Abstract
Schizophrenia is a mental disorder that affects approximately 1–2% of the population and develops in early adulthood. The disease is characterized by positive, negative, and cognitive symptoms. A large percentage of patients with schizophrenia have a treatment-resistant disease, and the risk of developing adverse effects is high. Many researchers have attempted to introduce new antipsychotic drugs to the clinic, but most of these treatments failed, and the diversity of schizophrenic symptoms is one of the causes of disappointing results. The present review summarizes the results of our latest papers, showing that the simultaneous activation of two receptors with sub-effective doses of their ligands induces similar effects as the highest dose of each compound alone. The treatments were focused on inhibiting the increased glutamate release responsible for schizophrenia arousal, without interacting with dopamine (D2) receptors. Ligands activating metabotropic receptors for glutamate, GABAB or muscarinic receptors were used, and the compounds were administered in several different combinations. Some combinations reversed all schizophrenia-related deficits in animal models, but others were active only in select models of schizophrenia symptoms (i.e., cognitive or negative symptoms).
1. Introduction
Schizophrenia is one of the most complicated mental disorders, and it is characterized by different symptoms that may enrich or disrupt normal behavior. Particular symptoms are not equally manifested in patients, and at least four groups of patients with schizophrenia have been described. However, diagnostic manuals (DSM-V and ICD-11) have recently abandoned the use of schizophrenia subtypes, as they are not stable over time, have low diagnostic value, and substantially reduce the heterogeneity of schizophrenia [1,2]. Separate diseases characterized by schizophrenia-like symptoms have also been specified. The manifestation, intensity, and occurrence of particular symptoms differ between groups (Table 1).

Table 1.
Groups of symptoms and symptom intensity in patients with schizophrenia, schizoaffective 31disorder, and psychotic disorder, where “−”no symptoms, “+”—very mild, “++”—mild, “+++”—moderate, “++++”—severe,” +++++”—very severe (based on [3]).
A large percentage of patients with schizophrenia suffer from cognitive impairments that substantially influence daily functioning. Patients with severe cases of schizophrenia or individuals with the predominant presentation of negative and cognitive symptoms are generally treatment-resistant. Other patients with schizophrenia, who respond relatively well to antipsychotic medications, develop adverse effects that lead to discontinuation of the treatment. These factors make living with schizophrenia difficult or impossible. In contrast to other mental diseases, such as depression or anxiety, the effectiveness of psychotherapy as an add-on treatment to antipsychotic medication is very limited [4,5].
Dopamine (D2) receptor blockade is the basic mechanism of action of currently used neuroleptic drugs. This receptor is responsible for drug efficacy and the development of adverse effects [6,7]. In contrast to typical neuroleptics with affinity for dopaminergic receptors only, the mechanisms of action of newer generations of drugs, also called atypical neuroleptics, involve a dopamine-based mechanism of action and antagonism or agonism towards serotonergic, adrenergic or histaminergic components [8]. However, atypical antipsychotics remain a heterogeneous group that exhibits different binding profiles, with risperidone being the least and clozapine the most atypical drug [9,10,11]. Diverse targets render atypical drugs slightly more effective and better tolerated [12], but the problem of drug resistance in patients with severe cases of schizophrenia, and the risk of the occurrence of adverse effects, remain relatively high.
The search for new treatment strategies for schizophrenia began years ago, but no spectacular achievements have been reported. This lack of success may be partially due to the ambiguous, unspecified, and complex causes of schizophrenia arousal. The specific changes responsible for schizophrenia development that contribute to the manifestation of particular symptoms have not been fully determined. For many years, the dopaminergic theory of schizophrenia dominated the field and indicated increased dopaminergic neurotransmission as the main factor responsible for the pathophysiology of the disease [8]. The theory was proposed based on observations that dopaminergic antagonists reversed the psychotic symptoms of schizophrenia [13,14,15]. The lack of effectiveness of dopamine-based drugs towards negative and cognitive symptoms of schizophrenia caused doubts regarding the theory and indicates obvious shortcomings of the hypothesis and limits of the treatment. Further research indicated that changes in dopaminergic neurotransmission were not necessarily crucial in schizophrenia arousal. At least two groups of patients were distinguished that differed in their responsiveness to treatment [16]. These groups were normodopaminergic and hyperdopaminergic subpopulations of patients. The latter group had a better response to neuroleptic medications [16]. Genetic predispositions were also indicated as important in successful treatment [17,18,19].
The observations that NMDA receptor antagonists, such as PCP, ketamine, or dizocilpine (MK-801), induced the full spectrum of schizophrenia symptoms prompted the development of the glutamatergic hypothesis of schizophrenia [20,21,22,23]. One of the first papers describing its more important relevance was released in 1987 by Javitt et al., who reviewed studies showing the induction of negative symptoms of schizophrenia in healthy subjects and animals after PCP administration and proposed a novel hypothesis of schizophrenia [24]. Other studies also presented this hypothesis and suggested that preferential hypofunction of NMDA receptors expressed on GABAergic postsynaptic sites led to a decrease in the sensitivity of these neurons to the stimulatory effect of glutamate [25,26]. Consequently, the synthesis and release of GABA becomes impaired, and the subsequent inhibitory control over glutamatergic neurons is lost. The resulting increase in glutamate release is the proposed primary cause of schizophrenia development and results from the hypofunction of NMDA receptors at critical sites in local circuits that modulate the function of a particular brain region or control projections from one region to another (e.g., hippocampal–cortical or thalamocortical projections) [25,26]. This increased glutamate efflux under specific conditions or individual predisposition results in subsequent changes in other neurotransmitters, e.g., dopamine [15].
The formulation of this theory provided new possibilities in the search for treatment strategies based on the reduction of enhanced glutamatergic transmission. Naturally occurring full or partial agonists at the glycine modulatory site of the NMDA receptor, such as glycine, d-serine, and d-cycloserine, and a glycine transporter inhibitor with low affinity, sarcosine, were investigated in add-on studies to ongoing antipsychotic treatment and primarily focused on persistent negative symptoms [27]. Improvements in negative symptoms, sometimes with improvements in cognitive and positive symptoms, were noted [28,29,30,31,32,33], although subsequent meta-analyses did not confirm these results [27,34]. However, the activation of NMDA-dependent pathways with dopaminergic system inhibition and the activation/inhibition of accidental receptors confound the therapeutic effect and increase the risk of adverse effects.
The discovery of metabotropic glutamate (mGlu) receptors in 1989 showed the possibility of regulating glutamatergic neurotransmission without directly targeting NMDA ion channels.
Extensive research on the therapeutic potency of mGlu receptors and their distribution within the CNS is summarized in a vast number of review papers. A PubMed search of “schizophrenia” and “metabotropic glutamate receptors” retrieved more than 100 review papers. The most important reviews are shown in Table 2.

Table 2.
Select reviews describing the role of metabotropic glutamate receptors in schizophrenia.
Despite the massive effort and financial resources invested to develop and introduce antipsychotic drugs with a mechanism of action based on the stimulation of mGlu receptors, a confirmed successful clinical trial has not been reported. After the controversial data published by Kinon et al. [56] and Patil et al. [57], clinical studies on a new generation of antipsychotics targeting mGlu receptor ligands were strongly limited but not completely discontinued. Therefore, innovative solutions focused on the inhibition of glutamatergic activity based on mGlu receptor signaling are desired. One possibility is as an add-on therapy based on the concomitant activation of other types of receptors involved in the regulation of the glutamatergic network.
2. Malfunction of Receptors in Patients with Schizophrenia
The causes of the pathophysiology of the disease and the subsequent changes that develop must be recognized and are fundamental to determining and introducing safe and effective treatments. Disrupted synaptic organization or impairments in receptor expression and function are important factors that may contribute to the success or failure of treatment.
According to some studies, patients with schizophrenia present diminished expression of the RGS4 mRNA [58,59,60,61], which is one of the 30 RGS molecules that function as GTPase activator proteins for Gα subunits. RGS4 is predominantly expressed in the brain [62], and a malfunction in RGS4 molecules translates into dysfunction of the G-protein-mediated signaling of metabotropic glutamate [63], GABAergic [64] and muscarinic acetylcholine receptors [65]. Available data and postmortem studies revealed robust changes in the expression of these receptors in patients with schizophrenia (Table 3A–C).
Most studies indicated decreased expression of mGlu2 receptors in the hippocampus of patients with schizophrenia, but increased expression in the cortex was also observed (Table 5C). Similarly, GABAB, M1, and M4 receptors were downregulated in most studies, and a few studies reported no changes (Table 3A,B). No changes in the expression of mGlu4 or the mGlu5 receptor were observed in postmortem studies (Table 3C). The functionality or excitability of these receptors is not known in patients with schizophrenia.
Statistical comparisons revealed robust changes and global trends in the population. Notably, individual features related to receptor expression and functionality made individual patients more susceptible to the development of specific symptoms of the disease and determined the responsiveness to treatment. Although the general trends of the population indicate the most plausible effective solutions, these solutions may fail in individual patients. Many different hypotheses have been proposed to explain why some individuals respond better than others to treatment, but the exact mechanisms of these discrepancies are not known [66,67]. However, differences in the expression and functionality of receptors between patients may underlie the differential responses.
The latest few papers published by our group proposed treatment strategies based on the bidirectional activation of select receptors. The strategy was to abolish glutamatergic arousal responsible for schizophrenia pathophysiology via activation of the most relevant pathways.

Table 3.
Expression of muscarinic (M1 and M4) (A), GABAB (B) and metabotropic glutamatergic receptors (mGlu5, mGlu2/3, mGlu2, mGlu4, and mGlu7) (C) in postmortem brain tissues from patients with schizophrenia.
Table 3.
Expression of muscarinic (M1 and M4) (A), GABAB (B) and metabotropic glutamatergic receptors (mGlu5, mGlu2/3, mGlu2, mGlu4, and mGlu7) (C) in postmortem brain tissues from patients with schizophrenia.
| (A) | ||||
| Receptor | Method | Brain Structure | Change | |
| M1/M4 | ||||
| [3H] pirenzepine binding | caudate-putamen | decrease | [68] | |
| [3H] pirenzepine binding | hippocampal formation | decrease | [69] | |
| [3H] pirenzepine binding | Brodmann area 9 | decrease | [70] | |
| [3H] pirenzepine binding | Brodmann area 40 | no change | [70] | |
| [3H] pirenzepine binding | Brodmann area 9 | decrease | [71] | |
| [3H] pirenzepine binding | Brodmann area 46 | decrease | [71] | |
| [3H] pirenzepine binding | anterior cingulate cortex | decrease | [72] | |
| [3H] pirenzepine binding | superior temporal gyrus | decrease | [73] | |
| [3H] pirenzepine binding | posterior cingulate cortex | decrease | [74] | |
| [3H] pirenzepine binding | hippocampal formation | decrease | [75] | |
| [3H] pirenzepine binding | Brodmann area 6 | decrease | [76] | |
| M1 | ||||
| in situ hybridization | caudate-putamen | no change | [77] | |
| in situ hybridization, Western blot | Brodmann area 9 | decrease | [70] | |
| in situ hybridization, Western blot | Brodmann area 40 | decrease no change | [70] | |
| cDNA | Brodmann area 6 | decrease | [78] | |
| in situ hybridization, Western blot | thalamus | no change | [79] | |
| in situ hybridization | hippocampal formation | no change | [75] | |
| immunohistochemistry | Brodmann area 9 | decrease | [80] | |
| immunohistochemistry | Brodmann area 17 | decrease | [80] | |
| immunohistochemistry | thalamus | no change | [80] | |
| immunohistochemistry | hippocampal formation | no change | [80] | |
| M4 | ||||
| in situ hybridization, Western blot | Brodmann area 9 | no change | [70] | |
| in situ hybridization, Western blot | Brodmann area 40 | decrease no change | [70] | |
| in situ hybridization, Western blot | thalamus | no change | [79] | |
| in situ hybridization | hippocampal formation | decrease | [75] | |
| M2/M4 | ||||
| [3H]AF-DX 384 | anterior cingulate cortex | no change | [81] | |
| (B) | ||||
| Receptor | Method | Brain Structure | Change | |
| GABAB | ||||
| immunohistochemistry | hippocampal formation | decrease (not quantified) | [82] | |
| immunohistochemistry | entorhinal cortex, inferior temporal cortex | decrease (not quantified) | [83] | |
| immunohistochemistry, Western blot | Brodmann area 9 | decrease (not quantified), decrease (GABAB1a) | [84] | |
| Western blot | lateral cerebellum | decrease | [85] | |
| Western blot | Brodmann area 9 | decrease | [86] | |
| (C) | ||||
| Receptor | Method | Brain Structure | Change | |
| mGlu5 | ||||
| [3H]MPEP binding | Brodmann area 46 | no change | [87] | |
| [3H]MPEP binding | Brodmann area 24 | no change | [88] | |
| in situ hybridization | Brodmann area 9 | no change | [89] | |
| in situ hybridization | Brodmann area 10 | no change | [89] | |
| in situ hybridization | Brodmann area 11 | increase | [89] | |
| in situ hybridization | hippocampal formation | no change | [90] | |
| in situ hybridization | parahippocampal gyrus | no change | [90] | |
| in situ hybridization | thalamus | no change | [91] | |
| Western blot | Brodmann area 9 | no change | [92] | |
| Western blot | Brodmann area 11 | no change | [92] | |
| Western blot | Brodmann area 32 | no change | [92] | |
| Western blot | Brodmann area 46 | no change | [92] | |
| Western blot | nucleus accumbens | no change | [92] | |
| Western blot | caudate nucleus | no change | [92] | |
| Western blot | putamen | no change | [92] | |
| Western blot | Brodmann area 10 | no change | [93] | |
| Western blot | lateral cerebellum | decrease (monomer) | [94] | |
| Western blot | Brodmann area 9 | decrease (monomer) | [94] | |
| Western blot | Brodmann area 46 | no change (monomer) | [87] | |
| Western blot | Brodmann area 46 | increase (total and dimer) | [95] | |
| RT-PCR | Brodmann area 9 | no change | [96] | |
| qRT-PCR | lateral cerebellum | decrease | [94] | |
| qRT-PCR | Brodmann area 46 | no change | [95] | |
| qPCR | Brodmann area 10 | no change | [97] | |
| qPCR | Brodmann area 46 | no change | [97] | |
| mGlu2/3 | ||||
| [3H]LY341495 binding | Brodmann area 24 | no change | [88] | |
| [3H]LY341495 binding | Brodmann area 17 | no change | [98] | |
| [3H]LY341495 binding | Brodmann area 24 | no change | [98] | |
| [3H]LY341495 binding | Brodmann area 46 | no change | [98] | |
| [3H]LY341495 binding | Brodmann area 46 | no change | [99] | |
| Western blot | Brodmann area 46 | no change | [100] | |
| Western blot | PFC | increase | [92] | |
| mGlu2 | ||||
| in situ hybridization | dentate gyrus | decrease | [101] | |
| in situ hybridization | CA3 | decrease | [101] | |
| in situ hybridization | CA2 | decrease | [101] | |
| in situ hybridization | subiculum | decrease | [101] | |
| in situ hybridization | parahipocampal gyrus | decrease | [101] | |
| in situ hybridization | thalamus | no change | [91] | |
| in situ hybridization | prefrontal cortex (white matter) | increase | [102] | |
| in situ hybridization | paranigral nucleus | increase | [102] | |
| Western blot | prefrontal cortex | no change | [103] | |
| Western blot | temporal cortex | no change | [103] | |
| Western blot | motor cortex | no change | [103] | |
| mGlu4 | ||||
| in situ hybridization | thalamus | no change | [91] | |
| Western blot | Brodmann area 9 | no change | [92] | |
| Western blot | Brodmann area 11 | no change | [92] | |
| Western blot | Brodmann area 32 | no change | [92] | |
| Western blot | Brodmann area 46 | no change | [92] | |
| Western blot | nucleus accumbens | no change | [92] | |
| Western blot | caudate nucleus | no change | [92] | |
| Western blot | putamen | no change | [92] | |
| mGlu7 | ||||
| in situ hybridization | thalamus | no change | [91] | |
3. Regulation of Glutamate Release
3.1. Glutamatergic Network in the Brain
Glutamate is the most abundant excitatory neurotransmitter in the brain, reaching high concentrations ranging from 5 to 15 µM per gram of tissue [104,105]. The activity of glutamatergic neurons is critical for the proper functioning of the cerebral cortex and the subcortical areas receiving glutamatergic projections.
Glutamatergic neurons are widely distributed across the CNS. At least five key glutamatergic pathways have been identified (Figure 1) [106]. Three pathways descend from the cortex to subcortical structures, such as the brainstem, thalamus, nucleus accumbens, and striatum. One pathway ascends from the thalamus to the cortex. Intracortical loops of glutamatergic interneurons that stabilize the activity of cortical networks have also been identified. Similar loops have been observed in other brain areas, such as the hippocampus.
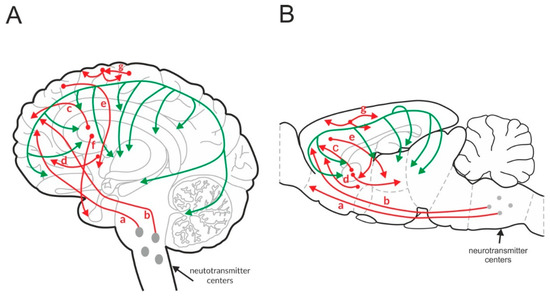
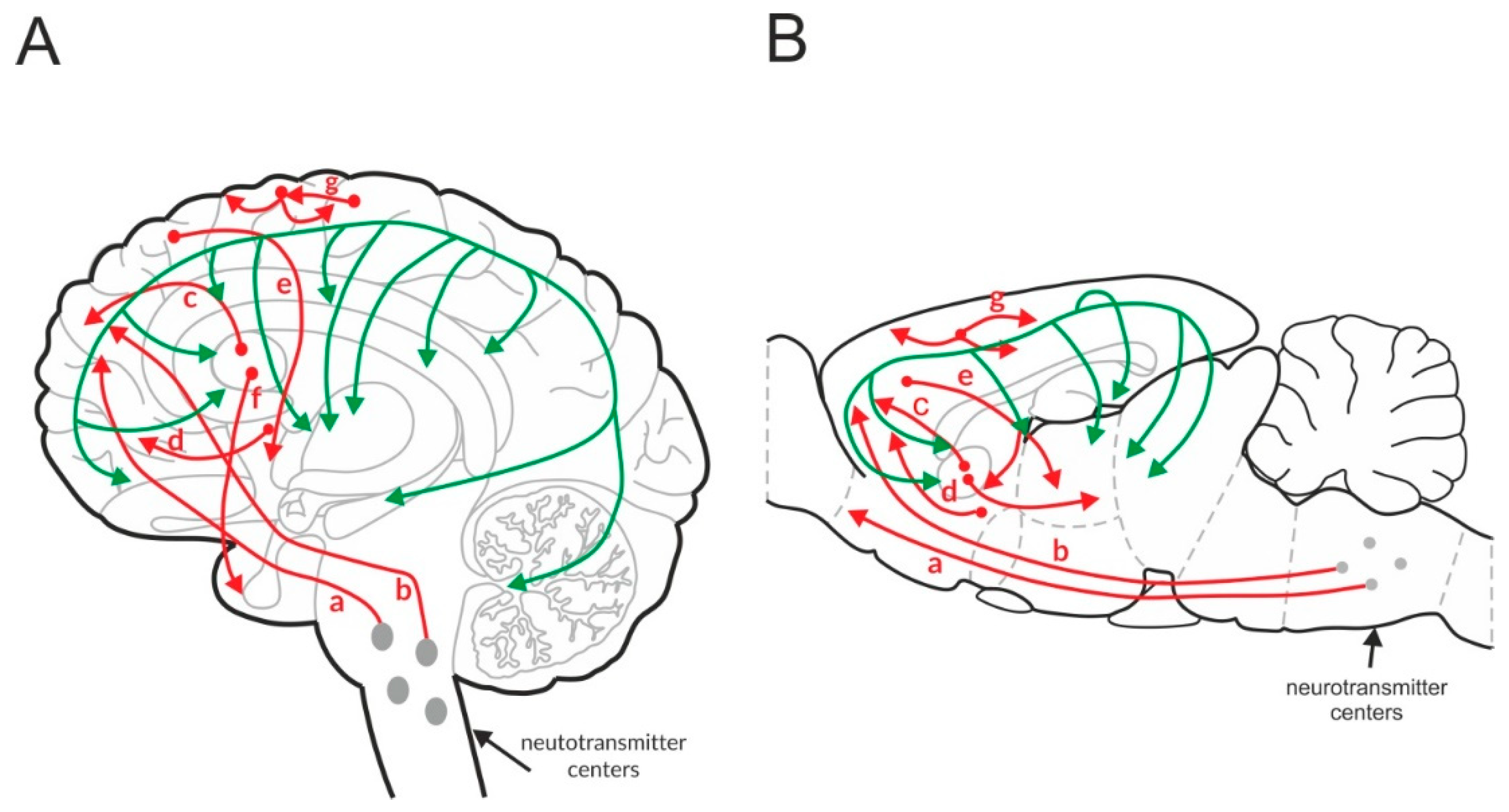
Figure 1.
Glutamatergic (red) and GABAergic (green) pathways in the human (A) and rat (B) brain. “a” and “b”—cortico-brainstem pathway, “c”—cortico-striatal pathway, “d”—cortico-accumbens pathway, “e”—cortico-thalamic pathway, “f”—thalamo-cortical pathway, and “g”—cortico-cortical pathway.
Based on these connections, glutamate is crucial in the integration of neurotransmission in the brain, including the regulation of monoaminergic nuclei located in the brainstem and cholinergic neurotransmission originating from the pedunculopontine and laterodorsal tegmental nucleus [106,107]. This excitatory system remains under the inhibitory control of GABAergic neurotransmission in a type of homeostatic balance.
GABAergic neurons are spread throughout the brain and form a network that connects with the excitatory system and regulates its functions (Figure 1) [108,109].
A variety of specific mechanisms regulate the release of neurotransmitters. One of the most important mechanisms is the presynaptic regulatory mechanism of receptors expressed on axon terminals, which may involve autoreceptors activated by the transmitters released from the host neuron or heteroreceptors activated by neurotransmitters that are synthesized by other neurons.
The activation or inhibition of receptors localized on dendritic shafts and cell bodies (postsynaptic receptors) triggers an electrical signal by regulating the activity of ion channels. The influx of ions changes the membrane potential of a neuron and results in a signal that is transmitted along the axon to regulate other neurons and the neuronal network.
The most important aspects of the pre- and postsynaptic regulation of glutamatergic networks are summarized below. Attention was placed on receptors that are likely targets for antipsychotic drug discovery.
3.2. Presynaptic Regulation of Glutamate Release—Autoreceptors
3.2.1. mGlu2 Receptors
The mGlu2 receptors are located at a distance from the synaptic cleft [110]. The glutamate potency at mGlu2 receptors is high—0.3–20 µM—but mGlu2 receptors are exposed to relatively low concentrations of glutamate under physiological conditions [110,111,112]. The receptors are negatively associated with adenyl cyclase activity, and their stimulation results in the inhibition of glutamate release [113].
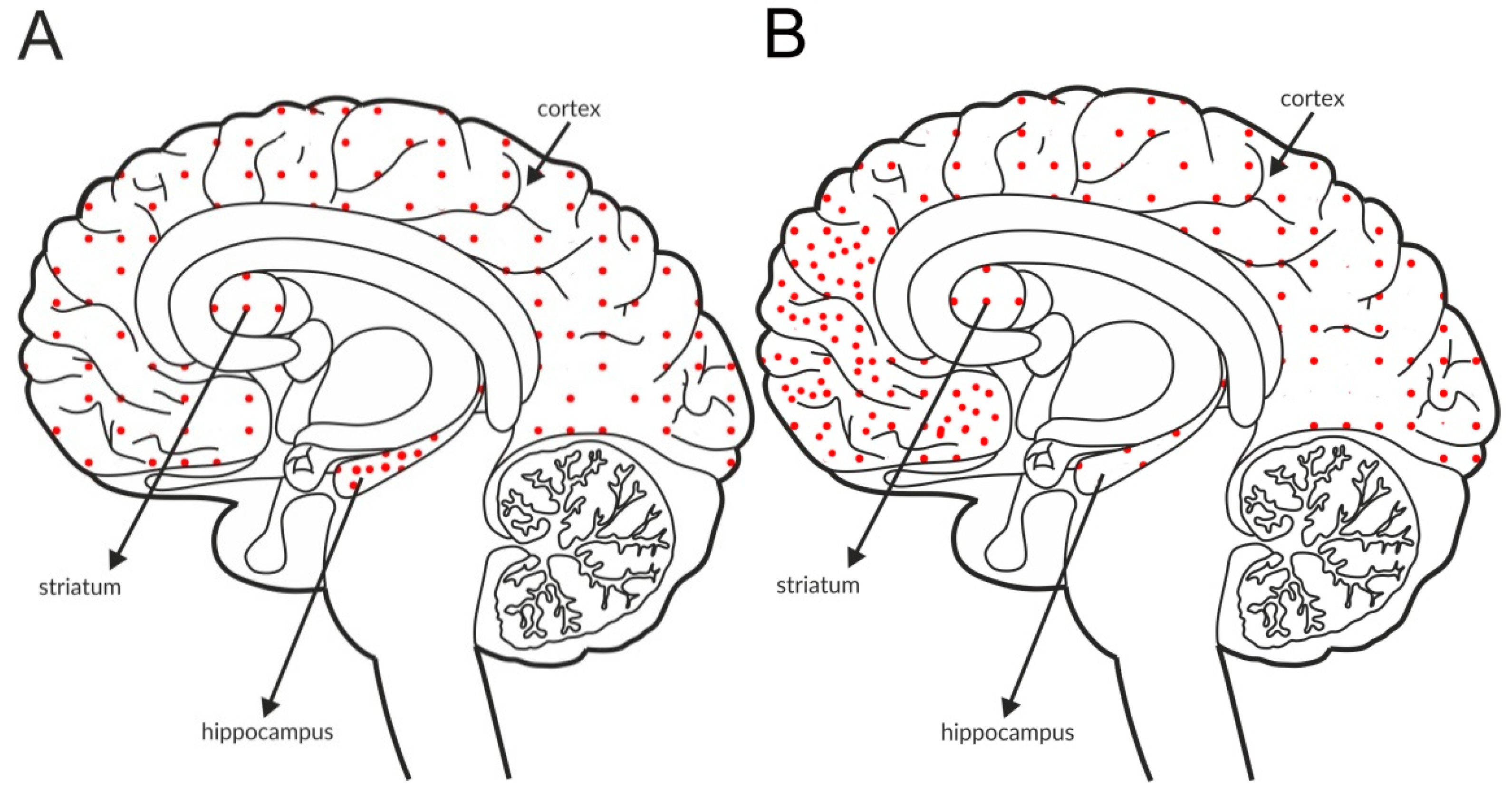
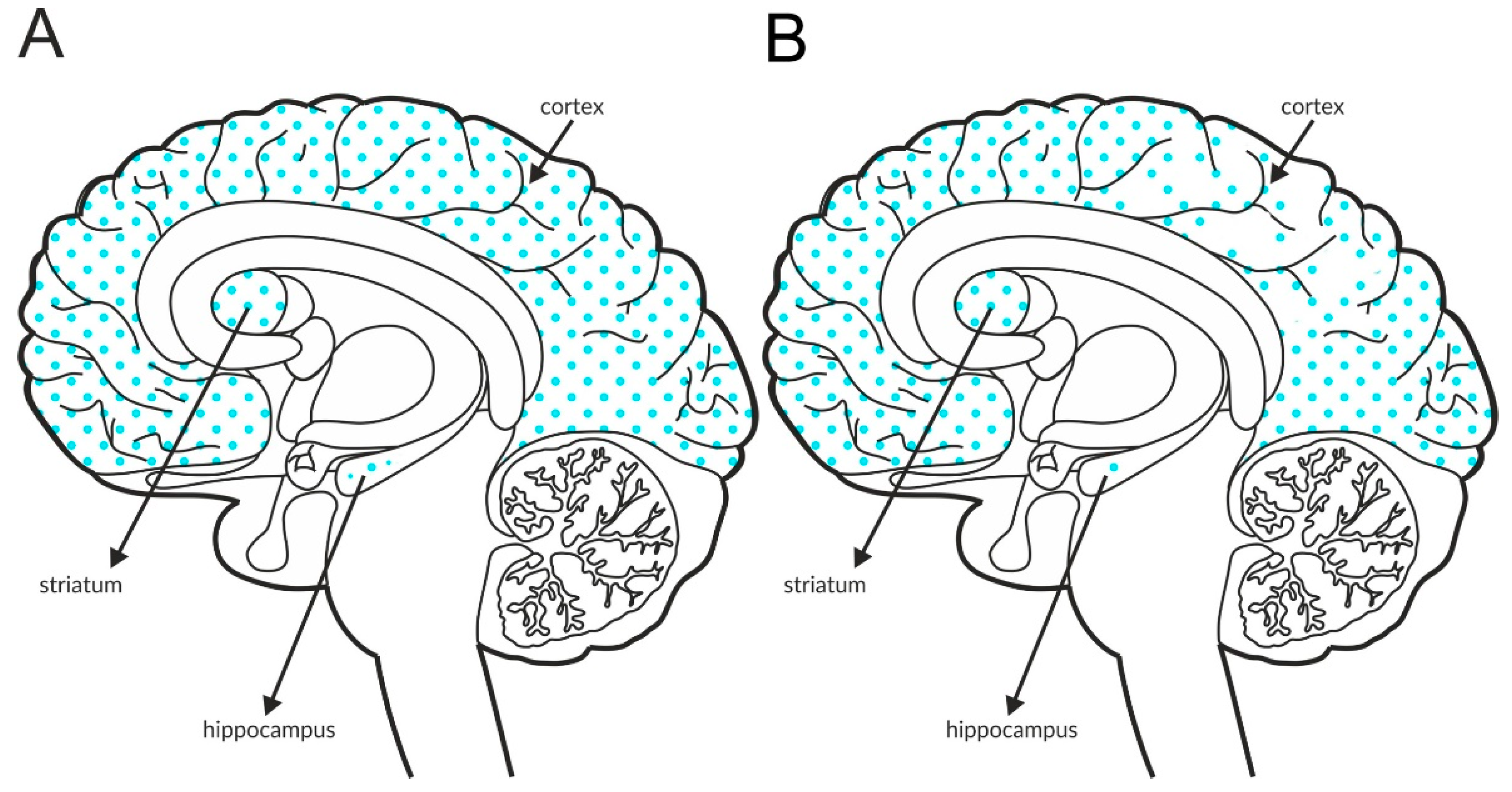
The most intense staining for mGlu2 receptors was detected in the neocortex and limbic cortical neurons, predominantly in the hippocampus, as shown in Figure 2A and Table 4A,B. The expression of the receptor at axon terminals was evident, but examples of postsynaptic expression of the receptor on the cell bodies and dendrites of Golgi cells in the cerebellum were also noticed [114].
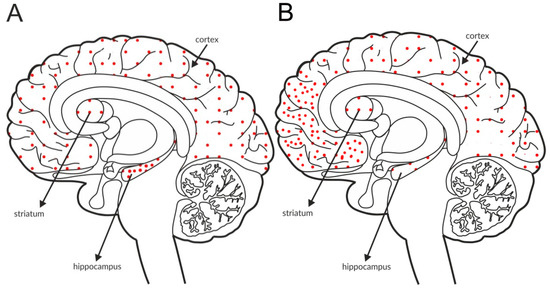
Figure 2.
Distribution of mGlu2 receptors in the brains of healthy individuals (A) and patients with schizophrenia (B). Dotted areas represent receptor expression in select structures. The expression intensity is indicated by the pattern density.
Some postmortem studies revealed a decrease in the expression of mGlu2 receptors in the hippocampus and increased expression in the prefrontal cortex of patients with schizophrenia (Figure 2B, Table 3C).
Ligands activating mGlu2 receptors inhibit the release of glutamate and have been extensively investigated as newer antipsychotics in animals and humans. A 2007 article showed the efficacy of a mGlu2/3 orthosteric agonist in patients with schizophrenia and provided hope for new treatment solutions [57], as described in the review “Schizophrenia drug says goodbye to dopamine” [115]. Unfortunately, the results from further clinical trials of mGlu2/3 orthosteric agonists were far from satisfactory, and work with the compound was ultimately discontinued. However, this decision may have been premature because the ligands displayed excellent activity in preclinical models [51,116] and some clinical studies [117,118].
The conflicting data may result from several factors, such as genetic diversity between humans or a prior history of antipsychotic treatment. Further studies with more homogenous groups of patients and/or without prior medical treatment are needed. Importantly, the poor oral bioavailability of the compounds due to their highly hydrophilic properties was shown to be one of the reasons for their poor efficacy in humans [57,119,120]. One of the solutions to improve the gastrointestinal absorption of compounds is to design prodrugs with better absorption properties. Peptide transporter 1 (PEPT1) regulates the bioavailability of various drugs, including some mGlu2/3 agonists; therefore, Eli Lilly designed prodrugs to be absorbed by PEPT1 (LY544344 for LY354740 and LY2140023 for LY404039) [119,121]. The generation of these prodrugs resulted in significantly higher bioavailability of the prototypes [119,122]. However, higher exposure may induce toxicity in patients [123]. An ester-based lipophilic prodrug of another mGlu2/3 agonist, MGS0008, was designed to avoid undesirable adverse effects [123]. MGS0274 besylate exhibited a 15-fold improvement in oral bioavailability compared to MGS0008, and its administration to patients was accompanied by fewer toxic effects caused by its unnecessary exposure [120,123,124].
3.2.2. Group III mGlu Receptors
The third group of mGlu receptors consists of the mGlu4, mGlu7, and mGlu8 subtypes. All of these receptors are expressed presynaptically and are negatively associated with adenyl cyclase activity [110]. The potency of glutamate at mGlu4 receptors is slightly lower than at mGlu2 receptors (3–38 µM), and these receptors are mainly located in the center of the synaptic cleft [110,111], near the site of fusion with synaptic vesicles. Therefore, these receptors are exposed to high glutamate concentrations [112].
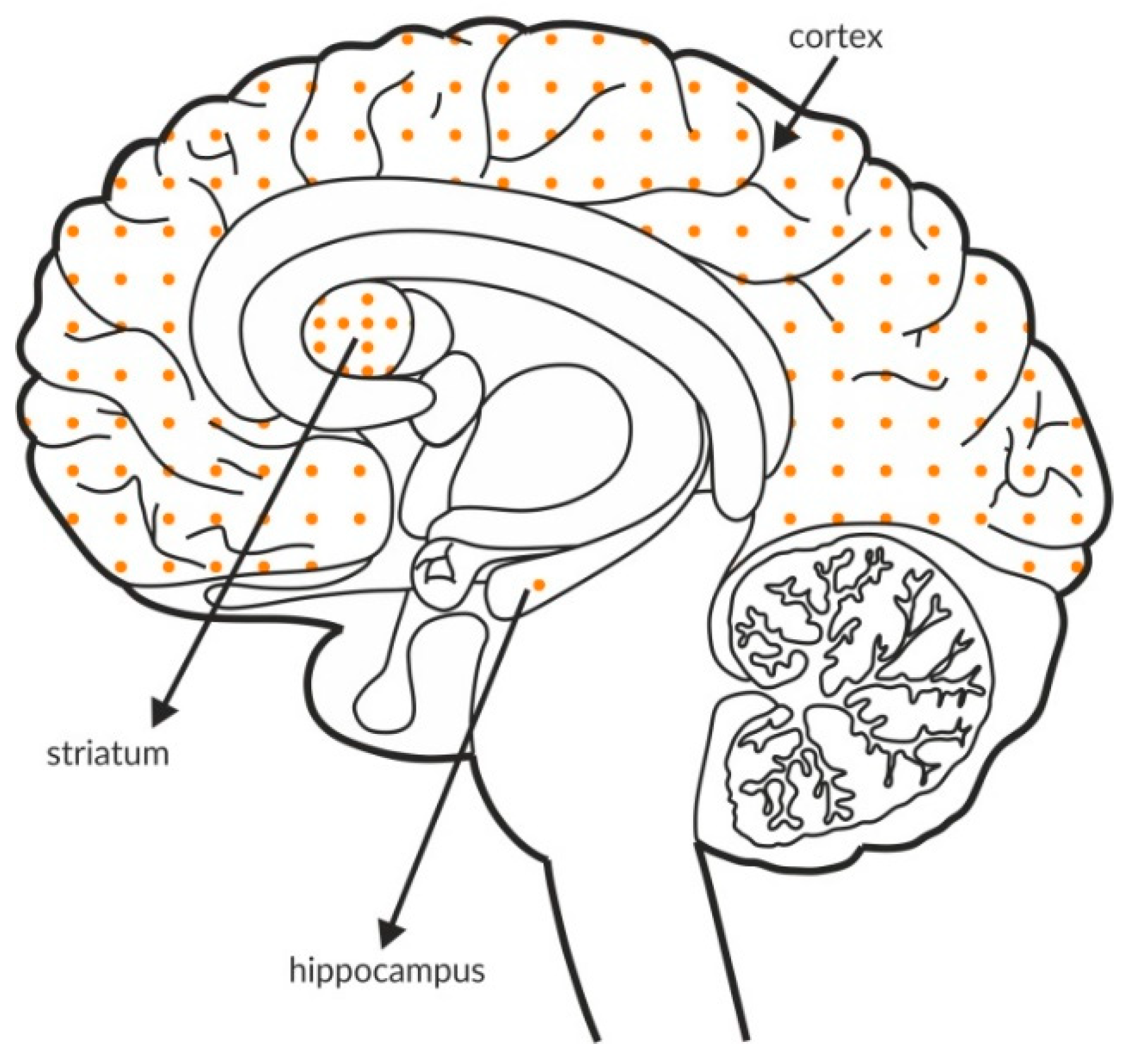
Similar to mGlu2 [125], the mGlu4 receptor is expressed predominantly on glutamatergic terminals that oppose other glutamatergic projection neurons [126,127]. At least two splice variants of mGlu4 receptors were identified [128], and stimulation of these receptors resulted in antipsychotic efficacy in several studies [51,53]. The receptor is expressed at relatively low levels in the hippocampus and cortex, and the most intense mGlu4 labeling is observed in the globus pallidus and cerebellum, as shown Figure 3 and Table 4A,B. Postmortem studies have not shown altered expression of mGlu4 receptors in patients with schizophrenia (Table 3C).
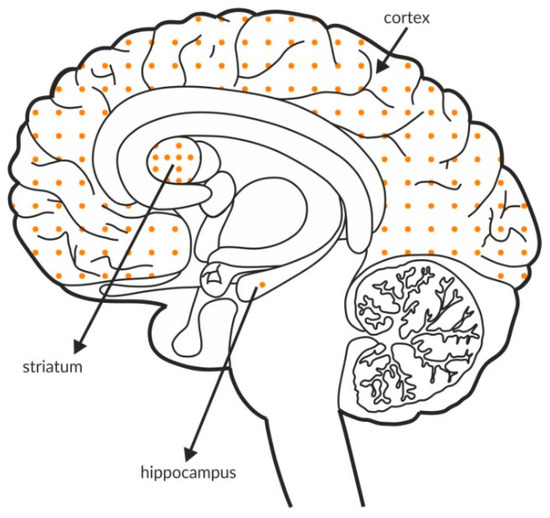
Figure 3.
Distribution of mGlu4 receptors in the brains of healthy individuals. Dotted areas represent receptor expression in select structures. The expression intensity is indicated by the pattern density.
The ability of mGlu2/3 and mGlu4 receptors to inhibit glutamate release in the cortex was confirmed in patch-clamp experiments, in which an orthosteric agonist or positive allosteric modulator (PAM) abolished the frequency (but not the amplitude) of DOI-induced spontaneous EPSCs [129,130,131].
The mGlu7 and mGlu8 receptors are the least recognized mGlu receptors. Five subtypes of mGlu7 [132] and three subtypes of the mGlu8 receptor were cloned [133]. Due to the limited number of available ligands activating or inhibiting these receptors, data on their pharmacological activity are scarce. Available publications indicate a lack of efficacy of activation of mGlu7 receptors in animal models of schizophrenia [134]. However, the only available mGlu7 PAM, AMN082, was only tested in MK-801-induced hyperactivity and DOI-induced head twitches. Therefore, the data are incomplete. In contrast, the efficacy of negative allosteric modulators of the mGlu7 receptor was observed in a wide range of tests [135].
The mGlu7 receptor is a presynaptic receptor located on glutamatergic axons. However, mGlu7-like immunoreactivity was also observed on GAD-expressing neurons in the islands of Calleja or striatum, suggesting that the receptor is also a heteroreceptor on GABAergic neurons [136]. The functional roles of these receptors are not clear because their low affinity for glutamate stimulation at distant synapses by a diluted signal is doubtful.
3.3. Presynaptic Regulation of Glutamate Release—Heteroreceptors
Heteroreceptors are activated by neurotransmitters other than those synthesized by the neurons on which the receptors are expressed.
The large number of heteroreceptors involved in the regulation of glutamate release makes a discussion of each type challenging. According to recent data, GABAB and muscarinic M4 receptors are of particular importance in the pathophysiology of schizophrenia and antipsychotic drug discovery.
3.3.1. GABAB Receptor
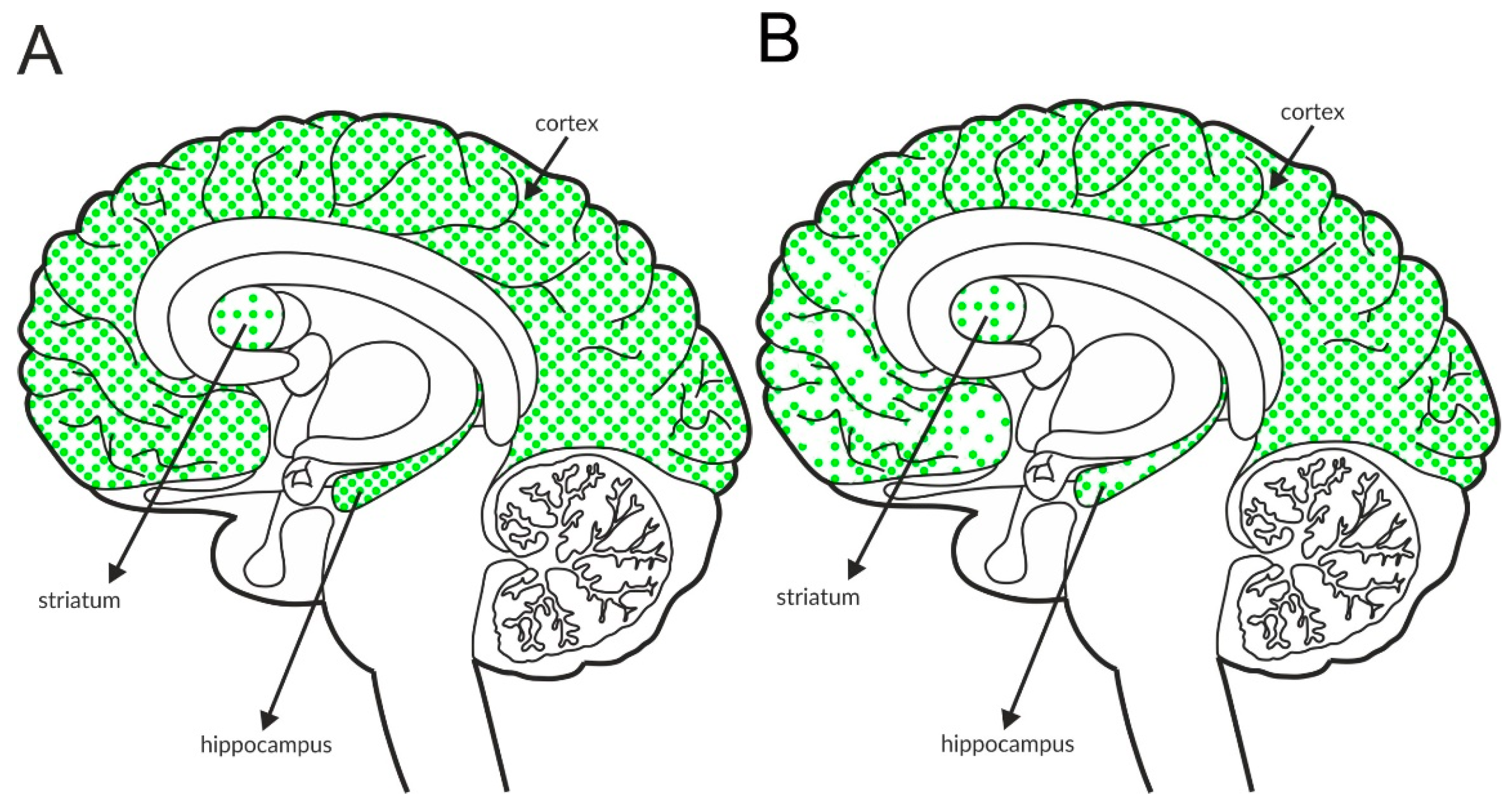
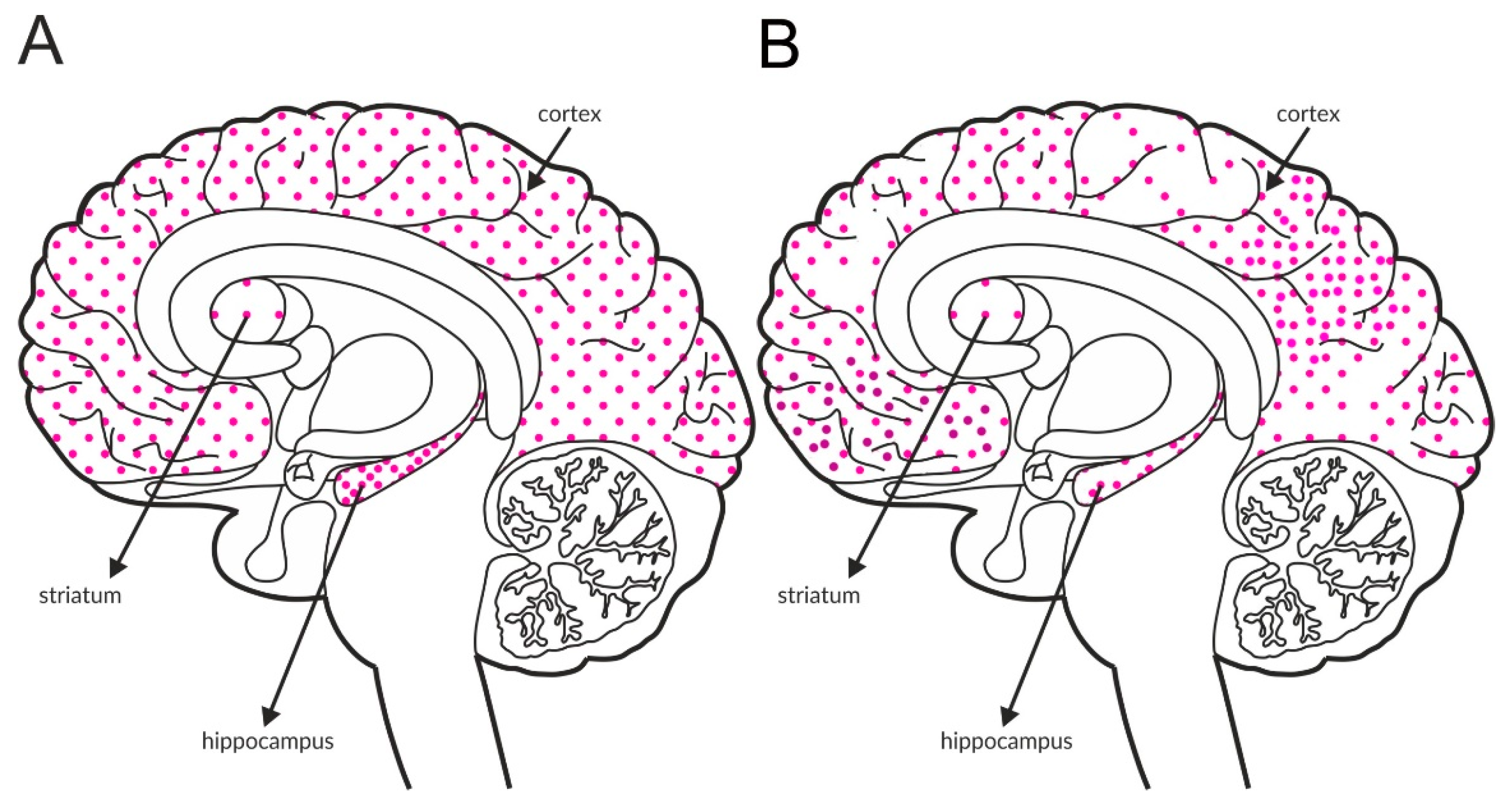
GABAB receptors, similar to group II and III mGlu receptors, are associated with adenyl cyclase activity and the inhibition of cAMP production. Glutamatergic terminals contain large numbers of this receptor, and its stimulation inhibits glutamate release [137]. Therefore, GABAB receptors, together with mGlu receptors, are one of the most important pathways regulating the release of glutamate. The GABAB receptor is found in all brain areas, and the receptor is expressed at relatively high levels in all brain structures. The labeling of the receptor is higher in the hippocampus and the cortex than in the striatum, with an additional increase in the hippocampus compared with the cortex (Figure 4A and Table 4A,B).
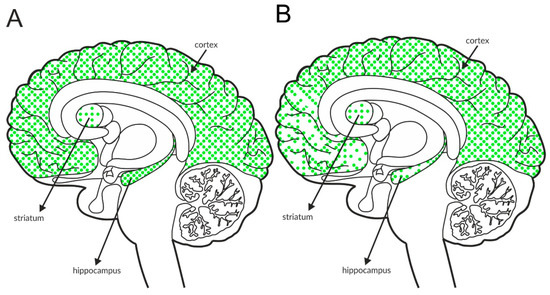
Figure 4.
Distribution of GABAB receptors in the brains of healthy individuals (A) and patients with schizophrenia (B). Dotted areas represent receptor expression in select structures. The expression intensity is indicated by the pattern density.
Available postmortem studies revealed decreased expression of GABAB receptors in both the hippocampus and prefrontal cortex of patients with schizophrenia (Figure 4B and Table 3B).
According to preclinical studies, the GABAB receptor is a promising target in antipsychotic drug discovery. The efficacy of PAMs of this receptor has been shown in animal models of positive, negative, and cognitive symptoms [137,138]. Notably, the use of PAMs instead of agonists is recommended because of the lower risk of developing adverse effects, such as myorelaxation or sedation, which may be induced after orthosteric agonist administration [139,140].
3.3.2. Muscarinic M4 Receptor
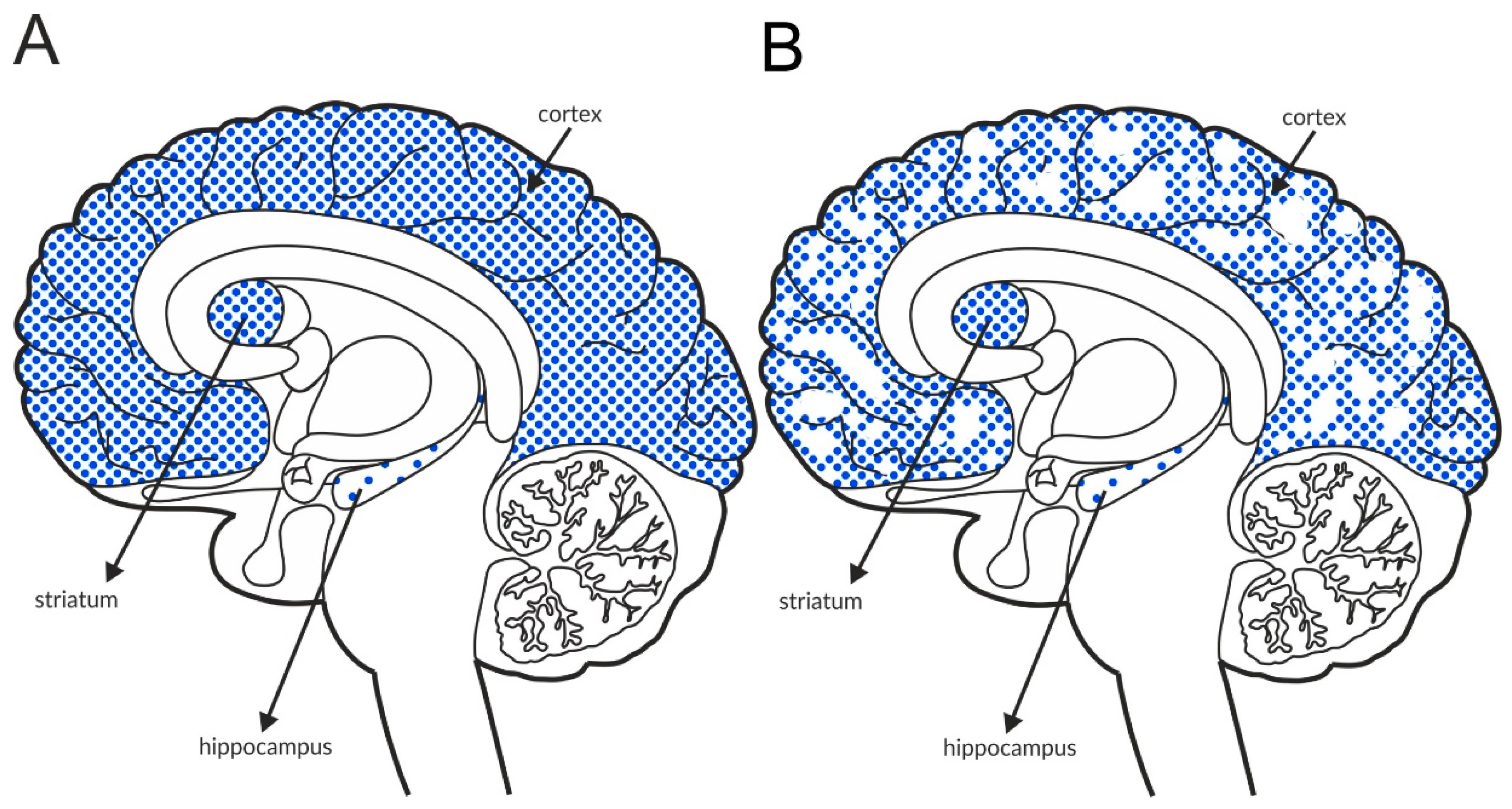
Recently, researchers investigating schizophrenia have focused on muscarinic receptors after the administration of xanomeline was reported to exhibit antipsychotic efficacy in patients with schizophrenia [141]. Xanomeline is a nonselective agonist of muscarinic receptors that preferentially binds to M1 and M4 receptors [142]. Therefore, this drug also induced adverse effects due to stimulation of peripherally expressed M2 and M3 receptors [143]. Treatment with selective ligands to activate muscarinic receptor subtypes that are preferentially expressed in the brain, such as M1, M4, or M5, should result in a lower risk of peripherally driven effects. The M4 subtype is located at presynaptic sites and may be a heteroreceptor on glutamatergic terminals [144,145].
The M4 receptor is negatively associated with adenyl cyclase activity. It functions as an autoreceptor in the striatum, but it is expressed as a heteroreceptor on glutamatergic axon terminals and regulates glutamate release, predominantly in the cortex and hippocampus [146,147,148,149,150,151]. Patch clamp recordings confirmed its ability to reduce excessive glutamate efflux in the cortex [152]. The expression of the receptor in the structures involved in schizophrenia pathophysiology is shown in Figure 5A and Table 4A,B. Postmortem studies indicate decreased expression of M4 receptors in the hippocampus and parietal cortex of patients with schizophrenia (Figure 5B and Table 3A).
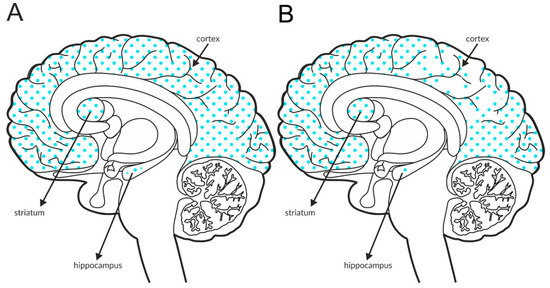
Figure 5.
Distribution of M4 receptors in the brains of healthy individuals (A) and patients with schizophrenia (B). Dotted areas represent receptor expression in select structures. The expression intensity is indicated by the pattern density.
3.4. Postsynaptic Regulation of Neuronal Circuits in Patients with Schizophrenia
The selection of receptors expressed on cell bodies and dendrites deserves attention in schizophrenia drug development. Their activation changes the neuronal potential and signal transduction along the axon terminal, which may affect distant neurons.
3.4.1. mGlu5 Receptor
The mGlu5 receptor is a member of the group I metabotropic glutamate receptor family, and it has three splice variants [153]. In contrast to the group II and group III receptors, this subtype interacts with phosphatase C and stimulates inositol production via Gαq signaling.
The mGlu5 receptor is expressed near NMDA receptors and is functionally linked via Shank and Homer proteins [154]. Therefore, the stimulation or inhibition of the mGlu5 receptor influences NMDA-mediated signaling [155,156,157], indicating that the pharmacological manipulation of this receptor represents a high risk. Fortunately, Conn and coworkers identified that the modulation of NMDA currents was not critical for mGlu5 pharmacology and discovered biased, selective potentiators of mGlu5 receptors coupled to Gαq-mediated signaling but not mGlu5 modulation of NMDAR currents or NMDAR-dependent synaptic plasticity in the rat hippocampus [158]. These ligands bind to sites distinct from the orthosteric (or endogenous) ligand, often with improved subtype selectivity and spatiotemporal control over receptor responses, which constitutes a novel therapeutic approach.
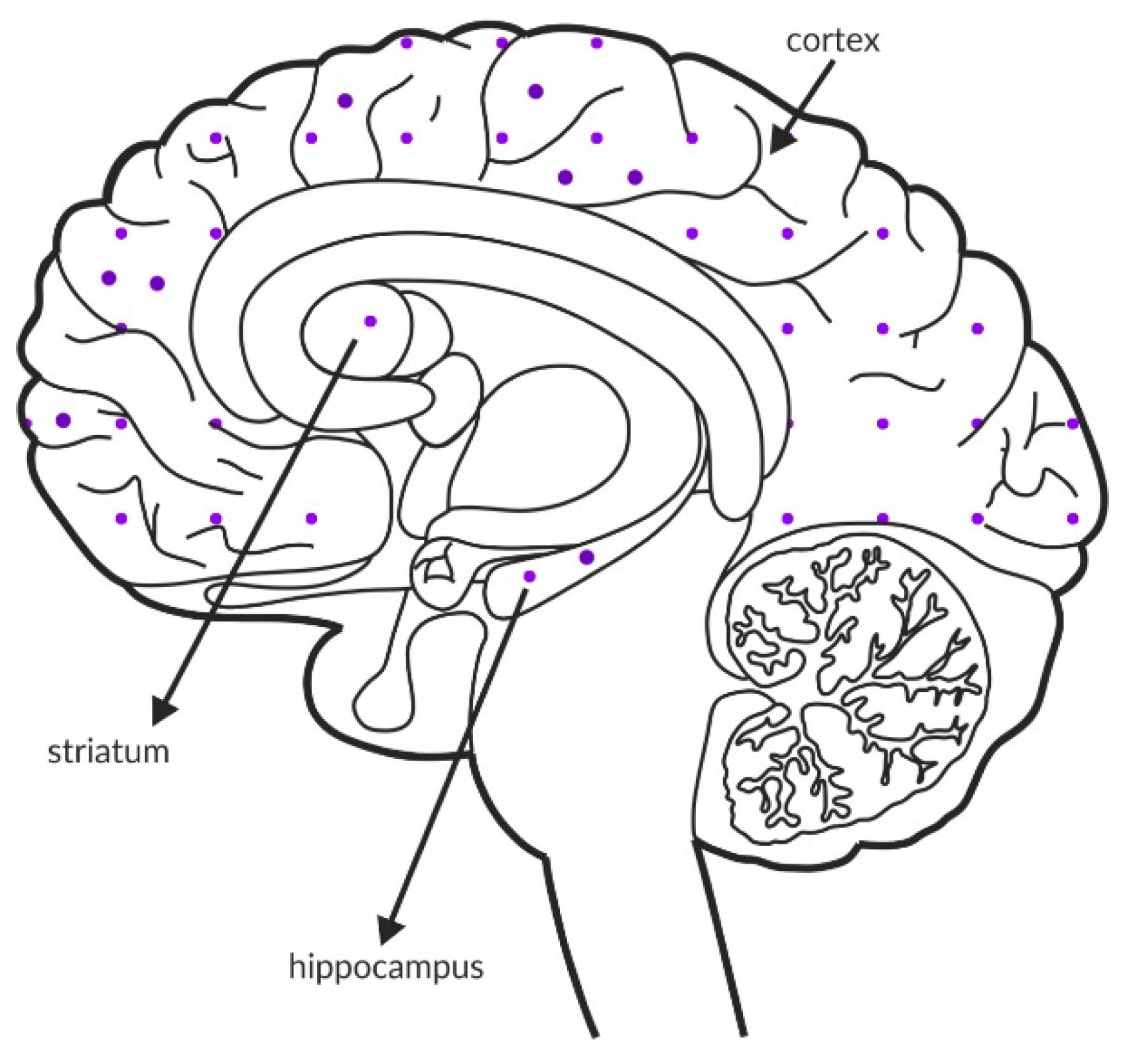
The mGlu5 receptors generally function as postsynaptic receptors on dendritic spines and shafts, but they were also detected presynaptically on axon terminals in the cortex and hippocampus. Electron microscopy and immunocytochemical studies indicated that these neurons may synthesize GABA [159,160]. The receptor is widely distributed across the brain, including structures that are critical in schizophrenia arousal. The most intense labeling was observed in the hippocampus, followed by the cortex, and the lowest expression was observed in the striatum. A schematic of the distribution of this receptor within these structures in the healthy brain is shown in Figure 6A and Table 4A,B. In postmortem studies, the expression of mGlu5 receptors was decreased in the prefrontal cortex and cerebellum (Figure 6B and Table 3C). The data from the frontal cortex are inconclusive, as the expression is increased in some regions and decreased in others.
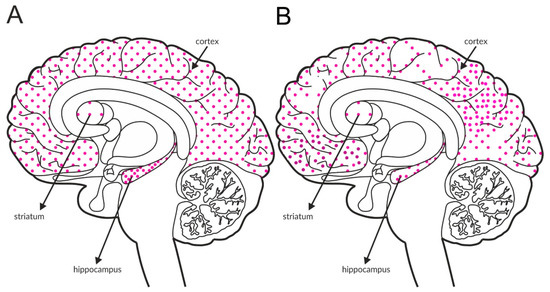
Figure 6.
Distribution of mGlu5 receptors in the brains of healthy individuals (A) and patients with schizophrenia (B). Dotted areas represent receptor expression in select structures. The expression intensity is indicated by the pattern density.
Stimulation of mGlu5 exerted antipsychotic-like activity in a vast range of animal models [51,53].
3.4.2. Muscarinic M1 Receptor
The M1 receptor is expressed in the cerebral cortex, hippocampus, thalamus, and striatum (Figure 7A and Table 4A,B) [161,162,163,164], and it activates phospholipase C and MAPK in the cerebral cortex in mice [165]. The M1 receptor colocalizes with NMDA receptors in hippocampal pyramidal neurons, and the simultaneous activation of the M1 and NMDA receptors increases NMDA currents [166]. Deletion of the M1 receptor results in a partial impairment of long-term potentiation in the hippocampus [166], which is also reflected in behavior [166,167]. Despite the presence of intact hippocampus-dependent memory, M1-/- mice show a deficit in consolidation over time during contextual fear conditioning, as well as impairments in win-shift and social discrimination learning, which suggests a role for the M1 receptor in cortex-dependent memory or hippocampal-cortical interaction [166]. M1 receptor deletion leads to elevated basal striatal dopamine release and locomotor activity, which is further enhanced by amphetamine challenge [167,168].
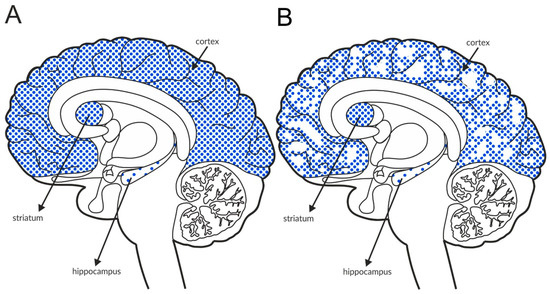
Figure 7.
Distribution of M1 receptors in the brains of healthy individuals (A) and patients with schizophrenia (B). Dotted areas represent receptor expression in select structures. The expression intensity is indicated by the pattern density.
The antipsychotic activity of M1 receptor ligands has not been extensively tested in preclinical studies. Our studies are some of the first to show activity in animal models of schizophrenia [169]. However, M1 ligand activity was observed in models of positive and cognitive, but not negative, symptoms of the disease [169,170].
3.4.3. Muscarinic M5 Receptor
The M5 receptor accounts for approximately 2% of all muscarinic receptors in the brain [164], and it is the least studied muscarinic receptor. It is expressed in the hippocampus, hypothalamus, cerebral cortex, striatum, substantia nigra pars compacta and ventral tegmental area (Figure 8 and Table 4A,B) [162,163,171]. It is also found on blood vessels in the brain [172,173]. The location of M5 receptors suggests a role in the regulation of dopamine release [174]. These receptors colocalize with D2 dopamine receptors in the substantia nigra pars compacta [171]. Due to the lack of selective M5 receptor ligands, the first preclinical studies were performed in mice lacking this receptor. The M5-/- mice showed no changes in motor coordination or basal locomotor activity, and no significant changes in locomotor activity were observed after amphetamine administration [175]. Deletion of the M5 receptor did not affect animal social interactions but weakened sensory motor gating processes [172,176]. M5-/- mice also showed a memory impairment in the new object recognition test and the Y maze [172]. The memory impairment may be partially explained by morphological (reduced number of dendritic spines) and physiological (reduced expression of NMDA, AMPA, and kainate receptor subunits, reduced frequency of spontaneous postsynaptic potentials, reduced LTP, and neurotransmitter release disturbances) changes within the hippocampal formation [172]. As shown in our previous studies, a PAM of the M5 receptor exerted antipsychotic-like effects on models of positive and cognitive, but not negative, symptoms of schizophrenia [169,170].
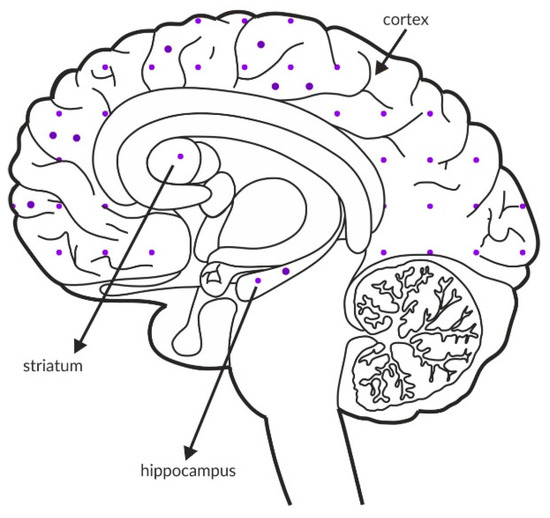
Figure 8.
Distribution of M5 receptors in the healthy brain. Dotted areas represent receptor expression in select structures. The expression intensity is indicated by the pattern density.
3.4.4. Comparative Assessment of Receptor Expression
Table 4A,B summarizes the available data on the expression of particular receptors in rodents and humans. Studies of protein expression were performed using immunohistochemistry, Western blotting and immunoprecipitation, and mRNA expression was investigated using in situ hybridization, PCR, or Northern blotting. All investigated receptors were widely expressed in structures that are important in schizophrenia arousal (e.g., cortex, hippocampus, and striatum).

Table 4.
The expression of muscarinic (M1, M4, and M5), GABA (GABAB), and metabotropic glutamate (mGlu2, mGlu5, mGlu4, mGlu7, and mGlu8) receptors in the rodent (A) or human brain (B). Protein expression was determined using immunohistochemistry, Western blotting, and immunoprecipitation. The mRNA levels were assessed using in situ hybridization, PCR, or Northern blotting.
Table 4.
The expression of muscarinic (M1, M4, and M5), GABA (GABAB), and metabotropic glutamate (mGlu2, mGlu5, mGlu4, mGlu7, and mGlu8) receptors in the rodent (A) or human brain (B). Protein expression was determined using immunohistochemistry, Western blotting, and immunoprecipitation. The mRNA levels were assessed using in situ hybridization, PCR, or Northern blotting.
| (A) | ||||
| Receptor | Protein | mRNA | ||
| M1 | cortex (including: mPFC, entorhinal cortex) | [162,170,177,178] | cortex (including piriform cortex, visual cortex) | [171,179,180,181,182,183] |
| nucleus accumbens | [171] | |||
| hippocampus | [162,170] | caudate-putamen | [171,179,183] | |
| caudate-putamen | [162] | basolateral amygdala | [182] | |
| nucleus accumbens | [162,184] | olfactory tubercule | [179] | |
| thalamus | [162] | primary olfactory cortex | [182,183] | |
| amygdala | [162] | hippocampus | [182] | |
| brainstem | [162] | olfactory nuclei | [182] | |
| olfactory tubercule | [162] | olfactory bulb | [171,182,183] | |
| M4 | cortex | [162,185] | ||
| caudate-putamen | [162,184,185] | |||
| nucleus accumbens | [162] | cortex (including primary olfactory cortex, visual cortex, piriform cortex) | [171,179,180,181,182,183,185] | |
| thalamus | [162] | nucleus accumbens | [171] | |
| hippocampus | [185] | caudate-putamen | [171,179,182,183,185] | |
| substantia nigra | [162] | hippocampus | [182,183,185] | |
| brainstem | [162] | olfactory tubercule | [171,179,183] | |
| olfactory tubercule | [162] | olfactory bulb | [182,185] | |
| olfactory bulb | [185] | |||
| islands of Calleja | [162] | |||
| M5 | brainstem | [162] | substantia nigra (pc) | [171,186] |
| ventral tegmental area | [171,186] | |||
| hippocampus (CA1) | [186] | |||
| ventral subiculum | [186] | |||
| GABAB | cortex | [187,188] | cortex (including piriform cortex) | [189] |
| caudate-putamen | [187,188] | hippocampus | [189] | |
| globus pallidus | [188] | nucleus accumbens | [189] | |
| nucleus accumbens | [188] | caudate-putamen | [189] | |
| amygdala | [188] | thalamus | [189] | |
| hippocampus | [187,188] | hypothalamus | [189] | |
| thalamus | [187,188] | substantia nigra (pc) | [189] | |
| hypothalamus | [188] | ventral tegmental area | [189] | |
| ventral tegmental area | [188] | cerebellum | [189] | |
| substantia nigra | [188] | pons | [189] | |
| cerebellum | [187,188] | |||
| olfactory bulb | [187] | (GABAB1) | ||
| medulla/pons | [187] | |||
| cortex (including piriform cortex) | [190,191] | |||
| (GABAB1A, GABAB1B, GABAB2) | ||||
| caudate-putamen | [190] | |||
| nucleus accumbens | [190] | |||
| globus pallidus | [190] | |||
| substantia nigra | [190] | |||
| amygdala | [190] | |||
| hippocampus | [190,191] | |||
| hypothalamus | [190] | |||
| thalamus | [190,191] | |||
| cerebellum | [190,191] | |||
| ventral tegmental area | [190] | |||
| pons | [190] | |||
| (GABAB2) | ||||
| cortex (including piriform cortex, frontal cortex, occipital cortex, retrosplenial cortex, | [192] | |||
| temporal cortex) | ||||
| hippocampus | [192] | |||
| thalamus | [192] | |||
| hypothalamus | [192] | |||
| striatum | [192] | |||
| nucleus accumbens | [192] | |||
| substantia nigra | [192] | |||
| amygdala, | [192] | |||
| cerebellum | [192] | |||
| (GABAB1A, GABAB2) | ||||
| mGlu5 | cortex (including piriform cortex) | [159,193] | cortex (including entorhinal cortex) | [194,195,196,197,198] |
| caudate-putamen | [159,193,199] | hippocampus | [194,195,196,197,198,200] | |
| nucleus accumbens | [159,193] | caudate-putamen | [194,195,196,197,198,201] | |
| hippocampus | [159,193,202,203] | nucleus accumbens | [196,197,198] | |
| thalamus | [159] | subiculum | [196,197] | |
| hypothalamus | [159] | thalamus | [196,198] | |
| subiculum | [159] | hypothalamus | [196] | |
| cerebellum | [159] | inferior and superior colliculi | [196,198] | |
| inferior colliculus | [193] | amygdala | [200] | |
| olfactory bulb | [159,193] | olfactory bulb | [196,198] | |
| olfactory tubercule | [159,193] | olfactory tubercule | [196,197] | |
| mGlu2 | cortex (including piriform cortex, entorhinal cortex) | [114,204,205] | ||
| hippocampus | [114,202,204,205] | cortex (including piriform cortex, entorhinal cortex) | [194,197,206,207] | |
| thalamus | [114,204,205] | hippocampus | [194,197,207] | |
| basolateral amygdala | [114,204] | thalamus | [197,206,207] | |
| caudate-putamen | [114,204,205] | basolateral amygdala | [206,207] | |
| nucleus accumbens | [114,204] | caudate-putamen | [206] | |
| globus pallidus | [204] | nucleus accumbens | [206] | |
| substantia nigra | [204] | globus pallidus | [206] | |
| ventral tegmental area | [114] | cerebellum | [194,197,206,208] | |
| cerebellum | [114,204] | olfactory tubercule | [206] | |
| olfactory bulb | [114,204] | |||
| olfactory tubercule | [114,204] | |||
| mGlu4 | cortex (including piriform cortex) | [209] | cortex (including entorhinal cortex) | [194,197,210,211,212] |
| caudate-putamen | [209] | caudate-putamen | [197,210,212,213] | |
| substantia nigra | [209] | substantia nigra | [197] | |
| hippocampus | [202,209] | nucleus accumbens | [197,212,213] | |
| thalamus | [209] | thalamus | [194,197,210,212,213,214] | |
| hypothalamus | [209] | hypothalamus | [212] | |
| amygdala | [209] | hippocampus | [194,210,214] | |
| superior colliculus | [209] | amygdala | [212] | |
| cerebellum | [209,215,216] | lateral septum | [210,214] | |
| olfactory bulb | [209] | cerebellum | [194,197,208,212,214] | |
| olfactory tubercule | [209] | olfactory bulb | [210,212,214] | |
| olfactory tubercule | [197,214] | |||
| mGlu7 | cortex (including piriform cortex) | [136,217] | ||
| caudate-putamen | [136] | |||
| nucleus accumbens | [136] | |||
| globus pallidus | [136] | |||
| substantia nigra | [136] | |||
| thalamus | [136] | |||
| hypothalamus | [136] | |||
| hippocampus | [136] | |||
| subiculum | [136] | |||
| amygdala | [136] | cortex | [212,218,219,220] | |
| ventral tegmental area | [136] | caudate-putamen | [212,213,218,219,220] | |
| olfactory bulb | [136] | globus pallidus | [212] | |
| olfactory tubercule | [217] | nucleus accumbens | [212,213,218,220] | |
| substantia nigra | [212] | |||
| (mGlu7a) | thalamus | [212,213,218,219,220] | ||
| cortex | [136] | hypothalamus | [212,219,220] | |
| hippocampus | [136] | amygdala | [212,220] | |
| substantia nigra | [136] | hippocampus | [218,219,220] | |
| globus pallidus | [136] | ventral tegmental area | [212] | |
| amygdala | [136] | superior and inferior colliculi | [219] | |
| cerebellum | [136] | locus coeruleus | [218] | |
| (mGlu7b) | cerebellum | [208,212,218,219,220] | ||
| cortex (including piriform cortex) | [221,222] | olfactory bulb | [212,218,219] | |
| hippocampus | [202,221,222,223] | olfactory tubercule | [219,220] | |
| thalamus | [222] | |||
| caudate-putamen | [222] | |||
| globus pallidus | [222] | |||
| nucleus accumbens | [222] | |||
| locus coeruleus | [222] | |||
| cerebellum | [222] | |||
| olfactory bulb | [221] | |||
| mGlu8 | cortex (including piriform cortex) | [218,224,225] | ||
| striatum | [213,218,225] | |||
| nucleus accumbens | [213,225] | |||
| globus pallidus | [225] | |||
| piriform cortex | [216] | substantia nigra | [225] | |
| entorhinal cortex | [216] | thalamus | [213,218,224,225] | |
| hippocampus | [202,226] | hypothalamus | [225] | |
| olfactory bulb | [216] | hippocampus | [218,224,225,226] | |
| amygdala | [218,225] | |||
| cerebellum | [208,218,224,225] | |||
| olfactory bulb | [218,224,227] | |||
| olfactory tubercule | [227] | |||
| (B) | ||||
| Receptor | Protein | mRNA | ||
| M1 | frontal cortex | [69,79,227] | ||
| parietal cortex | [70,228] | |||
| temporal cortex | [228] | frontal cortex | [70] | |
| occipital cortex | [228] | parietal cortex | [70] | |
| primary visual cortex | [80] | thalamus | [79] | |
| thalamus | [79,80] | hippocampus | [75] | |
| hippocampus | [80,228] | caudate-putamen | [77] | |
| nucleus basalis | [228] | |||
| putamen | [228] | |||
| M4 | frontal cortex | [70,228] | ||
| temporal cortex | [228] | |||
| parietal cortex | [70,228] | frontal cortex | [70] | |
| occipital cortex | [228] | parietal cortex | [70] | |
| thalamus | [79] | thalamus | [79] | |
| hippocampus | [228] | hippocampus | [75] | |
| nucleus basalis | [228] | |||
| putamen | [228] | |||
| M5 | frontal cortex | [228] | ||
| temporal cortex | [228] | |||
| parietal cortex | [228] | |||
| occipital cortex | [228] | |||
| nucleus basalis | [228] | |||
| GABAB | prefrontal cortex | [229] | ||
| frontal cortex | [192] | |||
| occipital cortex | [192] | |||
| temporal cortex | [192] | |||
| caudate nucleus | [192,229] | |||
| putamen | [192,229] | |||
| globus pallidus | [229] | |||
| substantia nigra | [192,229] | |||
| nucleus accumbens | [192] | |||
| entorhinal cortex | [230] | thalamus | [192] | |
| caudate | [230] | hypothalamus | [192] | |
| putamen | [230] | hippocampus | [192,229] | |
| globus pallidus | [230] | amygdala | [192] | |
| thalamus | [230] | corpus callosum | [192] | |
| hippocampus | [230] | cerebellum | [192,229] | |
| substantia nigra | [230] | |||
| cerebellum | [230] | |||
| cortex | [191] | |||
| (GABAB1, GABAB2) | putamen | [191] | ||
| caudate nucleus | [191] | |||
| substantia nigra | [191] | |||
| thalamus | [191] | |||
| hippocampus | [191] | |||
| amygdala | [191] | |||
| cerebellum | [191] | |||
| (GABAB2) | ||||
| mGlu5 | frontal cortex | [94] | cortex (including frontal cortex, prefrontal cortex) | [89,94,153] |
| hippocampus | [231] | hippocampus | [90,153] | |
| lateral cerebellum | [94] | parahippocampal gyrus | [90] | |
| cerebellum | [94,153] | |||
| mGlu2 | prefrontal cortex | [103] | prefrontal cortex | [102] |
| temporal cortex | [103] | thalamus | [91] | |
| dorsolateral prefrontal cortex | [100] | hippocampus | [101] | |
| motor cortex | [103] | ventral mesencephalon (including substantia nigra) | [102] | |
| hippocampus | [231] | |||
| mGlu4 | hippocampus | [231] | cortex | [232] |
| putamen | [232] | |||
| substantia nigra | [232] | |||
| caudate nucleus | [232] | |||
| thalamus | [91,232,233] | |||
| hypothalamus | [232,233] | |||
| hippocampus | [232,233] | |||
| amygdala | [232] | |||
| corpus callosum | [232] | |||
| cerebellum | [232,233,234] | |||
| mGlu7 | cortex (including entorhinal cortex) | [235] | ||
| thalamus | [91,234,235] | |||
| hypothalamus | [234] | |||
| hippocampus | [234,235] | |||
| caudate-putamen | [235] | |||
| cerebellum | [235] | |||
| mGlu8 | cortex | [133] | ||
| putamen | [133,225] | |||
| caudate nucleus | [133,225] | |||
| globus pallidus | [225] | |||
| nucleus accumbens | [225] | |||
| substantia nigra | [225] | |||
| cingulate gyrus | [225] | |||
| thalamus | [91,133,225] | |||
| hypothalamus | [225] | |||
| hippocampus | [225] | |||
| amygdala | [133,225] | |||
| locus coeruleus | [225] | |||
| cerebellum | [133,225] | |||
The quantitative analysis of the expression of the receptors differed between structures and comparisons, which may modulate the development of therapeutic effects and adverse effects. The quantitative analyses of the receptors in the brain structures most important for schizophrenia pathophysiology and treatment are summarized in Table 5.

Table 5.
Comparison of the expression of muscarinic (M1, M4, and M5), GABAB and metabotropic glutamate (mGlu2, mGlu4, and mGlu5) receptors in select brain structures: “0”—not detected, “+”—very low, “++”—low, “+++”—moderate, “++++”—high,” +++++”—intense, “nd”—no data.
Schematics of the rat and human brains with the expression of receptor proteins in outlined areas are also provided (as shown in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8), where the differences in the intensity of the expression of receptors are schematically visualized. These figures were constructed to show differences in the expression of individual receptors in different structures.
Comparisons of the intensity of receptor expression with the antipsychotic efficacy of ligands activating these receptors clearly show that the activity of the ligands does not necessarily correspond with the intensity of receptor expression in relevant structures. Therefore, orthosteric agonists or PAMs of mGlu4 receptors exhibit excellent activity in animal models of schizophrenia [130,236], but these receptors are expressed at the lowest levels in the cortex and hippocampus compared to other brain areas [209,232]. Instead, the high expression of mGlu4 receptors in the globus pallidus, where it is a heteroreceptor on GABAergic terminals, makes it a good target for anti-Parkinson drugs [237]. However, stimulation of these receptors may increase the risk of adverse effects on non-Parkinson patients. Much lower doses of mGlu4 PAMs/orthosteric agonists were active in animal models of schizophrenia than in models of Parkinson’s disease [237]. Therefore, the risk of inducing adverse effects during antipsychotic treatment appears to be relatively low.
The extensive expression of GABAB and mGlu5 receptors in cortical structures and the hippocampal formation [187,190] and their lower expression in deeper brain structures positively correlate with the activity of their ligands in animal models of schizophrenia and exclusively support the use of these receptors as targets for antipsychotic drugs. The functional connection of mGlu5 with NMDA receptors increases the risk of inducing adverse effects with activation of mGlu5 receptors, but biased ligands may be a solution [53].
Despite the initial hopes for mGlu2 receptors as antipsychotic drug targets, their expression in the cortex and hippocampus is relatively low [204].
The high expression of muscarinic receptors in structures related to schizophrenia arousal makes them excellent antipsychotic drug targets [163,238], and the efficacy of compounds activating these receptors was confirmed in animal models [152,169]. Of the three analyzed receptors, M1 was expressed at the highest levels.
The direct stimulation of post- or presynaptic sites results in the regulation of a particular neuron, which subsequently affects the neurons it innervates. The mechanisms engaged in the stabilization of inhibitory-excitatory balance in the CNS that are responsible for the antipsychotic effects of compounds are schematically shown in Figure 9.
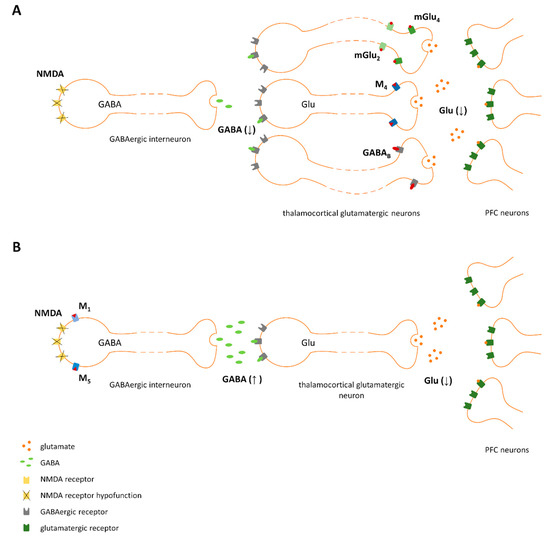
Figure 9.
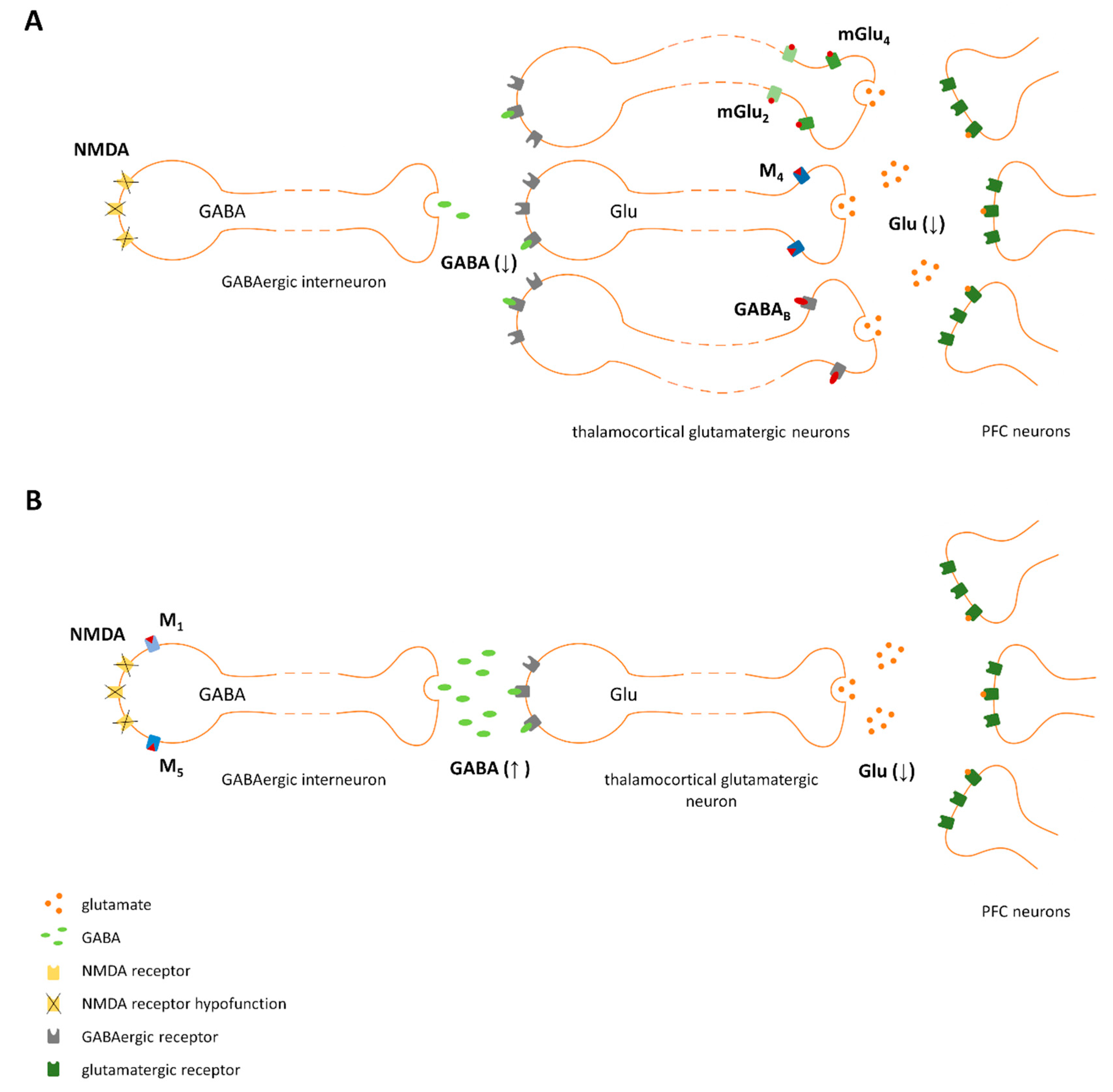
Proposed mechanism of action of ligands activating pre- (A) and postsynaptic receptors (B). NMDA receptor hypofunction results in decreased GABA release from GABAergic interneurons, which leads to disinhibition of thalamocortical glutamatergic neurons and increased glutamate release in the prefrontal cortex (PFC). A reduction in excess glutamate release in the PFC could be achieved directly (A) by the activation of presynaptic receptors expressed on thalamocortical glutamatergic terminals. (e.g., mGlu2, mGlu4, M4, or GABAB) or indirectly (B) by stimulating GABA release via the activation of postsynaptic receptors expressed on GABAergic interneurons (e.g., mGlu5, M1, or M5).
The aim of successive psychotropic treatment is to maintain homeostatic balance in the brain. Due to the extraordinary complexity of the central nervous system and its sensitivity to external factors, the precision and sensitivity of pharmacological manipulations must be considered to avoid adverse effects due to the unnecessary effects on the neuronal pathways responsible for other brain activities and functions.
4. Strategies Based on Bidirectional Inhibition of Glutamate Release
The individual differences between subjects, the complexity of microcircuits that regulate basic processes and the expression of receptors within these microcircuits have not been fully recognized in patients with schizophrenia and may determine the effectiveness and safety of treatment. Although several studies and clinical trials have been conducted, the treatment of negative and cognitive symptoms of schizophrenia remains unsatisfactory. Extensive research has been performed to develop new solutions, but spectacular success is lacking.
Exclusive stimulation of the receptors expressed in neuronal circuits involved in the pathophysiology of schizophrenia, without effects on dopaminergic neurotransmission and/or NMDA receptor-mediated signaling, should minimize the risk of adverse effects and improve the effectiveness of therapy. Our recent studies proposed a treatment based on the simultaneous stimulation of two receptors that are crucial for regulation of glutamatergic networks, and the results have been published [138,152,169,170,236,239]. In these studies, select combinations activating mGlu2/M1, mGlu2/M5, and mGlu4/M4 were not shown to alter prolactin levels or locomotor activity [152,170], prompting us to speculate that the use of sub-effective doses of at least two ligands may be safer than the highest dose of each compound alone or in combination with D2-based drugs [169,170].
The studies were performed using ligands that activate the receptors described in the first part of this review, e.g., muscarinic M1, M4 and M5, GABAB and metabotropic glutamate receptors (mGlu2, mGlu4 and mGlu5 receptors). Different combinations of ligands were used, and their efficacies were investigated by performing a vast range of tests in rodents that reflected the positive, negative, and cognitive symptoms of schizophrenia (Table 6).

Table 6.
Tests used to assess the antipsychotic activity of investigated ligands in rodents.
4.1. Simultaneous Administration of Ligands Activating Receptors Associated with Adenyl Cyclase Activity
The investigated combinations of ligands and their efficacies in animal models are shown in Table 7. The best working pair of compounds with evident efficacy in models of the positive, negative, and cognitive symptoms of schizophrenia were ligands that activated mGlu4/M4 receptors and mGlu2/M4 receptors (although these drugs were not tested in the models of positive symptoms) [152,239]. The simultaneous activation of GABAB receptors with mGlu4 or M4 receptors was not effective in models of negative symptoms and/or cognitive decline [169,236], and thus these combinations are less attractive for the reversal of negative and cognitive symptoms. However, the simultaneous activation of GABAB/M4 or mGlu4 receptors may be safer and more effective in patients with positive symptoms because the treatment of positive symptoms using current neuroleptic drugs carries a high risk of adverse effects.

Table 7.
Efficacy of the investigated combinations of ligands in tests assessing antipsychotic activity in rodents: “+”—compounds reversed the induced disruptions, “−/+”—compounds showed a trend toward reversing the induced disruptions, and “−”—compounds had no effect on the induced disruptions.
The synergistic effects of ligands with affinity for two different presynaptically located receptors may result from several factors:
The receptors are localized on one axon terminal, putatively a glutamatergic terminal. The concomitant stimulation results in the inhibition of glutamate release, and the ligands may complement the action of the other ligand. The receptors may act separately or through heterodimer formation (for a detailed description, see Section 4.1.1)
The receptors are localized on different nerve endings that innervate one brain area and/or several different structures. The receptors may complement the action of the other in that area, as shown in Figure 9.
4.1.1. Heterodimerization
As mentioned above, G protein-coupled receptors are known to form homo- and heteromeric structures. In the physiological state, mGlu receptors function as homodimers composed of two identical subunits, and each subunit may both bind the ligand and activate G-protein signaling (for a review see: Wieronska et al., 2016 [51]). The GABAB receptor functions as a heterodimer composed of two subunits, GABAB1 and GABAB2. The subunits depend on each other, i.e., GABAB1 binds the ligand and GABAB2 activates the signal transduction pathway [240].
According to numerous reports, G protein-coupled receptors may form heterodimers or oligomers with the same or other types of receptors, indicating strong multiple interactions between two or more receptors [241,242,243,244,245,246,247]. mGlu2-5-HT2A heterodimerization is one of the most important pathways implicated in schizophrenia [248,249,250]. Other forms of heterocomplexes in relation to schizophrenia have also been described, such as the mGlu5/D2/A2A oligomer [251,252]. Recently, mGlu2/mGlu4 heterodimers were described [253,254,255,256]. Therefore, the possible heterodimeric or oligomeric interactions of mGlu and muscarinic receptors are open for investigation and may possibly be implicated in the pathophysiology and treatment of schizophrenia.
4.2. Simultaneous Administration of Ligands Activating Receptors Associated with Adenyl Cyclase and the Inositol Phosphate Signaling Pathway
As shown in Table 8, the activity of the combined administration of sub-effective doses of an allosteric agonist of M1 or PAM of M5 receptors with sub-effective doses of PAMs of mGlu2 or GABAB receptors was observed in models of the cognitive symptoms of schizophrenia, but not in the models of positive symptoms [170]. No activity of the allosteric ligands of M1 or M5 receptors was observed in models of negative symptoms of schizophrenia [170]. Therefore, their combinations with ligands activating mGlu2 or GABAB receptors were not tested.

Table 8.
Efficacy of investigated combinations of ligands in tests assessing antipsychotic activity in rodents: “+”—compounds reversed the induced disruptions and “−”—compounds had no effect on the induced disruptions.
The costimulation of GABAB-mGlu5 receptors exhibited clear and evident efficacy in models of the positive, negative and cognitive symptoms of schizophrenia, which were comparable to the effects of the active dose of each ligand administered alone [138].
The expression of the receptors supports different mechanisms of the synergistic effects than the presynaptically expressed receptors.
Most likely, the postsynaptic receptors mGlu5, M1, and M5 are expressed on GABAergic neuron somata and dendrites [147,257,258,259], which enhances GABAergic inhibitory currents, and this activation indirectly counteracts GABAergic dysfunction due to NMDA hypofunction.
As indicated above, the activation of mGlu2 or GABAB receptors inhibits glutamate release. Therefore, the dual action involves an increase in the inhibition on the one hand and the inhibition of excitation on the other hand, which restores brain homeostasis.
5. Conclusions
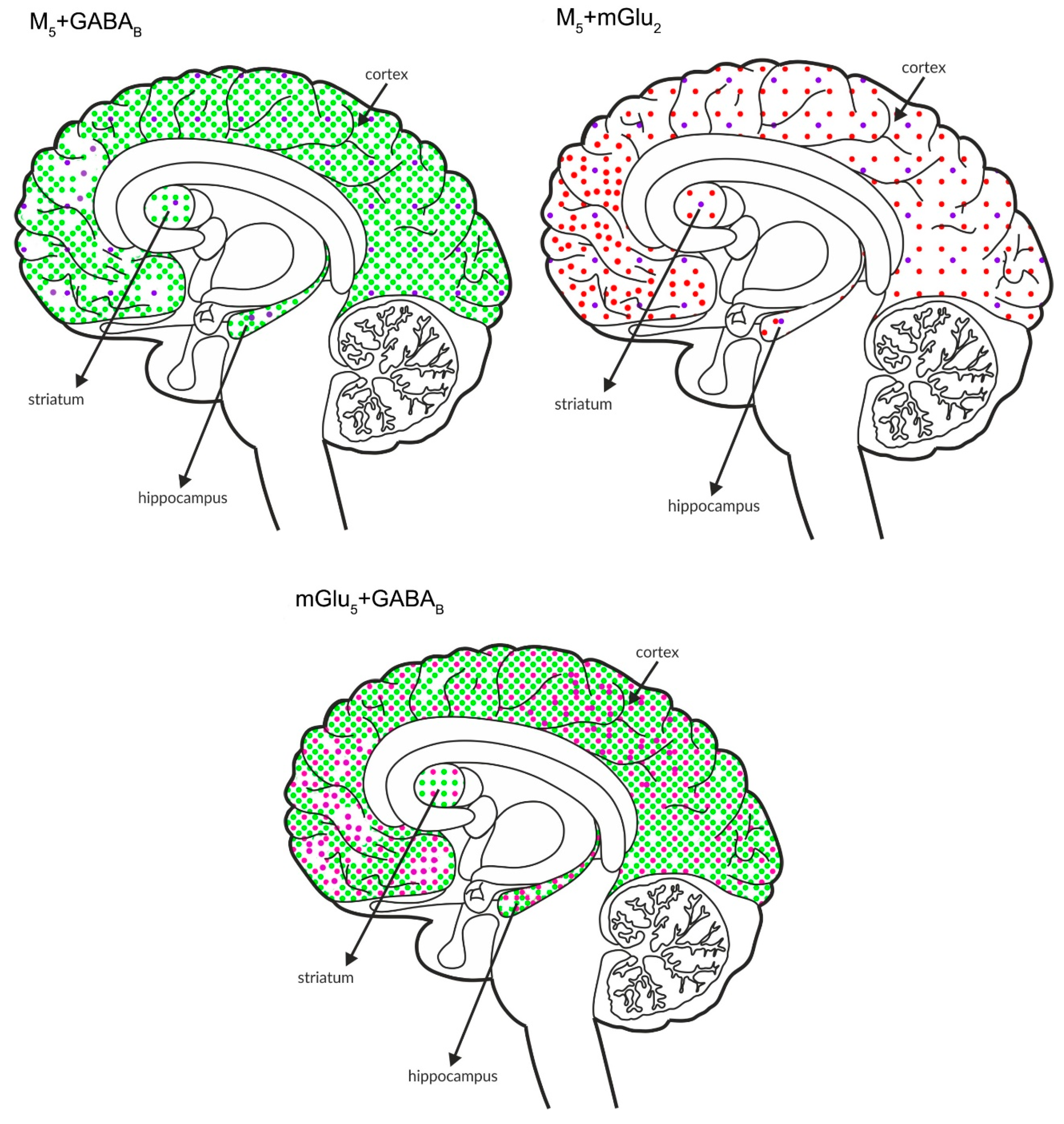
The figures shown below (Figure 10 and Figure 11) schematically illustrate the coexistence of particular types of receptors in select structures.
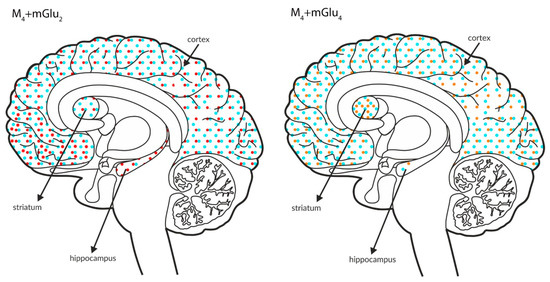
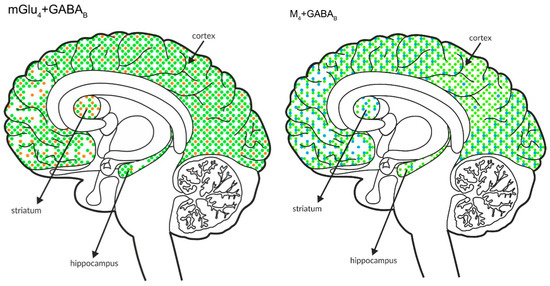
Figure 10.
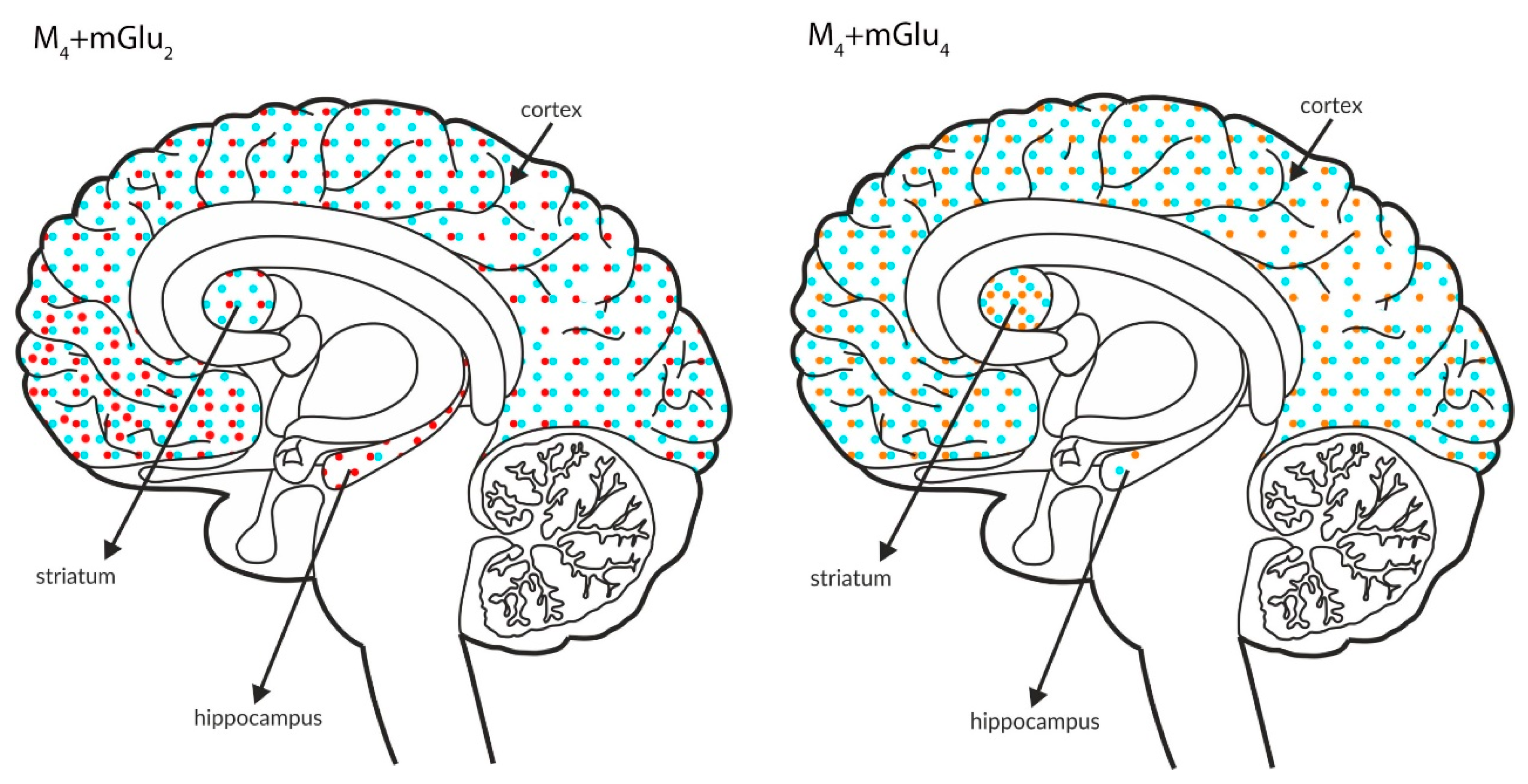
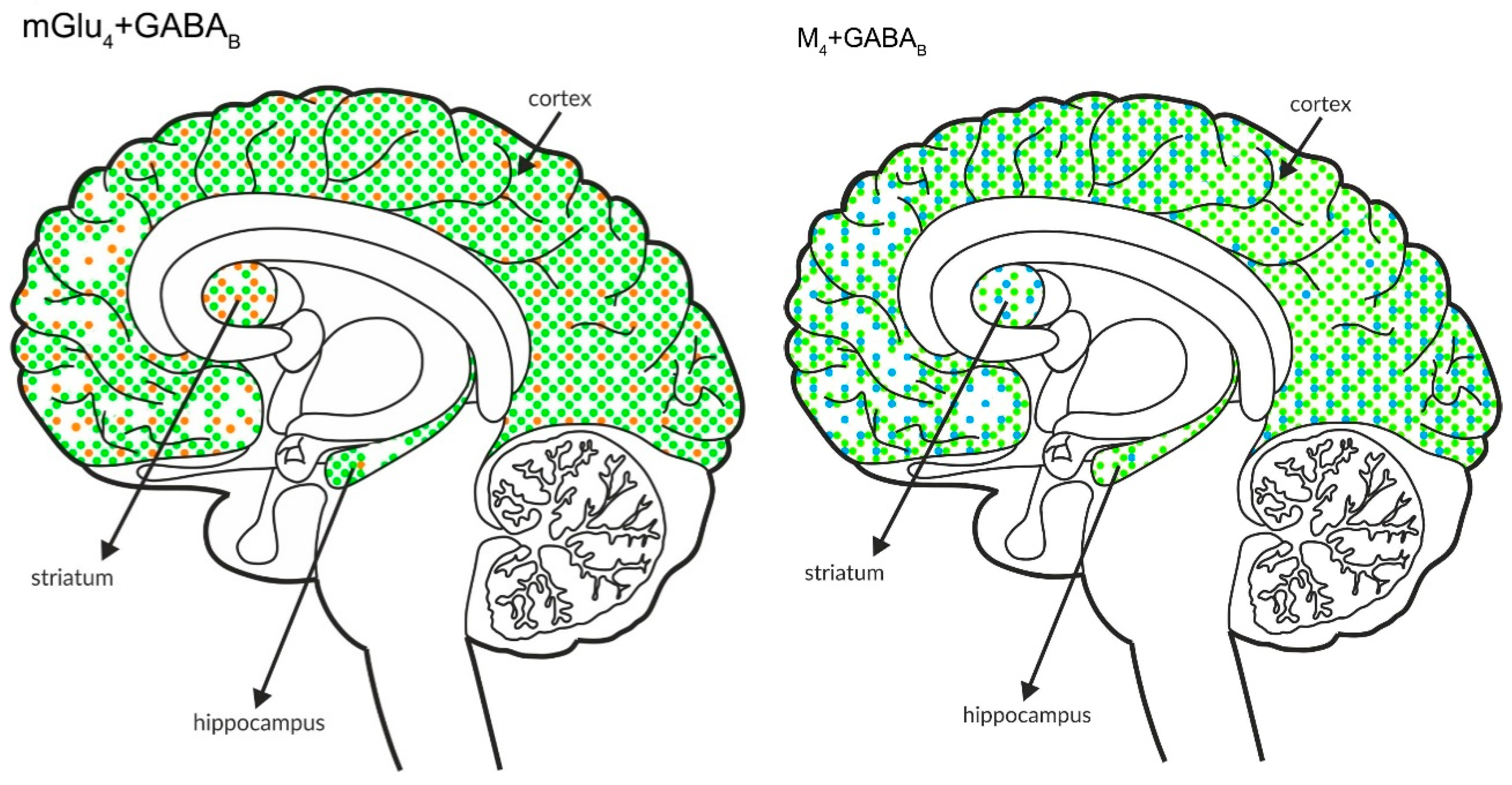
Simultaneous presynaptic effects on glutamate release. The coexpression of M4 receptors with mGlu2, GABAB or mGlu4 and mGlu4 with GABAB receptors in the cortex, hippocampus, and striatum of the human brain. M4 receptors are shown in light blue (•), mGlu2 is shown in red (•), mGlu4 is shown in orange (•), and GABAB is shown in neon green (•).
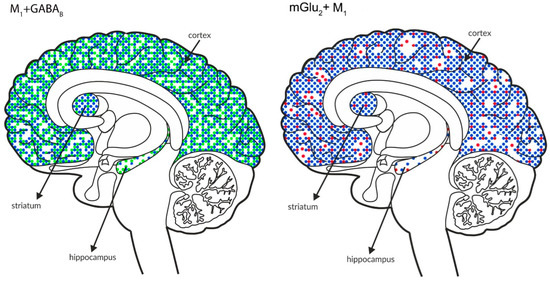
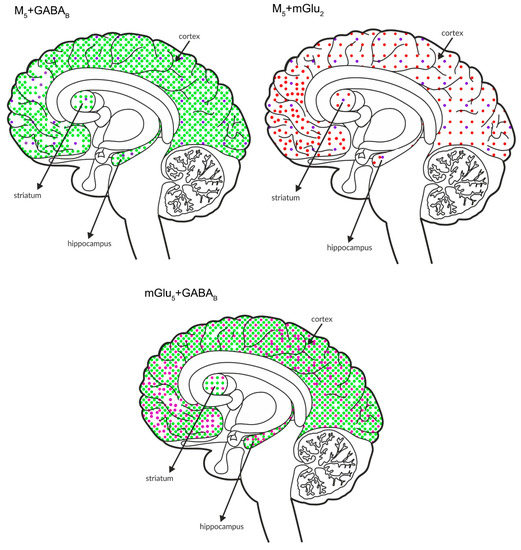
Figure 11.
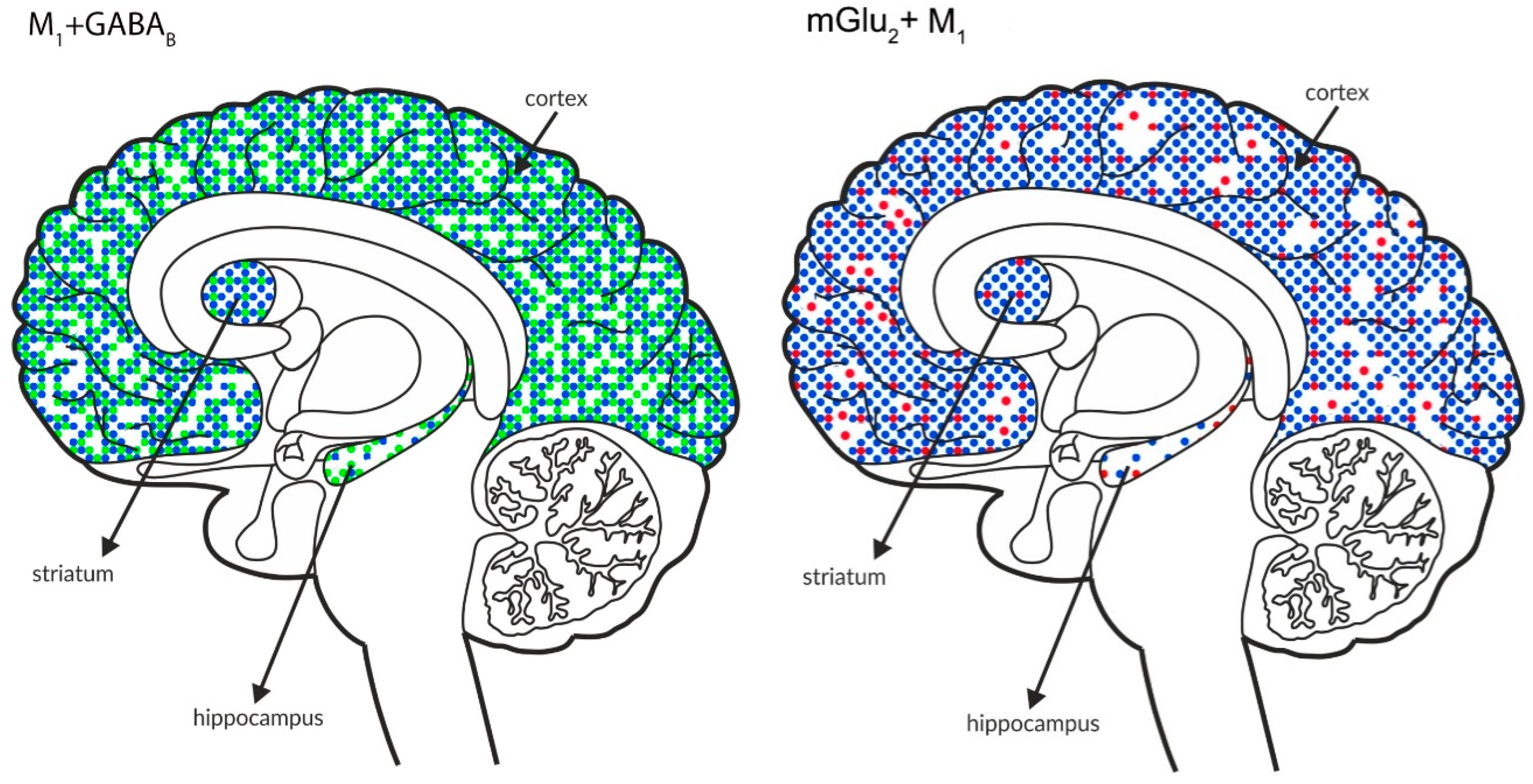
Simultaneous pre- and postsynaptic effects on glutamate release. The coexpression of M1 receptors with GABAB and mGlu2, M5 receptors with GABAB and mGlu2 receptors and mGlu5 receptors with GABAB receptors in the cortex, hippocampus, and striatum of the human brain. M1 receptors are shown in navy blue (•), M5 receptors are shown in violet (•), mGlu2 is shown in red (•), GABAB is shown in neon green (•), and mGlu5 is shown in neon pink (•).
The benefits and advantages of the combined activation of two selected receptors are sufficient to support the use of this approach in the treatment of schizophrenia.
Neither of the proposed treatments are based on the inhibition of dopaminergic receptors. Therefore, it may be speculated that the treatments are less burdened with the induction of adverse effects such as motor coordination and prolactin levels that are typical for presently used typical and second-generation neuroleptics. Preliminary experimental results supporting such conclusions can be found in Cieslik et al. 2018, Cieslik et al. 2019, and Cieslik et al. 2020 [152,169,170].
The results presented in the studies by Cieslik et al. 2018; 2020 indicate that the combined administration of the highest doses of the compounds or the administration of the highest dose of one compound with a subactive dose of the other does not produce additive effects [152,170]. Thus, the dosage does not need to be increased, and subsequently, the risk of unnecessary exposure to a treatment to obtain a therapeutic effect is relatively low. This finding might indicate the limited risk of unexpected events or toxic effects due to the accidental administration of a double dose of medications, which is particularly important for the mGlu4 or GABAB receptor. As stated above, the mGlu4 receptor, which is expressed in striatopallidal pathways, is considered an antiparkinsonian target [46,260]. The overstimulation of the receptor in these brain areas may result in undesired effects that counteract the putative antipsychotic efficacy. On the other hand, overstimulation of the GABAB receptor may exert adverse effects, such as sedation [139,140,261].
Analyses of the figures show overlap in the expression of particular receptors in select brain areas. The activation of receptors that are expressed at lower levels, such as mGlu2 or mGlu4, together with other types of receptors that are expressed at higher levels may complement the efficacy of the other receptor.
Overall conclusions obtained from the results discussed above and the consequences of the simultaneous administration of two compounds are as follows:
- −
- the dose of each compound may be reduced and the antipsychotic-like efficacy is the same as the highest dose of each compound administered alone (this approach may potentially allow us to avoid putative adverse effects or unnecessary exposure of the prodrug to patients, as shown previously for mGlu2/3 agonists);
- −
- the action of the combined treatment might be selective in specific areas and thus may target a specific group of symptoms;
- −
- the ligands administered in combinations may complement the action of the other ligand and compensate for possible receptor dysfunctions, activating both homodimers and heterodimers/heterocomplexes.
Funding
This work was supported by Statutory Funds from the Maj Institute of Pharmacology Polish Academy of Sciences in Krakow, Poland and by the grant no 2015/17/B/NZ7/02984 (OPUS) awarded to Joanna M Wierońska.
Conflicts of Interest
The authors have no conflict of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Tandon, R.; Gaebel, W.; Barch, D.M.; Bustillo, J.; Gur, R.E.; Heckers, S.; Malaspina, D.; Owen, M.J.; Schultz, S.; Tsuang, M.; et al. Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 2013, 150, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Braff, D.L.; Ryan, J.; Rissling, A.J.; Carpenter, W.T. Lack of use in the literature from the last 20 years supports dropping traditional schizophrenia subtypes from DSM-5 ICD-11. Schizophr. Bull. 2013, 39, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Mcglashan, T.H.; Fenton, W.S. Classical subtypes for schizophrenia: Literature review for DSM-IV. Schizophr. Bull. 1991, 17, 609–632. [Google Scholar] [CrossRef] [PubMed]
- Jauhar, S.; McKenna, P.J.; Radua, J.; Fung, E.; Salvador, R.; Laws, K.R. Cognitive-behavioural therapy for the symptoms of schizophrenia: Systematic review and meta-analysis with examination of potential bias. Br. J. Psychiatry 2014, 204, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.; Williams, C.; Billings, J.; Johnson, S. A systematic review and meta-analysis of cognitive behavioural informed psychological interventions for psychiatric inpatients with psychosis. Schizophr. Res. 2020, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D.A.; Moore, H.; Stott, L.; Holliday, N.; Javitch, J.A.; Robert Lane, J.; Charlton, S.J. Extrapyramidal side effects of antipsychotics are linked to their association kinetics at dopamine D2 receptors. Nat. Commun. 2017, 8, 763. [Google Scholar] [CrossRef]
- Seeman, P.; Lee, T.; Chau-Wong, M.; Wong, K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 1976, 261, 717–719. [Google Scholar] [CrossRef]
- Kapur, S.; Mamo, D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 1081–1090. [Google Scholar] [CrossRef]
- Aringhieri, S.; Carli, M.; Kolachalam, S.; Verdesca, V.; Cini, E.; Rossi, M.; McCormick, P.J.; Corsini, G.U.; Maggio, R.; Scarselli, M. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol. Ther. 2018, 192, 20–41. [Google Scholar] [CrossRef]
- Aringhieri, S.; Kolachalam, S.; Gerace, C.; Carli, M.; Verdesca, V.; Brunacci, M.G.; Rossi, C.; Ippolito, C.; Solini, A.; Corsini, G.U.; et al. Clozapine as the most efficacious antipsychotic for activating ERK 1/2 kinases: Role of 5-HT2A receptor agonism. Eur. Neuropsychopharmacol. 2017, 27, 383–398. [Google Scholar] [CrossRef]
- Schmid, C.L.; Streicher, J.M.; Meltzer, H.Y.; Bohn, L.M. Clozapine acts as an agonist at serotonin 2A receptors to counter MK-801-induced behaviors through a βarrestin2-independent activation of akt. Neuropsychopharmacology 2014, 39, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Corves, C.; Arbter, D.; Engel, R.R.; Li, C.; Davis, J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: A meta-analysis. Lancet 2009, 373, 31–41. [Google Scholar] [CrossRef]
- Campden-Main, B.C.; Wegielski, Z. The Control of Deviant Behavior in Chronically Disturbed Psychotic Patients by the Oral Administration of Reserpine. Ann. N. Y. Acad. Sci. 1955, 61, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kinross-Wright, V. Chlorpromazine and Reserpine in the Treatment of Psychoses. Ann. N. Y. Acad. Sci. 1955, 61, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.; McCutcheon, R.; Stone, J. Glutamate and dopamine in schizophrenia: An update for the 21st century. J. Psychopharmacol. 2015, 29, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Kapur, S. A neurobiological hypothesis for the classification of schizophrenia: Type a (hyperdopaminergic) and type b (normodopaminergic). Br. J. Psychiatry 2014, 205, 1–3. [Google Scholar] [CrossRef]
- Reynolds, G.P. The pharmacogenetics of symptom response to Antipsychotic drugs. Psychiatry Investig. 2012, 9, 1–7. [Google Scholar] [CrossRef]
- Stern, S.; Linker, S.; Vadodaria, K.C.; Marchetto, M.C.; Gage, F.H. Prediction of response to drug therapy in psychiatric disorders. Open Biol. 2018, 8, 180031. [Google Scholar] [CrossRef]
- Pouget, J.G.; Shams, T.A.; Tiwari, A.K.; Müller, D.J. Pharmacogenetics and outcome with antipsychotic drugs. Dialogues Clin. Neurosci. 2014, 16, 555–566. [Google Scholar]
- Luby, E.; Cohen, B.; Rosenbaum, G.; Gottlieb, J.S.; Kelley, R. Study Schizophrenomimetic Drug Sernyl. Arch. Neurol. Psychiatr. 1959, 81, 363–369. [Google Scholar] [CrossRef]
- Javitt, D.C.; Zukin, S.R. Recent Advances in the Phencyclidine Model of Schizophrenia. Am. J. Psychiatry 1991, 148, 1301–1308. [Google Scholar] [PubMed]
- Krystal, J.H.; Karper, L.P.; Seibyl, J.P.; Freeman, G.K.; Delaney, R.; Bremner, J.D.; Heninger, G.R.; Bowers, M.B.; Charney, D.S. Subanesthetic Effects of the Noncompetitive NMDA Antagonist, Ketamine, in Humans: Psychotomimetic, Perceptual, Cognitive, and Neuroendocrine Responses. Arch. Gen. Psychiatry 1994, 51, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Domino, E.F.; Luby, E.D. Phencyclidine/schizophrenia: One view toward the past, the other to the future. Schizophr. Bull. 2012, 38, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J. Clin. Psychiatry 1987, 9, 12–35. [Google Scholar] [PubMed]
- Conn, P.J.; Lindsley, C.W.; Jones, C.K. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol. Sci. 2009, 30, 25–31. [Google Scholar] [CrossRef]
- Coyle, J.T. Glutamate and Schizophrenia: Beyond the Dopamine Hypothesis. Cell. Mol. Neurobiol. 2006, 26, 4–6. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs 2011, 25, 859–885. [Google Scholar] [CrossRef]
- Coyle, J.T.; Tsai, G. The NMDA receptor glycine modulatory site: A therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology 2004, 174, 38. [Google Scholar] [CrossRef]
- Duncan, E.J.; Szilagyi, S.; Schwartz, M.P.; Bugarski-Kirola, D.; Kunzova, A.; Negi, S.; Stephanides, M.; Efferen, T.R.; Angrist, B.; Peselow, E.; et al. Effects of D-cycloserine on negative symptoms in schizophrenia. Schizophr. Res. 2004, 71, 239–248. [Google Scholar] [CrossRef]
- Evins, A.E.; Fitzgerald, S.M.; Wine, L.; Rosselli, R.; Goff, D.C. Placebo-controlled trial of glycine added to clozapine in schizophrenia. Am. J. Psychiatry 2000, 157, 826–828. [Google Scholar] [CrossRef]
- Goff, D.C.; Cather, C.; Gottlieb, J.D.; Evins, A.E.; Walsh, J.; Raeke, L.; Otto, M.W.; Schoenfeld, D.; Green, M.F. Once-weekly d-cycloserine effects on negative symptoms and cognition in schizophrenia: An exploratory study. Schizophr. Res. 2008, 106, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Heresco-Levy, U.; Javitt, D.C. Comparative effects of glycine and D-cycloserine on persistent negative symptoms in schizophrenia: A retrospective analysis. Schizophr. Res. 2004, 66, 89–96. [Google Scholar] [CrossRef]
- Javitt, D.C. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr. Opin. Psychiatry 2006, 19, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lane, H.Y.; Tseng, P.T.; Chen, S.J.; Liu, C.Y.; Lin, C.H. Effect of N-methyl-D-aspartate-receptor-enhancing agents on cognition in patients with schizophrenia: A systematic review and meta-analysis of double-blind randomised controlled trials. J. Psychopharmacol. 2019, 33, 436–448. [Google Scholar] [CrossRef]
- Chaki, S. Group II metabotropic glutamate receptor agonists as a potential drug for schizophrenia. Eur. J. Pharmacol. 2010, 639, 59–66. [Google Scholar] [CrossRef]
- Lesage, A.; Steckler, T. Metabotropic glutamate mGlu1 receptor stimulation and blockade: Therapeutic opportunities in psychiatric illness. Eur. J. Pharmacol. 2010, 639, 2–16. [Google Scholar] [CrossRef]
- Marek, G.J. Metabotropic glutamate 2/3 (mGlu2/3) receptors, schizophrenia and cognition. Eur. J. Pharmacol. 2010, 639, 81–90. [Google Scholar] [CrossRef]
- Yasuhara, A.; Chaki, S. Metabotropic glutamate receptors: Potential drug targets for psychiatric disorders. Open Med. Chem. J. 2010, 4, 20–36. [Google Scholar] [CrossRef]
- Chaki, S.; Hikichi, H. Targeting of Metabotropic Glutamate Receptors for the Treatment of Schizophrenia. Curr. Pharm. Des. 2011, 17, 94–102. [Google Scholar] [CrossRef]
- Gregory, K.J.; Dong, E.N.; Meiler, J.; Conn, P.J. Allosteric modulation of metabotropic glutamate receptors: Structural insights and therapeutic potential. Neuropharmacology 2011, 60, 66–81. [Google Scholar] [CrossRef]
- Nicoletti, F.; Bockaert, J.; Collingridge, G.L.; Conn, P.J.; Ferraguti, F.; Schoepp, D.D.; Wroblewski, J.T.; Pin, J.P. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology 2011, 60, 1017–1041. [Google Scholar] [CrossRef] [PubMed]
- Sheffler, D.J.; Gregory, K.J.; Rook, J.M.; Conn, P.J. Allosteric Modulation of Metabotropic Glutamate Receptors. Adv. Pharmacol. 2011, 62, 37–77. [Google Scholar] [PubMed]
- Fell, M.J.; McKinzie, D.L.; Monn, J.A.; Svensson, K.A. Group II metabotropic glutamate receptor agonists and positive allosteric modulators as novel treatments for schizophrenia. Neuropharmacology 2012, 62, 1473–1483. [Google Scholar] [CrossRef]
- Vinson, P.N.; Conn, P.J. Metabotropic glutamate receptors as therapeutic targets for schizophrenia. Neuropharmacology 2012, 62, 1461–1472. [Google Scholar] [CrossRef]
- Gregory, K.J.; Noetzel, M.J.; Niswender, C.M. Pharmacology of metabotropic glutamate receptor allosteric modulators: Structural basis and therapeutic potential for CNS disorders. Prog. Mol. Biol. Transl. Sci. 2013, 115, 61–121. [Google Scholar]
- Nickols, H.; Conn, P.J. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol. Dis. 2014, 61, 55–71. [Google Scholar] [CrossRef]
- Li, M.-L.; Hu, X.-Q.; Li, F.; Gao, W.-J. Perspectives on the mGluR2/3 agonists as a therapeutic target for schizophrenia: Still promising or a dead end? Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 60, 66–76. [Google Scholar] [CrossRef]
- Golubeva, A.V.; Moloney, R.D.; O’Connor, R.M.; Dinan, T.G.; Cryan, J.F. Metabotropic Glutamate Receptors in Central Nervous System Diseases. Curr. Drug Targets 2016, 17, 538–616. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.G.; Conn, P.J. Group I and group II metabotropic glutamate receptor allosteric modulators as novel potential antipsychotics. Curr. Opin. Pharmacol. 2015, 20, 40–45. [Google Scholar] [CrossRef]
- Muguruza, C.; Meana, J.J.; Callado, L.F. Group II metabotropic glutamate receptors as targets for novel antipsychotic drugs. Front. Pharmacol. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Wierońska, J.M.; Zorn, S.H.; Doller, D.; Pilc, A. Metabotropic glutamate receptors as targets for new antipsychotic drugs: Historical perspective and critical comparative assessment. Pharmacol. Ther. 2016, 157, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.J.; Conn, P.J. Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron 2017, 94, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Maksymetz, J.; Moran, S.P.; Conn, P.J. Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Mol. Brain 2017, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Orlando, R.; Menna, L.D.; Cannella, M.; Notartomaso, S.; Mascio, G.; Iacovelli, L.; Matrisciano, F.; Fazio, F.; Caraci, F.; et al. Targeting mGlu receptors for optimization of antipsychotic activity and disease-modifying effect in schizophrenia. Front. Psychiatry 2019, 10, 49. [Google Scholar] [CrossRef]
- Stansley, B.J.; Conn, P.J. Neuropharmacological Insight from Allosteric Modulation of mGlu Receptors. Trends Pharmacol. Sci. 2019, 40, 240–252. [Google Scholar] [CrossRef]
- Kinon, B.J.; Zhang, L.; Millen, B.A.; Osuntokun, O.O.; Williams, J.E.; Kollack-Walker, S.; Jackson, K.; Kryzhanovskaya, L.; Jarkova, N. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J. Clin. Psychopharmacol. 2011, 31, 349–355. [Google Scholar] [CrossRef]
- Patil, S.T.; Zhang, L.; Martenyi, F.; Lowe, S.L.; Jackson, K.A.L.; Andreev, B.V.; Avedisova, A.S.; Bardenstein, L.M.; Gurovich, I.Y.; Morozova, M.A.; et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: A randomized Phase 2 clinical trial. Nat. Med. 2007, 13, 1102–1107. [Google Scholar] [CrossRef]
- Erdely, H.A.; Tamminga, C.A.; Roberts, R.C.; Vogel, M.W. Regional alterations in RGS4 protein in Schizophrenia. Synapse 2006, 59, 472–479. [Google Scholar] [CrossRef]
- Bowden, N.A.; Scott, R.J.; Tooney, P.A. Altered expression of regulator of G-protein signalling 4 (RGS4) mRNA in the superior temporal gyrus in schizophrenia. Schizophr. Res. 2007, 89, 165–168. [Google Scholar] [CrossRef]
- Ding, L.; Hegde, A.N. Expression of RGS4 Splice Variants in Dorsolateral Prefrontal Cortex of Schizophrenic and Bipolar Disorder Patients. Biol. Psychiatry 2009, 65, 541–545. [Google Scholar] [CrossRef]
- Mirnics, K.; Middleton, F.A.; Stanwood, G.D.; Lewis, D.A.; Levitt, P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol. Psychiatry 2001, 6, 293–301. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.; Zheng, B.; Fischer, T.; Elenko, E.; Farquhar, M.G. The regulator of G protein signaling family. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 235–271. [Google Scholar] [CrossRef] [PubMed]
- Schwendt, M.; Sigmon, S.A.; McGinty, J.F. RGS4 overexpression in the rat dorsal striatum modulates mGluR5- and amphetamine-mediated behavior and signaling. Psychopharmacology 2012, 221, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Jung, S.; Son, H.; Kim, S.; Choi, J.; Lee, D.H.; Roh, G.S.; Kang, S.S.; Cho, G.J.; Choi, W.S.; et al. The GABAB receptor associates with regulators of G-protein signaling 4 protein in the mouse prefrontal cortex and hypothalamus. BMB Rep. 2014, 47, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Guzman, J.N.; Tkatch, T.; Chen, S.; Goldberg, J.A.; Ebert, P.J.; Levitt, P.; Wilson, C.J.; Hamm, H.E.; Surmeier, D.J. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat. Neurosci. 2006, 9, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Carbon, M.; Correll, C.U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 2014, 16, 505–524. [Google Scholar]
- Potkin, S.G.; Kane, J.M.; Correll, C.U.; Lindenmayer, J.P.; Agid, O.; Marder, S.R.; Olfson, M.; Howes, O.D. The neurobiology of treatment-resistant schizophrenia: Paths to antipsychotic resistance and a roadmap for future research. npj Schizophr. 2020, 6, 1. [Google Scholar] [CrossRef]
- Dean, B.; Crook, J.M.; Opeskin, K.; Hill, C.; Keks, N.; Copolov, D.L. The density of muscarinic M1 receptors is decreased in the caudate-putamen of subjects with schizophrenia. Mol. Psychiatry 1996, 1, 54–58. [Google Scholar]
- Crook, J.M.; Tomaskovic-Crook, E.; Copolov, D.L.; Dean, B. Decreased muscarinic receptor binding in subjects with schizophrenia: A study of the human hippocampal formation. Biol Psychiatry 2000, 48, 381–388. [Google Scholar] [CrossRef]
- Dean, B.; McLeod, M.; Keriakous, D.; McKenzie, J.; Scarr, E. Decreased muscarinic 1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 2002, 7, 1083–1091. [Google Scholar] [CrossRef]
- Crook, J.M.; Tomaskovic-Crook, E.; Copolov, D.L.; Dean, B. Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: A study of brodmann’s areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am. J. Psychiatry 2001, 158, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Zavitsanou, K.; Katsifis, A.; Mattner, F.; Huang, X.-F. Investigation of M1/M4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology 2004, 29, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Huang, X.F. Decreased density of muscarinic receptors in the superior temporal gyrus in schizophrenia. J. Neurosci. Res. 2005, 81, 883–890. [Google Scholar] [CrossRef]
- Newell, K.A.; Zavitsanou, K.; Jew, S.K.; Huang, X.-F. Alterations of muscarinic and GABA receptor binding in the posterior cingulate cortex in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 225–233. [Google Scholar] [CrossRef]
- Scarr, E.; Sundram, S.; Keriakous, D.; Dean, B. Altered Hippocampal Muscarinic M4, but Not M1, Receptor Expression from Subjects with Schizophrenia. Biol. Psychiatry 2007, 61, 1161–1170. [Google Scholar] [CrossRef]
- Dean, B.; Soulby, A.; Evin, G.M.; Scarr, E. Levels of [3H]pirenzepine binding in Brodmann’s area 6 from subjects with schizophrenia is not associated with changes in the transcription factor SP1 or BACE1. Schizophr. Res. 2008, 106, 229–236. [Google Scholar] [CrossRef]
- Dean, B.; Crook, J.M.; Pavey, G.; Opeskin, K.; Copolov, D.L. Muscarinic 1 and 2 receptor mRNA in the human caudate-putamen: No change in m1 mRNA in schizophrenia. Mol. Psychiatry 2000, 5, 203–207. [Google Scholar] [CrossRef]
- Mancama, D.; Arranz, M.J.; Landau, S.; Kerwin, R. Reduced expression of the muscarinic 1 receptor cortical subtype in schizophrenia. Am. J. Med. Genet. Neuropsychiatr. Genet. 2003, 119B, 2–6. [Google Scholar] [CrossRef]
- Dean, B.; Gray, L.; Keriakous, D.; Scarr, E. A comparison of M1 and M4 muscarinic receptors in the thalamus from control subjects and subjects with schizophrenia. Thalamus Relat. Syst. 2004, 2, 287–295. [Google Scholar] [CrossRef]
- Scarr, E.; Hopper, S.; Vos, V.; Suk Seo, M.; Everall, I.P.; Aumann, T.D.; Chana, G.; Dean, B. Low levels of muscarinic M1 receptor–positive neurons in cortical layers III and V in Brodmann areas 9 and 17 from individuals with schizophrenia. J. Psychiatry Neurosci. 2018, 43, 338–346. [Google Scholar] [CrossRef]
- Zavitsanou, K.; Katsifis, A.; Yu, Y.; Huang, X.F. M2/M4 muscarinic receptor binding in the anterior cingulate cortex in schizophrenia and mood disorders. Brain Res. Bull. 2005, 65, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, K.; Sasaki, M.; Ishikawa, M.; Iwakiri, M.; Hidaka, S.; Shiraishi, H.; Iritani, S. Immunohistochemical localization of gamma-aminobutyric acid(B) receptor in the hippocampus of subjects with schizophrenia. Neurosci. Lett. 2000, 283, 101–104. [Google Scholar] [CrossRef]
- Mizukami, K.; Ishikawa, M.; Hidaka, S.; Iwakiri, M.; Sasaki, M.; Iritani, S. Immunohistochemical localization of GABA B receptor in the entorhinal cortex and inferior temporal cortex of schizophrenic brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 393–396. [Google Scholar] [CrossRef]
- Ishikawa, M.; Mizukami, K.; Iwakiri, M.; Asada, T. Immunohistochemical and immunoblot analysis of γ-aminobutyric acid B receptor in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Neurosci. Lett. 2005, 383, 272–277. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D.; Thuras, P.D. Deficits in GABA(B) receptor system in schizophrenia and mood disorders: A postmortem study. Schizophr. Res. 2011, 128, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Folsom, T.D.; Thuras, P.D. GABAA and GABAB receptor dysregulation in superior frontal cortex of subjects with schizophrenia and bipolar disorder. Synapse 2017, 71, e21973. [Google Scholar] [CrossRef]
- Matosin, N.; Frank, E.; Deng, C.; Huang, X.F.; Newell, K.A. Metabotropic glutamate receptor 5 binding and protein expression in schizophrenia and following antipsychotic drug treatment. Schizophr. Res. 2013, 146, 1–3. [Google Scholar] [CrossRef]
- Matosin, N.; Fernandez-Enright, F.; Frank, E.; Deng, C.; Wong, J.; Huang, X.F.; Newell, K.A. Metabotropic glutamate receptor mGluR2/3 and mGluR5 binding in the anterior cingulate cortex in psychotic and nonpsychotic depression, bipolar disorder and schizophrenia: Implications for novel mGluR-based therapeutics. J. Psychiatry Neurosci. 2014, 39, 407–416. [Google Scholar] [CrossRef]
- Ohnuma, T.; Augood, S.J.; Arai, H.; McKenna, P.J.; Emson, P.C. Expression of the human excitatory amino acid transporter 2 and metabotropic glutamate receptors 3 and 5 in the prefrontal cortex from normal individuals and patients with schizophrenia. Mol. Brain Res. 1998, 56, 207–217. [Google Scholar] [CrossRef]
- Ohnuma, T.; Tessler, S.; Arai, H.; Faull, R.L.M.; McKenna, P.J.; Emson, P.C. Gene expression of metabotropic glutamate receptor 5 and excitatory amino acid transporter 2 in the schizophrenic hippocampus. Mol. Brain Res. 2000, 85, 24–31. [Google Scholar] [CrossRef]
- Richardson-Burns, S.M.; Haroutunian, V.; Davis, K.L.; Watson, S.J.; Meador-Woodruff, J.H. Metabotropic glutamate receptor mRNA expression in the schizophrenic thalamus. Biol. Psychiatry 2000, 47, 22–28. [Google Scholar] [CrossRef]
- Gupta, D.S.; McCullumsmith, R.E.; Beneyto, M.; Haroutunian, V.; Davis, K.L.; Meador-Woodruff, J.H. Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse 2005, 57, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Xuereb, J.H.; Crepaldi, L.; Corsi, M.; Michielin, F.; Ferraguti, F. Altered levels of glutamatergic receptors and Na+/K+ ATPase-α1 in the prefrontal cortex of subjects with schizophrenia. Schizophr. Res. 2011, 128, 7–14. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D.; Rooney, R.J.; Thuras, P.D. MRNA and protein expression for novel GABA A receptors θ and ρ2 are altered in schizophrenia and mood disorders; Relevance to FMRP-mGluR5 signaling pathway. Transl. Psychiatry 2013, 3, e271. [Google Scholar] [CrossRef] [PubMed]
- Matosin, N.; Fernandez-Enright, F.; Fung, S.J.; Lum, J.S.; Engel, M.; Andrews, J.L.; Huang, X.F.; Weickert, C.S.; Newell, K.A. Alterations of mGluR5 and its endogenous regulators Norbin, Tamalin and Preso1 in schizophrenia: Towards a model of mGluR5 dysregulation. Acta Neuropathol. 2015, 130, 119–129. [Google Scholar] [CrossRef]
- Volk, D.W.; Eggan, S.M.; Lewis, D.A. Alterations in metabotropic glutamate receptor 1α and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. Am. J. Psychiatry 2010, 167, 1489–1498. [Google Scholar] [CrossRef]
- Parkin, G.M.; Gibbons, A.; Udawela, M.; Dean, B. Excitatory amino acid transporter (EAAT)1 and EAAT2 mRNA levels are altered in the prefrontal cortex of subjects with schizophrenia. J. Psychiatr. Res. 2020, 123, 151–158. [Google Scholar] [CrossRef]
- McOmish, C.E.; Pavey, G.; Gibbons, A.; Hopper, S.; Udawela, M.; Scarr, E.; Dean, B. Lower [3H]LY341495 binding to mGlu2/3 receptors in the anterior cingulate of subjects with major depressive disorder but not bipolar disorder or schizophrenia. J. Affect. Disord. 2016, 190, 241–248. [Google Scholar] [CrossRef]
- Frank, E.; Newell, K.A.; Huang, X.-F. Density of metabotropic glutamate receptors 2 and 3 (mGluR2/3) in the dorsolateral prefrontal cortex does not differ with schizophrenia diagnosis but decreases with age. Schizophr. Res. 2011, 128, 56–60. [Google Scholar] [CrossRef]
- Crook, J.M.; Akil, M.; Law, B.C.W.; Hyde, T.M.; Kleinman, J.E. Comparative analysis of group II metabotropic glutamate receptor immunoreactivity in Brodmann’s area 46 of the dorsolateral prefrontal cortex from patients with schizophrenia and normal subjects. Mol. Psychiatry 2002, 7, 157–164. [Google Scholar] [CrossRef][Green Version]
- Eastwood, S.L.; McDonald, B.; Burnet, P.W.J.; Beckwith, J.P.; Kerwin, R.W.; Harrison, P.J. Decreased expression of mRNAs encoding non-NMDA glutamate receptors GluRl and GluR2 in medial temporal lobe neurons in schizophrenia. Mol. Brain Res. 1995, 29, 211–223. [Google Scholar] [CrossRef]
- Ghose, S.; Crook, J.M.; Bartus, C.L.; Sherman, T.G.; Herman, M.M.; Hyde, T.M.; Kleinman, J.E.; Akil, M. Metabotropic glutamate receptor 2 and 3 gene expression in the human prefrontal cortex and mesencephalon in schizophrenia. Int. J. Neurosci. 2008, 118, 1609–1627. [Google Scholar] [CrossRef][Green Version]
- Ghose, S.; Gleason, K.A.; Potts, B.W.; Lewis-Amezcua, K.; Tamminga, C.A. Differential expression of metabotropic glutamate receptors 2 and 3 in schizophrenia: A mechanism for antipsychotic drug action? Am. J. Psychiatry 2009, 166, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, A. Transport and metabolism of glutamate and gaba in neurons and glial cells. Int. Rev. Neurobiol. 1981, 22, 1–45. [Google Scholar] [PubMed]
- Conn, P.J.; Pin, J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.L.; Sachdeva, S.; Stahl, S.M. Glutamate neurocircuitry: Theoretical underpinnings in: Schizophrenia. Front. Pharmacol. 2012, 3, 195. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Waters, N.; Waters, S.; Carlsson, M.L. Network interactions in schizophrenia - Therapeutic implications. Brain Res. Rev. 2000, 31, 342–349. [Google Scholar] [CrossRef]
- Kehrer, C.; Maziashvili, N.; Dugladze, T.; Gloveli, T. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front. Mol. Neurosci. 2008, 1, 6. [Google Scholar] [CrossRef]
- Sohal, V.S.; Zhang, F.; Yizhar, O.; Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009, 459, 698–702. [Google Scholar] [CrossRef]
- Cartmell, J.; Schoepp, D.D. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 2000, 75, 889–907. [Google Scholar] [CrossRef]
- Schoepp, D.D.; Jane, D.E.; Monn, J.A. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology 1999, 38, 1431–1476. [Google Scholar] [CrossRef]
- Moussawi, K.; Riegel, A.; Nair, S.; Kalivas, P.W.; Bargas, J.; Nacional, U. Extracellular glutamate: Functional compartments operate in different concentration ranges. Front. Syst. Neurosci. 2011, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Pin, J.-P.; Duvoisin, R. The metabotropic glutamate receptors: Structure and functions. Neuropharmacology 1995, 34, 1–26. [Google Scholar] [CrossRef]
- Neki, A.; Ohishi, H.; Kaneko, T.; Shigemoto, R.; Nakanishi, S.; Mizuno, N. Pre- and postsynaptic localization of a metabotropic glutamate receptor, mGluR2, in the rat brain: An immunohistochemical study with a monoclonal antibody. Neurosci. Lett. 1996, 202, 197–200. [Google Scholar] [CrossRef]
- Weinberger, D.R. Schizophrenia drug says goodbye to dopamine. Nat. Med. 2007, 13, 1018–1019. [Google Scholar] [CrossRef] [PubMed]
- Connell, P.H. Amphetamine Psychosis. Maudsley Monographs, No. 5.; Oxford University Press: London, UK, 1958. [Google Scholar]
- Kinon, B.J.; Millen, B.A.; Zhang, L.; McKinzie, D.L. Exploratory Analysis for a Targeted Patient Population Responsive to the Metabotropic Glutamate 2/3 Receptor Agonist Pomaglumetad Methionil in Schizophrenia. Biol. Psychiatry 2015, 78, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Nisenbaum, L.K.; Downing, A.C.M.; Zhao, F.; Millen, B.A.; Munsie, L.; Kinon, B.J.; Adams, D.H.; Gomez, J.C.; Penny, M.A. Serotonin 2A Receptor SNP rs7330461 association with treatment response to pomaglumetad methionil in patients with schizophrenia. J. Pers. Med. 2016, 6, 9. [Google Scholar] [CrossRef]
- Bueno, A.B.; Collado, I.; De Dios, A.; Domínguez, C.; Martín, J.A.; Martín, L.M.; Martínez-Grau, M.A.; Montero, C.; Pedregal, C.; Catlow, J.; et al. Dipeptides as effective prodrugs of the unnatural amino acid (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740), a selective group II metabotropic glutamate receptor agonist. J. Med. Chem. 2005, 48, 5305–5320. [Google Scholar] [CrossRef]
- Urabe, H.; Miyakoshi, N.; Ohtake, N.; Nozoe, A.; Ochi, M.; Fukasawa, M.; Kinoshita, K.; Yamaguchi, J.; Marumo, T.; Hikichi, H.; et al. Discovery of MGS0274, an ester prodrug of a metabotropic glutamate receptor 2/3 agonist with improved oral bioavailability. Eur. J. Med. Chem. 2020, 203, 112521. [Google Scholar] [CrossRef]
- Annes, W.F.; Long, A.; Witcher, J.W.; Ayan-Oshodi, M.A.; Knadler, M.P.; Zhang, W.; Mitchell, M.I.; Cornelissen, K.; Hall, S.D. Relative contributions of presystemic and systemic peptidases to oral exposure of a novel metabotropic glutamate 2/3 receptor agonist (LY404039) after oral administration of prodrug Pomaglumetad methionil (LY2140023). J. Pharm. Sci. 2015, 104, 207–214. [Google Scholar] [CrossRef]
- Mezler, M.; Geneste, H.; Gault, L.; Marek, G.J. LY-2140023, a prodrug of the group II metabotropic glutamate receptor agonist LY-404039 for the potential treatment of schizophrenia. Curr. Opin. Investig. Drugs 2010, 11, 833–845. [Google Scholar] [PubMed]
- Kinoshita, K.; Ochi, M.; Iwata, K.; Fukasawa, M.; Yamaguchi, J. ichi Preclinical disposition of MGS0274 besylate, a prodrug of a potent group II metabotropic glutamate receptor agonist MGS0008 for the treatment of schizophrenia. Pharmacol. Res. Perspect. 2019, 7, e00520. [Google Scholar] [CrossRef]
- Watanabe, M.; Marcy, B.; Kinoshita, K.; Fukasawa, M.; Hikichi, H.; Chaki, S.; Okuyama, S.; Gevorkyan, H.; Yoshida, S. Safety and pharmacokinetic profiles of MGS0274 besylate (TS-134), a novel metabotropic glutamate 2/3 receptor agonist prodrug, in healthy subjects. Br. J. Clin. Pharmacol. 2020, 86, 2286–2301. [Google Scholar] [CrossRef] [PubMed]
- Bocchio, M.; Lukacs, I.P.; Stacey, R.; Plaha, P.; Apostolopoulos, V.; Livermore, L.; Sen, A.; Ansorge, O.; Gillies, M.J.; Somogyi, P.; et al. Group II metabotropic glutamate receptors mediate presynaptic inhibition of excitatory transmission in pyramidal neurons of the human cerebral cortex. Front. Cell. Neurosci. 2019, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Gereau IV, R.W.; Conn, P.J. Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J. Neurosci. 1995, 15, 6879–6889. [Google Scholar] [CrossRef]
- Jones, P.J.; Xiang, Z.; Conn, P.J. Metabotropic glutamate receptors mGluR4 and mGluR8 regulate transmission in the lateral olfactory tract-piriform cortex synapse. Neuropharmacology 2008, 55, 440–446. [Google Scholar] [CrossRef][Green Version]
- Thomsen, C.; Pekhletski, R.; Haldeman, B.; Gilbert, T.A.; O’Hara, P.; Hampson, D.R. Cloning and characterization of a metabotropic glutamate receptor, mGluR4b. Neuropharmacology 1997, 36, 21–30. [Google Scholar] [CrossRef]
- Woźniak, M.; Gołembiowska, K.; Noworyta-Sokołowska, K.; Acher, F.; Cieślik, P.; Kusek, M.; Tokarski, K.; Pilc, A.; Wierońska, J.M. Neurochemical and behavioral studies on the 5-HT1A-dependent antipsychotic action of the mGlu4 receptor agonist LSP4-2022. Neuropharmacology 2017, 115, 149–165. [Google Scholar] [CrossRef]
- Sławińska, A.; Wierońska, J.M.; Stachowicz, K.; Marciniak, M.; Łasoń-Tyburkiewicz, M.; Gruca, P.; Papp, M.; Kusek, M.; Tokarski, K.; Doller, D.; et al. The antipsychotic-like effects of positive allosteric modulators of metabotropic glutamate mGlu4 receptors in rodents. Br. J. Pharmacol. 2013, 169, 1824–1839. [Google Scholar] [CrossRef] [PubMed]
- Klodzinska, A.; Bijak, M.; Tokarski, K.; Pilc, A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol. Biochem. Behav. 2002, 73, 327–332. [Google Scholar] [CrossRef]
- Schulz, H.L.; Stohr, H.; Weber, B.H.F. Characterization of three novel isoforms of the metabotrobic glutamate receptor 7 (GRM7). Neurosci. Lett. 2002, 326, 37–40. [Google Scholar] [CrossRef]
- Malherbe, P.; Kratzeisen, C.; Lundstrom, K.; Richards, J.G.; Faull, R.L.M.; Mutel, V. Cloning and functional expression of alternative spliced variants of the human metabotropic glutamate receptor 8. Mol. Brain Res. 1999, 67, 201–210. [Google Scholar] [CrossRef]
- Wierońska, J.M.; Stachowicz, K.; Acher, F.; Lech, T.; Pilc, A. Opposing efficacy of group III mGlu receptor activators, LSP1-2111 and AMN082, in animal models of positive symptoms of schizophrenia. Psychopharmacology 2012, 220, 481–494. [Google Scholar]
- Cieślik, P.; Woźniak, M.; Kaczorowska, K.; Brański, P.; Burnat, G.; Chocyk, A.; Bobula, B.; Gruca, P.; Litwa, E.; Pałucha-Poniewiera, A.; et al. Negative Allosteric Modulators of mGlu7 Receptor as Putative Antipsychotic Drugs. Front. Mol. Neurosci. 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Shigemoto, R.; Ohishi, H.; Mizuno, N. Immunohistochemical Localization of Metabotropic Glutamate Receptors, mGluR7a and mGluR7b, in the Central Nervous System of the Adult Rat and Mouse: A Light and Electron Microscopic Study. J. Comp. Neurol. 1998, 352, 332–352. [Google Scholar] [CrossRef]
- Wierońska, J.M.; Kusek, M.; Tokarski, K.; Wabno, J.; Froestl, W.; Pilc, A. The GABA B receptor agonist CGP44532 and the positive modulator GS39783 reverse some behavioural changes related to positive syndromes of psychosis in mice. Br. J. Pharmacol. 2011, 163, 1034–1047. [Google Scholar] [CrossRef]
- Wierońska, J.M.; Kłeczek, N.; Woźniak, M.; Gruca, P.; Łasoń-Tyburkiewicz, M.; Papp, M.; Brański, P.; Burnat, G.; Pilc, A. mGlu5-GABAB interplay in animal models of positive, negative and cognitive symptoms of schizophrenia. Neurochem. Int. 2015, 88, 97–109. [Google Scholar] [CrossRef]
- May, C.R. Baclofen overdose. Ann. Emerg. Med. 1983, 12, 171–173. [Google Scholar] [CrossRef]
- Garabedian-Ruffalo, S.M.; Ruffalo, R.L. Adverse effects secondary to baclofen withdrawal. Drug Intell. Clin. Pharm. 1985, 19, 304–306. [Google Scholar] [CrossRef]
- Shekhar, A.; Potter, W.Z.; Lightfoot, J.; Lienemann, J.; Dubé, S.; Mallinckrodt, C.; Bymaster, F.P.; McKinzie, D.L.; Felder, C.C. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 2008, 165, 1033–1039. [Google Scholar] [CrossRef]
- Mirza, N.R.; Peters, D.; Sparks, R.G. Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists. CNS Drug Rev. 2003, 9, 159–186. [Google Scholar] [CrossRef] [PubMed]
- Melancon, B.J.; Tarr, J.C.; Panarese, J.D.; Wood, M.R.; Lindsley, C.W. Allosteric modulation of the M1 muscarinic acetylcholine receptor: Improving cognition and a potential treatment for schizophrenia and Alzheimer’s disease. Drug Discov. Today 2013, 18, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Pancani, T.; Foster, D.J.; Moehle, M.S.; Bichell, T.J.; Bradley, E.; Bridges, T.M.; Klar, R.; Poslusney, M.; Rook, J.M.; Daniels, J.S.; et al. Allosteric activation of M4 muscarinic receptors improve behavioral and physiological alterations in early symptomatic YAC128 mice. Proc. Natl. Acad. Sci. USA 2015, 112, 14078–14083. [Google Scholar] [CrossRef]
- Rouse, S.T.; Marino, M.J.; Potter, L.T.; Conn, P.J.; Levey, A.I. Muscarinic receptor subtypes involved in hippocampal circuits. Life Sci. 1999, 64, 501–509. [Google Scholar] [CrossRef]
- Klawonn, A.M.; Wilhelms, D.B.; Lindström, S.H.; Singh, A.K.; Jaarola, M.; Wess, J.; Fritz, M.; Engblom, D. Muscarinic M4 receptors on cholinergic and dopamine D1 receptor-expressing neurons have opposing functionality for positive reinforcement and influence impulsivity. Front. Mol. Neurosci. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Thorn, C.A.; Popiolek, M.; Stark, E.; Edgerton, J.R. Effects of M1 and M4 activation on excitatory synaptic transmission in CA1. Hippocampus 2017, 27, 794–810. [Google Scholar] [CrossRef]
- Dasari, S.; Gulledge, A.T. M1 and M4 Receptors Modulate Hippocampal Pyramidal Neurons. J. Neurophysiol. 2011, 105, 779–792. [Google Scholar] [CrossRef]
- Radnikow, G.; Feldmeyer, D. Layer- and cell type-specific modulation of excitatory neuronal activity in the neocortex. Front. Neuroanat. 2018, 12, 1–16. [Google Scholar] [CrossRef]
- Yang, D.; Günter, R.; Qi, G.; Radnikow, G.; Feldmeyer, D. Muscarinic and Nicotinic Modulation of Neocortical Layer 6A Synaptic Microcircuits Is Cooperative and Cell-Specific. Cereb. Cortex 2020, 30, 3528–3542. [Google Scholar] [CrossRef]
- Amar, M.; Lucas-Meunier, E.; Baux, G.; Fossier, P. Blockade of different muscarinic receptor subtypes changes the equilibrium between excitation and inhibition in rat visual cortex. Neuroscience 2010, 169, 1610–1620. [Google Scholar] [CrossRef]
- Cieślik, P.; Woźniak, M.; Rook, J.M.; Tantawy, M.N.; Conn, P.J.; Acher, F.; Tokarski, K.; Kusek, M.; Pilc, A.; Wierońska, J.M. Mutual activation of glutamatergic mGlu4 and muscarinic M4 receptors reverses schizophrenia-related changes in rodents. Psychopharmacology 2018, 235, 2897–2913. [Google Scholar]
- Malherbe, P.; Kew, J.N.C.; Richards, J.G.; Knoflach, F.; Kratzeisen, C.; Zenner, M.T.; Faull, R.L.M.; Kemp, J.A.; Mutel, V. Identification and characterization of a novel splice variant of the metabotropic glutamate receptor 5 gene in human hippocampus and cerebellum. Mol. Brain Res. 2002, 109, 168–178. [Google Scholar] [CrossRef]
- Tu, J.C.; Xiao, B.; Naisbitt, S.; Yuan, J.P.; Petralia, R.S.; Brakeman, P.; Doan, A.; Aakalu, V.K.; Lanahan, A.A.; Sheng, M.; et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 1999, 23, 583–592. [Google Scholar] [CrossRef]
- Doherty, A.J.; Palmer, M.J.; Henley, J.M.; Collingridge, G.L.; Jane, D.E. (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology 1997, 36, 265–267. [Google Scholar] [CrossRef]
- Attucci, S.; Carlá, V.; Mannaioni, G.; Moroni, F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br. J. Pharmacol. 2001, 132, 799–806. [Google Scholar] [CrossRef]
- Mannaioni, G.; Marino, M.J.; Valenti, O.; Traynelis, S.F.; Conn, P.J. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J. Neurosci. 2001, 21, 5925–5934. [Google Scholar] [CrossRef] [PubMed]
- Rook, J.M.; Xiang, Z.; Lv, X.; Ghoshal, A.; Dickerson, J.W.; Bridges, T.M.; Johnson, K.A.; Foster, D.J.; Gregory, K.J.; Vinson, P.N.; et al. Biased mGlu5-Positive Allosteric Modulators Provide InVivo Efficacy without Potentiating mGlu5 Modulation of NMDAR Currents. Neuron 2015, 86, 1029–1040. [Google Scholar] [CrossRef]
- Romano, C.; Sesma, M.A.; McDonald, C.T.; O’malley, K.; van den Pol, A.N.; Olney, J.W. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol. 1995, 355, 455–469. [Google Scholar] [CrossRef]
- Bragina, L.; Bonifacino, T.; Bassi, S.; Milanese, M.; Bonanno, G.; Conti, F. Differential expression of metabotropic glutamate and GABA receptors at neocortical glutamatergic and GABAergic axon terminals. Front. Cell. Neurosci. 2015, 9, 345. [Google Scholar] [CrossRef]
- Levey, A.I.; Edmunds, S.M.; Koliatsos, V.; Wiley, R.G.; Heilman, C.J. Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 4077–4092. [Google Scholar] [CrossRef]
- Levey, A.I.; Kitt, C.A.; Simonds, W.F.; Price, D.L.; Brann, M.R. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci. Off. J. Soc. Neurosci. 1991, 11, 3218–3226. [Google Scholar] [CrossRef]
- Wei, J.; Walton, E.A.; Milici, A.; Buccafusco, J.J. m1-m5 Muscarinic Receptor Distribution in Rat CNS by RT-PCR and HPLC. J. Neurochem. 1994, 63, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, R.P.; Ciesla, W.; Flores, L.R.; Wall, S.J.; Li, M.; Satkus, S.A.; Weisstein, J.S.; Spagnola, B.V.; Wolfe, B.B. Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: Distribution of m4 and m5 receptors in rat brain. Mol. Pharmacol. 1993, 43, 149–157. [Google Scholar]
- Hamilton, S.E.; Nathanson, N.M. The M1 Receptor is Required for Muscarinic Activation of Mitogen-activated Protein (MAP) Kinase in Murine Cerebral Cortical Neurons. J. Biol. Chem. 2001, 276, 15850–15853. [Google Scholar] [CrossRef] [PubMed]
- Anagnostaras, S.G.; Murphy, G.G.; Hamilton, S.E.; Mitchell, S.L.; Rahnama, N.P.; Nathanson, N.M.; Silva, A.J. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat. Neurosci. 2003, 6, 51–58. [Google Scholar] [CrossRef]
- Miyakawa, T.; Yamada, M.; Duttaroy, A.; Wess, J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J. Neurosci. 2001, 21, 5239–5250. [Google Scholar] [CrossRef]
- Gerber, D.J.; Sotnikova, T.D.; Gainetdinov, R.R.; Huang, S.Y.; Caron, M.G.; Tonegawa, S. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 15312–15317. [Google Scholar] [CrossRef]
- Cieślik, P.; Woźniak, M.; Tokarski, K.; Kusek, M.; Pilc, A.; Płoska, A.; Radulska, A.; Pelikant-Małecka, I.; Żołnowska, B.; Sławiński, J.; et al. Simultaneous activation of muscarinic and GABA B receptors as a bidirectional target for novel antipsychotics. Behav. Brain Res. 2019, 359, 671–685. [Google Scholar] [CrossRef]
- Cieślik, P.; Domin, H.; Chocyk, A.; Gruca, P.; Litwa, E.; Płoska, A.; Radulska, A.; Pelikant-Małecka, I.; Brański, P.; Kalinowski, L.; et al. Simultaneous activation of mGlu2 and muscarinic receptors reverses MK-801-induced cognitive decline in rodents. Neuropharmacology 2020, 174, 107866. [Google Scholar] [CrossRef]
- Weiner, D.M.; Levey, A.I.; Brann, M.R. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc. Natl. Acad. Sci. USA 1990, 87, 7050–7054. [Google Scholar] [CrossRef]
- Araya, R.; Noguchi, T.; Yuhki, M.; Kitamura, N.; Higuchi, M.; Saido, T.C.; Seki, K.; Itohara, S.; Kawano, M.; Tanemura, K.; et al. Loss of M5 muscarinic acetylcholine receptors leads to cerebrovascular and neuronal abnormalities and cognitive deficits in mice. Neurobiol. Dis. 2006, 24, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Bymaster, F.P.; McKinzie, D.L.; Felder, C.C.; Wess, J. M1-M5 muscarinic receptor knockout mice as novel tools to study the physiological roles of the muscarinic cholinergic system. Neurochem. Res. 2003, 28, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.J.; Gentry, P.R.; Lizardi-Ortiz, J.E.; Bridges, T.M.; Wood, M.R.; Niswender, C.M.; Sulzer, D.; Lindsley, C.W.; Xiang, Z.; Conn, P.J. M5 Receptor Activation Produces Opposing Physiological Outcomes in Dopamine Neurons Depending on the Receptor’s Location. J. Neurosci. 2014, 34, 3253–3262. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Lamping, K.G.; Duttaroy, A.; Zhang, W.; Cui, Y.; Bymaster, F.P.; McKinzie, D.L.; Felder, C.C.; Deng, C.X.; Faraci, F.M.; et al. Cholinergic dilation of cerebral blood vessels is abolished in M5 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA 2001, 98, 14096–14101. [Google Scholar] [CrossRef]
- Thomsen, M.; Wörtwein, G.; Fink-Jensen, A.; Woldbye, D.P.D.; Wess, J.; Caine, S.B. Decreased prepulse inhibition and increased sensitivity to muscarinic, but not dopaminergic drugs in M5 muscarinic acetylcholine receptor knockout mice. Psychopharmacology 2007, 192, 97–110. [Google Scholar] [CrossRef]
- Rouse, S.T.; Levey, A.I. Expression of m1-m4 muscarinic acetylcholine receptor immunoreactivity in septohippocampal neurons and other identified hippocampal afferents. J. Comp. Neurol. 1996, 375, 406–416. [Google Scholar] [CrossRef]
- Oda, S.; Tsuneoka, Y.; Yoshida, S.; Adachi-Akahane, S.; Ito, M.; Kuroda, M.; Funato, H. Immunolocalization of muscarinic M1 receptor in the rat medial prefrontal cortex. J. Comp. Neurol. 2018, 526, 1329–1350. [Google Scholar] [CrossRef]
- Vilaró, M.T.; Wiederhold, K.H.; Palacios, J.M.; Mengod, G. Muscarinic cholinergic receptors in the rat caudate-putamen and olfactory tubercle belong predominantly to the m4 class: In situ hybridization and receptor autoradiography evidence. Neuroscience 1991, 159–167. [Google Scholar] [CrossRef]
- Roßner, S.; Kues, W.; Witzemann, V.; Schliebs, R. Laminar expression of m1-, m3- and m4-muscarinic cholinergic receptor genes in the developing rat visual cortex using in situ hybridization histochemistry. Effect of monocular visual deprivation. Int. J. Dev. Neurosci. 1993, 11, 369–378. [Google Scholar]
- Schliebs, R.; Rossner, S.; Kumar, A.; Bigl, V. Muscarinic acetylcholine receptor subtypes in rat visual cortex—A comparative study using quantitative receptor autoradiography and in situ hybridization. Indian J. Exp. Biol. 1994, 32, 25–30. [Google Scholar]
- Buckley, N.J.; Bonner, T.I.; Brann, M.R. Localization of a family of muscarinic receptor mRNAs in rat brain. J. Neurosci. 1988, 8, 4646–4652. [Google Scholar] [CrossRef] [PubMed]
- Brann, M.R.; Buckley, N.J.; Bonner, T.I. The striatum and cerebral cortex express different muscarinic receptor mRNAs. FEBS Lett. 1988, 230, 90–94. [Google Scholar] [CrossRef]
- Hersch, S.M.; Gutekunst, C.A.; Rees, H.D.; Heilman, C.J.; Levey, A.I. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: Light and electron microscopic immunocytochemistry using subtype-specific antibodies. J. Neurosci. 1994, 14, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Gomeza, J.; Zhang, L.; Kostenis, E.; Felder, C.; Bymaster, F.; Brodkin, J.; Shannon, H.; Xia, B.; Deng, C.; Wess, J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 10483–10488. [Google Scholar] [CrossRef] [PubMed]
- Vilaró, M.T.; Palacios, J.M.; Mengod, G. Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci. Lett. 1990, 114, 154–159. [Google Scholar] [CrossRef]
- Benke, D.; Honer, M.; Michel, C.; Bettler, B.; Mohler, H. γ-Aminobutyric acid type B receptor splice variant proteins GBR1a and GBR1b are both associated with GBR2 in situ and display differential regional and subcellular distribution. J. Biol. Chem. 1999, 274, 27323–27330. [Google Scholar] [CrossRef]
- Charles, K.J.; Evans, M.L.; Robbins, M.J.; Calver, A.R.; Leslie, R.A.; Pangalos, M.N. Comparative immunohistochemical localisation of GABAB1a, GABAB1b and GABAB2 subunits in rat brain, spinal cord and dorsal root ganglion. Neuroscience 2001, 106, 447–467. [Google Scholar] [CrossRef]
- Lu, X.Y.; Ghasemzadeh, M.B.; Kalivas, P.W. Regional distribution and cellular localization of γ-aminobutyric acid subtype 1 receptor mRNA in the rat brain. J. Comp. Neurol. 1999, 407, 166–182. [Google Scholar] [CrossRef]
- Durkin, M.M.; Gunwaldsen, C.A.; Borowsky, B.; Jones, K.A.; Branchek, T.A. An in situ hybridization study of the distribution of the GABA(B2) protein mRNA in the rat CNS. Mol. Brain Res. 1999, 71, 185–200. [Google Scholar] [CrossRef]
- Clark, J.A.; Mezey, É.; Lam, A.S.; Bonner, T.I. Distribution of the GABAB receptor subunit gb2 in rat CNS. Brain Res. 2000, 860, 41–52. [Google Scholar] [CrossRef]
- Calver, A.R.; Medhurst, A.D.; Robbins, M.J.; Charles, K.J.; Evans, M.L.; Harrison, D.C.; Stammers, M.; Hughes, S.A.; Hervieu, G.; Couve, A.; et al. The expression of GABA(B1) and GABA(B2) receptor subunits in the CNS differs from that in peripheral tissues. Neuroscience 2000, 100, 155–170. [Google Scholar] [CrossRef]
- Shigemoto, R.; Nomura, S.; Ohishi, H.; Sugihara, H.; Nakanishi, S.; Mizuno, N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci. Lett. 1993, 163, 53–57. [Google Scholar] [CrossRef]
- Fotuhi, M.; Standaert, D.G.; Testa, C.M.; Penney, J.B.; Young, A.B. Differential expression of metabotropic glutamate receptors in the hippocampus and entorhinal cortex of the rat. Mol. Brain Res. 1994, 21, 283–292. [Google Scholar] [CrossRef][Green Version]
- Kerner, J.A.; Standaert, D.G.; Penney, J.B.; Young, A.B.; Landwehrmeyer, G.B. Expression of group one metabotropic glutamate receptor subunit mRNAs in neurochemically identified neurons in the rat neostriatum, neocortex, and hippocampus. Mol. Brain Res. 1997, 48, 259–269. [Google Scholar] [CrossRef]
- Abe, T.; Sugihara, H.; Nawa, H.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J. Biol. Chem. 1992, 267, 13361–13368. [Google Scholar]
- Testa, C.M.; Standaert, D.G.; Young, A.B.; Penney, J.B. Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J. Neurosci. 1994, 14, 3005–3018. [Google Scholar] [CrossRef]
- Mutel, V.; Ellis, G.J.; Adam, G.; Chaboz, S.; Nilly, A.; Messer, J.; Bleuel, Z.; Metzler, V.; Malherbe, P.; Schlaeger, E.J.; et al. Characterization of [3H]quisqualate binding to recombinant rat metabotropic glutamate 1a and 5a receptors and to rat and human brain sections. J. Neurochem. 2000, 75, 2590–2601. [Google Scholar] [CrossRef]
- Tallaksen-Greene, S.J.; Kaatz, K.W.; Romano, C.; Albin, R.L. Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain Res. 1998, 780, 210–217. [Google Scholar] [CrossRef]
- Śmiałowska, M.; Wierońska, J.M.; Brański, P.; Obuchowicz, E.; Pilc, A. MPEP, mGLU5 receptor antagonist, regulates NPYmRNA expression in hippocampal and amygdalar neurons. Pol. J. Pharmacol. 2004, 56, 709–718. [Google Scholar]
- Testa, C.M.; Standaert, D.G.; Landwehrmeyer, G.B.; Penney, J.B.; Young, A.B. Differential expression of mGluR5 metabotropic glutamate receptor mRNA by rat striatal neurons. J. Comp. Neurol. 1995, 354, 241–252. [Google Scholar] [CrossRef]
- Shigemoto, R.; Kinoshita, A.; Wada, E.; Nomura, S.; Ohishi, H.; Takada, M.; Flor, P.J.; Neki, A.; Abe, T.; Nakanishi, S.; et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 1997, 17, 7503–7522. [Google Scholar] [CrossRef] [PubMed]
- Luján, R.; Nusser, Z.; Roberts, J.D.B.; Shigemoto, R.; Somogyi, P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci. 1996, 8, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, H.; Neki, A.; Mizuno, N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: An immunohistochemical study with a monoclonal antibody. Neurosci. Res. 1998, 30, 65–82. [Google Scholar] [CrossRef]
- Venkatadri, P.S.; Lee, C.C. Differential Expression of mGluR2 in the Developing Cerebral Cortex of the Mouse. J. Biomed. Sci. Eng. 2014, 7, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, H.; Shigemoto, R.; Nakanishi, S.; Mizuno, N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience 1993, 53, 1009–1018. [Google Scholar] [CrossRef]
- McOmish, C.E.; Demireva, E.Y.; Gingrich, J.A. Developmental expression of mGlu2 and mGlu3 in the mouse brain. Gene Expr. Patterns 2016, 22, 46–53. [Google Scholar] [CrossRef]
- Berthele, A.; Platzer, S.; Laurie, D.J.; Weis, S.; Sommer, B.; Zieglgänsberger, W.; Conrad, B.; Tölle, T.R. Expression of metabotropic glutamate receptor subtype mRNA (mGluR1-8) in human cerebellum. Neuroreport 1999, 10, 3861–3867. [Google Scholar] [CrossRef]
- Corti, C.; Aldegheri, L.; Somogyi, P.; Ferraguti, F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience 2002, 110, 403–420. [Google Scholar] [CrossRef]
- Kristensen, P.; Suzdak, P.D.; Thomsen, C. Expression pattern and pharmacology of the rat type IV metabotropic glutamate receptor. Neurosci. Lett. 1993, 155, 159–162. [Google Scholar] [CrossRef]
- Thomsen, C.; Kristensen, P.; Mulvihill, E.; Haldeman, B.; Suzdak, P.D. L-2-amino-4-phosphonobutyrate (L-AP4) is an agonist at the type iv metabotropic glutamate receptor which is negatively coupled to adenylate cyclase. Eur. J. Pharmacol. Mol. Pharmacol. 1992, 227, 361–362. [Google Scholar] [CrossRef]
- Ohishi, H.; Akazawa, C.; Shigemoto, R.; Nakanishi, S.; Mizuno, N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J. Comp. Neurol. 1995, 360, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Messenger, M.J.; Dawson, L.G.; Duty, S. Changes in metabotropic glutamate receptor 1-8 gene expression in the rodent basal ganglia motor loop following lesion of the nigrostriatal tract. Neuropharmacology 2002, 43, 261–271. [Google Scholar] [CrossRef]
- Tanabe, Y.; Nomura, A.; Masu, M.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J. Neurosci. 1993, 13, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Mateos, J.M.; Azkue, J.; Sarría, R.; Kuhn, R.; Grandes, P.; Knöpfel, T. Localization of the mGlu4a metabotropic glutamate receptor in rat cerebellar cortex. Histochem. Cell Biol. 1998, 109, 135–139. [Google Scholar] [CrossRef]
- Kinoshita, A.; Ohishi, H.; Nomura, S.; Shigemoto, R.; Nakanishi, S.; Mizuno, N. Presynaptic localization of a metabotropic glutamate receptor, mGluR4a, in the cerebellar cortex: A light and electron microscope study in the rat. Neurosci. Lett. 1996, 207, 199–202. [Google Scholar] [CrossRef]
- Wada, E.; Shigemoto, R.; Kinoshita, A.; Ohishi, H.; Mizuno, N. Metabotropic glutamate receptor subtypes in axon terminals of projection fibers from the main and accessory olfactory bulbs: A light and electron microscopic immunohistochemical study in the rat. J. Comp. Neurol. 1998, 393, 493–504. [Google Scholar] [CrossRef]
- Corti, C.; Restituito, S.; Rimland, J.M.; Brabet, I.; Corsi, M.; Pin, J.P.; Ferraguti, F. Cloning and characterization of alternative mRNA forms for the rat metabotropic glutamate receptors mGluR7 and mGluR8. Eur. J. Neurosci. 1998, 10, 3629–3641. [Google Scholar] [CrossRef]
- Okamoto, N.; Hori, S.; Akazawa, C.; Hayashi, Y.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J. Biol. Chem. 1994, 269, 1231–1236. [Google Scholar]
- Kinzie, J.M.; Saugstad, J.A.; Westbrook, G.L.; Segerson, T.P. Distribution of metabotropic glutamate receptor 7 messenger RNA in the developing and adult rat brain. Neuroscience 1995, 69, 167–176. [Google Scholar] [CrossRef]
- Kinzie, J.M.; Shinohara, M.M.; Van Den Pol, A.N.; Westbrook, G.L.; Segerson, T.P. Immunolocalization of metabotropic glutamate receptor 7 in the rat olfactory bulb. J. Comp. Neurol. 1997, 385, 372–384. [Google Scholar] [CrossRef]
- Bradley, S.R.; Rees, H.D.; Yi, H.; Levey, A.I.; Conn, P.J. Distribution and developmental regulation of metabotropic glutamate receptor 7a in rat brain. J. Neurochem. 1998, 71, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto, R.; Kulik, A.; Roberts, J.D.B.; Ohishi, H.; Nusser, Z.; Kaneko, T.; Somogyi, P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature 1996, 381, 523–525. [Google Scholar] [CrossRef]
- Saugstad, J.A.; Kinzie, J.M.; Shinohara, M.M.; Segerson, T.P.; Westbrook, G.L. Cloning and expression of rat metabotropic glutamate receptor 8 reveals a distinct pharmacological profile. Mol. Pharmacol. 1997, 51, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.J.; Starr, K.R.; Honey, A.; Soffin, E.M.; Rourke, C.; Jones, G.A.; Kelly, F.M.; Strum, J.; Melarange, R.A.; Harris, A.J.; et al. Evaluation of the mGlu8 receptor as a putative therapeutic target in schizophrenia. Brain Res. 2007, 1152, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, F.; Klausberger, T.; Cobden, P.; Baude, A.; Roberts, J.D.B.; Szucs, P.; Kinoshita, A.; Shigemoto, R.; Somogyi, P.; Dalezios, Y. Metabotropic glutamate receptor 8-expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. J. Neurosci. 2005, 25, 10520–10536. [Google Scholar] [CrossRef] [PubMed]
- Duvoisin, R.M.; Zhang, C.; Ramonell, K. A novel metabotropic glutamate receptor expressed in the retina and olfactory bulb. J. Neurosci. 1995, 15, 3075–3083. [Google Scholar] [CrossRef]
- Flynn, D.D.; Ferrari-DiLeo, G.; Mash, D.C.; Levey, A.I. Differential Regulation of Molecular Subtypes of Muscarinic Receptors in Alzheimer’s Disease. J. Neurochem. 1995, 64, 1888–1891. [Google Scholar] [CrossRef]
- Berthele, A.; Platzer, S.; Weis, S.; Conrad, B.; Tölle, T.R. Expression of GABA(B1) and GABA(B2) mRNA in the human brain. Neuroreport 2001, 12, 3269–3275. [Google Scholar] [CrossRef]
- Billinton, A.; Ige, A.O.; Wise, A.; White, J.H.; Disney, G.H.; Marshall, F.H.; Waldvogel, H.J.; Faull, R.L.M.; Emson, P.C. GABA(B) receptor heterodimer-component localisation in human brain. Mol. Brain Res. 2000, 77, 111–124. [Google Scholar] [CrossRef]
- Blümcke, I.; Behle, K.; Malitschek, B.; Kuhn, R.; Knöpfel, T.; Wolf, H.K.; Wiestler, O.D. Immunohistochemical distribution of metabotropic glutamate receptor subtypes mGluR1b, mGluR2/3, mGluR4a and mGluR5 in human hippocampus. Brain Res. 1996, 736, 217–226. [Google Scholar] [CrossRef]
- Makoff, A.; Lelchuk, R.; Oxer, M.; Harrington, K.; Emson, P. Molecular characterization and localization of human metabotropic glutamate receptor type 4. Mol. Brain Res. 1996, 37, 239–248. [Google Scholar] [CrossRef]
- Flor, P.J.; Lukic, S.; Rüegg, D.; Leonhardt, T.; Knöpfel, T.; Kühn, R. Molecular cloning, functional expression and pharmacological characterization of the human metabotropic glutamate receptor type 4. Neuropharmacology 1995, 34, 149–155. [Google Scholar] [CrossRef]
- Flor, P.J.; Van Der Putten, H.; Rüegg, D.; Lukic, S.; Leonhardt, T.; Bence, M.; Sansig, G.; Knöpfel, T.; Kuhn, R. A novel splice variant of a metabotropic glutamate receptor, human mGluR7b. Neuropharmacology 1997, 36, 153–159. [Google Scholar] [CrossRef]
- Makoff, A.; Pilling, C.; Harrington, K.; Emson, P. Human metabotropic glutamate receptor type 7: Molecular cloning and mRNA distribution in the CNS. Mol. Brain Res. 1996, 40, 165–170. [Google Scholar] [CrossRef]
- Woźniak, M.; Acher, F.; Marciniak, M.; Lasoń-Tyburkiewicz, M.; Gruca, P.; Papp, M.; Pilc, A.; Wierońska, J.M. Involvement of GABAB Receptor Signaling in Antipsychotic-like Action of the Novel Orthosteric Agonist of the mGlu4 Receptor, LSP4-2022. Curr. Neuropharmacol. 2016, 14, 413–426. [Google Scholar] [CrossRef]
- Charvin, D. mGlu4 allosteric modulation for treating Parkinson’s disease. Neuropharmacology 2018, 135, 308–315. [Google Scholar] [CrossRef]
- Levey, A.I. Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993, 52, 441–448. [Google Scholar] [CrossRef]
- Cieślik, P.; Radulska, A.; Pelikant-Małecka, I.; Płoska, A.; Kalinowski, L.; Wierońska, J.M. Reversal of MK-801-Induced Disruptions in Social Interactions and Working Memory with Simultaneous Administration of LY487379 and VU152100 in Mice. Int. J. Mol. Sci. 2019, 20, 2781. [Google Scholar] [CrossRef]
- Jones, K.A.; Borowsky, B.; Tamm, J.A.; Craig, D.A.; Durkin, M.M.; Dai, M.; Yao, W.J.; Johnson, M.; Gunwaldsen, C.; Huang, L.Y.; et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 1998, 396, 674–679. [Google Scholar] [CrossRef]
- Carriba, P.; Navarro, G.; Ciruela, F.; Ferré, S.; Casadó, V.; Agnati, L.; Cortés, A.; Mallol, J.; Fuxe, K.; Canela, E.I.; et al. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat. Methods 2008, 5, 727–733. [Google Scholar] [CrossRef]
- Dupré, D.J.; Robitaille, M.; Éthier, N.; Villeneuve, L.R.; Mamarbachi, A.M.; Hébert, T.E. Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J. Biol. Chem. 2006, 281, 34561–34573. [Google Scholar] [CrossRef] [PubMed]
- Gandia, J.; Galino, J.; Amaral, O.B.; Soriano, A.; Lluís, C.; Franco, R.; Ciruela, F. Detection of higher-order G protein-coupled receptor oligomers by a combined BRET-BiFC technique. FEBS Lett. 2008, 582, 2979–2984. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Urizar, E.; Kralikova, M.; Mobarec, J.C.; Shi, L.; Filizola, M.; Javitch, J.A. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 2008, 27, 2293–2304. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gimenez, J.F.; Canals, M.; Pediani, J.D.; Milligan, G. The α1b-adrenoceptor exists as a higher-order oligomer: Effective oligomerization is required for receptor maturation, surface delivery, and function. Mol. Pharmacol. 2007, 71, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Carriba, P.; Gandía, J.; Ciruela, F.; Casadó, V.; Cortés, A.; Mallol, J.; Canela, E.I.; Lluis, C.; Franco, R. Detection of heteromers formed by cannabinoid CB1, dopamine D2, and adenosine A2A G-protein-coupled receptors by combining bimolecular fluorescence complementation and bioluminescence energy transfer. Sci. World J. 2008, 8, 1088–1097. [Google Scholar] [CrossRef]
- Vidi, P.A.; Chemel, B.R.; Hu, C.D.; Watts, V.J. Ligand-dependent oligomerization of dopamine D2 and adenosine A2A receptors in living neuronal cells? Mol. Pharmacol. 2008, 74, 544–551. [Google Scholar] [CrossRef]
- González-Maeso, J.; Ang, R.; Yuen, T.; Chan, P.; Weisstaub, N.V.; López-Giménez, J.F.; Zhou, M.; Okawa, Y.; Callado, L.F.; Milligan, G.; et al. Identification of a Novel Serotonin/Glutamate Receptor Complex Implicated in Psychosis. Nature 2008, 452, 93–97. [Google Scholar] [CrossRef]
- Shah, U.H.; González-Maeso, J. Serotonin and glutamate interactions in preclinical schizophrenia models. ACS Chem. Neurosci. 2019, 10, 3068–3077. [Google Scholar] [CrossRef]
- Hideshima, K.S.; Hojati, A.; Saunders, J.M.; On, D.M.; de la Fuente Revenga, M.; Shin, J.M.; Sánchez-González, A.; Dunn, C.M.; Pais, A.B.; Pais, A.C.; et al. Role of mGlu2 in the 5-HT 2A receptor-dependent antipsychotic activity of clozapine in mice. Psychopharmacology 2018, 235, 3149–3165. [Google Scholar] [CrossRef]
- Cabello, N.; Gandía, J.; Bertarelli, D.C.G.; Watanabe, M.; Lluís, C.; Franco, R.; Ferré, S.; Luján, R.; Ciruela, F. Metabotropic glutamate type 5, dopamine D2 and adenosine A 2a receptors form higher-order oligomers in living cells. J. Neurochem. 2009, 109, 1497–1507. [Google Scholar] [CrossRef]
- Fuxe, K.; Marcellino, D.; Leo, G.; Agnati, L.F. Molecular integration via allosteric interactions in receptor heteromers. A working hypothesis. Curr. Opin. Pharmacol. 2010, 10, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.M.; Møller, T.C.; Ster, J.; Giraldo, J.; Maurel, D.; Rovira, X.; Scholler, P.; Zwier, J.M.; Perroy, J.; Durroux, T.; et al. Pharmacological evidence for a metabotropic glutamate receptor heterodimer in neuronal cells. eLife 2017, 6, e25233. [Google Scholar] [CrossRef] [PubMed]
- Fulton, M.G.; Loch, M.T.; Cuoco, C.A.; Rodriguez, A.L.; Days, E.; Vinson, P.N.; Kozek, K.A.; Weaver, C.D.; Blobaum, A.L.; Conn, P.J.; et al. Challenges in the Discovery and Optimization of mGlu2/4 Heterodimer Positive Allosteric Modulators. Lett. Drug Des. Discov. 2019, 16, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Z.; Moreno-Delgado, D.; Dalton, J.A.R.; Rovira, X.; Trapero, A.; Goudet, C.; Llebaria, A.; Giraldo, J.; Yuan, Q.; et al. Allosteric control of an asymmetric transduction in a g protein-coupled receptor heterodimer. eLife 2017, 6, e26985. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Noetzel, M.J.; Johnson, K.A.; Zamorano, R.; Jalan-Sakrikar, N.; Gregory, K.J.; Jeffrey Conn, P.; Niswender, C.M. Selective actions of novel allosteric modulators reveal functional heteromers of Metabotropic glutamate receptors in the CNS. J. Neurosci. 2014, 34, 79–94. [Google Scholar] [CrossRef]
- Colavita, M.; Terral, G.; Lemercier, C.E.; Drago, F.; Marsicano, G.; Massa, F. Layer-specific potentiation of network GABAergic inhibition in the CA1 area of the hippocampus. Sci. Rep. 2016, 6, 28454. [Google Scholar] [CrossRef]
- González, J.C.; Albiñana, E.; Baldelli, P.; García, A.G.; Hernández-Guijo, J.M. Presynaptic muscarinic receptor subtypes involved in the enhancement of spontaneous GABAergic postsynaptic currents in hippocampal neurons. Eur. J. Neurosci. 2011, 33, 69–81. [Google Scholar] [CrossRef]
- Yi, F.; Ball, J.; Stoll, K.E.; Satpute, V.C.; Mitchell, S.M.; Pauli, J.L.; Holloway, B.B.; Johnston, A.D.; Nathanson, N.M.; Deisseroth, K.; et al. Direct excitation of parvalbumin-positive interneurons by M1muscarinic acetylcholine receptors: Roles in cellular excitability, inhibitory transmission and cognition. J. Physiol. 2014, 592, 3463–3494. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Leo, R.J.; Baer, D. Delirium associated with baclofen withdrawal: A review of common presentations and management strategies. Psychosomatics 2005, 46, 503–507. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).