Investigation on Microstructures and Mechanical Properties of Isotactic Polypropylene Parts Fabricated by Different Process Conditions with Different Aging Periods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Injection Molding Experiments
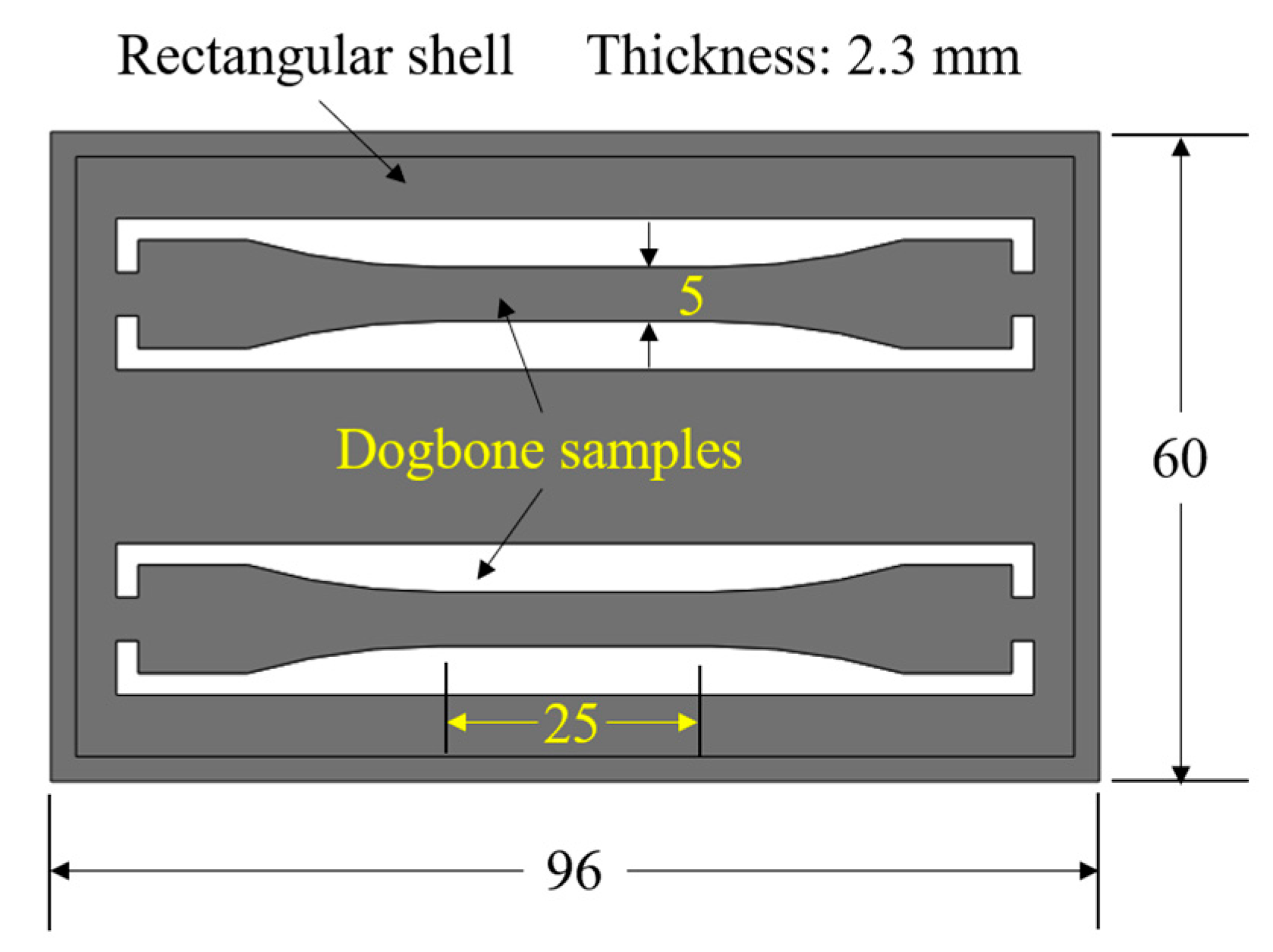
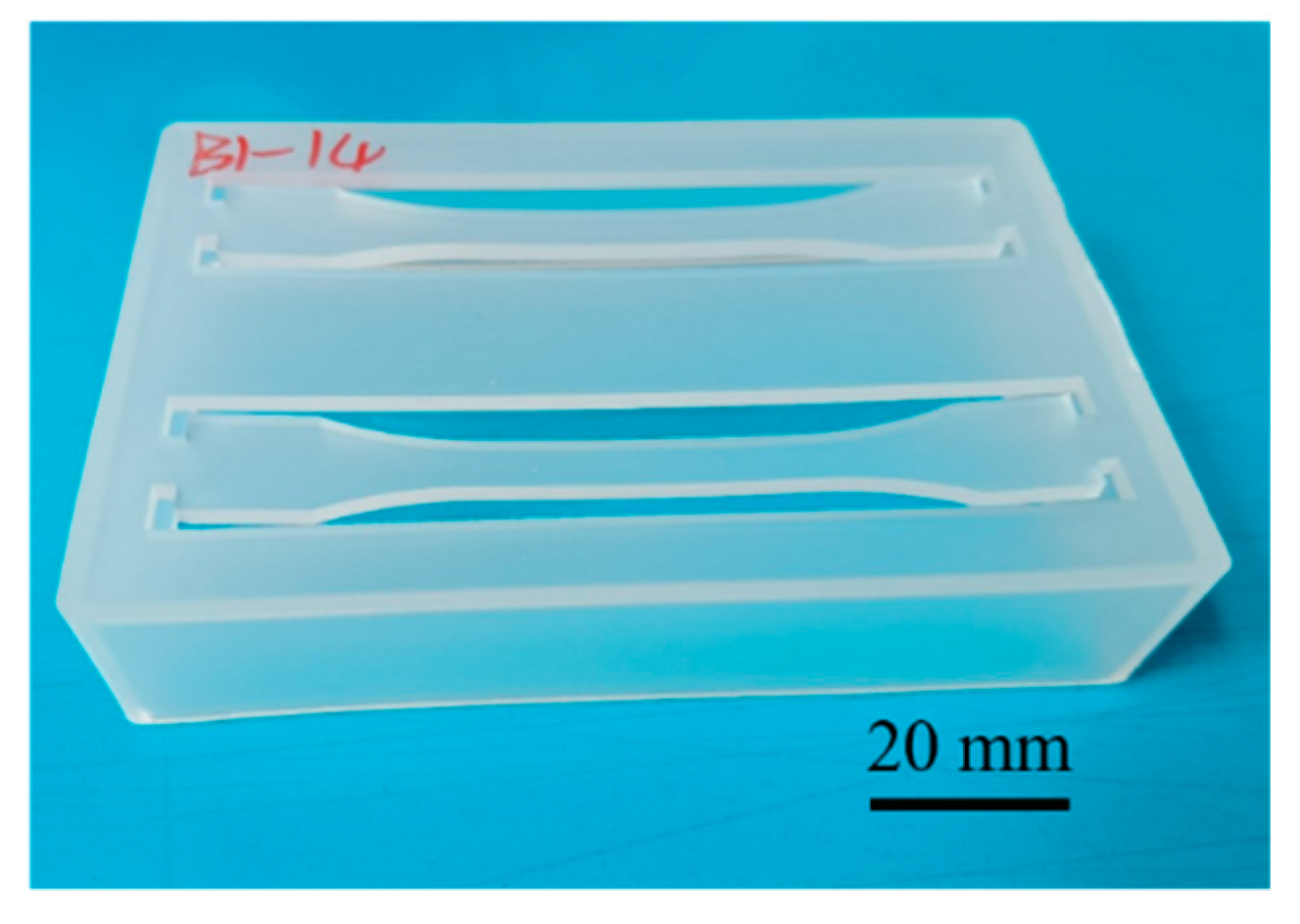
2.1.1. Parts Design and Materials
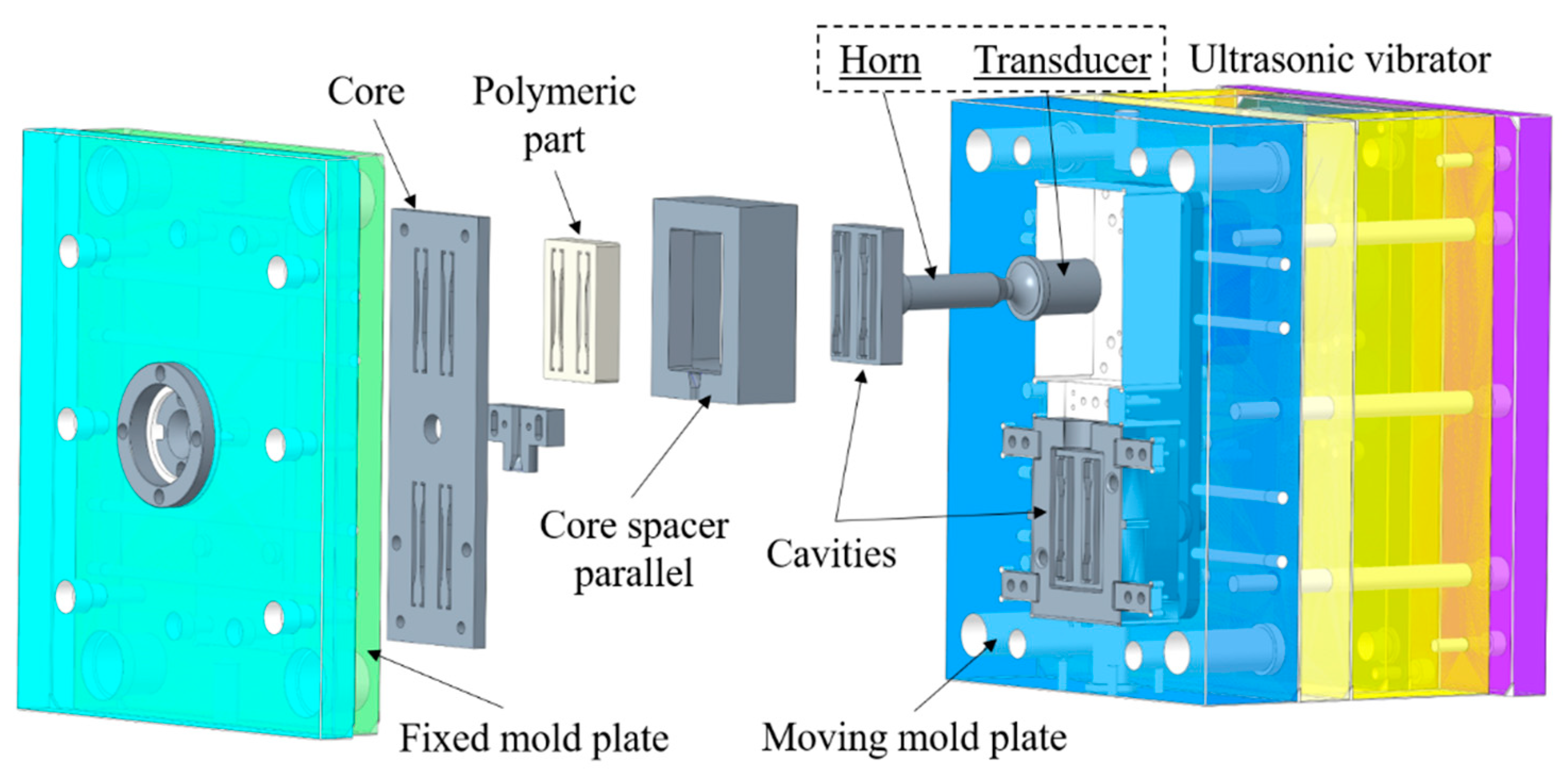
2.1.2. Injection Molding System and Experimental Design
2.1.3. Natural Aging Experiments
2.2. Characterization of Fabricated iPP Parts
2.2.1. X-ray Diffraction Tests
2.2.2. Fourier Transform Infrared Analysis
2.2.3. Scanning Electron Microscope Imaging
2.2.4. Tensile Tests
2.2.5. Statistical Analysis.
3. Results
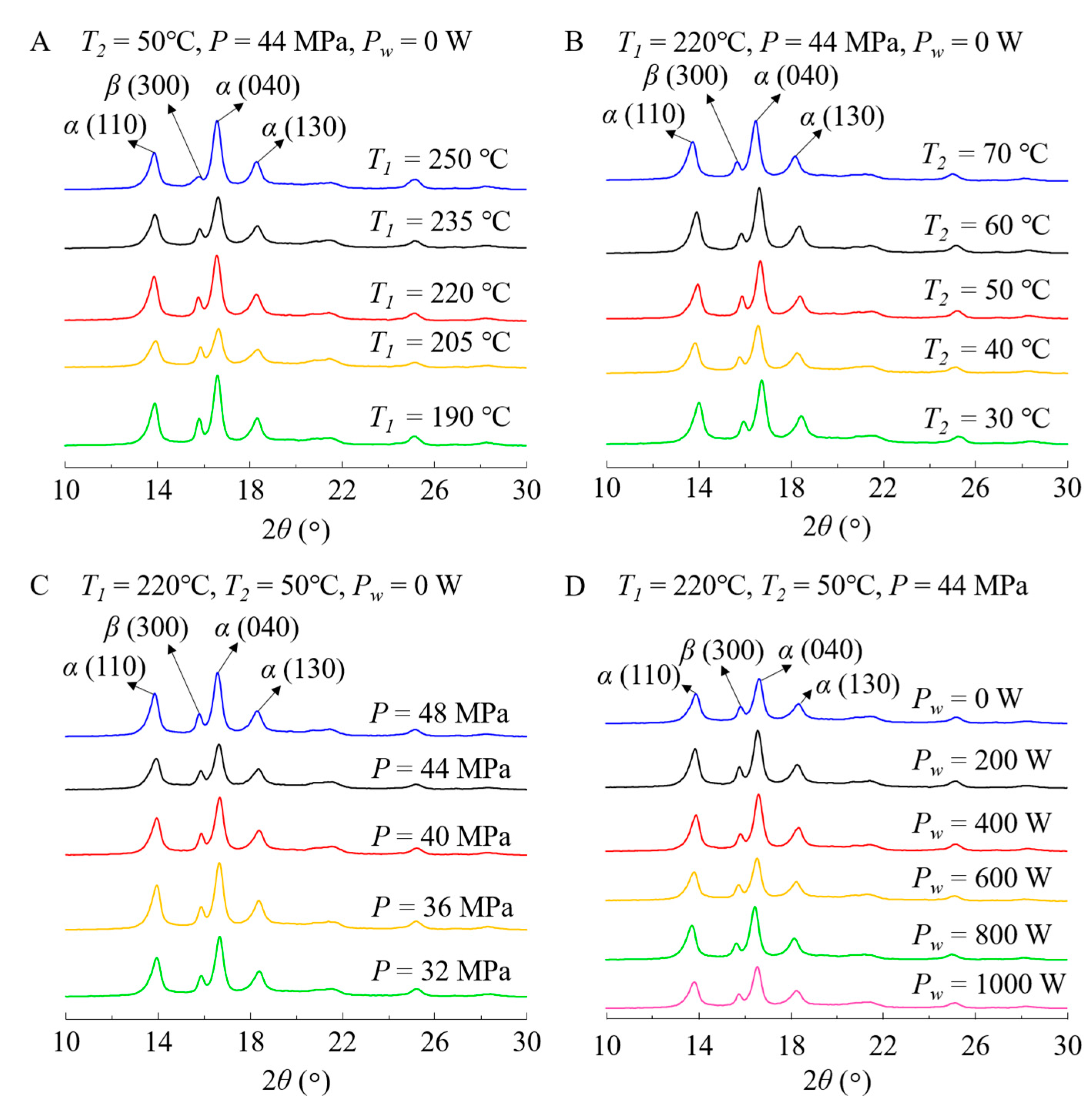
3.1. Effects of Process Conditions on Crystallinity
3.2. Comparison of Crystal Forms before and after Aging
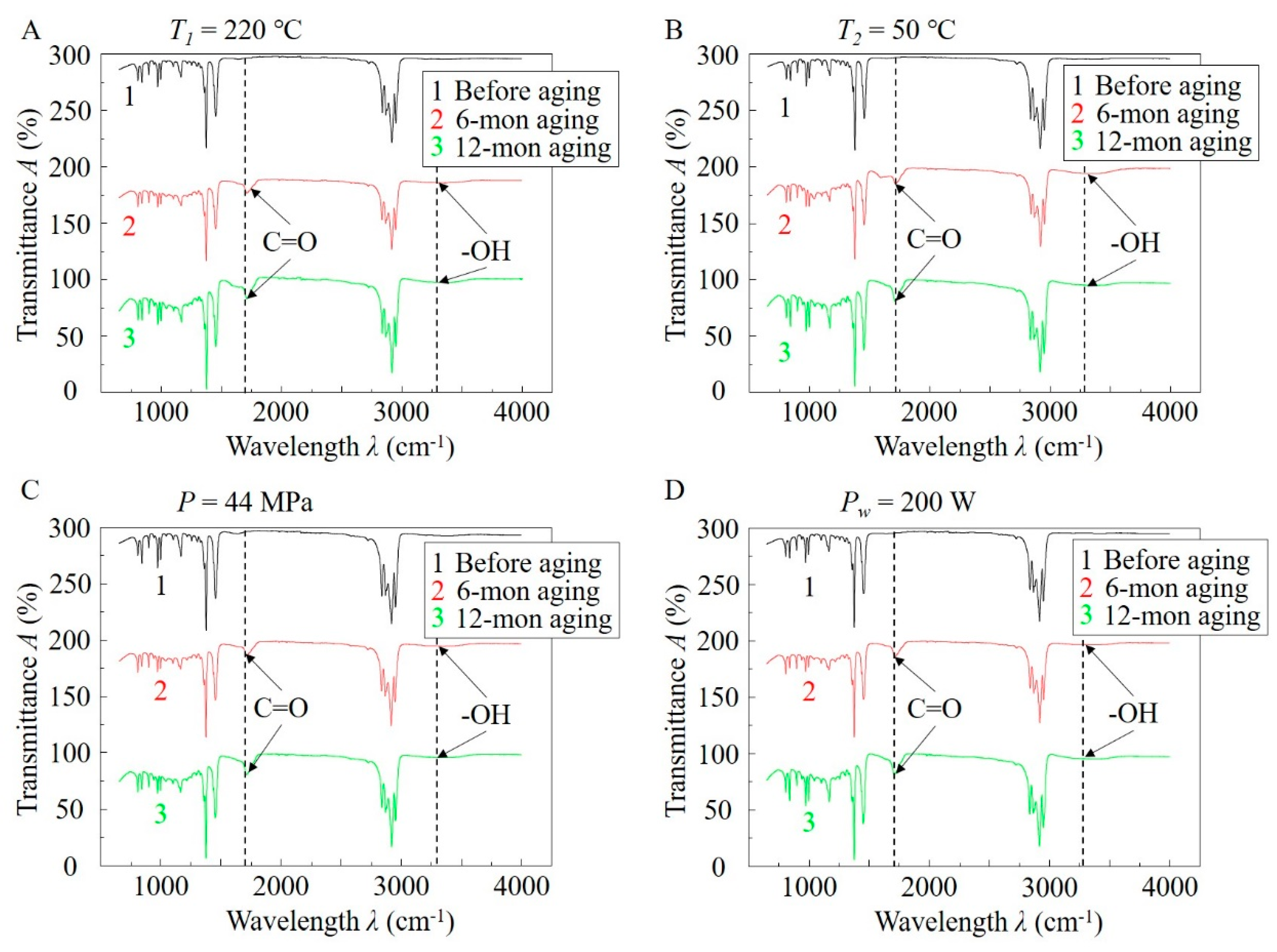
3.3. Comparison of Molecular Structures of the Samples before and after Aging
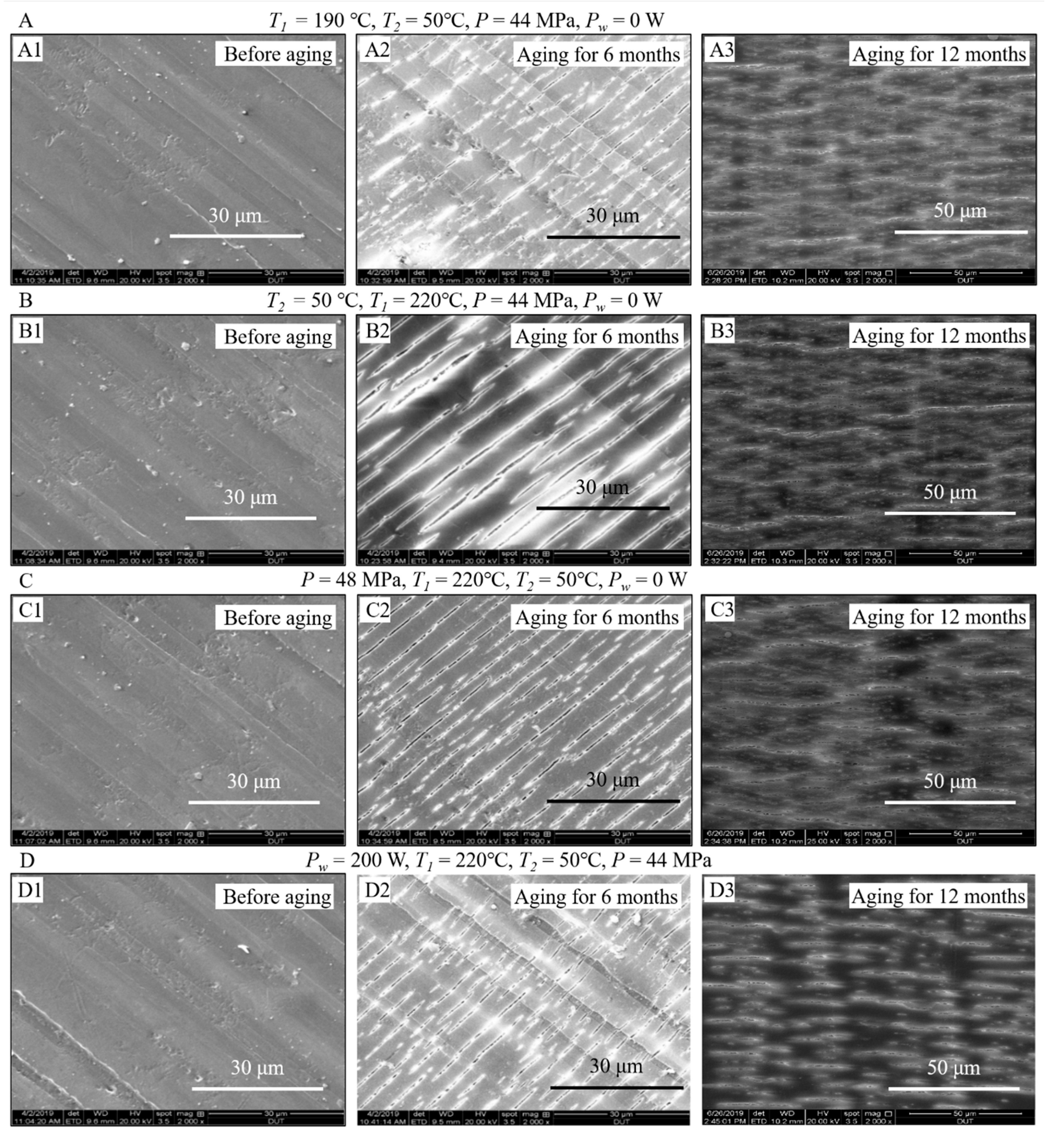
3.4. Comparison of Surface Morphologies of the Samples before and after Aging
3.5. Comparison of Tensile Behaviors of the Samples before and after Aging
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hodge, I.M. Physical aging in polymer glasses. Science 1995, 267, 1945–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celina, M.; Gillen, K.T.; Assink, R.A. Accelerated aging and lifetime prediction: Review of non-Arrhenius behaviour due to two competing processes. Polym. Degrad. Stab. 2005, 90, 395–404. [Google Scholar] [CrossRef]
- Celina, M.C. Review of polymer oxidation and its relationship with materials performance and lifetime prediction. Polym. Degrad. Stab. 2013, 98, 2419–2429. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Akcelrud, L. Correlations between structure and accelerated artificial ageing of XLPE. Eur. Polym. J. 2006, 42, 553–562. [Google Scholar] [CrossRef]
- Roy, P.K.; Surekha, P.; Raman, R.; Rajagopal, C. Investigating the role of metal oxidation state on the degradation behaviour of LDPE. Polym. Degrad. Stab. 2009, 94, 1033–1039. [Google Scholar] [CrossRef]
- Ge, S.; Kang, X.; Zhao, Y. One-year biodegradation study of UHMWPE as artificial joint materials: Variation of chemical structure and effect on friction and wear behavior. Wear 2011, 271, 2354–2363. [Google Scholar] [CrossRef]
- Matuana, L.M.; Jin, S.; Stark, N.M. Ultraviolet weathering of HDPE/wood-flour composites coextruded with a clear HDPE cap layer. Polym. Degrad. Stab. 2011, 96, 97–106. [Google Scholar] [CrossRef]
- Turnbull, L.; Liggat, J.J.; MacDonald, W.A. Ageing of poly (ethylene terephthalate) and poly (ethylene naphthalate) under moderately accelerated conditions. J. Appl. Polym. Sci. 2012, 124, 4517–4529. [Google Scholar] [CrossRef]
- Leong, Y.W.; Abu Bakar, M.B.; Mohd Ishak, Z.A.; Ariffin, A. Filler treatment effects on the weathering of talc-, CaCO3-and kaolin-filled polypropylene hybrid composites. Compos. Interfaces 2006, 13, 659–684. [Google Scholar] [CrossRef]
- Li, J.; Yang, R.; Yu, J.; Liu, Y. Natural photo-aging degradation of polypropylene nanocomposites. Polym. Degrad. Stab. 2008, 93, 84–89. [Google Scholar] [CrossRef]
- Obadal, M.; Čermák, R.; Raab, M.; Verney, V.; Commereuc, S.; Fraïsse, F. Structure evolution of α-and β-polypropylenes upon UV irradiation: A multiscale comparison. Polym. Degrad. Stab. 2005, 88, 532–539. [Google Scholar] [CrossRef]
- Výchopňová, J.; Čermák, R.; Obadal, M.; Raab, M.; Verney, V.; Commereuc, S. The role of specific nucleation in polypropylene photodegradation. Polym. Degrad. Stab. 2007, 92, 1763–1768. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Hao, J.; Wang, W. Ultraviolet weathering performance of high-density polyethylene/wood-flour composites with a basalt-fiber-included shell. Polymers 2018, 10, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Sánchez, X.; Hernández-Avila, M.; Elizalde, L.E.; Martínez, O.; Ferrer, I.; Elías-Zuñiga, A. Micro injection molding processing of UHMWPE using ultrasonic vibration energy. Mater. Des. 2017, 132, 1–12. [Google Scholar] [CrossRef]
- Sacristan, M.; Planta, X.; Morell, M.; Puiggali, J. Effects of ultrasonic vibration on the micro-molding processing of polylactide. Ultrason. Sonochem. 2014, 21, 376–386. [Google Scholar] [CrossRef]
- Li, D.; Xin, Y.; Song, Y.; Dong, T.; Ben, H.; Yu, R.; Zhang, Y. Crystalline modification of isotactic polypropylene with a rare earth nucleating agent based on ultrasonic vibration. Polymers 2019, 11, 1777. [Google Scholar] [CrossRef] [Green Version]
- Somani, R.H.; Yang, L.; Sics, I.; Hsiao, B.S.; Pogodina, N.V.; Winter, H.H.; Agarwal, P.; Fruitwala, H.; Tsou, A. Orientation-induced crystallization in isotactic polypropylene melt by shear deformation. Macromol. Symp. 2002, 185, 105–117. [Google Scholar] [CrossRef]
- Somani, R.H.; Yang, L.; Hsiao, B.S.; Fruitwala, H. Nature of shear-induced primary nuclei in iPP melt. J. Macromol. Sci. Phys. 2003, 42, 515–531. [Google Scholar] [CrossRef]
- Liu, P.; Hu, A.; Wang, S.; Shi, M.; Ye, G.; Xu, J. Evaluation of nonisothermal crystallization kinetic models for linear poly (phenylene sulfide). J. Appl. Polym. Sci. 2011, 121, 14–20. [Google Scholar] [CrossRef]
- Lee, W.I.; Talbott, M.F.; Springer, G.S.; Berglund, L.A. Effects of cooling rate on the crystallinity and mechanical properties of thermoplastic composites. J. Reinf. Plast. Compos. 1987, 6, 2–12. [Google Scholar] [CrossRef]
- Rizvi, S.J.A. Effect of injection molding parameters on crystallinity and mechanical properties of isotactic polypropylene. Int. J. Plast. Technol. 2017, 21, 404–426. [Google Scholar] [CrossRef]
- Ameli, A.; Nofar, M.; Jahani, D.; Rizvi, G.; Park, C.B. Development of high void fraction polylactide composite foams using injection molding: Crystallization and foaming behaviors. Chem. Eng. J. 2015, 262, 78–87. [Google Scholar] [CrossRef]
- Pantani, R.; Coccorullo, I.; Speranza, V.; Titomanlio, G. Morphology evolution during injection molding: Effect of packing pressure. Polymer 2007, 48, 2778–2790. [Google Scholar] [CrossRef]
- Le, M.C.; Belhabib, S.; Nicolazo, C.; Vachot, P.; Mousseau, P.; Sarda, A.; Deterre, R. Pressure influence on crystallization kinetics during injection molding. J. Mater. Process. Technol. 2011, 211, 1757–1763. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Li, H.; Lai, S.Y.; Jow, J. Physical and chemical effects of ultrasound vibration on polymer melt in extrusion. Ultrason. Sonochem. 2010, 17, 66–71. [Google Scholar] [CrossRef]
- Ávila-Orta, C.; Espinoza-González, C.; Martínez-Colunga, G.; Bueno-Baqués, D.; Maffezzoli, A.; Lionetto, F. An overview of progress and current challenges in ultrasonic treatment of polymer melts. Adv. Polym. Technol. 2013, 32, E582–E602. [Google Scholar] [CrossRef]
- Zabihi, F.; Eslamian, M. Effect of the ultrasonic substrate vibration on nucleation and crystallization of PbI2 crystals and thin films. Crystals 2018, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Chen, B.; Zhang, X. Study on the formation of β-crystal during the crystallization process of polypropylene reactor granule. Polymer 2007, 48, 5480–5483. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Z.; Ding, Q.; Wang, C.; Mai, K. Influence of different β-nucleating agent on crystallization behavior, morphology, and melting characteristic of multiwalled carbon nanotube-filled isotactic polypropylene nanocomposites. Polym. Compos. 2015, 36, 635–643. [Google Scholar] [CrossRef]
- Dou, Q. Effect of the composition ratio of pimelic acid/calcium stearate bicomponent nucleator and crystallization temperature on the production of β crystal form in isotactic polypropylene. J. Appl. Polym. Sci. 2008, 107, 958–965. [Google Scholar] [CrossRef]
- Kang, J.; Chen, J.; Cao, Y.; Li, H. Effects of ultrasound on the conformation and crystallization behavior of isotactic polypropylene and β-isotactic polypropylene. Polymer 2010, 51, 249–256. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, C.; Wang, C.; Guo, J.; Mai, K. Nonisothermal crystallization kinetics of isotactic polypropylene nucleated with a novel supported β-nucleating agent. J. Therm. Anal. Calorim. 2011, 103, 311–318. [Google Scholar] [CrossRef]
- Madeleine-Perdrillat, C.; Delor-Jestin, F.; Bussiere, P.O.; de Sainte Claire, P.; Pilichowski, J.F.; Baba, M. Simultaneous UV or thermal exposure and IR detection of evolved vapours: New tool for studying polymer photo-degradation. J. Photochem. Photobiol. A 2014, 278, 53–59. [Google Scholar] [CrossRef]
- He, P.; Xiao, Y.; Zhang, P.; Xing, C.; Zhu, N.; Zhu, X.; Yan, D. Thermal degradation of syndiotactic polypropylene and the influence of stereoregularity on the thermal degradation behaviour by in situ FTIR spectroscopy. Polym. Degrad. Stab. 2005, 88, 473–479. [Google Scholar] [CrossRef]
- Islam, N.Z.M.; Othman, N.; Ahmad, Z.; Ismail, Z. Effect of pro-degradant additive on photo-oxidative aging of polypropylene film. Sains. Malays 2011, 40, 803–808. [Google Scholar]
- Gupta, S.; McDonald, B.; Carrizosa, S.B.; Price, C. Microstructure, residual stress, and intermolecular force distribution maps of graphene/polymer hybrid composites: Nanoscale morphology-promoted synergistic effects. Compos. B Eng. 2016, 92, 175–192. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, Y.; Yang, J.; Kong, M.; Yang, H.; Zhao, J.; Li, G. Outdoor and accelerated laboratory weathering of polypropylene: A comparison and correlation study. Polym. Degrad. Stab. 2015, 112, 145–159. [Google Scholar] [CrossRef]
- Vadori, R.; Mohanty, A.K.; Misra, M. The effect of mold temperature on theperformance of injection molded poly (lactic acid)-based bioplastic. Macromol. Mater. Eng. 2013, 298, 981–990. [Google Scholar] [CrossRef]
- Tordjeman, P.; Robert, C.; Marin, G.; Gerard, P. The effect of α, β crystalline structure on the mechanical properties of polypropylene. Eur. Phys. J. E. Soft Matter 2001, 4, 459–465. [Google Scholar] [CrossRef]
- Peshkovskii, S.L.; Friedman, M.L.; Tukachinskii, A.I.; Vinogradov, G.V.; Enikolopian, N.S. Acoustic cavitation and its effect on flow in polymers and filled systems. Polym. Compos. 1983, 4, 126–134. [Google Scholar] [CrossRef]
- Pawlak, A.; Galeski, A.; Rozanski, A. Cavitation during deformation of semicrystalline polymers. Prog. Polym. Sci. 2014, 39, 921–958. [Google Scholar] [CrossRef]



| Process Parameters | Variables | ||||
|---|---|---|---|---|---|
| Melt temperature (°C) | 190 | 205 | 220 | 235 | 250 |
| Mold temperature (°C) | 30 | 40 | 50 | 60 | 70 |
| Holding pressure (MPa) | 32 | 36 | 40 | 44 | 48 |
| Ultrasound power (W) | 200 | 400 | 600 | 800 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhu, T.; Bi, J.; Hua, W.; Yu, T.; Jin, Y.; Zhao, D. Investigation on Microstructures and Mechanical Properties of Isotactic Polypropylene Parts Fabricated by Different Process Conditions with Different Aging Periods. Polymers 2020, 12, 2828. https://doi.org/10.3390/polym12122828
Liu Y, Zhu T, Bi J, Hua W, Yu T, Jin Y, Zhao D. Investigation on Microstructures and Mechanical Properties of Isotactic Polypropylene Parts Fabricated by Different Process Conditions with Different Aging Periods. Polymers. 2020; 12(12):2828. https://doi.org/10.3390/polym12122828
Chicago/Turabian StyleLiu, Ying, Tieli Zhu, Jie Bi, Weijian Hua, Tongmin Yu, Yifei Jin, and Danyang Zhao. 2020. "Investigation on Microstructures and Mechanical Properties of Isotactic Polypropylene Parts Fabricated by Different Process Conditions with Different Aging Periods" Polymers 12, no. 12: 2828. https://doi.org/10.3390/polym12122828
APA StyleLiu, Y., Zhu, T., Bi, J., Hua, W., Yu, T., Jin, Y., & Zhao, D. (2020). Investigation on Microstructures and Mechanical Properties of Isotactic Polypropylene Parts Fabricated by Different Process Conditions with Different Aging Periods. Polymers, 12(12), 2828. https://doi.org/10.3390/polym12122828







