Appetite Control across the Lifecourse: The Acute Impact of Breakfast Drink Quantity and Protein Content. The Full4Health Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures and Protocol
2.3. Anthropometric Measures
2.4. Test Drinks
2.5. Ad Libitum EI
2.6. Subjective Appetite Assessment
2.7. Test Drink Pleasantness and Satisfaction
2.8. Food Reward: Leeds Food Preference Questionnaire (LFPQ)
2.9. Blood Sampling and Processing
2.10. Biomarker Analysis
2.10.1. Appetite Hormones
2.10.2. Glucose Homeostasis
2.11. Statistical Analysis
3. Results
3.1. Participants
3.2. Test Drinks
3.3. Ad Libitum EI
3.4. Subjective Appetite Assessment
3.5. Test Drink Pleasantness and Satisfaction
3.6. Food Reward: LFPQ
3.7. Biomarkers
3.7.1. Appetite Hormones
3.7.2. Glucose Homeostasis
4. Discussion
4.1. Ad Libitum EI and Subjective Appetite
4.2. Food Reward: LFPQ
4.3. Biomarkers
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases. Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series No. 916; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Darnton-Hill, I.; Nishida, C.; James, W.P.T. A life course approach to diet, nutrition and the prevention of chronic diseases. Public Health Nutr. 2004, 7, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, A.I.C.; Johnstone, A.M.; de Roos, B.; Myint, P.K. Role of protein in healthy ageing. Eur. J. Integr. Med. 2018, 23, 32–36. [Google Scholar] [CrossRef]
- Zac-Varghese, S.; Tan, T.; Bloom, S.R. Hormonal interactions between gut and brain. Discov. Med. 2010, 10, 543–552. [Google Scholar] [PubMed]
- Bilman, E.; van Kleef, E.; van Trijp, H. External cues challenging the internal appetite control system—Overview and practical implications. Crit. Rev. Food Sci. Nutr. 2017, 57, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G.; Bloom, S.R. Gut hormones in the control of appetite. Exp. Physiol. 2004, 89, 507–516. [Google Scholar] [CrossRef]
- Simpson, K.A.; Bloom, S.R. Appetite and Hedonism: Gut Hormones and the Brain. Endocrinol. Metab. Clin. 2010, 39, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef]
- Rendina, D.; Campanozzi, A.; De Filippo, G. Methodological approach to the assessment of the obesogenic environment in children and adolescents: A review of the literature. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 561–571. [Google Scholar] [CrossRef]
- Marschner, A.; Mell, T.; Wartenburger, I.; Villringer, A.; Reischies, F.M.; Heekeren, H.R. Reward-based decision-making and aging. Brain Res. Bull. 2005, 67, 382–390. [Google Scholar] [CrossRef]
- Dreher, J.-C.; Meyer-Lindenberg, A.; Kohn, P.; Berman, K.F. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proc. Natl. Acad. Sci. USA 2008, 105, 15106–15111. [Google Scholar] [CrossRef]
- Pelchat, M.L. Food Cravings in Young and Elderly Adults. Appetite 1997, 28, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.M. The New Mexico Aging Process Study. Nutr. Rev. 1994, 52, S34–S37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Devine, A.; Suleska, A.; Tan, C.Y.; Toh, C.Z.J.; Kerr, D.; Prince, R.L. Adequacy and change in nutrient and food intakes with aging in a seven-year cohort study in elderly women. J. Nutr. Health Aging 2010, 14, 723–729. [Google Scholar] [CrossRef]
- Morley, J.E. Anorexia of aging: Physiologic and pathologic. Am. J. Clin. Nutr. 1997, 66, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Briefel, R.R.; McDowell, M.A.; Alaimo, K.; Caughman, C.R.; Bischof, A.L.; Carroll, M.D.; Johnson, C.L. Total energy intake of the US population: The third National Health and Nutrition Examination Survey, 1988-1991. Am. J. Clin. Nutr. 1995, 62, 1072S–1080S. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.G.; Johnstone, A.M.; Halford, J.C.G. Approaches to influencing food choice across the age groups: From children to the elderly. Proc. Nutr. Soc. 2015, 74, 149–157. [Google Scholar] [CrossRef]
- Nickols-Richardson, S.M.; Coleman, M.D.; Volpe, J.J.; Hosig, K.W. Perceived Hunger Is Lower and Weight Loss Is Greater in Overweight Premenopausal Women Consuming a Low-Carbohydrate/High-Protein vs High-Carbohydrate/Low-Fat Diet. J. Am. Diet. Assoc. 2005, 105, 1433–1437. [Google Scholar] [CrossRef]
- Lejeune, M.P.; Westerterp, K.R.; Adam, T.C.; Luscombe-Marsh, N.D.; Westerterp-Plantenga, M.S. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am. J. Clin. Nutr. 2006, 83, 89–94. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef]
- Wolfe, R.R. The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br. J. Nutr. 2012, 108 (Suppl. 2), S88–S93. [Google Scholar] [CrossRef]
- Veldhorst, M.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanisms of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Bales-Voelker, L.I.; Harris, C.T. A protein-rich beverage consumed as a breakfast meal leads to weaker appetitive and dietary responses v. a protein-rich solid breakfast meal in adolescents. Br. J. Nutr. 2011, 106, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Nguo, K.; Bonham, M.; Truby, H.; Barber, E.; Brown, J.; Huggins, C. Effect of Macronutrient Composition on Appetite Hormone Responses in Adolescents with Obesity. Nutrients 2019, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Belza, A.; Ritz, C.; Sørensen, M.Q.; Holst, J.J.; Rehfeld, J.F.; Astrup, A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am. J. Clin. Nutr. 2013, 97, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Murison, S.D.; Duncan, J.S.; Rance, K.A.; Speakman, J.R. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am. J. Clin. Nutr. 2005, 82, 941–948. [Google Scholar] [CrossRef]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee; Technical Report Series no. 854; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39 (Suppl. 1), 5–41. [Google Scholar]
- Chowdhury, E.A.; Richardson, J.D.; Tsintzas, K.; Thompson, D.; Betts, J.A. Carbohydrate-rich breakfast attenuates glycaemic, insulinaemic and ghrelin response to ad libitum lunch relative to morning fasting in lean adults. Br. J. Nutr. 2015, 114, 98–107. [Google Scholar] [CrossRef]
- Leidy, H.J.; Racki, E.M. The addition of a protein-rich breakfast and its effects on acute appetite control and food intake in ‘breakfast-skipping’ adolescents. Int. J. Obes. 2010, 34, 1125–1133. [Google Scholar] [CrossRef]
- McCance, R.A.; Widdowson, E.M. McCance and Widdowson’s the Composition of Foods, 7th ed.; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Anderson, G.H.; Catherine, N.L.A.; Woodend, D.M.; Wolever, T.M.S. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am. J. Clin. Nutr. 2002, 76, 1023–1030. [Google Scholar] [CrossRef]
- Jansen, A.; Theunissen, N.; Slechten, K.; Nederkoorn, C.; Boon, B.; Mulkens, S.; Roefs, A. Overweight children overeat after exposure to food cues. Eat. Behav. 2003, 4, 197–209. [Google Scholar] [CrossRef]
- Finlayson, G.; King, N.; Blundell, J. The role of implicit wanting in relation to explicit liking and wanting for food: Implications for appetite control. Appetite 2008, 50, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Oustric, P.; Thivel, D.; Dalton, M.; Beaulieu, K.; Gibbons, C.; Hopkins, M.; Blundell, J.; Finlayson, G. Measuring food preference and reward: Application and cross-cultural adaptation of the Leeds Food Preference Questionnaire in human experimental research. Food Qual. Prefer. 2020, 80, 103824. [Google Scholar] [CrossRef]
- Griffioen-Roose, S.; Mars, M.; Siebelink, E.; Finlayson, G.; Tomé, D.; de Graaf, C. Protein status elicits compensatory changes in food intake and food preferences. Am. J. Clin. Nutr. 2011, 95, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Griffioen-Roose, S.; Mars, M.; Finlayson, G.; Blundell, J.E.; de Graaf, C. The effect of within-meal protein content and taste on subsequent food choice and satiety. Br. J. Nutr. 2011, 106, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Cole, R.E.; Berryman, C.E.; Finlayson, G.; Radcliffe, P.N.; Kominsky, M.T.; Murphy, N.E.; Carbone, J.W.; Rood, J.C.; Young, A.J.; et al. Appetite Suppression and Altered Food Preferences Coincide with Changes in Appetite-Mediating Hormones During Energy Deficit at High Altitude, But Are Not Affected by Protein Intake. High Alt. Med. Biol. 2018, 19, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Alvarez-Guaita, A.; Melvin, A.; Rimmington, D.; Dattilo, A.; Miedzybrodzka, E.L.; Cimino, I.; Maurin, A.-C.; Roberts, G.P.; Meek, C.L.; et al. GDF15 Provides an Endocrine Signal of Nutritional Stress in Mice and Humans. Cell Metab. 2019, 29, 707–718.e8. [Google Scholar] [CrossRef]
- Cheke, L.G.; Bonnici, H.M.; Clayton, N.S.; Simons, J.S. Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia 2017, 96, 137–149. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Giezenaar, C.; Trahair, L.G.; Rigda, R.; Hutchison, A.T.; Feinle-Bisset, C.; Luscombe-Marsh, N.D.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; et al. Lesser suppression of energy intake by orally ingested whey protein in healthy older men compared with young controls. Am. J. Physiol. Integr. Comp. Physiol. 2015, 309, R845–R854. [Google Scholar] [CrossRef]
- Brindal, E.; Baird, D.; Danthiir, V.; Wilson, C.; Bowen, J.; Slater, A.; Noakes, M. Ingesting breakfast meals of different glycaemic load does not alter cognition and satiety in children. Eur. J. Clin. Nutr. 2012, 66, 1166–1171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chandarana, K.; Drew, M.E.; Emmanuel, J.; Karra, E.; Gelegen, C.; Chan, P.; Cron, N.J.; Batterham, R.L. Subject Standardization, Acclimatization, and Sample Processing Affect Gut Hormone Levels and Appetite in Humans. Gastroenterology 2009, 136, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Naska, A.; Costacou, T. DAFNE III Group Disparities in food habits across Europe. Proc. Nutr. Soc. 2002, 61, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Yannakoulia, M.; Karayiannis, D.; Terzidou, M.; Kokkevi, A.; Sidossis, L.S. Nutrition-related habits of Greek adolescents. Eur. J. Clin. Nutr. 2004, 58, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Rumm-Kreuter, D. Comparison of the eating and cooking habits of northern Europe and the Mediterranean countries in the past, present and future. Int. J. Vitam. Nutr. Res. 2001, 71, 141–148. [Google Scholar] [CrossRef]
- Zandstra, E.H.; de Graaf, C. Sensory perception and pleasantness of orange beverages from childhood to old age. Food Qual. Prefer. 1998, 9, 5–12. [Google Scholar] [CrossRef]
- Wadhera, D.; Capaldi-Phillips, E.D. A review of visual cues associated with food on food acceptance and consumption. Eat. Behav. 2014, 15, 132–143. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Astrup, A.; Holst, J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Investig. 1998, 101, 515–520. [Google Scholar] [CrossRef]
- Adrian, T.E.; Ferri, G.-L.; Bacarese-Hamilton, A.J.; Fuessl, H.S.; Polak, J.M.; Bloom, S.R. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985, 89, 1070–1077. [Google Scholar] [CrossRef]
- De Silva, A.; Salem, V.; Long, C.J.; Makwana, A.; Newbould, R.D.; Rabiner, E.A.; Ghatei, M.A.; Bloom, S.R.; Matthews, P.M.; Beaver, J.D.; et al. The Gut Hormones PYY3-36 and GLP-17-36 amide Reduce Food Intake and Modulate Brain Activity in Appetite Centers in Humans. Cell Metab. 2011, 14, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Schwartz, T.W.; Lovgreen, N.A.; Pedersen, O.; Beck-Nielsen, H. Diurnal profile of pancreatic polypeptide, pancreatic glucagon, gut glucagon and insulin in human morbid obesity. Int. J. Obes. 1983, 7, 529–538. [Google Scholar] [PubMed]
- Näslund, E.; Barkeling, B.; King, N.; Gutniak, M.; Blundell, J.E.; Holst, J.J.; Rössner, S.; Hellström, P.M. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int. J. Obes. 1999, 23, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, M.A.; Borque, M.; Martinez-Sarmiento, J.; Aparicio, E.; Hernández, C.; Cabrerizo, L.; Fernández-Represa, J.A. Peptide YY Secretion in Morbidly Obese Patients Before and After Vertical Banded Gastroplasty. Obes. Surg. 2002, 12, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Withers, D.J.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Inhibition of food intake in obese subjects by peptide YY3-36. N. Engl. J. Med. 2003, 349, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Krarup, T.; Sonne, J.; Madsbad, S.; Vølund, A.; Juul, A.G.; Holst, J.J. Incretin Secretion in Relation to Meal Size and Body Weight in Healthy Subjects and People with Type 1 and Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2003, 88, 2706–2713. [Google Scholar] [CrossRef]
- Kim, B.J.; Carlson, O.D.; Jang, H.J.; Elahi, D.; Berry, C.; Egan, J.M. Peptide YY is secreted after oral glucose administration in a gender-specific manner. J. Clin. Endocrinol. Metab. 2005, 90, 6665–6671. [Google Scholar] [CrossRef]
- Vazquez Roque, M.I.; Camilleri, M.; Stephens, D.A.; Jensen, M.D.; Burton, D.D.; Baxter, K.L.; Zinsmeister, A.R. Gastric Sensorimotor Functions and Hormone Profile in Normal Weight, Overweight, and Obese People. Gastroenterology 2006, 131, 1717–1724. [Google Scholar] [CrossRef]
- Morley, J.E.; Silver, A.J. Anorexia in the elderly. Neurobiol. Aging 1988, 9, 9–16. [Google Scholar] [CrossRef]
- Datz, F.L.; Christian, P.E.; Moore, J. Gender-related differences in gastric emptying. J. Nucl. Med. 1987, 28, 1204–1207. [Google Scholar]
- Giezenaar, C.; Trahair, L.G.; Luscombe-Marsh, N.D.; Hausken, T.; Standfield, S.; Jones, K.L.; Lange, K.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of randomized whey-protein loads on energy intake, appetite, gastric emptying, and plasma gut-hormone concentrations in older men and women. Am. J. Clin. Nutr. 2017, 106, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.P.; Adrian, T.E.; Carolan, G.; Chatterjee, V.K.; Bloom, S.R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 1987, 28, 166–170. [Google Scholar] [CrossRef]
- Brennan, I.M.; Feltrin, K.L.; Nair, N.S.; Hausken, T.; Little, T.J.; Gentilcore, D.; Wishart, J.M.; Jones, K.L.; Horowitz, M.; Feinle-Bisset, C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, 602–610. [Google Scholar] [CrossRef]
- Hirschberg, A.L. Sex hormones, appetite and eating behaviour in women. Maturitas 2012, 71, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Campolier, M.; Thondre, S.P.; Clegg, M.; Shafat, A.; Mcintosh, A.; Lightowler, H. Changes in PYY and gastric emptying across the phases of the menstrual cycle and the influence of the ovarian hormones. Appetite 2016, 107, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.M.; Ange, B.A.; Anderson, C.A.M.; Miller, E.R., III; Holbrook, J.T.; Appel, L.J. Characteristics Associated With Fasting Appetite Hormones (Obestatin, Ghrelin, and Leptin). Obesity 2009, 17, 349–354. [Google Scholar] [CrossRef]
- Sturm, K.; MacIntosh, C.G.; Parker, B.A.; Wishart, J.; Horowitz, M.; Chapman, I.M. Appetite, food intake, and plasma concentrations of cholecystokinin, ghrelin, and other gastrointestinal hormones in undernourished older women and well-nourished young and older women. J. Clin. Endocrinol. Metab. 2003, 88, 3747–3755. [Google Scholar] [CrossRef]
- Bertoli, S.; Magni, P.; Krogh, V.; Ruscica, M.; Dozio, E.; Testolin, G.; Battezzati, A. Is ghrelin a signal of decreased fat-free mass in elderly subjects? Eur. J. Endocrinol. 2006, 155, 321–330. [Google Scholar] [CrossRef][Green Version]
- Di Francesco, V.; Zamboni, M.; Zoico, E.; Mazzali, G.; Dioli, A.; Omizzolo, F.; Bissoli, L.; Fantin, F.; Rizzotti, P.; Solerte, S.B.; et al. Unbalanced serum leptin and ghrelin dynamics prolong postprandial satiety and inhibit hunger in healthy elderly: Another reason for the “anorexia of aging”. Am. J. Clin. Nutr. 2006, 83, 1149–1152. [Google Scholar] [CrossRef]
- Schneider, S.M.; Al-Jaouni, R.; Caruba, C.; Giudicelli, J.; Arab, K.; Suavet, F.; Ferrari, P.; Mothe-Satney, I.; Van Obberghen, E.; Hébuterne, X. Effects of age, malnutrition and refeeding on the expression and secretion of ghrelin. Clin. Nutr. 2008, 27, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; Luscombe-Marsh, N.D.; Hutchison, A.T.; Standfield, S.; Feinle-Bisset, C.; Horowitz, M.; Chapman, I.; Soenen, S. Dose-dependent effects of randomized intraduodenal whey-protein loads on glucose, gut hormone, and amino acid concentrations in healthy older and younger men. Nutrients 2018, 10, 78. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Pincelli, A.I.; Corrá, B.; Viarengo, R.; Bonomo, S.M.; Galimberti, D.; Scacchi, M.; Scarpini, E.; Cavagnini, F.; Müller, E.E. Plasma ghrelin concentrations in elderly subjects: Comparison with anorexic and obese patients. J. Endocrinol. 2002, 175, R1–R5. [Google Scholar] [CrossRef]
- Di Francesco, V.; Fantin, F.; Residori, L.; Bissoli, L.; Micciolo, R.; Zivelonghi, A.; Zoico, E.; Omizzolo, F.; Bosello, O.; Zamboni, M. Effect of age on the dynamics of acylated ghrelin in fasting conditions and in response to a meal. J. Am. Geriatr. Soc. 2008, 56, 1369–1370. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Haack, A.; Winning, K.; Wirth, R.; Fischer, B.; Uter, W.; Erdmann, J.; Schusdziarra, V.; Sieber, C.C. Impaired postprandial response of active ghrelin and prolonged suppression of hunger sensation in the elderly. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Farhy, L.S.; Liu, J.; Pezzoli, S.S.; Johnson, M.L.; Gaylinn, B.D.; Thorner, M.O. Age-dependent decline in acyl-ghrelin concentrations and reduced association of acyl-ghrelin and growth hormone in healthy older adults. J. Clin. Endocrinol. Metab. 2014, 99, 602–608. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Small, C.J.; Ward, H.L.; Murphy, K.G.; Dakin, C.L.; Taheri, S.; Kennedy, A.R.; Roberts, G.H.; Morgan, D.G.A.; Ghatei, M.A.; et al. The Novel Hypothalamic Peptide Ghrelin Stimulates Food Intake and Growth Hormone Secretion. Endocrinology 2000, 141, 4325–4328. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, G.; Cabral, A.; Cornejo, M.P.; De Francesco, P.N.; Garcia-Romero, G.; Reynaldo, M.; Perello, M. Des-Acyl Ghrelin Directly Targets the Arcuate Nucleus in a Ghrelin-Receptor Independent Manner and Impairs the Orexigenic Effect of Ghrelin. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef]
- Könner, A.C.; Klöckener, T.; Brüning, J.C. Control of energy homeostasis by insulin and leptin: Targeting the arcuate nucleus and beyond. Physiol. Behav. 2009, 97, 632–638. [Google Scholar] [CrossRef]
- Serra-Prat, M.; Palomera, E.; Clave, P.; Puig-Domingo, M. Effect of age and frailty on ghrelin and cholecystokinin responses to a meal test. Am. J. Clin. Nutr. 2009, 89, 1410–1417. [Google Scholar] [CrossRef]
- Doucet, E.; St-Pierre, S.; Alméras, N.; Mauriège, P.; Després, J.-P.; Richard, D.; Bouchard, C.; Tremblay, A. Fasting Insulin Levels Influence Plasma Leptin Levels Independently from the Contribution of Adiposity: Evidence from Both a Cross-Sectional and an Intervention Study1. J. Clin. Endocrinol. Metab. 2000, 85, 4231–4237. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.F.; Bernaba, B.; Hwu, C.-M.; Jinagouda, S.; Fahmi, S.; Kogosov, E.; Boyadjian, R. Insulin Regulates Plasma Ghrelin Concentration. J. Clin. Endocrinol. Metab. 2002, 87, 3997–4000. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.F.; Khan, A.; Sharma, A.; Michael, R.; Riad-Gabriel, M.G.; Boyadjian, R.; Jinagouda, S.D.; Steil, G.M.; Kamdar, V. Physiological insulinemia acutely modulates plasma leptin. Diabetes 1998, 47, 544–549. [Google Scholar] [CrossRef]
- Ellis, A.C.; Chandler-Laney, P.; Casazza, K.; Goree, L.L.; McGwin, G.; Gower, B.A. Circulating ghrelin and GLP-1 are not affected by habitual diet. Regul. Pept. 2012, 176, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, J.; Lippl, F.; Schusdziarra, V. Differential effect of protein and fat on plasma ghrelin levels in man. Regul. Pept. 2003, 116, 101–107. [Google Scholar] [CrossRef]
- Veedfald, S.; Wu, T.; Bound, M.; Grivell, J.; Hartmann, B.; Rehfeld, J.F.; Deacon, C.F.; Horowitz, M.; Holst, J.J.; Rayner, C.K. Hyperosmolar Duodenal Saline Infusion Lowers Circulating Ghrelin and Stimulates Intestinal Hormone Release in Young Men. J. Clin. Endocrinol. Metab. 2018, 103, 4409–4418. [Google Scholar] [CrossRef] [PubMed]
- Blom, W.A.M.; Lluch, A.; Stafleu, A.; Vinoy, S.; Holst, J.J.; Schaafsma, G.; Hendriks, H.F.J. Effect of a high-protein breakfast on the postprandial ghrelin response. Am. J. Clin. Nutr. 2006, 83, 211–220. [Google Scholar] [CrossRef]
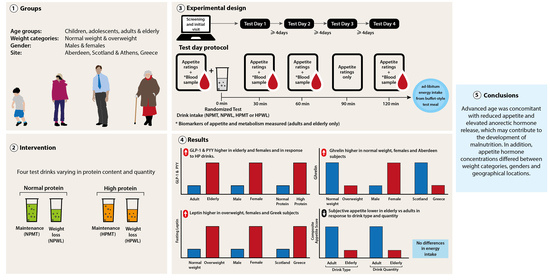
 anthropometric measurements,
anthropometric measurements,  LFPQ,
LFPQ,  appetite ratings (Likert scale, children and adolescents; VAS, adult and elderly),
appetite ratings (Likert scale, children and adolescents; VAS, adult and elderly),  blood sampling (adult and elderly cohorts only),
blood sampling (adult and elderly cohorts only),  randomized test drink intake (NPMT, NPWL, HPMT or HPWL),
randomized test drink intake (NPMT, NPWL, HPMT or HPWL),  ad libitum food intake from buffet-style test meal; (HPMT) high-protein maintenance, HPWL: high-protein weight loss, LFPQ: Leeds Food Preference Questionnaire, NPMT: normal-protein maintenance, NPWL: normal-protein weight loss, VAS: visual analog scale.
ad libitum food intake from buffet-style test meal; (HPMT) high-protein maintenance, HPWL: high-protein weight loss, LFPQ: Leeds Food Preference Questionnaire, NPMT: normal-protein maintenance, NPWL: normal-protein weight loss, VAS: visual analog scale.
 anthropometric measurements,
anthropometric measurements,  LFPQ,
LFPQ,  appetite ratings (Likert scale, children and adolescents; VAS, adult and elderly),
appetite ratings (Likert scale, children and adolescents; VAS, adult and elderly),  blood sampling (adult and elderly cohorts only),
blood sampling (adult and elderly cohorts only),  randomized test drink intake (NPMT, NPWL, HPMT or HPWL),
randomized test drink intake (NPMT, NPWL, HPMT or HPWL),  ad libitum food intake from buffet-style test meal; (HPMT) high-protein maintenance, HPWL: high-protein weight loss, LFPQ: Leeds Food Preference Questionnaire, NPMT: normal-protein maintenance, NPWL: normal-protein weight loss, VAS: visual analog scale.
ad libitum food intake from buffet-style test meal; (HPMT) high-protein maintenance, HPWL: high-protein weight loss, LFPQ: Leeds Food Preference Questionnaire, NPMT: normal-protein maintenance, NPWL: normal-protein weight loss, VAS: visual analog scale.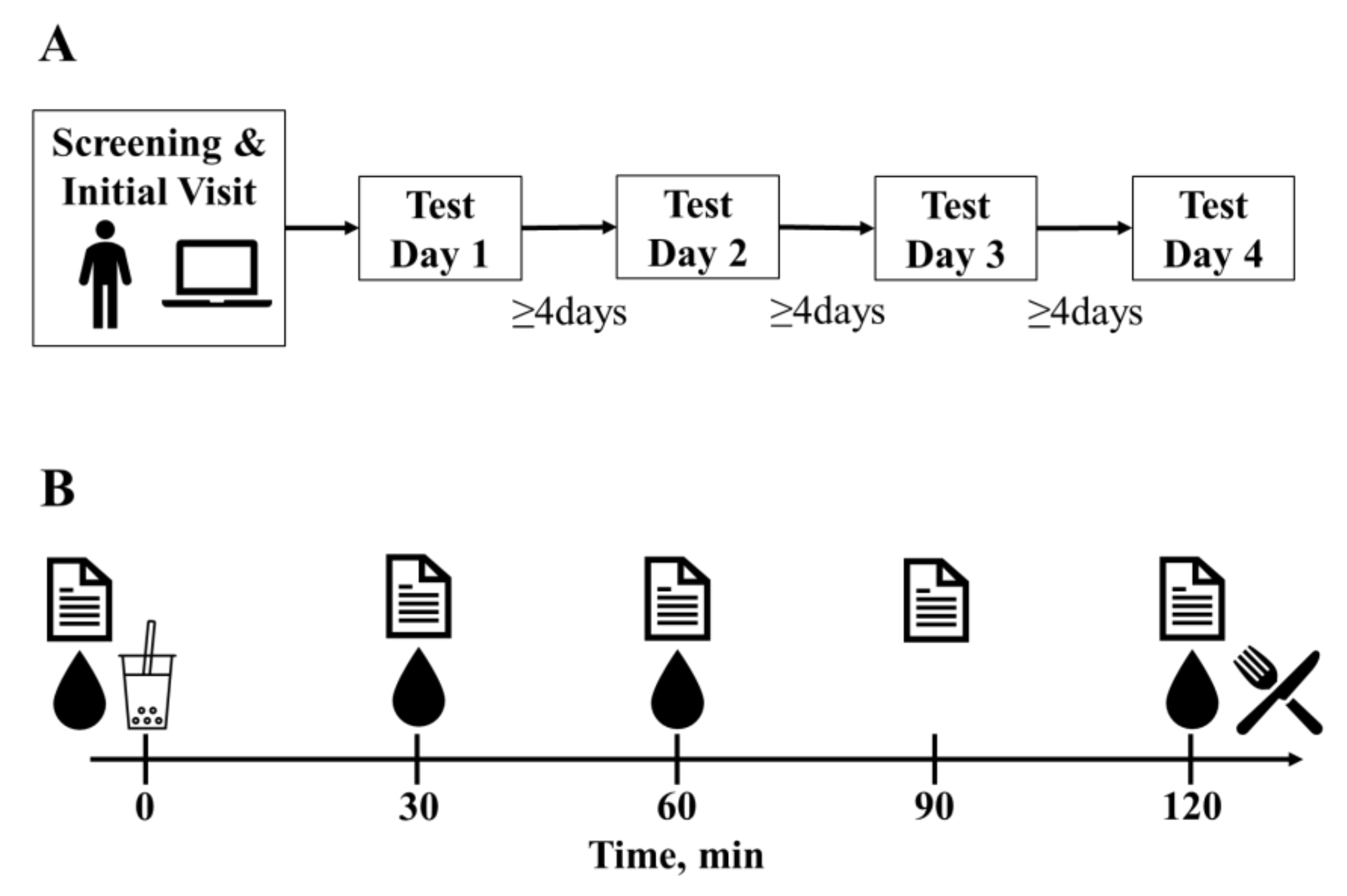



| Product (per 100 mL) | HP Drink | NP Drink | Whole Milk | |
|---|---|---|---|---|
| Total Energy | (kcal) | 130 | 130 | 63 |
| Protein | (g) | 10.0 | 5.0 | 3.4 |
| Energy (%) | 30.7 | 15.3 | 21.9 | |
| Casein (g) | 8.0 | 4.0 | 2.7 | |
| Whey (g) | 2.0 | 1.0 | 0.7 | |
| Fat | (g) | 3.5 | 3.5 | 3.6 |
| Energy (%) | 24.2 | 24.2 | 50.6 | |
| Carbohydrate | (g) | 14.7 | 19.7 | 4.6 |
| Energy (%) | 45.1 | 60.5 | 27.9 | |
| Lactose (g) | <0.06 | <0.06 | 4.6 | |
| Children | Adolescents | Adults | Elderly | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABDN (n = 39) | HUA (n = 64) | All (n = 103) | ABDN (n = 45) | HUA (n = 64) | All (n = 109) | ABDN (n = 46) | HUA (n = 51) | All (n = 97) | ABDN (n = 36) | HUA (n = 46) | All (n = 82) | |
| Age (years) | 8.72 ± 0.69 | 9.20 ± 0.65 | 9.02 ± 0.70 | 15.4 ± 1.28 | 14.5 ± 1.33 | 14.9 ± 1.37 | 29.9 ± 7.34 | 32.8 ± 6.71 | 31.4 ± 7.13 | 68.0 ± 3.82 | 68.5 ± 3.88 | 68.3 ± 3.84 |
| Height (m) | 1.37 ± 0.08 | 1.40 ± 0.06 | 1.39 ± 0.07 | 1.65 ± 0.09 | 1.67 ± 0.09 | 1.66 ± 0.09 | 1.71 ± 0.09 | 1.71 ± 0.10 | 1.71 ± 0.09 | 1.64 ± 0.08 | 1.62 ± 0.08 | 1.62 ± 0.08 |
| Weight (kg) | 31.6 ± 6.94 | 38.3 ± 8.39 | 35.7 ± 8.49 | 60.3 ± 12.3 | 65.7 ± 10.4 | 63.4 ± 11.5 | 71.0 ± 14.2 | 76.1 ± 14.4 | 73.7 ± 14.5 | 68.6 ± 11.9 | 75.7 ± 14.4 | 72.6 ± 13.7 |
| BMI (kg/m2) | 16.7 ± 2.41 | 19.5 ± 3.33 | 18.4 ± 3.31 | 22.2 ± 4.23 | 23.5 ± 2.82 | 23.0 ± 3.51 | 24.3 ± 4.07 | 25.9 ± 4.33 | 25.1 ± 4.27 | 25.5 ± 3.58 | 28.9 ± 4.71 | 27.4 ± 4.55 |
| BMR 2 (MJ) | 4.93 ± 0.62 | 5.52 ± 0.82 | 5.30 ± 0.8 | 6.70 ± 1.02 | 7.14 ± 1.04 | 6.96 ± 1.05 | 6.67 ± 1.04 | 7.18 ± 1.17 | 6.94 ± 1.13 | 5.73 ± 0.74 | 6.54 ± 1.00 | 6.19 ± 0.98 |
| Body fat 3 (%) | 22.8 ± 5.70 | 25.4 ± 5.93 | 24.4 ± 5.95 | 24.8 ± 8.47 | 24.8 ± 7.54 | 24.8 ± 7.90 | 23.6 ± 10.1 | 26.2 ± 9.95 | 24.9 ± 10.1 | 32.4 ± 6.78 | 35.4 ± 6.78 | 34.2 ± 6.90 |
| Waist Circumference 4 (cm) | 64.5 ± 8.56 | 71.8 ± 12.4 | 69.6 ± 11.8 | 81.6 ± 10.9 | 84.9 ± 9.75 | 83.7 ± 10.3 | 91.4 ± 13.0 | 96.9 ± 12.4 | 94.4 ± 12.9 | 100 ± 14.0 | 109 ± 12.8 | 106 ± 14.0 |
| Visceral Fat 4 (%) | 3.67 ± 2.23 | 4.95 ± 3.25 | 4.58 ± 3.03 | 5.43 ± 3.67 | 6.05 ± 3.28 | 5.81 ± 3.43 | 7.72 ± 4.92 | 9.64 ± 4.64 | 8.76 ± 4.84 | 10.4 ± 4.25 | 15.1 ± 6.76 | 13.2 ± 6.29 |
| NPWL | NPMT | HPWL | HPMT | SEDtype | ptype2 | ||
|---|---|---|---|---|---|---|---|
| Energy (kcal) | Children (n = 102) | 306 | 425 | 304 | 425 | 10 | <0.001 |
| Adolescents (n = 108) | 406 | 565 | 402 | 557 | |||
| Adults (n = 97) | 399 | 553 | 395 | 548 | |||
| Elderly (n = 82) | 348 | 483 | 343 | 478 | |||
| Protein (g) | Children (n = 102) | 11.4 | 15.9 | 23.3 | 32.6 | 0.6 | <0.001 |
| Adolescents (n = 108) | 15.2 | 21.1 | 30.8 | 42.7 | |||
| Adults (n = 97) | 14.9 | 20.6 | 30.3 | 42.0 | |||
| Elderly (n = 82) | 13.0 | 18.0 | 26.3 | 36.6 | |||
| Group | NPWL | NPMT | HPWL | HPMT | SEDtype | ptype2 | NP | HP | SEDcomposition | pcomposition2 | WL | MT | SEDquantity | pquantity2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Children (n = 103) | 718 | 641 | 679 | 646 | 29 | 0.483 | 679 | 663 | 21 | 0.941 | 699 | 644 | 21 | 0.074 |
| Adolescents (n = 109) | 950 | 876 | 940 | 852 | 914 | 895 | 945 | 864 | |||||||
| Adults (n = 97) | 672 | 603 | 641 | 627 | 639 | 634 | 658 | 615 | |||||||
| Elderly (n = 82) | 526 | 522 | 478 | 500 | 524 | 490 | 502 | 510 | |||||||
| BMI | Normal Weight (n = 221) | 701 | 644 | 656 | 636 | 21 | 0.928 | 672 | 646 | 15 | 0.700 | 677 | 639 | 15 | 0.858 |
| Overweight (n = 170) | 732 | 677 | 715 | 675 | 706 | 696 | 725 | 677 | |||||||
| Gender | Males (n = 171) | 825 | 751 | 773 | 744 | 21 | 0.166 | 789 | 758 | 15 | 0.220 | 799 | 749 | 15 | 0.123 |
| Females (n = 220) | 608 | 569 | 598 | 567 | 589 | 581 | 603 | 567 | |||||||
| Site | HUA (n = 225) | 806 | 766 | 797 | 756 | 21 | 0.245 | 787 | 778 | 15 | 0.272 | 801 | 761 | 15 | 0.897 |
| ABDN (n = 166) | 627 | 555 | 572 | 555 | 591 | 565 | 600 | 555 | |||||||
| Group | NPWL | NPMT | HPWL | HPMT | SEDtype | ptype2 | NP | HP | SEDcomposition | pcomposition2 | WL | MT | SEDquantity | pquantity2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Children (n = 103) | 1022 | 1075 | 991 | 1077 | 50 | 0.562 | 1049 | 1034 | 46 | 0.793 | 1007 | 1076 | 46 | 0.143 |
| Adolescents (n = 109) | 1372 | 1461 | 1352 | 1416 | 1416 | 1384 | 1362 | 1438 | |||||||
| Adults (n = 97) | 1078 | 1168 | 1048 | 1186 | 1123 | 1117 | 1063 | 1177 | |||||||
| Elderly (n = 82) | 885 | 1022 | 835 | 993 | 953 | 914 | 860 | 1008 | |||||||
| BMI | Normal Weight (n = 221) | 1235 | 1325 | 1,85 | 1314 | 31 | 0.625 | 1280 | 1249 | 29 | 0.499 | 1210 | 1319 | 29 | 0.940 |
| Overweight (n = 170) | 943 | 1038 | 928 | 1023 | 991 | 975 | 936 | 1030 | |||||||
| Gender | Males (n = 171) | 1044 | 1126 | 996 | 1112 | 31 | 0.775 | 1085 | 1054 | 29 | 0.751 | 1020 | 1119 | 29 | 0.455 |
| Females (n = 220) | 1134 | 1237 | 1117 | 1225 | 1185 | 1171 | 1125 | 1231 | |||||||
| Site | HUA (n = 225) | 995 | 1075 | 942 | 1063 | 30 | 0.287 | 1035 | 1002 | 28 | 0.239 | 968 | 1069 | 28 | 0.862 |
| ABDN (n = 166) | 1183 | 1288 | 1171 | 1274 | 1236 | 1222 | 1177 | 1281 | |||||||
| Time (mins) | NPWL | NPMT | HPWL | HPMT | SEDtype | Type.Time Interaction | NP | HP | SEDcomposition | Composition.Time Interaction | WL | MT | SEDquantity | Quantity.Time Interaction | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p3 | p3 | p3 | ||||||||||||||
| Fullness | Children | 0 | 2.25 | 2.02 | 1.77 | 2.19 | 0.21 | 0.252 | 2.13 | 1.98 | 0.16 | 0.220 | 2.01 | 2.10 | 0.16 | 0.554 |
| 30 | 3.73 | 3.86 | 3.37 | 3.71 | 3.79 | 3.54 | 3.55 | 3.78 | ||||||||
| 60 | 3.06 | 3.14 | 3.02 | 2.83 | 3.10 | 2.92 | 3.04 | 2.98 | ||||||||
| 90 | 2.60 | 2.75 | 2.52 | 2.51 | 2.68 | 2.51 | 2.56 | 2.63 | ||||||||
| 120 | 2.13 | 2.28 | 2.33 | 2.19 | 2.21 | 2.26 | 2.23 | 2.23 | ||||||||
| Adolescents | 0 | 2.69 | 2.90 | 2.95 | 2.95 | 0.18 | 2.80 | 2.95 | 0.14 | 2.82 | 2.92 | 0.14 | ||||
| 30 | 4.49 | 4.85 | 5.06 | 5.13 | 4.67 | 5.10 | 4.78 | 4.99 | ||||||||
| 60 | 4.15 | 4.44 | 4.52 | 4.59 | 4.29 | 4.56 | 4.33 | 4.52 | ||||||||
| 90 | 3.65 | 3.94 | 3.91 | 4.06 | 3.80 | 3.98 | 3.78 | 4.00 | ||||||||
| 120 | 3.28 | 3.53 | 3.59 | 3.59 | 3.40 | 3.59 | 3.43 | 3.56 | ||||||||
| PFC | Children | 0 | 6.61 | 6.89 | 7.05 | 7.16 | 0.19 | 0.332 | 6.75 | 7.10 | 0.16 | 0.209 | 6.83 | 7.02 | 0.16 | 0.653 |
| 30 | 6.00 | 6.00 | 6.22 | 5.90 | 6.00 | 6.06 | 6.11 | 5.95 | ||||||||
| 60 | 6.70 | 6.55 | 6.54 | 6.96 | 6.62 | 6.75 | 6.62 | 6.76 | ||||||||
| 90 | 7.14 | 7.14 | 7.05 | 7.26 | 7.14 | 7.16 | 7.10 | 7.20 | ||||||||
| 120 | 7.62 | 7.65 | 7.55 | 7.60 | 7.64 | 7.57 | 7.58 | 7.63 | ||||||||
| Adolescents | 0 | 6.19 | 6.06 | 6.05 | 5.90 | 0.21 | 6.13 | 5.98 | 0.14 | 6.12 | 5.98 | 0.14 | ||||
| 30 | 5.08 | 4.63 | 4.58 | 4.31 | 4.85 | 4.44 | 4.83 | 4.47 | ||||||||
| 60 | 5.47 | 5.05 | 5.08 | 4.96 | 5.26 | 5.02 | 5.27 | 5.01 | ||||||||
| 90 | 5.85 | 5.66 | 5.61 | 5.61 | 5.76 | 5.61 | 5.73 | 5.64 | ||||||||
| 120 | 6.42 | 6.08 | 6.07 | 6.10 | 6.25 | 6.09 | 6.25 | 6.09 | ||||||||
| Time (mins) | NPWL | NPMT | HPWL | HPMT | SEDtype | Type.Time Interaction | NP | HP | SEDcomposition | Composition.Time Interaction | WL | MT | SEDquantity | Quantity.Time Interaction | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p3 | p3 | p3 | ||||||||||||||
| Appetite Score (mm) | Adults | 0 | 63.2 | 65.6 | 61.5 | 62.8 | 2.1 | 0.004 | 64.4 | 62.1 | 1.6 | 0.624 | 62.3 | 64.2 | 1.6 | < 0.001 |
| 30 | 42.6 | 35.5 | 41.2 | 34.8 | 39.0 | 38.0 | 41.9 | 35.1 | ||||||||
| 60 | 46.7 | 40.3 | 44.6 | 37.9 | 43.5 | 41.3 | 45.7 | 39.1 | ||||||||
| 90 | 52.0 | 44.8 | 49.6 | 42.4 | 48.4 | 46.0 | 50.8 | 43.6 | ||||||||
| 120 | 55.9 | 50.9 | 54.6 | 48.1 | 53.4 | 51.4 | 55.3 | 49.5 | ||||||||
| Elderly | 0 | 44.5 | 43.7 | 45.4 | 43.9 | 2.7 | 44.1 | 44.6 | 2.1 | 44.9 | 43.8 | 2.1 | ||||
| 30 | 31.4 | 28.6 | 29.7 | 31.3 | 30.0 | 30.5 | 30.5 | 30.0 | ||||||||
| 60 | 35.2 | 33.7 | 34.7 | 33.0 | 34.4 | 33.9 | 35.0 | 33.3 | ||||||||
| 90 | 40.9 | 37.5 | 39.1 | 36.5 | 39.2 | 37.8 | 40.0 | 37.0 | ||||||||
| 120 | 44.3 | 41.9 | 43.2 | 38.3 | 43.1 | 40.8 | 43.7 | 40.1 | ||||||||
| Drink | Time (min) | GLP-1 (pg/mL) | SED | Drink.Time Interaction, p 2 | PYY (pg/mL) | SED | Drink.Time Interaction, p 2 | Ghrelin (pg/mL) | SED | Drink.Time Interaction, p 2 |
|---|---|---|---|---|---|---|---|---|---|---|
| NPWL | 0 | 12.7 | 1.3 | <0.001 | 46.1 | 1.9 | <0.001 | 773 | 12 | <0.005 |
| 30 | 33.1 | 68.3 | 651 | |||||||
| 60 | 26.6 | 69.6 | 596 | |||||||
| 120 | 21.8 | 65.2 | 604 | |||||||
| NPMT | 0 | 11.9 | 43.5 | 762 | ||||||
| 30 | 37.1 | 71.3 | 659 | |||||||
| 60 | 31.3 | 74.3 | 584 | |||||||
| 120 | 28.7 | 73.4 | 574 | |||||||
| HPWL | 0 | 12.1 | 44.9 | 767 | ||||||
| 30 | 31.3 | 63.2 | 661 | |||||||
| 60 | 27.6 | 63.4 | 603 | |||||||
| 120 | 26.3 | 64.2 | 622 | |||||||
| HPMT | 0 | 12.5 | 45.2 | 763 | ||||||
| 30 | 33.9 | 66.4 | 653 | |||||||
| 60 | 31.1 | 67.3 | 578 | |||||||
| 120 | 28.7 | 66.5 | 572 | |||||||
| NP | 0 | 12.3 | 1.1 | <0.001 | 44.8 | 1.5 | <0.001 | 767 | 9 | 0.710 |
| 30 | 35.1 | 69.8 | 655 | |||||||
| 60 | 29.0 | 72.0 | 590 | |||||||
| 120 | 25.2 | 69.3 | 589 | |||||||
| HP | 0 | 12.3 | 45.1 | 765 | ||||||
| 30 | 32.6 | 64.8 | 657 | |||||||
| 60 | 29.4 | 65.4 | 591 | |||||||
| 120 | 27.5 | 65.3 | 597 | |||||||
| WL | 0 | 12.4 | 1.1 | <0.001 | 45.5 | 1.5 | <0.001 | 770 | 9 | < 0.001 |
| 30 | 32.2 | 65.7 | 656 | |||||||
| 60 | 27.1 | 66.5 | 599 | |||||||
| 120 | 24.1 | 64.7 | 613 | |||||||
| MT | 0 | 12.2 | 44.4 | 763 | ||||||
| 30 | 35.5 | 68.8 | 656 | |||||||
| 60 | 31.2 | 70.8 | 581 | |||||||
| 120 | 28.7 | 69.9 | 573 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crabtree, D.R.; Buosi, W.; Fyfe, C.L.; Horgan, G.W.; Manios, Y.; Androutsos, O.; Giannopoulou, A.; Finlayson, G.; Beaulieu, K.; Meek, C.L.; et al. Appetite Control across the Lifecourse: The Acute Impact of Breakfast Drink Quantity and Protein Content. The Full4Health Project. Nutrients 2020, 12, 3710. https://doi.org/10.3390/nu12123710
Crabtree DR, Buosi W, Fyfe CL, Horgan GW, Manios Y, Androutsos O, Giannopoulou A, Finlayson G, Beaulieu K, Meek CL, et al. Appetite Control across the Lifecourse: The Acute Impact of Breakfast Drink Quantity and Protein Content. The Full4Health Project. Nutrients. 2020; 12(12):3710. https://doi.org/10.3390/nu12123710
Chicago/Turabian StyleCrabtree, Daniel R., William Buosi, Claire L. Fyfe, Graham W. Horgan, Yannis Manios, Odysseas Androutsos, Angeliki Giannopoulou, Graham Finlayson, Kristine Beaulieu, Claire L. Meek, and et al. 2020. "Appetite Control across the Lifecourse: The Acute Impact of Breakfast Drink Quantity and Protein Content. The Full4Health Project" Nutrients 12, no. 12: 3710. https://doi.org/10.3390/nu12123710
APA StyleCrabtree, D. R., Buosi, W., Fyfe, C. L., Horgan, G. W., Manios, Y., Androutsos, O., Giannopoulou, A., Finlayson, G., Beaulieu, K., Meek, C. L., Holst, J. J., Van Norren, K., Mercer, J. G., Johnstone, A. M., & on behalf of the Full4Health-Study Group. (2020). Appetite Control across the Lifecourse: The Acute Impact of Breakfast Drink Quantity and Protein Content. The Full4Health Project. Nutrients, 12(12), 3710. https://doi.org/10.3390/nu12123710