Stability and Antiglycoxidant Potential of Bilberry Anthocyanins in Simulated Gastrointestinal Tract Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
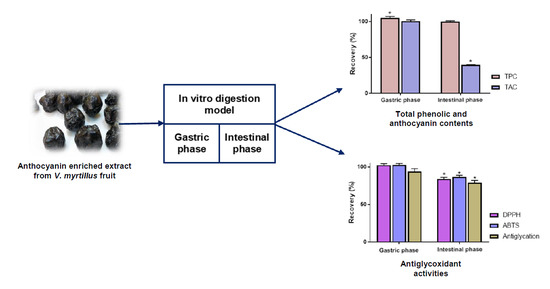
2.2. In Vitro Gastrointestinal Digestion Procedure
2.2.1. Gastric Phase
2.2.2. Intestinal Phase
2.2.3. Sample Management
2.3. Spectrometric Evaluations of Total Phenolic Content, Total Anthocyanin Content, Radical Scavenging, and Antiglycation Activities
2.4. HPLC Analysis of AEVM
2.5. Statistical Analyses
3. Results and Discussion
3.1. Recovery Index of Total Anthocyanin and Total Phenolic Contents
3.2. Individual Stability of Anthocyanin Constituents during Simulated In Vitro Gastrointestinal Digestion
3.3. Impact of Simulated Digestion on Radical Scavenging and Antiglycation Activities of AEVM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Popović, D.; Đukić, D.; Katić, V.; Jović, Z.; Jović, M.; Lalić, J.; Golubović, I.; Stojanović, M.; Ulrih, N.P.; Stanković, M.; et al. Antioxidant and proapoptotic effects of anthocyanins from bilberry extract in rats exposed to hepatotoxic effects of carbon tetrachloride. Life Sci. 2016, 157, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Giusti, M.M. Anthocyanins—Nature’s Bold, Beautiful, and Health-Promoting Colors. Foods 2019, 8, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Nutrigenomic analysis of the protective effects of bilberry anthocyanin-rich extract in apo E-deficient mice. Genes Nutr. 2010, 5, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraisse, D.; Bred, A.; Felgines, C.; Senejoux, F. Screening and Characterization of Antiglycoxidant Anthocyanins from Vaccinium myrtillus Fruit Using DPPH and Methylglyoxal Pre-Column HPLC Assays. Antioxidants 2020, 9, 512. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinonen, M. Antioxidant Activity of Anthocyanins and Their Aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Wang, Q.; Li, W.; Liu, L. Effect of Vaccinium myrtillus Extract Supplement on Advanced Glycation End-products: A Pilot Study (P06-098-19). Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Habánová, M.; Saraiva, J.A.; Haban, M.; Schwarzova, M.; Chlebo, P.; Predna, L.; Gažo, J.; Wyka, J. Intake of bilberries (Vaccinium myrtillus L.) reduced risk factors for cardiovascular disease by inducing favorable changes in lipoprotein profiles. Nutr. Res. 2016, 36, 1415–1422. [Google Scholar] [CrossRef]
- Han, F.; Yang, P.; Wang, H.; Fernandesd, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, Y.; Tao, C.; Liu, M.; Pan, Y.; Lv, Z. Effect of temperature and pH on stability of anthocyanin obtained from blueberry. J. Food Meas. Charact. 2018, 12, 1744–1753. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, C.; Wang, H.; Han, F.; Liu, Y.; Wang, L.; Liu, Y. Stability of Anthocyanins and Their Degradation Products from Cabernet Sauvignon Red Wine under Gastrointestinal pH and Temperature Conditions. Molecules 2018, 23, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; E Kulling, S. Stability and biotransformation of various dietary anthocyanins in vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised staticin vitrodigestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Nehring, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds, minerals, and antioxidant capacity of Mimosa scabrella Bentham honeydew honeys. Food Res. Int. 2017, 99, 670–678. [Google Scholar] [CrossRef]
- Su, D.; Li, N.; Chen, M.; Yuan, Y.; He, S.; Wang, Y.; Wu, Q.; Li, L.; Yang, H.; Zeng, Q. Effects ofin vitrodigestion on the composition of flavonoids and antioxidant activities of the lotus leaf at different growth stages. Int. J. Food Sci. Technol. 2018, 53, 1631–1639. [Google Scholar] [CrossRef]
- Senejoux, F.; Ndoye, S.; Fraisse, D.; Akendengue, B.; Dioum, M.; Gueye, R.; Sall, C.; Seck, I.; Felgines, C.; Seck, M. Antioxidant and antiglycation properties of two mango (Mangifera indica L.) cultivars from Senegal. Asian Pac. J. Trop. Biomed. 2018, 8, 137. [Google Scholar] [CrossRef]
- Bilberry Fresh Fruit. In European Pharmacopoeia, 9th ed.; Council of Europe: Strasbourg, France, 2016; p. 1546.
- Meda, N.; Fraisse, D.; Gnoula, C.; Vivier, M.; Felgines, C.; Senejoux, F. Characterization of antioxidants from Detarium microcarpum Guill. et Perr. leaves using HPLC-DAD coupled with pre-column DPPH assay. Eur. Food Res. Technol. 2017, 3, 367–1666. [Google Scholar] [CrossRef]
- Nunes, R.; Anastácio, A.; De Carvalho, I.S. Antioxidant and Free Radical Scavenging Activities of Different Plant Parts from TwoEricaSpecies. J. Food Qual. 2012, 35, 307–314. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Séro, L.; Sanguinet, L.; Blanchard, P.; Dang, B.T.; Morel, S.; Richomme, P.; Seraphin, D.; Derbré, S. Tuning a 96-Well Microtiter Plate Fluorescence-Based Assay to Identify AGE Inhibitors in Crude Plant Extracts. Molecules 2013, 18, 14320–14339. [Google Scholar] [CrossRef] [Green Version]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red wine—Their stability under simulated gastrointestinal digestion. Phytochemistry 2005, 66, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red cabbage—Stability to simulated gastrointestinal digestion. Phytochemistry 2007, 68, 1285–1294. [Google Scholar] [CrossRef]
- David, L.; Danciu, V.; Moldovan, B.; Filip, G.A. Effects of in Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants 2019, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Grijalva, E.P.; Angulo-Escalante, M.A.; León-Félix, J.; Heredia, J.B. Effect of in Vitro Digestion on the Total Antioxidant Capacity and Phenolic Content of 3 Species of Oregano (Hedeoma patens, Lippia graveolens, Lippia palmeri). J. Food Sci. 2017, 82, 2832–2839. [Google Scholar] [CrossRef]
- Peixoto, F.M.; Fernandes, I.; Gouvêa, A.C.M.; Santiago, M.C.; Borguini, R.G.; Mateus, N.; Freitas, V.; Godoy, R.L.; Ferreira, I.M. Simulation of in vitro digestion coupled to gastric and intestinal transport models to estimate absorption of anthocyanins from peel powder of jabuticaba, jamelão and jambo fruits. J. Funct. Foods 2016, 24, 373–381. [Google Scholar] [CrossRef]
- Ali, H.M.; Almagribi, W.; Al-Rashidi, M.N. Antiradical and reductant activities of anthocyanidins and anthocyanins, structure–activity relationship and synthesis. Food Chem. 2016, 194, 1275–1282. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Yeh, W.-J.; Hsia, S.-M.; Lee, W.-H.; Wu, C.-H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, Ø.M. Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, C.S.; Cuerrier, A.; Lamont, E.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A.L.; Johns, T. Investigating Wild Berries as a Dietary Approach to Reducing the Formation of Advanced Glycation Endproducts: Chemical Correlates of in Vitro Antiglycation Activity. Plant Foods Hum. Nutr. 2014, 69, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Li, X. Antioxidant Structure–Activity Relationship Analysis of Five Dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Assay | Undigested Matrix | Gastric Phase | Intestinal Phase |
|---|---|---|---|
| Total Phenolic Content (mg Gallic acid equivalent/g) | 434.8 ± 1.9 a | 456.6 ± 7.5 b | 438.7 ± 2.1 a |
| Total Anthocyanin Content (mg Cyanidin 3-O-glucoside equivalent/g) | 321.8 ± 2.0 a | 323.2 ± 5.0 a | 127.4 ± 2.4 b |
| Peak Number | Compound | Gastric Phase (Recovery, %) | Intestinal Phase (Recovery, %) |
|---|---|---|---|
| 1 | Delphinidin 3-O-galactoside | 97.6 ± 1.6 | 18.4 ± 0.4 |
| 2 | Delphinidin 3-O-glucoside | 96.7 ± 1.5 | 16.1 ± 0.4 |
| 3 | Cyanidin 3-O-galactoside | 98.0 ± 1.1 | 40.9 ± 0.7 |
| 4 | Delphinidin 3-O-arabinoside | 98.5 ± 2.2 | 17.4 ± 0.2 |
| 5 | Cyanidin 3-O-glucoside | 98.1 ± 1.5 | 38.2 ± 0.6 |
| 6 | Petunidin 3-O-galactoside | 99.2 ± 0.7 | 30.1 ± 1.4 |
| 7 | Cyanidin 3-O-arabinoside | 100.1 ± 2.9 | 40.0 ± 0.5 |
| 8 | Petunidin 3-O-glucoside | 97.7 ± 1.5 | 30.5 ± 0.5 |
| 9 | Peonidin 3-O-galactoside | 98.3 ± 2.9 | 40.5 ± 0.7 |
| 10 | Petunidin 3-O-arabinoside | 98.8 ± 2.6 | 29.8 ± 0.3 |
| 11 | Peonidin 3-O-glucoside | 98.7 ± 1.4 | 39.3 ± 1.2 |
| 11′ | Malvidin 3-O-galactoside | ||
| 12 | Malvidin 3-O-glucoside | 98.3 ± 1.6 | 39.2 ± 1.1 |
| 13 | Malvidin 3-O-arabinoside | 98.4 ± 1.5 | 41.2 ± 1.4 |
| Assay | Undigested Matrix | Gastric Phase | Intestinal Phase |
|---|---|---|---|
| DPPH scavenging activity (μmol of Trolox eq/g) | 2696.5 ± 26.5 a | 2758.1 ± 30.0 a | 2259.5 ± 70.9 b |
| ABTS scavenging activity (μmol of Trolox eq/g) | 4732.6 ± 54.5 a | 4862.5 ± 57.4 a | 4102.8 ± 83.5 b |
| Antiglycation activity (IC50, mg/L) | 70.41 ± 4.38 a | 75.10 ± 3.15 a | 89.04 ± 5.24 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraisse, D.; Bred, A.; Felgines, C.; Senejoux, F. Stability and Antiglycoxidant Potential of Bilberry Anthocyanins in Simulated Gastrointestinal Tract Model. Foods 2020, 9, 1695. https://doi.org/10.3390/foods9111695
Fraisse D, Bred A, Felgines C, Senejoux F. Stability and Antiglycoxidant Potential of Bilberry Anthocyanins in Simulated Gastrointestinal Tract Model. Foods. 2020; 9(11):1695. https://doi.org/10.3390/foods9111695
Chicago/Turabian StyleFraisse, Didier, Alexis Bred, Catherine Felgines, and François Senejoux. 2020. "Stability and Antiglycoxidant Potential of Bilberry Anthocyanins in Simulated Gastrointestinal Tract Model" Foods 9, no. 11: 1695. https://doi.org/10.3390/foods9111695
APA StyleFraisse, D., Bred, A., Felgines, C., & Senejoux, F. (2020). Stability and Antiglycoxidant Potential of Bilberry Anthocyanins in Simulated Gastrointestinal Tract Model. Foods, 9(11), 1695. https://doi.org/10.3390/foods9111695