One-Step Catalytic or Photocatalytic Oxidation of Benzene to Phenol: Possible Alternative Routes for Phenol Synthesis?
Abstract
:1. Introduction
- (1)
- PHENOLIC RESINS: by the reaction of phenol or substituted phenol with formaldehyde, phenol–formaldehyde resins or phenolic resins can be obtained. The first example was Bakelite as a commercial synthetic resin [2].
- (2)
- POLYCARBONATES (a very pure phenol feed is required): polycarbonates are thermoplastic polymers containing carbonate groups in their chains [3].
- (3)
- EPOXY RESINS: epoxy phenolic resins are resins modified at the phenolic hydroxyl group to include an epoxide functional group. This addition increases the ability of the resin to crosslink, creating a stronger polymer [4].
- (4)
- INTERMEDIATE FOR CAPROLACTAM (nylon production): caprolactam is a monomer for nylon production. Among the routes for its manufacture, one is via cyclohexanone and cyclohexanone oxime. Cyclohexanone can be prepared either from phenol or from cyclohexane. The phenol route is a two-stage process, in which the first stage foresees the reaction among phenol and hydrogen in the presence of a nickel catalyst at around 180 °C to form cyclohexanol, subsequently dehydrogenated at around 400 °C in the presence of a copper catalyst to yield the cyclohexanone [5].
2. Homogeneous and Heterogeneous Catalysts for the One-Step Catalytic Oxidation of Benzene to Phenol in Liquid Phase
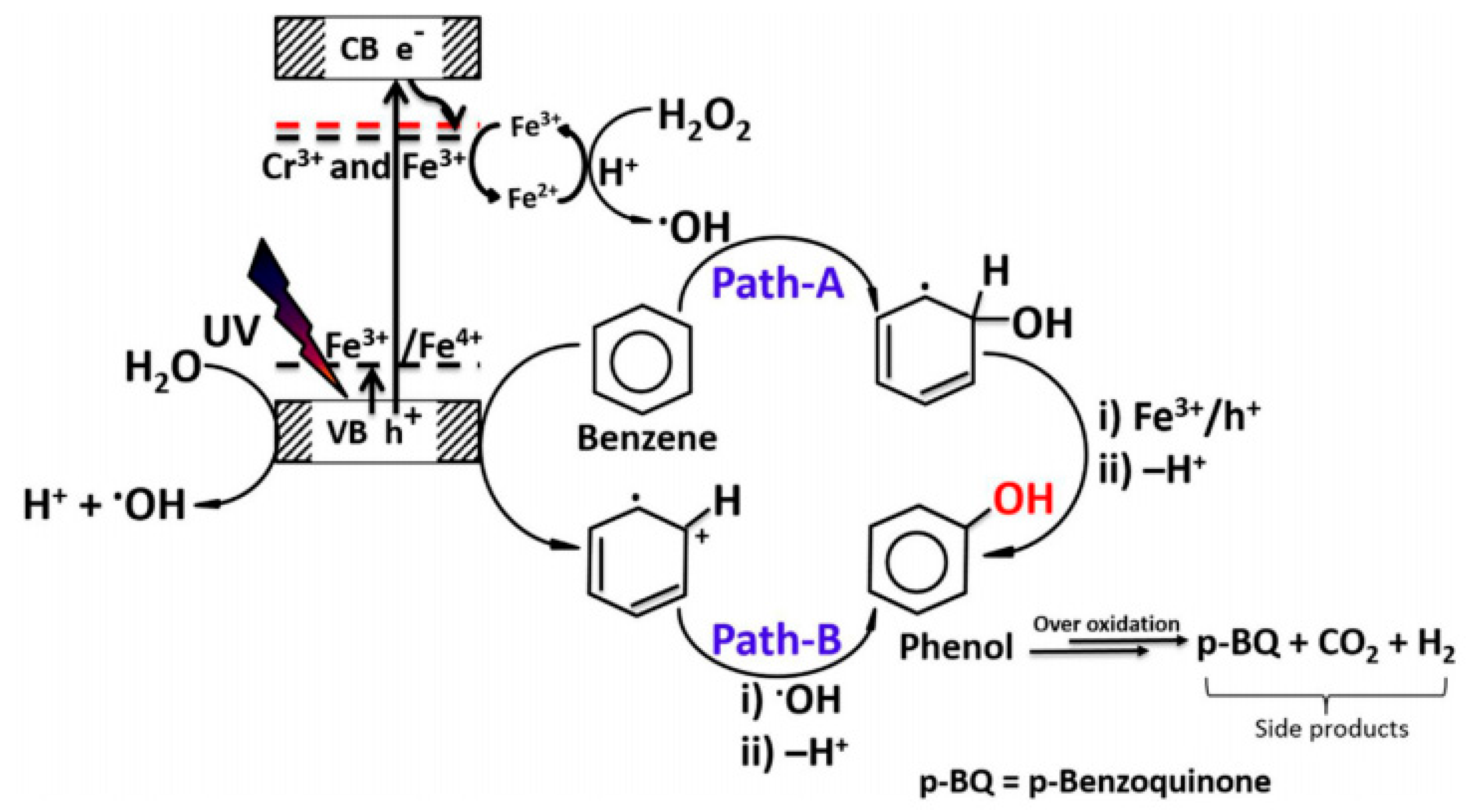
3. Homogeneous and Heterogeneous Photocatalysts for the One-Step Catalytic Oxidation of Benzene to Phenol in Liquid Phase
- (1)
- hydroxylation of benzene with water;
- (2)
- coupling of benzene;
- (3)
- reduction of the produced phenol and successive oxidation of the produced cyclohexanol to cyclohexanone;
- (4)
- decomposition of benzene with water.
4. Concluding Remarks and Perspectives
- the immobilization of the catalysts or photocatalysts on macroscopic supports (i.e., the development of structured catalysts) to avoid the separation of catalyst powders from the liquid phase containing phenol at the end of the oxidation step;
- the development of structured catalysts or photocatalysts with high stability and which are easily recyclable;
- the development of novel selective oxidation systems (e.g., highly efficient photoanodes for the photoelectrocatalytic oxidation of benzene to phenol);
- the design of efficient and low-cost systems to recover the produced phenol from the liquid phase.
Author Contributions
Funding
Conflicts of Interest
References
- Schmidt, R.J. Industrial catalytic processes—Phenol production. Appl. Catal. A Gen. 2005, 280, 89–103. [Google Scholar] [CrossRef]
- Solyman, W.S.; Nagiub, H.M.; Alian, N.A.; Shaker, N.O.; Kandil, U.F. Synthesis and characterization of phenol/formaldehyde nanocomposites: Studying the effect of incorporating reactive rubber nanoparticles or Cloisite-30B nanoclay on the mechanical properties, morphology and thermal stability. J. Radiat. Res. Appl. Sci. 2017, 10, 72–79. [Google Scholar] [CrossRef]
- Pryde, C.; Hellman, M. Solid state hydrolysis of bisphenol-A polycarbonate. I. Effect of phenolic end groups. J. Appl. Polym. Sci. 1980, 25, 2573–2587. [Google Scholar] [CrossRef]
- Takeichi, T.; Furukawa, N. Epoxy Resins and Phenol-Formaldehyde Resins. In Polymer Science: A Comprehensive Reference; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 723–751. [Google Scholar]
- Brydson, J.A. Plastics Materials; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Zakoshansky, V. The cumene process for phenol-acetone production. Pet. Chem. 2007, 47, 273–284. [Google Scholar] [CrossRef]
- Fortuin, J.; Waterman, H. Production of phenol from cumene. Chem. Eng. Sci. 1953, 2, 182–192. [Google Scholar] [CrossRef]
- The Essential Chemical Industry—Online. Available online: https://www.essentialchemicalindustry.org/chemicals/phenol.html (accessed on 2 October 2020).
- Park, H.; Choi, W. Photocatalytic conversion of benzene to phenol using modified TiO2 and polyoxometalates. Catal. Today 2005, 101, 291–297. [Google Scholar] [CrossRef]
- Molinari, R.; Poerio, T. Remarks on studies for direct production of phenol in conventional and membrane reactors. Asia Pac. J. Chem. Eng. 2010, 5, 191–206. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K. One-Step Selective Hydroxylation of Benzene to Phenol. Asian J. Org. Chem. 2015, 4, 836–845. [Google Scholar] [CrossRef]
- Ottenbacher, R.V.; Talsi, E.P.; Bryliakov, K.P. Recent progress in catalytic oxygenation of aromatic C–H groups with the environmentally benign oxidants H2O2 and O2. Appl. Organomet. Chem. 2020, 34, e5900. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, W.; Han, B. Catalytic hydroxylation of benzene to phenol with hydrogen peroxide using catalysts based on molecular sieves. New J. Chem. 2013, 37, 1654–1664. [Google Scholar] [CrossRef]
- Wanna, W.H.; Janmanchi, D.; Thiyagarajan, N.; Ramu, R.; Tsai, Y.-F.; Yu, S.S. Selective Oxidation of Simple Aromatics Catalyzed by Nano-Biomimetic Metal Oxide Catalysts: A Mini Review. Front. Chem. 2020, 8, 589178. [Google Scholar] [CrossRef] [PubMed]
- Yuranov, I.; Bulushev, D.A.; Renken, A.; Kiwi-Minsker, L. Benzene to phenol hydroxylation with N2O over Fe-Beta and Fe-ZSM-5: Comparison of activity per Fe-site. Appl. Catal. A Gen. 2007, 319, 128–136. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Z.; Mei, F.; Zhu, D.; Xiong, H.; Yin, G. Redox Inactive Metal Ion Promoted C H Activation of Benzene to Phenol with PdII (bpym): Demonstrating New Strategies in Catalyst Designs. Chem. Asian J. 2013, 8, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, C.; Ye, L.; Wu, Y.; Yue, B.; Chen, X.; He, H. Direct hydroxylation of benzene to phenol using H2O2 as an oxidant over vanadium-containing mesoporous carbon catalysts. Appl. Catal. A Gen. 2015, 504, 440–447. [Google Scholar] [CrossRef]
- Parida, K.; Rath, D. Structural properties and catalytic oxidation of benzene to phenol over CuO-impregnated mesoporous silica. Appl. Catal. A Gen. 2007, 321, 101–108. [Google Scholar] [CrossRef]
- Niwa, S.-i.; Eswaramoorthy, M.; Nair, J.; Raj, A.; Itoh, N.; Shoji, H.; Namba, T.; Mizukami, F. A one-step conversion of benzene to phenol with a palladium membrane. Science 2002, 295, 105–107. [Google Scholar] [CrossRef]
- Al-Sabagh, A.; Yehia, F.; Eshaq, G.; ElMetwally, A. Eclectic hydroxylation of benzene to phenol using ferrites of Fe and Zn as durable and magnetically retrievable catalysts. ACS Sustain. Chem. Eng. 2017, 5, 4811–4819. [Google Scholar] [CrossRef]
- Monfared, H.H.; Amouei, Z. Hydrogen peroxide oxidation of aromatic hydrocarbons by immobilized iron (III). J. Mol. Catal. A Chem. 2004, 217, 161–164. [Google Scholar] [CrossRef]
- Lyu, Y.-J.; Qi, T.; Yang, H.-Q.; Hu, C.-W. Performance of edges on carbon for the catalytic hydroxylation of benzene to phenol. Catal. Sci. Technol. 2018, 8, 176–186. [Google Scholar] [CrossRef]
- Yamada, M.; Karlin, K.D.; Fukuzumi, S. One-step selective hydroxylation of benzene to phenol with hydrogen peroxide catalysed by copper complexes incorporated into mesoporous silica–alumina. Chem. Sci. 2016, 7, 2856–2863. [Google Scholar] [CrossRef] [Green Version]
- Wanna, W.H.; Ramu, R.; Janmanchi, D.; Tsai, Y.-F.; Thiyagarajan, N.; Yu, S.S.-F. An efficient and recyclable copper nano-catalyst for the selective oxidation of benzene to p-benzoquinone (p-BQ) using H2O2 (aq) in CH3CN. J. Catal. 2019, 370, 332–346. [Google Scholar] [CrossRef]
- Peng, J.; Shi, F.; Gu, Y.; Deng, Y. Highly selective and green aqueous–ionic liquid biphasic hydroxylation of benzene to phenol with hydrogen peroxide. Green Chem. 2003, 5, 224–226. [Google Scholar] [CrossRef]
- Nomiya, K.; Yagishita, K.; Nemoto, Y.; Kamataki, T.-a. Functional action of Keggin-type mono-vanadium (V)-substituted heteropolymolybdate as a single species on catalytic hydroxylation of benzene in the presence of hydrogen peroxide. J. Mol. Catal. A Chem. 1997, 126, 43–53. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, W.; Li, H.; Chao, Y.; Xun, S.; Chang, Y.; Liu, H.; Zhao, Z. Mechanism and optimization for oxidative desulfurization of fuels catalyzed by Fenton-like catalysts in hydrophobic ionic liquid. J. Mol. Catal. A Chem. 2014, 382, 8–14. [Google Scholar] [CrossRef]
- Anandababu, K.; Muthuramalingam, S.; Velusamy, M.; Mayilmurugan, R. Single-step benzene hydroxylation by cobalt (ii) catalysts via a cobalt (iii)-hydroperoxo intermediate. Catal. Sci. Technol. 2020, 10, 2540–2548. [Google Scholar] [CrossRef]
- Muthuramalingam, S.; Anandababu, K.; Velusamy, M.; Mayilmurugan, R. One step phenol synthesis from benzene catalysed by nickel (ii) complexes. Catal. Sci. Technol. 2019, 9, 5991–6001. [Google Scholar] [CrossRef]
- You, X.; Wei, Z.; Wang, H.; Li, D.; Liu, J.; Xu, B.; Liu, X. Synthesis of two copper clusters and their catalysis towards the oxidation of benzene into phenol. RSC Adv. 2014, 4, 61790–61798. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Lamaty, F.; Bantreil, X.; Villemejeanne, B. Si10Cu6N4 cage hexacoppersilsesquioxanes containing N ligands: Synthesis, structure, and high catalytic activity in peroxide oxidations. Inorg. Chem. 2017, 56, 15026–15040. [Google Scholar] [CrossRef]
- Tsuji, T.; Zaoputra, A.A.; Hitomi, Y.; Mieda, K.; Ogura, T.; Shiota, Y.; Yoshizawa, K.; Sato, H.; Kodera, M. Specific enhancement of catalytic activity by a dicopper core: Selective hydroxylation of benzene to phenol with hydrogen peroxide. Angew. Chem. Int. Ed. 2017, 129, 7887–7890. [Google Scholar] [CrossRef]
- Conde, A.; Diaz-Requejo, M.M.; Pérez, P.J. Direct, copper-catalyzed oxidation of aromatic C–H bonds with hydrogen peroxide under acid-free conditions. Chem. Commun. 2011, 47, 8154–8156. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Muthuramalingam, S.; Dhara, A.K.; Singh, U.; Mayilmurugan, R.; Ghosh, K. Cu (I) complexes obtained via spontaneous reduction of Cu (II) complexes supported by designed bidentate ligands: Bioinspired Cu (I) based catalysts for aromatic hydroxylation. Dalton Trans. 2020, 49, 13829–13839. [Google Scholar] [CrossRef] [PubMed]
- Kudrik, E.V.; Sorokin, A.B. N-Bridged Diiron Phthalocyanine Catalyzes Oxidation of Benzene with H2O2 via Benzene Oxide with NIH Shift Evidenced by Using 1, 3, 5-[D3] Benzene as a Probe. Chem. Eur. J. 2008, 14, 7123–7126. [Google Scholar] [PubMed]
- Raba, A.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. Catalytic hydroxylation of benzene and toluene by an iron complex bearing a chelating di-pyridyl-di-NHC ligand. Chem. Commun. 2014, 50, 11454–11457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramu, R.; Wanna, W.H.; Janmanchi, D.; Tsai, Y.-F.; Liu, C.-C.; Mou, C.-Y.; Yu, S.S.-F. Mechanistic study for the selective oxidation of benzene and toluene catalyzed by Fe (ClO4) 2 in an H2O2-H2O-CH3CN system. Mol. Catal. 2017, 441, 114–121. [Google Scholar] [CrossRef]
- Yalymov, A.I.; Bilyachenko, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Shul’pina, L.S.; Dorovatovskii, P.V.; Es’kova, M.A.; Lamaty, F.; Bantreil, X. High catalytic activity of heterometallic (Fe6Na7 and Fe6Na6) cage silsesquioxanes in oxidations with peroxides. Catalysts 2017, 7, 101. [Google Scholar] [CrossRef]
- Vinogradov, M.M.; Kozlov, Y.N.; Nesterov, D.S.; Shul’pina, L.S.; Pombeiro, A.J.; Shul’pin, G.B. Oxidation of hydrocarbons with H2O2/O2 catalyzed by osmium complexes containing p-cymene ligands in acetonitrile. Catal. Sci. Technol. 2014, 4, 3214–3226. [Google Scholar] [CrossRef]
- Sarma, B.B.; Carmieli, R.; Collauto, A.; Efremenko, I.; Martin, J.M.; Neumann, R. Electron transfer oxidation of benzene and aerobic oxidation to phenol. ACS Catal. 2016, 6, 6403–6407. [Google Scholar] [CrossRef]
- Li, X.; Xue, H.; Lin, Q.; Yu, A. Amphiphilic poly (ionic liquid)/Wells–Dawson-type phosphovanadomolybdate ionic composites as efficient and recyclable catalysts for the direct hydroxylation of benzene with H2O2. Appl. Organomet. Chem. 2020, 34, e5606. [Google Scholar] [CrossRef]
- Carneiro, L.; Silva, A.R. Selective direct hydroxylation of benzene to phenol with hydrogen peroxide by iron and vanadyl based homogeneous and heterogeneous catalysts. Catal. Sci. Technol. 2016, 6, 8166–8176. [Google Scholar] [CrossRef]
- Dong, Y.; Niu, X.; Song, W.; Wang, D.; Chen, L.; Yuan, F.; Zhu, Y. Facile synthesis of vanadium oxide/reduced graphene oxide composite catalysts for enhanced hydroxylation of benzene to phenol. Catalysts 2016, 6, 74. [Google Scholar] [CrossRef]
- Shijina, A.V.; Renuka, N.K. Single step conversion of benzene to phenol using hydrogen peroxide over modified V2O5–Al2O3 systems. React. Kinet. Catal. Lett. 2009, 98, 139–147. [Google Scholar] [CrossRef]
- Peng, G.; Fu, Z.; Yin, D.; Zhong, S.; Yang, Y.; Yu, N.; Yin, D. A Promising Coupled Process of Pd/γ-Al2O3–NH4VO3 Catalyzing the Hydroxylation of Benzene with Hydrogen Peroxide Produced In Situ by an Anthraquinone Redox Route. Catal. Lett. 2007, 118, 270–274. [Google Scholar] [CrossRef]
- Jiang, W.-F.; Wang, W.; Wang, H.-L.; Li, Z.-Q. Photooxidation of benzene to phenol by Al2O3-supported Fe (III)-5-sulfosalicylic acid (ssal) complex. Catal. Lett. 2009, 130, 463–469. [Google Scholar] [CrossRef]
- Miyahara, T.; Kanzaki, H.; Hamada, R.; Kuroiwa, S.; Nishiyama, S.; Tsuruya, S. Liquid-phase oxidation of benzene to phenol by CuO–Al2O3 catalysts prepared by co-precipitation method. J. Mol. Catal. A Chem. 2001, 176, 141–150. [Google Scholar] [CrossRef]
- Masumoto, Y.-k.; Hamada, R.; Yokota, K.; Nishiyama, S.; Tsuruya, S. Liquid-phase oxidation of benzene to phenol by vanadium catalysts in aqueous solvent with high acetic acid concentration. J. Mol. Catal. A Chem. 2002, 184, 215–222. [Google Scholar] [CrossRef]
- Miyake, T.; Hamada, M.; Sasaki, Y.; Oguri, M. Direct synthesis of phenol by hydroxylation of benzene with oxygen and hydrogen. Appl. Catal. A Gen. 1995, 131, 33–42. [Google Scholar] [CrossRef]
- Lemke, K.; Ehrich, H.; Lohse, U.; Berndt, H.; Jähnisch, K. Selective hydroxylation of benzene to phenol over supported vanadium oxide catalysts. Appl. Catal. A Gen. 2003, 243, 41–51. [Google Scholar] [CrossRef]
- Tanarungsun, G.; Kiatkittipong, W.; Praserthdam, P.; Yamada, H.; Tagawa, T.; Assabumrungrat, S. Ternary metal oxide catalysts for selective oxidation of benzene to phenol. J. Ind. Eng. Chem. 2008, 14, 596–601. [Google Scholar] [CrossRef]
- Tanarungsun, G.; Kiatkittipong, W.; Praserthdam, P.; Yamada, H.; Tagawa, T.; Assabumrungrat, S. Hydroxylation of benzene to phenol on Fe/TiO2 catalysts loaded with different types of second metal. Catal. Commun. 2008, 9, 1886–1890. [Google Scholar] [CrossRef]
- Lee, C.W.; Lee, W.J.; Park, Y.K.; Park, S.-E. Catalytic hydroxylation of benzene over vanadium-containing molecular sieves. Catal. Today 2000, 61, 137–141. [Google Scholar] [CrossRef]
- He, J.; Xu, W.-p.; Evans, D.G.; Duan, X.; Li, C.-y. Role of pore size and surface properties of Ti-MCM-41 catalysts in the hydroxylation of aromatics in the liquid phase. Microporous Mesoporous Mater. 2001, 44, 581–586. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Zhao, X.-H.; Zhang, G.-R.; Ding, H.-M.; Shan, Y.-K. Selective hydroxylation of benzene using dioxygen activated by vanadium–copper oxide catalysts supported on SBA-15. Appl. Catal. A Gen. 2007, 328, 150–155. [Google Scholar] [CrossRef]
- Jourshabani, M.; Badiei, A.; Shariatinia, Z.; Lashgari, N.; Mohammadi Ziarani, G. Fe-supported SBA-16 type cagelike mesoporous silica with enhanced catalytic activity for direct hydroxylation of benzene to phenol. Ind. Eng. Chem. Res. 2016, 55, 3900–3908. [Google Scholar] [CrossRef]
- Abbo, H.S.; Titinchi, S.J. Di-, tri-and tetra-valent ion-exchanged NaY zeolite: Active heterogeneous catalysts for hydroxylation of benzene and phenol. Appl. Catal. A Gen. 2009, 356, 167–171. [Google Scholar] [CrossRef]
- Yang, J.-H.; Sun, G.; Gao, Y.; Zhao, H.; Tang, P.; Tan, J.; Lu, A.-H.; Ma, D. Direct catalytic oxidation of benzene to phenol over metal-free graphene-based catalyst. Energy Environ. Sci. 2013, 6, 793–798. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.; Yue, B.; Chen, X.; He, H. Direct hydroxylation of benzene to phenol using H2O2 as an oxidant over vanadium-containing nitrogen doped mesoporous carbon catalysts. RSC Adv. 2016, 6, 87656–87664. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.; Yue, B.; Chen, X.; He, H. Vanadium-containing mesoporous carbon and mesoporous carbon nanoparticles as catalysts for benzene hydroxylation reaction. Mater. Today Commun. 2017, 11, 61–67. [Google Scholar] [CrossRef]
- Pezhman, A.; Badiei, A.; Koolivand, A.; Ziarani, G.M. Direct hydroxylation of benzene to phenol over Fe3O4 supported on nanoporous carbon. Chin. J. Catal. 2011, 32, 258–263. [Google Scholar]
- Xu, J.; Jiang, Q.; Chen, T.; Wu, F.; Li, Y.-X. Vanadia supported on mesoporous carbon nitride as a highly efficient catalyst for hydroxylation of benzene to phenol. Catal. Sci. Technol. 2015, 5, 1504–1513. [Google Scholar] [CrossRef]
- Wang, C.; Hu, L.; Wang, M.; Yue, B.; He, H. Cerium promoted Vg-C3N4 as highly efficient heterogeneous catalysts for the direct benzene hydroxylation. R. Soc. Open Sci. 2018, 5, 180371. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.; Cai, M.; Jin, L.; Wang, X.; Yu, J.; Chen, Y.; Dai, L. Amorphous Cr-doped gC 3 N 4 as an efficient catalyst for the direct hydroxylation of benzene to phenol. New J. Chem. 2019, 43, 16169–16175. [Google Scholar] [CrossRef]
- Yu, Z.-H.; Gan, Y.-L.; Xu, J.; Xue, B. Direct Catalytic Hydroxylation of Benzene to Phenol Catalyzed by FeCl 3 Supported on Exfoliated Graphitic Carbon Nitride. Catal. Lett. 2020, 150, 301–311. [Google Scholar] [CrossRef]
- Tu, T.N.; Nguyen, H.T.; Nguyen, H.T.; Nguyen, M.V.; Nguyen, T.D.; Tran, N.T.; Lim, K.T. A new iron-based metal–organic framework with enhancing catalysis activity for benzene hydroxylation. RSC Adv. 2019, 9, 16784–16789. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhang, D.; Han, X.; Dong, T.; Guo, X.; Song, C.; Si, R.; Liu, W.; Liu, Y.; Zhao, Z. Preassembly strategy to single Cu-N3 sites inlaid porous hollow carbonitride spheres for selective oxidation of benzene to phenol. J. Am. Chem. Soc. 2018, 140, 16936–16940. [Google Scholar] [CrossRef]
- Ito, S.; Mitarai, A.; Hikino, K.; Hirama, M.; Sasaki, K. Deactivation reaction in the hydroxylation of benzene with Fenton’s reagent. J. Org. Chem. 1992, 57, 6937–6941. [Google Scholar] [CrossRef]
- Bal, R.; Tada, M.; Sasaki, T.; Iwasawa, Y. Direct phenol synthesis by selective oxidation of benzene with molecular oxygen on an interstitial-N/Re cluster/zeolite catalyst. Angew. Chem. Int. Ed. 2006, 118, 462–466. [Google Scholar] [CrossRef]
- Xia, H.; Sun, K.; Sun, K.; Feng, Z.; Li, W.X.; Li, C. Direct spectroscopic observation of Fe (III)—Phenolate complex formed from the reaction of benzene with peroxide species on Fe/ZSM-5 at room temperature. J. Phys. Chem. C 2008, 112, 9001–9005. [Google Scholar] [CrossRef]
- Borah, P.; Ma, X.; Nguyen, K.T.; Zhao, Y. A vanadyl complex grafted to periodic mesoporous organosilica: A green catalyst for selective hydroxylation of benzene to phenol. Angew. Chem. Int. Ed. 2012, 124, 7876–7881. [Google Scholar] [CrossRef]
- Chen, P.; Chen, L.; Zeng, Y.; Ding, F.; Jiang, X.; Liu, N.; Au, C.-T.; Yin, S.-F. Three-dimension hierarchical heterostructure of CdWO4 microrods decorated with Bi2WO6 nanoplates for high-selectivity photocatalytic benzene hydroxylation to phenol. Appl. Catal. B Environ. 2018, 234, 311–317. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Fu, X.; Antonietti, M.; Wang, X. Fe-g-C3N4-catalyzed oxidation of benzene to phenol using hydrogen peroxide and visible light. J. Am. Chem. Soc. 2009, 131, 11658–11659. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Isupova, L.A.; Ciambelli, P. Photo-Fenton oxidation of acetic acid on supported LaFeO3 and Pt/LaFeO3 perovskites. Chem. Eng. Trans. 2011, 25, 1013–1018. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, Y.; Li, H.; Chen, Z.; Wang, Y. Selective oxidation of benzene to phenol by FeCl3/mpg-C3N4 hybrids. RSC Adv. 2013, 3, 5121–5126. [Google Scholar] [CrossRef]
- Ohkubo, K.; Kobayashi, T.; Fukuzumi, S. Direct oxygenation of benzene to phenol using quinolinium ions as homogeneous photocatalysts. Angew. Chem. Int. Ed. 2011, 123, 8811–8814. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Q.; Zang, D.; Huang, Y.; Yu, H.; Wei, Y. Light-Induced Efficient Hydroxylation of Benzene to Phenol by Quinolinium and Polyoxovanadate-Based Supramolecular Catalysts. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Ciambelli, P.; Sannino, D.; Palma, V.; Vaiano, V.; Eloy, P.; Dury, F.; Gaigneaux, E.M. Tuning the selectivity of MoOx supported catalysts for cyclohexane photo oxidehydrogenation. Catal. Today 2007, 128, 251–257. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Ciambelli, P.; Carotenuto, G.; Di Serio, M.; Santacesaria, E. Enhanced performances of grafted VOx on titania/silica for the selective photocatalytic oxidation of ethanol to acetaldehyde. Catal. Today 2013, 209, 159–163. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Ciambelli, P.; Hidalgo, M.C.; Murcia, J.J.; Navío, J.A. Oxidative dehydrogenation of ethanol over Au/TiO2 photocatalysts. J. Adv. Oxid. Technol. 2012, 15, 284–293. [Google Scholar] [CrossRef]
- Vaiano, V.; Sarno, G.; Sacco, O.; Sannino, D. Degradation of terephthalic acid in a photocatalytic system able to work also at high pressure. Chem. Eng. J. 2017, 312, 10–19. [Google Scholar] [CrossRef]
- Fessi, N.; Nsib, M.F.; Cardenas, L.; Guillard, C.; Dappozze, F.; Houas, A.; Parrino, F.; Palmisano, L.; Ledoux, G.; Amans, D.; et al. Surface and Electronic Features of Fluorinated TiO2 and Their Influence on the Photocatalytic Degradation of 1-Methylnaphthalene. J. Phys. Chem. C 2020, 124, 11456–11468. [Google Scholar] [CrossRef]
- Žerjav, G.; Scandura, G.; Garlisi, C.; Palmisano, G.; Pintar, A. Sputtered vs. sol-gel TiO2-doped films: Characterization and assessment of aqueous bisphenol A oxidation under UV and visible light radiation. Catal. Today 2020, 357, 380–391. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J. Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Stoller, M.; Ciambelli, P.; Chianese, A. Photocatalytic removal of phenol by ferromagnetic N-TiO2/SiO2/Fe3O4 nanoparticles in presence of visible light irradiation. Chem. Eng. Trans. 2016, 47, 235–240. [Google Scholar] [CrossRef]
- Devaraji, P.; Jo, W.-K. Noble metal free Fe and Cr dual-doped nanocrystalline titania (Ti1− x− yMx+ yO2) for high selective photocatalytic conversion of benzene to phenol at ambient temperature. Appl. Catal. A Gen. 2018, 565, 1–12. [Google Scholar] [CrossRef]
- Gupta, N.; Bansal, P.; Pal, B. Metal ion-TiO2 nanocomposites for the selective photooxidation of benzene to phenol and cycloalkanol to cycloalkanone. J. Exp. Nanosci. 2015, 10, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, Y.; Saito, N.; Hirai, T. Adsorption-driven photocatalytic activity of mesoporous titanium dioxide. J. Am. Chem. Soc. 2005, 127, 12820–12822. [Google Scholar] [CrossRef]
- Yuzawa, H.; Aoki, M.; Otake, K.; Hattori, T.; Itoh, H.; Yoshida, H. Reaction mechanism of aromatic ring hydroxylation by water over platinum-loaded titanium oxide photocatalyst. J. Phys. Chem. C 2012, 116, 25376–25387. [Google Scholar] [CrossRef]
- Ide, Y.; Nakamura, N.; Hattori, H.; Ogino, R.; Ogawa, M.; Sadakane, M.; Sano, T. Sunlight-induced efficient and selective photocatalytic benzene oxidation on TiO2-supported gold nanoparticles under CO2 atmosphere. Chem. Commun. 2011, 47, 11531–11533. [Google Scholar] [CrossRef]
- Tomita, O.; Abe, R.; Ohtani, B. Direct synthesis of phenol from benzene over platinum-loaded tungsten (VI) oxide photocatalysts with water and molecular oxygen. Chem. Lett. 2011, 40, 1405–1407. [Google Scholar] [CrossRef] [Green Version]
- Tanarungsun, G.; Kiatkittipong, W.; Assabumrungrat, S.; Yamada, H.; Tagawa, T.; Praserthdam, P. Multi transition metal catalysts supported on TiO2 for hydroxylation of benzene to phenol with hydrogen peroxide. J. Ind. Eng. Chem. 2007, 13, 870–877. [Google Scholar]
- Devaraji, P.; Jo, W.-K. Natural leaf-assisted dual-phase two-dimensional leaf TiO2 and Cu(OH)2 co-catalyst for photocatalytic conversion of benzene to phenol. Mater. Res. Bull. 2019, 110, 67–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. Stabilizing CuPd bimetallic alloy nanoparticles deposited on holey carbon nitride for selective hydroxylation of benzene to phenol. J. Catal. 2019, 379, 154–163. [Google Scholar] [CrossRef]
- Verma, S.; Nasir Baig, R.; Nadagouda, M.N.; Varma, R.S. Hydroxylation of Benzene via C–H activation using bimetallic CuAg@ g-C3N4. ACS Sustain. Chem. Eng. 2017, 5, 3637–3640. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, G.; Badiei, A.; Ziarani, G.M.; Jafarabadi, M.; Hamzehloo, M. Photocatalytic synthesis of phenol by direct hydroxylation of benzene by a modified nanoporous silica (LUS-1) under sunlight. Chin. J. Catal. 2012, 33, 1347–1353. [Google Scholar] [CrossRef]
- Ye, X.; Cui, Y.; Qiu, X.; Wang, X. Selective oxidation of benzene to phenol by Fe-CN/TS-1 catalysts under visible light irradiation. Appl. Catal. B Environ. 2014, 152, 383–389. [Google Scholar] [CrossRef]
- Wang, D.; Wang, M.; Li, Z. Fe-based metal–organic frameworks for highly selective photocatalytic benzene hydroxylation to phenol. ACS Catal. 2015, 5, 6852–6857. [Google Scholar] [CrossRef]
- Dasireddy, V.D.; Likozar, B. Selective photocatalytic oxidation of benzene to phenol using carbon nanotube (CNT)-supported Cu and TiO2 heterogeneous catalysts. J. Taiwan Inst. Chem. Eng. 2018, 82, 331–341. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Ding, Z.; Mahadi, A.H.; Zhao, Y.; Song, Y.-F. Photocatalytic selective oxidation of benzene to phenol in water over layered double hydroxide: A thermodynamic and kinetic perspective. Chem. Eng. J. 2020, 388, 124248. [Google Scholar] [CrossRef]
- Bui, T.D.; Kimura, A.; Higashida, S.; Ikeda, S.; Matsumura, M. Two routes for mineralizing benzene by TiO2-photocatalyzed reaction. Appl. Catal. B Environ. 2011, 107, 119–127. [Google Scholar] [CrossRef]
- Devaraji, P.; Sathu, N.K.; Gopinath, C.S. Ambient oxidation of benzene to phenol by photocatalysis on Au/Ti0.98V0.02O2: Role of holes. ACS Catal. 2014, 4, 2844–2853. [Google Scholar] [CrossRef]
- Einaga, H.; Ibusuki, T.; Futamura, S. Improvement of catalyst durability by deposition of Rh on TiO2 in photooxidation of aromatic compounds. Environ. Sci. Technol. 2004, 38, 285–289. [Google Scholar] [CrossRef]
- Nishikawa, M.; Shiroishi, W.; Honghao, H.; Suizu, H.; Nagai, H.; Saito, N. Probability of Two-Step Photoexcitation of Electron from Valence Band to Conduction Band through Doping Level in TiO2. J. Phys. Chem. A 2017, 121, 5991–5997. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 2002, 98, 13669–13679. [Google Scholar] [CrossRef]
- Kunai, A.; Hata, S.; Ito, S.; Sasaki, K. The role of oxygen in the hydroxylation reaction of benzene with Fenton’s reagent. Oxygen 18 tracer study. J. Am. Chem. Soc. 1986, 108, 6012–6016. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.D.; Kimura, A.; Ikeda, S.; Matsumura, M. Determination of oxygen sources for oxidation of benzene on TiO2 photocatalysts in aqueous solutions containing molecular oxygen. J. Am. Chem. Soc. 2010, 132, 8453–8458. [Google Scholar] [CrossRef]








| Component | ∆H° at 298 K (kJ/mol) | ∆G° at 298 K (kJ/mol) | |
|---|---|---|---|
| Reagent | Benzene (l) | 48.99464 | 124.34848 |
| Benzene (g) | 82.92688 | 129.66216 | |
| Oxidant | H2O2 (l) | −136.10552 | −105.47864 |
| N2O (g) | 82.04824 | 104.1816 | |
| O2(g) | |||
| H2(g) | |||
| Product | Phenol (s) | −165.01696 | −50.4172 |
| Phenol (g) | −96.35752 | −32.88624 | |
| H2O | −285.82996 | −237.178408 |
| Catalyst | t (h) | T (°C) | P (atm) | Operating Conditions | Benzene Conversion (%) X | Phenol Yield (%) η | Selectivity to Phenol (%) Sp | Ref. |
|---|---|---|---|---|---|---|---|---|
| CuO/Al2O3 | - | 80 | 1 | 80 vol% acetic acid, benzene: 22.5 mmol; ascorbic acid: 4 mmol. | - | 1.2 | - | [47] |
| V/Al2O3 | - | 30 | 4 | 80 vol% acetic acid; benzene: 5.6 mmol; ascorbic acid: 1 mmol. | - | 8.4 | - | [48] |
| V2O5–Al2O3 | 6 | 60 | 1 | Catalyst: 0.2 g (14 wt%V2O5); benzene: 1.46 mmol; acetonitrile: 4 mL; H2O2: 11.68 mmol. | 13 | - | 100 | [44] |
| Fe3+–Al2O3 | 6 | 60 | 1 | Catalyst: 0.20 g; acetonitrile: 4 mL; benzene: 1.24 mmol; H2O2: 6 mmol. | 12 | 12 | - | [21] |
| Ru/SiO2 Rh/SiO2 Pd/SiO2 Ir/SiO2 Pt/SiO2 | - | 20 | 1 | Catalyst: 0.5 wt.-% metal/SiO2:1.0 g; H2/O2 = 3; benzene: 20 mL; acetic acid: 25 mL. | - | - | 0 99.7 88.2 64.5 63.9 | [49] |
| Ru/SiO2 Rh/SiO2 Pd/SiO2 Ir/SiO2 Pt/SiO2 | - | 60 | 1 | Catalyst: 0.5 wt.-%; metal: 20wt.-%; V2O5/SiO2: 1.0 g; H2/O2: 3; benzene: 20 mL; acetic acid: 25 mL. | - | - | 100 100 99.7 100 100 | [49] |
| 0.1%V/SiO2 | - | 70 | 1 | Catalyst: 0.204 g; benzene: 40 mmol benzene/H2O2 mole ratio: 1; acetonitrile: - mL. | 10 | - | 81 | [50] |
| Fe5V2.5Cu2.5/TiO2 | 4 | 30 | 1 | Catalyst: 0.2 g; benzene: 11 mL; benzene/H2O2 mole ratio: 0.5; acetonitrile: 40 mL. | 9.8 | 7.154 | 73 | [51] |
| FePt/TiO2 (5%;1%) | 4 | 30 | 1 | Catalyst: 0.2 g; benzene: 11 mL; benzene/H2O2 mole ratio: 0.5; acetonitrile: 40 mL. | 6.5 | 5.92 | 91 | [52] |
| V/MCM-41 [Si/V = 1/9.4] | 6 | 60 | 1 | Catalyst: 0.05 g; benzene: 6 mL; benzene/H2O2 mole ratio: 1/1.15; acid acetic: 6 mL. | 1.4 | - | 93 | [53] |
| 4%Cu/MCM-41 | 1.6 | 30 | 1 | Catalyst: 0.05 g; benzene: 1 mL; benzene/H2O2 mole ratio: 1/2; acid acetic: 7.5 mL. | 21 | 19.7 | 94 | [18] |
| Ti-MCM-41 [Si/Ti = 25] | 3.5 | 65 | 1 | Catalyst: 0.05 g; benzene: 0.045 mol; benzene/H2O2 mole ratio: 1/3; acetone: 15 g. | 98 | - | >95 | [54] |
| VOx/FeSBA-15 VOx/CoSBA-15 VOx/NiSBA-15 VOx/CrSBA-15 VOx/MnSBA-15 VOx/ZnSBA-15 VOx/AgSBA-15 VOx/CuSBA-15 | 5 | 80 | 1 | Catalyst: 0.05 g; benzene: 1mL; solvent (acetic acid/H2O v/v): 36 mL; ascorbic acid: 11.9 mmol. | - | 12.8 11.3 15.8 10.2 17.2 17.9 18.1 24.7 | - | [55] |
| Fe/SBA-16 | 8 | 65 | 1 | Catalyst: 0.1 g; benzene: 1 mL; H2O2: 2 mL; acetonitrile: 20 mL; | 12.1 | 11.7 | 96.4 | [56] |
| 1.4wt%Cu(II)-NaY | 6 | 70 | 1 | Catalyst: 0.025 g; benzene: 0.02 mol; H2O2: 0.02 mol. | 33.2 | - | 100 | [57] |
| Graphene (CCG) | 16 | 60 | 1 | Catalyst: 0.02 g; benzene: 130 mg; H2O2: 2.4 mL; acetonitrile: 1.2 mL. | 17.8 | 17 | > 99 | [58] |
| 4.2V/NC-600 | 3 | 70 | 1 | Catalyst: 0.02 g; benzene: 0.4 mL; H2O2: 1.4 mL; acetic acid: 5 mL. | 27.7 | 26.8 | 96.7 | [59] |
| 4V/MCN-S | 3 | 70 | 1 | Catalyst: 0.02 g; benzene: 0.4 mL; H2O2: 1.4 mL; acetic acid: 5 mL. | 38.2 | 36.7 | 96.1 | [60] |
| Fe3O4/CMK-3 | 4 | 60 | 1 | Catalyst: 0.02 g; benzene: 1 mL; H2O2: 2 mL; acetonitrile: 6 mL. | 18 | - | 92 | [61] |
| 10V/mp-C3N4 | 3 | 60 | 1 | Catalyst: 0.06 g; benzene: 1.5 mL; H2O2: 3 mL; acetonitrile: 6 mL. | 18 | 18 | 93 | [62] |
| Ce0.07-0.07V-g-C3N4 | 4 | 70 | Catalyst: 0.04 g; benzene: 1 mL; H2O2: 3.5 mL; acid acetic: 10 mL. | 33.7 | 32.3 | 95.9 | [63] | |
| Cr/g-C3N4-300 | 7 | 65 | Catalyst: 0.04 g; benzene: 3.36 mmol; H2O2: 1.2 mL; acetonitrile: 2 mL. | 31.1 | 30.9 | 99.5 | [64] | |
| FeCl3/eg-C3N4 | 3 | 60 | 1 | Catalyst: 0.05 g; benzene: 11.2 mmol; H2O2: 3 mL; acetonitrile: 5 mL. | 22 | 22 | 99 | [65] |
| Fe-TBAPy | 3 | 60 | 1 | Catalyst: 0.05 g; benzene: 11.2 mmol; H2O2: 3 mL; acetonitrile: 5 mL. | - | 64.5 | 92.9 | [66] |
| Cu-SA/HCNS | 12 | 60 | Catalyst: 0.05 g; benzene: 0.4 mL; H2O2: 6 mL; acetonitrile: 6 mL. | 86 | - | 96.7 | [67] |
| Photocatalyst | t * (h) | Light Source | Operating Conditions | Benzene Conversion (%) X | Phenol Yield (%) η | Selectivity to Phenol (%) Sp | Ref. |
|---|---|---|---|---|---|---|---|
| nTiO2 mTiO2 mTiO2 | 2 2 6 | Hg lamp λ > 320 nm | Photocatalyst: 10 mg + nitrogen flow H2O: 10 mL benzene: 20 μmol pH 7 | 26 23 42 | 2 19 34 | 8 83 81 | [88] |
| TiO2 | 6 | 450 W Xe arc lamp | Photocatalyst:25 mg Benzene: 20 mM [Fe3+]: 1.47 mM [Ag+ ]: 0.98 mM H2O2: 9.4 mM | - | <1 | 96 | [9] |
| Pt-TiO2 | 1.5 | λ > 385 nm | Photocatalyst: 0.2 g benzene: 0.05 mL H2O: 4 mL | - | 2.1 | 91 | [89] |
| Au-P25: in 100 kPa air in 230 kPa CO2 P25: in 100 kPa air in 230 kPa CO2 | 24 | Solar simulator | Photocatalyst: 60 mg aqueous benzene solution: 20 mL C0benzene: 600 ppm dry ice:0-200 mg closed container: 50 mL | 13 14 34 31 | 8 13 7 7 | 62 89 21 22 | [90] |
| Au-V-TiO2 | 18 | 400 W Hg lamp λ = 200−400 nm | Photocatalyst: 30 mg CH3CN: 2 mL benzene: 1 mL (25 wt%) H2O2: 2 mL | 18 | 16 | 88 | [86] |
| Pt/WO3-K | a 1 b 4 e 0.25 | 300 W Xe lamp λ>300 nm c λ>400 nm | Photocatalyst: 50 mg C0benzene: 2.5 mmolL−1 H2O: 7.5 mL 279 K O2 dAr | [91] | |||
| Pt/WO3-K | |||||||
| WO3-K | 16.4 b | 84.6 b | |||||
| Pt/WO3-Y | 40.6a | 58.8 a | |||||
| Pt/WO3-S | 32.4 a | 48.7 a | |||||
| Pt/TiO2-P25 | |||||||
| TiO2-P25 | 85.2 b | 20.6 b | |||||
| Pt/TiO2-M | 43 a | 31 a | |||||
| Pt/TiO2-J. | |||||||
| Fe3+ impregnated TiO2 | 1–2 | 125 W Hg lamp UV light | Photocatalyst: 50 mg aqueous benzene (1 to 20 mM): 5 mL | - | 9–15 | 80–86 | [87] |
| Fe-Cr-TiO2 | 12 | 450 W mercury lamp λ = 200–400 nm | Photocatalyst: 30 mg CH3CN: 2 mL benzene: 1 mL (25 wt%) H2O2: 2 mL | 28 ± 0.5 | 25.2 ± 0.5 | 90 ± 0.5 | [86] |
| Fe-V-Cu supported on TiO2 | 4 | black light blue fluorescent bulb (8W) | Photocatalyst: 0.2 g benzene: 11 cm3 benzene/H2O2 mole ratio: 0.5 (30 wt%) H2O2: 30 cm3 solvent: 40 cm3 acetone a, acetonitrile b, pyridine c ascorbic acid: 0.5 | 18.61 a 14.27 b 7.9 c | 9.68 a 9.7 b 7.11 c | 52 a 68 b 90 c | [92] |
| LT-550 LT-750 Cu(OH)2/LT-550 Cu(OH)2/LT-750 Cu(OH)2/LT-750a Cu(OH)2/LT-750b | 6 | UV light | Photocatalyst: 5 mg Benzene: 100 μL CH3CN: 500 μL H2O: 13 mL (30 wt%) H2O2: 87 μL | 38.7 47.1 42 49.9 a 55.0 b 41 | 36.3 45.2 40.7 48.4 a 47.9 b 36.5 | 94 96 97 97 a 87 b 89 | [93] |
| CuPd/g-C3N4 | 1.5 | solar simulator | Solution A: -photocatalyst: 20 mg - deionized water: 30 mL Solution B: - benzene: 0.5 mL - acetonitrile: 30 mL. (30 wt%) H2O2: 5 mmol added to the two mixed solutions. | 98.1 | 87.8 | 89.6 | [94] |
| Fe2O3/g-C3N4 Pd/g-C3N4 Cu/g-C3N4 Ni/g-C3N4 Ag/g-C3N4 FePd/g-C3N4 FeCu/g-C3N4 FeAg/g-C3N4 FeNi/g-C3N4 PdCu/g-C3N4 PdNi/g-C3N4 PdAg/g-C3N4 CuNi/g-C3N4 CuAg/g-C3N4 CuAg/g-C3N4a CuAg/g-C3N4b CuAg/g-C3N4c CuAg/g-C3N4d CuAg/g-C3N4e CuAg/g-C3N4f | 12 12 12 12 12 12 12 12 12 12 12 12 12 0.5 0.5 0.5 3 0.5 0.5 0.5 | Visible light 20 W domestic bulb | Photocatalyst: 100 mg Benzene:1 mmol CH3CN: 5.0 mL (30 wt%) H2O2: 1.1 mmol a50 mg of catalyst b 25 mg of catalyst c 15 mg of catalyst d methanol as a solvent e water as a solvent f ethanol as a solvent | 15 43 39 20 32 70 67 41 29 81 72 77 57 99 99 a 99 b 99 c 86 d 83 e 99 f | − | − | [95] |
|
mpg-C3N4 3%FeCl3/mpg-C3N4 5%FeCl3/mpg-C3N4 10%FeCl3/mpg-C3N4 20%FeCl3/mpg-C3N4 5%FeCl3/mpg-C3N4a 5%FeCl3/mpg-C3N4b 5%FeCl3/mpg-C3N4c 5%FeCl3/mpg-C3N4d 5%FeCl3/mpg-C3N4e | 4 | 100 W mercury lamp λ > 420nm | Photocatalyst: 25 mg benzene: 4.5 mmol (30 wt%) H2O2: 0.255 mL 60 °C a T = 25 °C b T = 40 °C c T = 80 °C d H2O2: 0.510 mL e H2O2: 0.765 mL | 2 17 38 23 25 4 a 10 b 21 c 44 d 47 e | − | 95 98 97 94 80 99 a 96 b 81 c 85 d 60 e | [75] |
| g-C3N4 mpg-C3N4 FeCl3 5%Fe-g-C3N4 10%Fe-g-C3N4 20%Fe-g-C3N4Cu-g-C3N4 Ti-g-C3N4 Ni-g-C3N4 Zn-g-C3N4 Fe/SBA-15 g-C3N4/SBA-15 Fe-g-C3N4/SBA-15 | 4 | 500 W Xenon lamp λ > 420 nm | Photocatalyst: 50 mg CH3CN: 4 mL benzene: 0.8 mL H2O: 4 mL (30 wt%)H2O2: 0.51 mL | − | 0 2.0 0.5 1.8 4.8 2.5 1.4 0.1 0.1 0.1 1.0 0.1 11.9 | − | [73] |
| 10%Fe-g-C3N4 20%Fe-g-C3N4 30%Fe-g-C3N4 10%Fe-g-C3N4-LUS-1 20%Fe-g-C3N4-LUS-1 | 4 | sunlight | Photocatalyst: 0.05 g benzene: 1 mL CH3CN: 4 mL H2O2: 0.5 mL T = 60 °C | − | 6.5 8 10.5 10 16 | >90 ~90 ~ 90 >90 >90 | [96] |
| Fe-CN TS-1 Fe-CN/TS-1–1 a Fe-CN/TS-1–2 b Fe-CN/TS-1–3 c Fe-CN/TS-1–4 d Fe-CN/TS-1–5 e Fe-CN/TS-1–6 f Fe-CN/TS-1–2 g Fe-CN/TS-1–7 h Fe-CN/TS-1–8 i Fe/TS-1 | 4 | 300 W Xenon lamp λ> 420 nm | CH3CN: 4 mL benzene: 0.8 mL H2O: 4 mL (30 wt%) H2O2: 0.51 mL 60 °C pH = 7 Fe-CN/TS-1-X a X = 1 for 10% dicyandiamide/TS-1 b X = 2 for 20% dicyandiamide/TS-1 c X = 3 for 50% dicyandiamide/TS-1 d X = 4 for 100% dicyandiamide/TS-1 e X = 5 for 200% dicyandiamide/TS-1 Fe-CN/TS-1-X f X = 6 for 5% FeCl3/dicyandiamide g X = 2 for 10% FeCl3/dicyandiamide h X = 7 for 20% FeCl3/dicyandiamide i X = 8 for 50% FeCl3/dicyandiamide | − | 1.1 2.4 2.8 a 10 b 8.8 c 1.3 d 0.1 e 1.4 f 10 g 5 h 1.6 i 7.6 | − | [97] |
| MIL-100(Fe) MIL-68(Fe)i | 8 | Visible light irradiation λ≥ 420 nm | Photocatalyst: 10 mg H2O2: 0.5 mmol Solvent: 4 mL a CH3CN solvent H2O2:benzene(1:2) b Acetone solvent H2O2:benzene(1:2) c H2O solvent H2O2:benzene(1:2) d DMF solvent H2O2:benzene(1:2) e CH3CN:H2O (1:1) H2O2:benzene(1:2) f CH3CN:H2O (1:1) H2O2:benzene(3:4) g CH3CN:H2O (1:1) H2O2:benzene(2:2) h CH3CN:H2O (1:1) H2O2:benzene(3:2) i CH3CN:H2O (1:1) H2O2:benzene(3:4) | 10.3 a 2.4 b 8.3 c 3.3 d 13.6 e 20.1 f 21.7 g 22.5 h 14i | 10.3 a 2.38 b 7.1 c 2.5 d 13.3 e 14.77 f 20.8 g 31.05 h 9.45 i | >99 a 99 b 85 c 76 d 98 e 98 f 96 g 92 h 90 i | [98] |
| Ti/CNT Cu/Ti/CNT | 0.75 | Low-pressure mercury lamp | Photocatalyst: 100 mg benzene: 20 mL H2O: 20 mL | 53.8 68.3 | 35.1 51.8 | 65.3 75.8 | [99] |
| Zn-Ti-LDH | 3 | 300 W Xenon lamp | Photocatalyst: 20 mg Benzene: 0.2 mmol H2O: 20 mL | 5.65 | 4.59 | 87.18 | [100] |
| Bi2WO6/CdWO4 composite | 3 | 300 W Xe lamp λ ≥4 00nm | Photocatalyst: 50 mg benzene: 0.5 mmol CH3CN: 3 mL H2O: 100 μL O2: 3 mL min−1 | 5.8 | − | >99 | [72] |
| QuCN+ ion | 1 | 500 W xenon lamp λ = 290–600 nm | [QuCN+]: 2.0 mM [C6H6]: 30 mM [H2O]: 3.0 M | 31 | 30 | 98 | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, A.; Sacco, O.; Sannino, D.; Venditto, V.; Vaiano, V. One-Step Catalytic or Photocatalytic Oxidation of Benzene to Phenol: Possible Alternative Routes for Phenol Synthesis? Catalysts 2020, 10, 1424. https://doi.org/10.3390/catal10121424
Mancuso A, Sacco O, Sannino D, Venditto V, Vaiano V. One-Step Catalytic or Photocatalytic Oxidation of Benzene to Phenol: Possible Alternative Routes for Phenol Synthesis? Catalysts. 2020; 10(12):1424. https://doi.org/10.3390/catal10121424
Chicago/Turabian StyleMancuso, Antonietta, Olga Sacco, Diana Sannino, Vincenzo Venditto, and Vincenzo Vaiano. 2020. "One-Step Catalytic or Photocatalytic Oxidation of Benzene to Phenol: Possible Alternative Routes for Phenol Synthesis?" Catalysts 10, no. 12: 1424. https://doi.org/10.3390/catal10121424
APA StyleMancuso, A., Sacco, O., Sannino, D., Venditto, V., & Vaiano, V. (2020). One-Step Catalytic or Photocatalytic Oxidation of Benzene to Phenol: Possible Alternative Routes for Phenol Synthesis? Catalysts, 10(12), 1424. https://doi.org/10.3390/catal10121424









