photoproducts by high-resolution mass spectrometry. Environ. Sci. Technol. 2017, 51, 3148–3156. [Google Scholar] [CrossRef]
- Alexy, R.; Kümpel, T.; Kümmerer, K. Assessment of degradation of 18 antibiotics in the Closed Bottle Test. Chemosphere 2004, 57, 505–512. [Google Scholar] [CrossRef]
- Kim, S.; Eichhorn, P.; Jensen, J.N.; Weber, A.S.; Aga, D.S. Removal of antibiotics in wastewater: Effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environ. Sci. Technol. 2005, 39, 5816–5823. [Google Scholar] [CrossRef]
- Ingerslev, F.; Sørensen, B.H. Biodegradability properties of sulfonamides in activated sludge. Environ. Toxicol. Chem. 2000, 19, 2467–2473. [Google Scholar] [CrossRef]
- Maki, T.; Hasegawa, H.; Kitami, H.; Fumoto, K.; Munekage, Y.; Ueda, K. Bacterial degradation of antibiotic residues in marine fish farm sediments of Uranouchi Bay and phylogenetic analysis of antibiotic-degrading bacteria using 16S rDNA sequences. Fish. Sci. 2006, 72, 811–820. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the presence of sulfonamides in the environment and their influence on human health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Adamek, E.; Baran, W.; Sobczak, A. Assessment of the biodegradability of selected sulfa drugs in two polluted rivers in Poland: Effects of seasonal variations, accidental contamination, turbidity and salinity. J. Hazard. Mater. 2016, 313, 147–158. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, S.C.; Chander, Y.; Singh, A.K. Antibiotic use in agriculture and its impact on the terrestrial environment. Adv. Agron. 2005, 87, 1–54. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Sengelov, B.; Tjornelund, J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Zhang, H.; Shen, G.; Yuan, Z.; Zhang, W. Degradation kinetics and mechanism of sulfadiazine and sulfamethoxazole in an agricultural soil system with manure application. Sci. Total Environ. 2017, 607–608, 1348–1356. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H. Adsorption behavior of antibiotic in soil environment: A critical review. Front. Environ. Sci. Eng. 2015, 9, 565–574. [Google Scholar] [CrossRef]
- Vithanage, M.; Rajapaksha, A.U.; Tang, X.; Thiele-Bruhn, S.; Kim, K.H.; Lee, S.E.; Ok, Y.S. Sorption and transport of sulfamethazine in agricultural soils amended with invasive-plant-derived biochar. J. Environ. Manag. 2014, 141, 95–103. [Google Scholar] [CrossRef]
- Park, J.Y.; Huwe, B. Effect of pH and soil structure on transport of sulphonamide antibiotics in agricultural soils. Environ. Pollut. 2016, 213, 561–570. [Google Scholar] [CrossRef]
- Kurwadkar, S.T.; Adamas, C.D.; Meyer, M.T.; Kolpin, D.W. Comparative mobility of sulfonamides and bromide tracer in three soils. J. Environ. Manag. 2011, 92, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hu, X.; Yin, D.; Zhang, H.; Yu, Z. Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere 2011, 82, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, Y.; Yang, C.; Liu, X.; Zhang, J.; Li, E.; Zhang, Q.; Wang, X. Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci. Total Environ. 2017, 609, 1423–1432. [Google Scholar] [CrossRef]
- Wei, R.; Ge, F.; Huang, S.; Chen, M.; Wang, R. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere 2011, 82, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, Y.; Tong, L.; Li, Y.; Deng, Y.; Guo, W.; Gan, Y. Seasonal variation of antibiotics concentration in the aquatic environment: A case study at Jianghan Plain, central China. Sci. Total Environ. 2015, 527–528, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lin, L.; Luo, Z.; Yan, C.; Zhang, X. Occurrence of selected antibiotics in Jiulongjiang River in various seasons, South China. J. Environ. Monit. 2011, 13, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere 2012, 89, 1308–1315. [Google Scholar] [CrossRef]
- Huang, F.; Zou, S.; Deng, D.; Lang, H.; Liu, F. Antibiotics in a typical karst river system in China: Spatiotemporal variation and environmental risks. Sci. Total Environ. 2019, 650, 1348–1355. [Google Scholar] [CrossRef]
- Yan, C.; Yang, Y.; Zhou, J.; Liu, M.; Nie, M.; Shi, H.; Gu, L. Antibiotics in the Surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environ. Pollut. 2013, 175, 22–29. [Google Scholar] [CrossRef]
- Azanu, D.; Styrishave, B.; Darko, G.; Weisser, J.J.; Abaidoo, R.C. Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Sci. Total Environ. 2018, 622–623, 293–305. [Google Scholar] [CrossRef]
- Pailler, J.-Y.; Krein, A.; Pfister, L.; Hoffmann, L.; Guignard, C. Solid phase extraction coupled to liquid chromatography-tandem mass spectrometry analysis of sulfonamides, tetracyclines, analgesics and hormones in surface water and wastewater in Luxembourg. Sci. Total Environ. 2009, 407, 4736–4743. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cruz, M.S.; García-Galán, M.J.; Barceló, D. Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography-quadrupole linear ion trap-mass spectrometry. J. Chromatogr. A 2008, 1193, 50–59. [Google Scholar] [CrossRef] [PubMed]
- López-Serna, R.; Petrović, M.; Barceló, D. Development of a fast instrumental method for the analysis of pharmaceuticals in environmental and wastewaters based on ultra high performance liquid chromatography (UHPLC)–tandem mass spectrometry (MS/MS). Chemosphere 2011, 85, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, A.; Nebot, C.; Vázquez, B.I.; Miranda, J.M.; Abuín, C.M.F.; Cepeda, A. Detection of veterinary drug residues in Surface waters collected nearby farming areas in Galicia, North of Spain. Environ. Sci. Pollut. Res. 2014, 21, 2367–2377. [Google Scholar] [CrossRef]
- Kim, S.-C.; Carlson, K. Quantification of human and veterinary antibiotics in wáter and sediment using SPE/LC/MS/MS. Anal. Bioanal. Chem. 2007, 387, 1301–1315. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, M.E.; Meyer, M.; Thurman, E.M. Analysis of trace levels of sulfonamide and tetracycline antimicrobials in groundwater and surface water using solid-phase extraction and liquid chromatography/mass spectrometry. Anal. Chem. 2001, 73, 4640–4646. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Wu, M.; Li, Z.; Liu, X. Occurrences and regional distributions of 20 antibiotics in water bodies during groundwater recharge. Sci. Total Environ. 2015, 518–519, 498–506. [Google Scholar] [CrossRef]
- Kivits, T.; Broers, H.P.; Beeltje, H.; van Vliet, M.; Griffioen, J. Presence and fate of veterinary antibiotics in age-dated groundwater in areas with intensive livestock farming. Environ. Pollut. 2018, 241, 988–998. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Garrido, T.; Fraile, J.; Ginebreda, A.; Díaz-Cruz, M.S.; Barceló, D. Simultaneous occurrence of nitrates and sulfonamide antibiotics in two ground wáter bodies of Catalonia (Spain). J. Hydrol. 2010, 383, 93–101. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.-G.; Singer, A.C.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, Z.H.; Yang, Y.; Huang, Y.L.; Zou, S.C.; Luan, T.G. A preliminary investigation on the occurrence and distribution of antibiotic resistance genes in the Beijiang River, South China. J. Environ. Sci. 2013, 25, 1656–1661. [Google Scholar] [CrossRef]
- Luo, Y.; Mao, D.Q.; Rysz, M.; Zhou, D.X.; Zhang, H.J.; Xu, L.; Alvarez, P.J.J. Trends in antibiotic resistance genes occurrence in the Haihe River, China. Environ. Sci. Technol. 2010, 44, 7220–7225. [Google Scholar] [CrossRef] [PubMed]
- Harnisz, M.; Korzeniewska, E.; Ciesielski, S.; Gołaś, I. tet genes as indicators of changes in the water environment: Relationships between culture-dependent and culture-independent approaches. Sci. Total Environ. 2015, 505, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Pomati, F.; Netting, A.G.; Calamari, D.; Neilan, B.A. Effects of erythromycin, tetracycline and ibuprofen on the growth of Synechocystis sp. And Lemna minor. Aquat. Toxicol. 2004, 67, 387–396. [Google Scholar] [CrossRef]
- Gao, L.; Shi, L.J.; Yuan, T. Growth inhibitive effect of typical antibiotics and their mixtures on Selenastrum capricornutum. J. Environ. Health 2013, 30, 475–478. [Google Scholar]
- Brain, R.A.; Johnson, D.J.; Richards, S.M.; Sanderson, H.; Sibley, P.K.; Solomon, K.R. Effects of 25 pharmaceutical compounds to Lemna gibba using a seven-day static-renewal test. Environ. Toxicol. Chem. 2004, 23, 371–382. [Google Scholar] [CrossRef]
- Nunes, B.; Antunes, S.C.; Gomes, R.; Campos, J.C.; Braga, M.R.; Ramos, A.S.; Correia, A.T. Acute effects of tetracycline exposure in the freshwater fish Gambusia Holbrooki: Antioxidant effects, neurotoxicity and histological alterations. Arch. Environ. Contam. Toxicol. 2015, 68, 371–381. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, M.J.; Yu, S.H.; Kim, S.D. The individual and population effects of tetracycline on Daphnia magna in multigenerational exposure. Ecotoxicology 2012, 21, 993–1002. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wu, S.C.; Nie, X.P.; Yediler, A.; Wong, M.H. The effects of residual tetracycline on soil enzymatic activities and plant growth. J. Environ. Sci. Heal. 2009, 44, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Niu, D.; Li, Z.; Liang, Y.; Zhang, S. Effectos of antibiotics oxytetracycline on soil enzyme activities and microbial biomass in wheat rhizosphere. Sci. Agric. Sinica 2010, 43, 721–728. [Google Scholar]
- Liu, F.; Ying, G.-G.; Tao, R.; Zhao, J.-L.; Yang, J.-F.; Zhao, L.-F. Effects of six selected antibiotics on plant grwth and soil microbial and enzymatic activities. Environ. Pollut. 2009, 157, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Kotzerke, A.; Sharma, S.; Schauss, K.; Heuer, H.; Thiele-Bruhn, S.; Smalla, K.; Wilke, B.M.; Schloter, M. Alterations in soil microbial activity and N-transformation processes due to sulfadiazine loads in pig manure. Environ. Pollut. 2008, 153, 315–322. [Google Scholar] [CrossRef]
- Toth, J.D.; Feng, Y.; Dou, Z. Veterinary antibiotics at environmentally relevant concentrations inhibit soil iron reduction and nitrification. Soil Biol. Biochem. 2011, 43, 2470–2472. [Google Scholar] [CrossRef]
- Klaver, A.L.; Matthews, R.A. Effects of oxytetracycline on nitrification in a model aquatic system. Aquaculture 1994, 123, 237–247. [Google Scholar] [CrossRef]
- Ahmad, M.; Vithanage, M.; Kim, K.; Cho, J.-S.; Lee, Y.H.; Joo, Y.K.; Lee, S.S.; Ok, Y.S. Inhibitory ffect of veterinary antibiotics on denitrification in groundwater: A microcosm approach. Sci. World, J. 2014, 2014, 879831. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Yu, W.; Ma, Q.; Wang, J.; Zhou, H.; Jiang, C. The combined effect of sulfadiazine and copper on soil microbial activity and community structure. Ecotoxicol. Environ. Safe. 2016, 134, 43–52. [Google Scholar] [CrossRef]
- Li, D.; Qi, R.; Yang, M.; Zhang, Y.; Yu, T. Bacterial community characteristics under long-term antibiotic selection pressures. Water Res. 2011, 45, 6063–6073. [Google Scholar] [CrossRef]
- Schmitt, H.; Stoob, K.; Hamscher, G.; Smit, E.; Seinen, W. Tetracyclines and tetracycline resistance in agricultural soils: Microcosm and field studies. Microb. Ecol. 2006, 51, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, V.; Nam, H.-M.; Sawant, A.A.; Headrick, S.I.; Nguyen, L.-T.; Oliver, S.P. Distribution of tetracycline and streptomycin resistance genes and class 1 integrons in enterobacteriaceae isolated from dairy and nondairy farm soils. Microb. Ecol. 2008, 55, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Qiao, M.; Zhang, B.; Cheng, W.-D.; Zhu, Y.-G. Abundance and diversity of tetracycline resistance genes in soils adjacent to representative swine feedlots in China. Environ. Sci. Technol. 2010, 44, 6933–6939. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Hao, Y.; Shen, M.; Zhao, Q.; Li, Q.; Hu, J. Impacts of supplementing chemical fertilizers with organic fertilizers manufactured using pig manure as a substrate on the spread of tetracycline resistance genes in soil. Ecotoxicol. Environ. Saf. 2016, 130, 279–288. [Google Scholar] [CrossRef]
- Gao, F.-Z.; He, L.-Y.; He, L.-X.; Zou, H.-Y.; Zhang, M.; Wu, D.-L.; Liu, Y.-S.; Shi, Y.-J.; Bai, H.; Ying, G.-G. Untreated swine wastes changed antibiotic resistance and microbial community in the soils and impacted abundances of antibiotic resistance genes in the vegetables. Sci. Total Environ. 2020, 741, 140482. [Google Scholar] [CrossRef]
- Macedo, G.; Hernandez-Leal, L.; van der Maas, P.; Heederik, D.; Mevius, D.; Schmitt, H. The impact of manure and soil texture on antimicrobial resistance gene levels in farmlands and adjacent ditches. Sci. Total Environ. 2020, 737, 139563. [Google Scholar] [CrossRef]
- Heuer, H.; Smalla, K. Manure and sulfadiazine synergistically increased bacterial antibiotic resistance in soil over at least two months. Environ. Microbiol. 2007, 9, 657–666. [Google Scholar] [CrossRef]
- Heuer, H.; Focks, A.; Lamshöft, M.; Smalla, K.; Matthies, M.; Spiteller, M. Fate of sulfadiazine administered to pigs and its quantitative effect on the dynamics of bacterial resistance genes in manure and manured soil. Soil Biol. Biochem. 2008, 40, 1892–1900. [Google Scholar] [CrossRef]
- Selvam, A.; Xu, D.; Zhao, Z.; Wong, J.W.C. Fate of tetracycline, sulfonamide and fluoroquinolone resistance genes and the changes in bacterial diversity during composting of swine manure. Bioresour. Technol. 2012, 126, 383–390. [Google Scholar] [CrossRef]
- Hsu, J.-T.; Chen, C.-Y.; Young, C.-W.; Chao, W.-L.; Li, M.-H.; Liu, Y.-H.; Lin, C.-M.; Ying, C. Prevalence of sulfonamide-resistant bacteria, resistance genes and integron-associated horizontal gene transfer in natural water bodies and soils adjacent to a swine feedlot in northern Taiwan. J. Hazard. Mater. 2014, 277, 4–43. [Google Scholar] [CrossRef]
- Lin, H.; Chapman, S.J.; Freitag, T.E.; Kyle, C.; Ma, J.; Yang, Y.; Zhang, Z. Fate of tetracycline and sulfonamide resistance genes in a grassland soil amended with different organic fertilizers. Ecotoxicol. Environ. Safe. 2019, 170, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Zhao, L.-X.; Li, Y.-T.; Su, J.-Q. Manure fertilization increase antibiotic resistance in soils from typical greenhouse vegetable production bases, China. J. Hazard. Mater. 2020, 391, 122267. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Jin, H.; Ye, X.; Liu, W.; Li, D.; Shah, G.M.; Zhu, Y. Fate and driving factors of antibiotic resistance genes in an integrated swine wastewater treatment system: From wastewater to soil. Sci. Total Environ. 2020, 721, 137654. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Shi, M.; Xing, L.; Wang, X.; Gao, H.; Sun, Y. Sulfamethoxazole affects the microbial composition and antibiotic resistance gene abundance in soil and accumulates in lettuce. Environ. Sci. Poll. Res. 2020, 27, 29257–29265. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.A.; Dantas, G. The shared antibiotic resistome of soil bacteria and human pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Rensing, C.; Holm, P.E.; Virta, M.; Brandt, K.K. Comparison of metals and tetracycline as selective agents for development of tetracycline resistant bacterial communities in agricultural soil. Environ. Sci. Technol. 2017, 51, 3040–3047. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Beck, I.-C. Effects of sulfonamide and tetracycline antibiotics on soil microbial activity and microbial biomass. Chemosphere 2005, 59, 457–465. [Google Scholar] [CrossRef]
- Ma, J.; Lin, H.; Sun, W.; Wang, Q.; Yu, Q.; Zhao, Y.; Fu, J. Soil microbial systems respond differentially to tetracycline, sulfamonomethoxine, and ciprofloxacin entering soil under pot experimental conditions alone and in combination. Environ. Sci. Pollut. Res. 2014, 21, 7436–7448. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S. Microbial inhibition by pharmaceutical antibiotics in different soils-Dose-response relations determined with the iron(III) reduction test. Environ. Toxicol. Chem. 2005, 24, 869–876. [Google Scholar] [CrossRef]
- Chen, W.; Liu, W.; Pan, N.; Jiao, W.; Wang, M. Oxytetracycline on functions and structure of soil microbial community. J. Soil Sci. Plant Nutr. 2013, 13, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Unger, I.M.; Goyne, K.W.; Kennedy, A.C.; Kremer, R.J.; McLain, J.E.T.; Williams, C.F. Antibiotic effects on microbial community characteristics in soils under conservation management practices. Soil Sci. Soc. Am. J. 2013, 77, 100–112. [Google Scholar] [CrossRef]
- Rousk, J.; Demoling, L.A.; Bahr, A.; Bååth, E. Examining the fungal and bacterial niche overlap using selective inhibitors in soil. FEMS Microbiol. Ecol. 2008, 63, 350–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousk, J.; Demoling, L.A.; Bååth, E. Contrasting short-term antibiotic effects on respiration and bacterial growth compromises the validity of the selective respiratory inhibition technique to distinguish fungi and bacteria. Microb. Ecol. 2009, 58, 75–85. [Google Scholar] [CrossRef]
- Santás-Miguel, V.; Arias-Estévez, M.; Díaz-Raviña, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A.; Fernández-Calviño, D. Interactions between soil properties and tetracycline toxicity affecting to bacterial community growth in agricultural soil. Appl. Soil Ecol. 2020, 147, 103437. [Google Scholar] [CrossRef]
- Santás-Miguel, V.; Arias-Estévez, M.; Díaz-Raviña, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A.; Fernández-Calviño, D. Effect of oxytetracycline and chlortetracycline on bacterial community growth in agricultural soils. Agronomy 2020, 10, 1011. [Google Scholar] [CrossRef]
- Brandt, K.K.; Sjøholm, O.R.; Krogh, K.A.; Halling-Sørensen, B.; Nybroe, O. Increased pollution-induced bacterial community tolerance to sulfadiazine in soil hotspots amended with artificial root exudates. Environ. Sci. Technol. 2009, 43, 2963–2968. [Google Scholar] [CrossRef]
- Zielezny, Y.; Groeneweg, J.; Vereecken, H.; Tappe, W. Impact of sulfadiazine and chlorotetracycline on soil bacterial community structure and respiratory activity. Soil Biol. Biochem. 2006, 38, 2372–2380. [Google Scholar] [CrossRef]
- Molaei, A.; Lakzian, A.; Haghnia, G.; Astaraei, A.; Rasouli-Sadaghiani, M.; Ceccherini, M.T.; Datta, R. Assessment of some cultural experimental methods to study the effects of antibiotics on microbial activities in a soil: An incubation study. PLoS ONE 2017, 12, e0180663. [Google Scholar] [CrossRef]
- Awad, Y.M.; Ok, Y.S.; Igalavithana, A.D.; Lee, Y.H.; Sonn, Y.-K.; Usman, A.R.A.; Al-Wabel, M.I.; Lee, S.S. Sulphamethazine in poultry manure changes carbon and nitrogen mineralisation in soils. Chem. Ecol. 2016, 32, 899–918. [Google Scholar] [CrossRef]
- Hammesfahr, U.; Bierl, R.; Thiele-Bruhn, S. Combined effects of the antibiotic sulfadiazine and liquid manure on the soil microbialcommunity structure and functions. J. Plant Nutr. Soil Sci. 2011, 174, 614–623. [Google Scholar] [CrossRef]
- Gutiérrez, I.R.; Watanabe, N.; Harter, T.; Glaser, B.; Radke, M. Effect of sulfonamide antibiotics on microbial diversity and activity in a Californian Mollic Haploxeralf. J. Soils Sediments 2010, 10, 537–544. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sarmah, A.K. Dissipation of sulfamethoxazole in pasture soils as affected by soil and environmental factors. Sci. Total Environ. 2014, 479–480, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Havelkova, B.; Beklova, M.; Kovacova, V.; Hlavkova, D.; Pikula, J. Ecotoxicity of selected antibiotics for organisms of aquatic and terrestrial ecosystems. Neuroendocrinol. Lett. 2016, 37, 38–44. [Google Scholar]
- Litskas, V.D.; Karamanlis, X.N.; Prousali, S.P.; Koveos, D.S. The xenobiotic doxycycline affects nitrogen transformations in soil and impacts earthworms and cultivated plants. J. Environ. Sci. Health A 2019, 54, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, M.N. Antibiotics trace in the aquatic environment: Persistence and adverse environmental impact. Curr. Opin. Environ. Sci. Health 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, S.C.; Baidoo, S.K.; Chander, Y.; Rosen, C.J. Antibiotic uptake by plants from soil fertilized with animal manure. J. Environ. Qual. 2005, 34, 2082–2085. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.; Wong, C.K.C.; Chu. L.M. Distribution of antibiotics in wastewater-irrigated soils and their accumulation in vegetable crops in the Pearl River Delta, Southern China. J. Agric. Food Chem. 2014, 62, 11062–11069. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Liu, H.; Chen, J.; Sun, Y. The accumulation and distribution of five antibiotics from soil in 12 cultivars of pak choi. Environ. Pollut. 2019, 254, 113115. [Google Scholar] [CrossRef]
- Hussain, S.; Naeem, M.; Chaudhry, M.N.; Iqbal, M.A. Accumulation of residual antibiotics in the vegetables irrigated by pharmaceutical wastewater. Expo. Health 2016, 8, 107–115. [Google Scholar] [CrossRef]
- Mullen, R.A.; Hurst, J.J.; Naas, K.M.; Sassoubre, L.M.; Aga, D.S. Assessing uptake of antimicrobials by Zea mays L. and prevalence of antimicrobial resistance genes in manure-fertilized soil. Sci. Total Environ. 2019, 646, 409–415. [Google Scholar] [CrossRef]
- Kang, D.H.; Gupta, S.; Rosen, C.; Fritz, V.; Singh, A.; Chander, Y.; Murray, H.; Rohwer, C. Antibiotic uptake by vegetable crops from manure-applied soils. J. Agric. Food Chem. 2013, 61, 9992–10001. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, C.; Domínguez, C.; Pérez-Babace, L.; Cañameras, N.; Comas, J.; Bayona, J.M. Estimate of uptake and translocation of emerging organic contaminants from irrigation water concentration in lettuce grown under controlled conditions. J. Hazard. Mater. 2016, 305, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, L.; Chen, L.; Li, S.; Sun, L. Bioaccumulation of antibiotics in crops under long-term manure application: Occurrence, biomass response and human exposure. Chemosphere 2019, 219, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.; Shenker, M.; Chefetz, B. Insights into the uptake processes of wastewater-borne pharmaceuticals by vegetables. Environ. Sci. Technol. 2014, 48, 593–600. [Google Scholar] [CrossRef]
- Grote, M.; Schwake-Anduschus, C.; Michel, R.; Stevens, H.; Heyser, W.; Langenkämper, G.; Betsche, T.; Freitag, M. Incorporation of veterinary antibiotics into crops from manured soil. Landbauforschung Völkenrode 2007, 57, 25–32. [Google Scholar]
- Franklin, A.M.; Williams, C.F.; Andrews, D.M.; Woodward, E.E.; Watson, J.E. Uptake of three antibiotics and an antiepileptic drug by wheat crops spray irrigated with wastewater treatment plant effluent. J. Environ. Qual. 2016, 45, 546–554. [Google Scholar] [CrossRef]
- Bártíková, H.; Podlipná, R.; Skálová, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef]
- Opriş, O.; Copaciu, F.; Soran, M.J.; Ristoiu, D.; Niinemets, Ü.; Copolovici, L. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: Leaf volatiles as a promising new tool to assess toxicity. Ecotoxicol. Environ. Safe 2013, 87, 70–79. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.H.; Liu, C.X.; Wang, Z.; Dong, J.; Zhu, G.F.; Huang, X. Potential effect and accumulation of veterinary antibiotics in Phradmites australis under hydroponic conditions. Ecol. Eng. 2013, 53, 138–143. [Google Scholar] [CrossRef]
- EC. Regulation (EC) N o 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. O.J.L. 2003, 268, 29–43. [Google Scholar]

| Antibiotic Class | Mode of Action | Examples | Main Use |
|---|---|---|---|
| Aminoglycosides | Inhibition of protein synthesis (Inhibits translation) | Amikacin | Veterinary |
| Apramycin | Veterinary | ||
| Gentamycin | Human, Veterinary, Plants | ||
| Neomycin | Human, Veterinary | ||
| Streptomycin | Veterinary, Plants | ||
| β-Lactams | Inhibition of cell wall synthesis | Amoxicillin | Veterinary |
| Cloxacilin | Veterinary | ||
| Cefuroxime | Human | ||
| Oxacillin | Veterinary | ||
| Glycopeptides | Acting on the wall or membrane cell, inhibits peptidoglycan synthesis | Bleomycin | Human |
| Polymyxins | Human, Veterinary | ||
| Teicoplanin | Human | ||
| Vancomycin | Veterinary | ||
| Lincosamides | Inhibition of protein synthesis by reversibly binding to the 50S ribosomal subunit | Clindamycin | Humans |
| Lincomycin | Veterinary | ||
| Macrolides | Inhibition of protein synthesis by reversibly binding to the 50S ribosomal subunit | Azithromycin | Human |
| Clarithromycin | Human | ||
| Erythromycin | Human, Veterinary | ||
| Roxythromycin | Human | ||
| Tylosin | Veterinary | ||
| Quinolones and Fluoroquinolones | Inhibition of DNA replication and transcription | Ciprofloxacin | Human |
| Enrofloxacin | Veterinary | ||
| Flumequine | Human | ||
| Ofloxacin | Human | ||
| Sulfonamides | Inhibition of the folic acid synthesis | Sulfachloropyridazine | Human, Veterinary |
| Sulfadiazine | Veterinary | ||
| Sulfamethazine | Veterinary | ||
| Sulfamethoxazole | Veterinary | ||
| Sulfapyridine | Human | ||
| Tetracyclines | Inhibition of protein synthesis | Chlortetracycline | Veterinary |
| Doxycycline | Human, Veterinary | ||
| Oxytetracycline | Human, Veterinary, Plants | ||
| Tetracycline | Human, Veterinary |
| Common Name | Chemical Structure | Chemical Formula | Molecular Weight (g mol−1) | Log KOW [10] | pKa [11] | Water Solubility (mg L−1) [10] |
|---|---|---|---|---|---|---|
| Tetracycline | 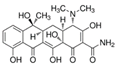 | C22H24N2O8 | 444.4 | −1.30 | 3.3–7.8–9.6 | 231 |
| Oxytetracycline |  | C22H24N2O9 | 460.4 | −0.90 | 3.2–7.5–8.9 | 313 |
| Chlortetracycline |  | C22H23ClN2O8 | 478.9 | −0.62 | 3.3–7.6–9.3 | 630 |
| Common Name | Chemical Structure | Chemical Formula | Molecular Weight (g mol−1) | Log KOW [10] | pKa [11] | Water Solubility (mg L−1) [10] |
|---|---|---|---|---|---|---|
| Sulfadiazine |  | C10H10N4O2S | 250.3 | −0.09 | 2.10–6.28 | 77 |
| Sulfachlorpyridazine |  | C10H9ClN4O2S | 284.7 | 0.31 | 1.87–5.45 | 35 |
| Sulfamethazine |  | C12H14N4O2S | 278.3 | 0.89 | 2.07–7.49 | 1500 |
| Compound | Animal Type | Administration Way | Excretion Ratio (%) | References |
|---|---|---|---|---|
| Chlortetracycline | cattle | Oral (feed) | 75 | [26] |
| Tetracycline | swine | Oral (feed) | 42–72 | [27] |
| Oxytetracycline | cattle | Injection | 20 | [28] |
| Sulfadiazine | swine | Oral (gelatin capsules) | 44 | [29] |
| Sulfamerazine | swine | Oral (feed) | 41–44 | [30] |
| Sulfachloropyridazine | swine | Oral (feed) | 57–66 | [30] |
| Sulfadimoxine | swine | Oral (feed) | 36–39 | [30] |
| Sulfaquinolaxine | swine | Oral (feed) | 83–87 | [30] |
| Sulfamethazine | swine | Injection | 25 | [31] |
| Country | TC | OTC | CTC | DC | SDZ | SMT | SCP | SMX | Reference |
|---|---|---|---|---|---|---|---|---|---|
| China | 22.0 | 423.0 | 120.0 | 0.6 | 1.7 | 1.2 | [38] | ||
| China | 105.0 | 2683.0 | 1079.0 | 2.5 | 0.9 | [39] | |||
| China | 189.8 | 613.2 | 2668.9 | [40] | |||||
| China | 153 | 571.4 | 10,967.0 | 495.0 | 3.2 | 177.9 | 52.9 | 58.1 | [41] |
| China | 74.4 | 79.7 | 104.6 | 85.5 | 74.0 | 54.5 | [42] | ||
| China | 60.4 | 415 | 222.0 | 0.7 | 2.58 | 9.3 | [43] | ||
| China | 25.7 | 31.9 | 161.5 | 184.8 | [44] | ||||
| China | 976.2 | 1398.5 | 1590.2 | 870.5 | 760.1 | 11.5 | [45] | ||
| China | 197.0 | 530.0 | 105.0 | 0.9 | 0.6 | [46] | |||
| Denmark | 15.5 | [47] | |||||||
| Germany | 443.0 | 27.0 | 93.0 | 4.5 | [48] | ||||
| Korea | 177.6 | 0.7 | 0.9 | 1.1 | 1.1 | [49] | |||
| Korea | 2.9 | 3.8 | 0.9 | 28.4 | 5.4 | [50] | |||
| Malaysia | 365.0 | [51] | |||||||
| Spain | 64.3 | 105.4 | 34.4 | 45.7 | [52] | ||||
| Spain | 4.3 | 20.4 | 2.6 | [53] | |||||
| Spain | 600 | 200 | 100 | 200 | [54] | ||||
| UK | 305.0 | 0.8 | [55] |
| Compound | pH/OC/eCEC/Clay | Kd (L kg−1) | Reference |
|---|---|---|---|
| Tetracyclines | |||
| TC | 5.5–6.2/1.1–3.9/13.7–19.9/26–49 | 450–15,278 | [60] |
| TC | 3.8–7.5/0.5–2.9/11.3–26.5/nd | 3102–312,447 | [61] |
| OTC | 5.6–6.3/1.1–1.6/6.7–35.3/5.2–16.9 | 417–1026 | [62] |
| OTC | 3.8–7.5/0.5–2.9/11.3–26.5/nd | 1229–269,097 | [61] |
| OTC | 4.0–7.1/0.8–4.4/16.9–20.2/32–78 | 650–2191 | [63] |
| OTC | 4.7–7.8/0.2–41.3/2.5–40.9/0.4–36.1 | 550–15,849 | [64] |
| OTC | 3.4–7.4/2.2–12.2/1.6–39.3/0.2–52 | 950–7200 | [65] |
| CTC | 3.8–7.5/0.5–2.9/11.3–26.5/nd | 5706–164,973 | [61] |
| CTC | 7.4/2.0/18.7/47.3 | 298 | [66] |
| Sulfonamides | |||
| SDZ | 3.7–6.8/0.67–21.34/0.7–13.8/6.0–68.4 | 0.8–14.3 | [67] |
| SDZ | 4.4–6.7/0.5–2.9/7.4–14.8/16–43 | 0.1–24.3 | [68] |
| SMT | 4.7–7.8/0.2–41.3/2.5–40.9/0.4–36.1 | 1.1–26.3 | [64] |
| SMT | 5.1–6.9/1.1–8.2/7.6–35.6/2–37 | 1.0–5.1 | [69] |
| SMT | 3.7–6.8/0.67–21.34/0.7–13.8/6.0–68.4 | 1.0–32.0 | [67] |
| SMT | 7.4/2.0/18.7/47.3 | 8.2 | [66] |
| SCP | 3.7–6.8/0.67–21.34/0.7–13.8/6.0–68.4 | 0.7–70.1 | [67] |
| SCP | 5.1–6.9/1.1–8.2/7.6–35.6/2–37 | 19–10.6 | [69] |
| SCP | 4.1–5.0/0.9–1.9/1.9–6.6/6–55 | 1.0–4.5 | [70] |
| SCP | 3.4–7.4/2.2–12.2/1.6–39.3/0.2–52 | 0.4–35.0 | [65] |
| SMP | 4.1–7.3/0.3–10.9/3.0–27.7/9–29 | 0.9–26.0 | [71] |
| SMX | 3.7–6.8/0.67–21.34/0.7–13.8/6.0–68.4 | 0.7–28.5 | [67] |
| SMX | 5.1–6.9/1.1–8.2/7.6–35.6/2–37 | 1.1–3.1 | [69] |
| STZ | 3.7–6.8/0.67–21.34/0.7–13.8/6.0–68.4 | 1.0–67.1 | [67] |
| SPY | 7.5/1.6/nd/nd | 1.0 | [72] |
| Compound | pH/Organic Carbon (%) | KF (Ln mg1−n kg−1) | Csmax (mg kg−1) | Reference |
|---|---|---|---|---|
| Tetracyclines | ||||
| TC | 4.1–7.1/1.1–10.9 | 731–7130 | 3904–13,243 | [80] |
| TC | 3.9–8.2/1.0–8.8 | 240–1601 | 412–2144 | [77] |
| TC | 7.7–8.6/0.9–3.4 | 778–2375 | [76] | |
| TC | 8.3/0.5 | 180 | 6810 | [79] |
| OTC | 3.9–8.2/1.0–8.8 | 105–1362 | 327–2874 | [77] |
| OTC | 5.3–8.3/0.3–5.9 | 53–928 | 1330–6050 | [79] |
| OTC | 4.1–7.1/1.1–10.9 | 735–7944 | 3656–13,554 | [83] |
| CTC | 3.9–8.2/1.0–8.8 | 323–1988 | 418–1197 | [77] |
| CTC | 8.3/0.5 | 302 | 3210 | [79] |
| CTC | 4.1–7.1/1.1–10.9 | 912–9465 | 5039–14,541 | [83] |
| Sulfonamides | ||||
| SDZ | 4.1–7.1/1.1–10.9 | 1.9–11.0 | [90] | |
| SDZ | 4.0–5.0/1.3–3.4 | 0.45–2.6 | [91] | |
| SDZ | 5.1–7.3/1.0–4.3 | 3.0–9.6 | [92] | |
| SMT | 4.1–7.1/1.1–10.9 | 2.9–15.0 | [93] | |
| SMT | 5.1–6.9/1.1–8.2 | 0.83–5.98 | [69] | |
| SMT | 4.0–5.0/1.3–3.4 | 0.9–3.7 | [91] | |
| SMT | 5.1–7.3/1.0–4.3 | 4.6–6.6 | [92] | |
| SCP | 4.1–7.1/1.1–10.9 | 0.5–23.2 | [93] | |
| SCP | 5.1–6.9/1.1–8.2 | 3.27–14.49 | [69] | |
| SCP | 4.0–5.0/1.3–3.4 | 1.9–5.6 | [91] | |
| SMX | 5.1–6.9/1.1–8.2 | 2.37–6.75 | [69] | |
| SMX | 5.3–8.7/0.1–3.2 | 0.1–4.8 | [94] | |
| SMX | 5.8–8.6/0.2–1.4 | 0.1–3.1 | [95] | |
| SMX | 5.1–7.3/1.0–4.3 | 2.8–12.1 | [92] | |
| SFX | 5.6–6.7/1.2–8.7 | 0.2–2.6 | 7.4–12.4 | [96] |
| Compound | pH/Organic Carbon (%) | Kd(des) | KF(des) | %des | HI | Reference |
|---|---|---|---|---|---|---|
| Tetracyclines | ||||||
| TC | 4.1–7.1/1.1–10.9 | 0.0–8.9 | [80] | |||
| TC | 4.4–4.5/2.7–22.7 | 8.0–9.0 | [102] | |||
| TC | 4.7/2.8 | 0.5 | [112] | |||
| TC | 5.5–6.2/0.6–2.2 | 1820–13,183 | 0.9–1.4 | [113] | ||
| TC | 6.2/2.2 | 1963 | 1.0 | [78] | ||
| OTC | 4.1–7.1/1.1–10.9 | 948–13,695 | 0–9.9 | [83] | ||
| OTC | 4.4–4.5/2.7–22.7 | 12.0–18.0 | [102] | |||
| OTC | 3.2–7.5/0.04–8.9 | 520–10983 | 3.0–20.0 | [114] | ||
| OTC | 5.4/1.2–1.5 | 1169–3572 | [115] | |||
| CTC | 4.1–7.1/1.1–10.9 | 1800–33,431 | 0.0–5.7 | [83] | ||
| CTC | 4.4–4.5/2.7–22.7 | 7.0–14.0 | [102] | |||
| DC | 4.1–7.3/0.3–10.9 | 0.0–2.1 | [101] | |||
| DC | 6.9/2.1 | 1079 | 2.6 | [116] | ||
| Sulfonamides | ||||||
| SDZ | 4.1–7.1/1.1–10.9 | 1.6–29.3 | 7.0–59.0 | 0.2–2.1 | [90] | |
| SDZ | 4.4–6.7/0.5–2.9 | 1.2–90.4 | 3.2–32.5 | 0.8–1.1 | [68] | |
| SDZ | 4.3–6.5/1.0–1.1 | 0.5–2.4 | 0.9–2.2 | 10.8–38.9 | [107] | |
| SDZ | 4.1–5.0/0.9–1.9 | 0.2–5.0 | 0.7–0.9 | [108] | ||
| SDZ | 6.1–7.6/0.5–1.9 | 4.6–11.6 | 30.3–52.1 | [84] | ||
| SDZ | 4.1–5.0/0.9–1.9 | 0.5–5.0 | 0.7–0.9 | [91] | ||
| SDZ | 5.1–7.3/1.0–4.3 | 0.0–14.9 | [92] | |||
| SMT | 4.1–7.1/1.1–10.9 | 3.2–133.9 | 3.0–37.0 | [93] | ||
| SMT | 4.1–5.0/0.9–1.9 | 3.9–12.9 | 1.2–1.4 | [111] | ||
| SMT | 5.2–7.4/1.2–3.0 | 46.0–85.0 | [117] | |||
| SMT | 5.1–7.3/1.0–4.3 | 3.2–6.4 | [92] | |||
| SCP | 4.1–7.1/1.1–10.9 | 5.3–65.4 | 4.0–33.0 | [93] | ||
| SCP | 4.1–5.0/0.9–1.9 | 1.7–29.0 | 1.0–16.3 | [70] | ||
| SCP | 4.1–5.0/0.9–1.9 | 18.0–37.0 | 1.4–1.7 | [91] | ||
| SDM | 4.1–5.0/0.9–1.9 | 0.9–7.8 | 0.9–1.1 | [111] | ||
| SDM | 4.1–5.0/0.9–1.9 | 0.8–7.8 | 0.9–1.1 | [91] | ||
| SQX | 4.1–5.0/0.9–1.9 | 6.8–27.0 | 1.0–1.1 | [111] | ||
| SMX | 6.1–7.6/0.5–1.9 | 5.4–6.5 | 43.7–48.3 | [84] | ||
| SMX | 5.1–7.3/1.0–4.3 | 7.7–35.9 | [92] | |||
| Compound | pH | Light Source | Light Intensity (W m−2) | k (min−1) | t1/2 (min) | Reference |
|---|---|---|---|---|---|---|
| TC | 4 | Xenon lamp | 550 | 0.0030 | 229 | [146] |
| TC | 5.5 | Xenon lamp | 550 | 0.0060 | 126 | [146] |
| TC | 7.2 | Xenon lamp | 550 | 0.0500 | 14 | [146] |
| TC | 4 | Mercury lamp | 500 | 0.0020 | 347 | [144] |
| TC | 6 | Mercury lamp | 500 | 0.0025 | 277 | [144] |
| TC | 7 | Mercury lamp | 500 | 0.0253 | 27 | [144] |
| TC | 9 | Mercury lamp | 500 | 0.1801 | 4 | [144] |
| TC | 4 | Xenon lamp | 500 | 0.0008 | 866 | [139] |
| TC | 6 | Xenon lamp | 500 | 0.0022 | 315 | [139] |
| TC | 7 | Xenon lamp | 500 | 0.0042 | 165 | [139] |
| TC | 8 | Xenon lamp | 500 | 0.0071 | 98 | [139] |
| TC | 10 | Xenon lamp | 500 | 0.0249 | 28 | [139] |
| OTC | 4 | Xenon lamp | 550 | 0.0070 | 101 | [146] |
| OTC | 5.5 | Xenon lamp | 550 | 0.0170 | 42 | [146] |
| OTC | 7.2 | Xenon lamp | 550 | 0.0390 | 18 | [146] |
| OTC | 4 | Mercury lamp | 500 | 0.0068 | 102 | [143] |
| OTC | 6 | Mercury lamp | 500 | 0.0089 | 78 | [143] |
| OTC | 7 | Mercury lamp | 500 | 0.0186 | 37 | [143] |
| OTC | 9 | Mercury lamp | 500 | 0.0692 | 10 | [143] |
| CTC | 4 | Xenon lamp | 550 | 0.0050 | 134 | [146] |
| CTC | 5.5 | Xenon lamp | 550 | 0.0070 | 104 | [146] |
| CTC | 7.2 | Xenon lamp | 550 | 0.0630 | 11 | [146] |
| CTC | 7.3 | Xenon lamp | 150 | 0.0081 | 86 | [165] |
| Compound | pH | Light Source | Light Intensity (W m−2) | k (min−1) | t1/2 (min) | Reference |
|---|---|---|---|---|---|---|
| SDZ | 4 | Xenon lamp | 550 | 0.0002 | 4230 | [151] |
| SDZ | 5.5 | Xenon lamp | 550 | 0.0006 | 1146 | [151] |
| SDZ | 7.2 | Xenon lamp | 550 | 0.0009 | 792 | [151] |
| SDZ | 5.5 | Xenon lamp | 250 | 606 | [135] | |
| SDZ | 8 | Xenon lamp | 500 | 0.0006 | 1124 | [161] |
| SDZ | 7.9 | Xenon lamp | 500 | 0.0015 | 462 | [161] |
| SDZ | 8.1 | Xenon lamp | 500 | 0.0022 | 320 | [161] |
| SDZ | 8.3 | Xenon lamp | 500 | 0.0037 | 189 | [161] |
| SDZ | 7 | Xenon lamp | 500 | 1920 | [161] | |
| SDZ | 6.7 | Xenon lamp | 500 | 0.0043 | 161 | [166] |
| SDZ | 4 | Xenon lamp | 500 | 0.0015 | 478 | [155] |
| SDZ | 8 | Xenon lamp | 500 | 0.0061 | 114 | [155] |
| SMT | 4 | Xenon lamp | 550 | 0.0001 | 5064 | [151] |
| SMT | 5.5 | Xenon lamp | 550 | 0.0004 | 1950 | [151] |
| SMT | 7.2 | Xenon lamp | 550 | 0.0012 | 564 | [151] |
| SMT | 5.5 | Xenon lamp | 250 | 690 | [135] | |
| SMT | 6.7 | Xenon lamp | 500 | 0.0044 | 159 | [166] |
| SMT | 4 | Xenon lamp | 500 | 0.0005 | 1386 | [155] |
| SMT | 8 | Xenon lamp | 500 | 0.0029 | 243 | [155] |
| SCP | 4 | Xenon lamp | 550 | 0.0095 | 72 | [151] |
| SCP | 5.5 | Xenon lamp | 550 | 0.0043 | 162 | [151] |
| SCP | 7.2 | Xenon lamp | 550 | 0.0050 | 138 | [151] |
| SCP | 5.5 | Xenon lamp | 250 | 420 | [135] | |
| SMX | 6.7 | Xenon lamp | 500 | 0.0031 | 227 | [166] |
| SMX | 5.5 | Xenon lamp | 750 | 0.0508 | 14 | [136] |
| STZ | 5.5 | Xenon lamp | 250 | 183 | [135] | |
| STZ | 6.7 | Xenon lamp | 500 | 0.0093 | 75 | [166] |
| SDM | 5.5 | Xenon lamp | 250 | 738 | [135] | |
| SMR | 5.5 | Xenon lamp | 250 | 672 | [135] | |
| SMP | 6.7 | Xenon lamp | 500 | 0.0085 | 82 | [166] |
| SND | 6.7 | Xenon lamp | 500 | 0.0258 | 27 | [166] |
| SGD | 6.7 | Xenon lamp | 500 | 0.0050 | 127 | [166] |
| Country | TC | OTC | CTC | DC | SDZ | SMT | SCP | SMX | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Surface water | |||||||||
| China | 114 | 85 | 17 | 47 | 41 | 623 | 54 | 55 | [180] |
| China | 1451 | 2797 | 876 | 500 | 172 | 715 | [181] | ||
| China | 810 | 2200 | 2420 | 1000 | 4660 | 560 | [182] | ||
| China | 100 | 63 | 48 | 33 | 25 | 28 | 133 | [183] | |
| China | 190 | 221 | 1037 | 326 | 776 | 58 | [184] | ||
| China | 16 | 940 | [185] | ||||||
| China | 101 | 55 | 5 | 2 | [186] | ||||
| China | 2 | 23 | 4 | 6 | 72 | 89 | 57 | [187] | |
| Ghana | 30 | 26 | 44 | 68 | 2861 | [188] | |||
| Luxembourg | 8 | 7 | 22 | [189] | |||||
| Spain | 2312 | 6192 | 1488 | [190] | |||||
| Spain | 87 | 27 | 18 | 21 | 23 | 55 | [191] | ||
| Spain | 56 | 64 | 2 | [192] | |||||
| UK | 4490 | 4130 | [55] | ||||||
| USA | 20 | 10 | 40 | 20 | 80 | [193] | |||
| USA | 110 | 340 | 690 | 220 | 1900 | [194] | |||
| USA | 110 | 1340 | 150 | 220 | [195] | ||||
| Groundwater | |||||||||
| China | 23 | 19 | 31 | 20 | 2 | 40 | [183] | ||
| China | 48 | 39 | 76 | 39 | 49 | 117 | 250 | [196] | |
| Netherlands | 2 | 13 | 18 | [197] | |||||
| Spain | 7 | 107 | 312 | [198] | |||||
| USA | 220 | [195] | |||||||
| Crop | Country | TC | OTC | CTC | DC | SDZ | SMT | SCP | SMX | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Cabbage | China | 10.1 | 1.2 | [255] | ||||||
| Cabbage | China | 5.9 | [256] | |||||||
| Cabbage | USA | 11.4 | [254] | |||||||
| Carrot | Pakistan | 0.8 | 0.8 | [257] | ||||||
| Celery | China | 3.1 | nd | 12.6 | 0.1 | nd | [39] | |||
| Coriander | China | 5.6 | 330.0 | 532.0 | 0.3 | nd | [39] | |||
| Corn | Spain | 300.0 | 200.0 | 100.0 | 100.0 | 400.0 | 600.0 | 200.0 | [54] | |
| Corn | China | 6.6 | nd | [255] | ||||||
| Corn | USA | 3652.0 | 3335.0 | 372.0 | 423.0 | 3.0 | nd | nd | [258] | |
| Corn | USA | 3.8 | [259] | |||||||
| Grass | Spain | 100.0 | 100.0 | 100.0 | nd | 100.0 | 100.0 | 200.0 | [54] | |
| Lettuce | USA | 1.0 | [259] | |||||||
| Lettuce | Spain | 495.0 | [260] | |||||||
| Onion | USA | 14.4 | [254] | |||||||
| Peanut | China | 20.1 | 22.7 | 21.8 | 19.2 | 5.3 | 3.6 | [261] | ||
| Radish | China | 9.2 | 4.2 | [255] | ||||||
| Radish | China | 1.4 | 57.0 | 18.0 | 0.5 | 2.7 | [39] | |||
| Radish | USA | 3.0 | [259] | |||||||
| Rape | China | 1.8 | 187.0 | 3.3 | nd | 0.2 | [39] | |||
| Rice | China | 8.5 | nd | [255] | ||||||
| Spinach | China | 6.3 | 1.7 | [255] | ||||||
| Spinach | Pakistan | 0.8 | 0.8 | [257] | ||||||
| Spinach | USA | 3.0 | [259] | |||||||
| Spinach | USA | 5.0 | [259] | |||||||
| Spinach | Spain | 1.7 | [260] | |||||||
| Tomato | Israel | 2.0 | [262] | |||||||
| Wheat | Spain | nd | nd | nd | nd | nd | 100.0 | 100.0 | [54] | |
| Wheat | Germany | 404.0 | 487.0 | [263] | ||||||
| Wheat | Pakistan | 0.5 | 0.6 | [257] | ||||||
| Wheat | USA | 0.5 | [264] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conde-Cid, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks. Processes 2020, 8, 1479. https://doi.org/10.3390/pr8111479
Conde-Cid M, Núñez-Delgado A, Fernández-Sanjurjo MJ, Álvarez-Rodríguez E, Fernández-Calviño D, Arias-Estévez M. Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks. Processes. 2020; 8(11):1479. https://doi.org/10.3390/pr8111479
Chicago/Turabian StyleConde-Cid, Manuel, Avelino Núñez-Delgado, María José Fernández-Sanjurjo, Esperanza Álvarez-Rodríguez, David Fernández-Calviño, and Manuel Arias-Estévez. 2020. "Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks" Processes 8, no. 11: 1479. https://doi.org/10.3390/pr8111479
APA StyleConde-Cid, M., Núñez-Delgado, A., Fernández-Sanjurjo, M. J., Álvarez-Rodríguez, E., Fernández-Calviño, D., & Arias-Estévez, M. (2020). Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks. Processes, 8(11), 1479. https://doi.org/10.3390/pr8111479










