Ginseng Stem-Leaf Saponins in Combination with Selenium Promote the Immune Response in Neonatal Mice with Maternal Antibody
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethical Statement
2.3. Vaccines and Adjuvant
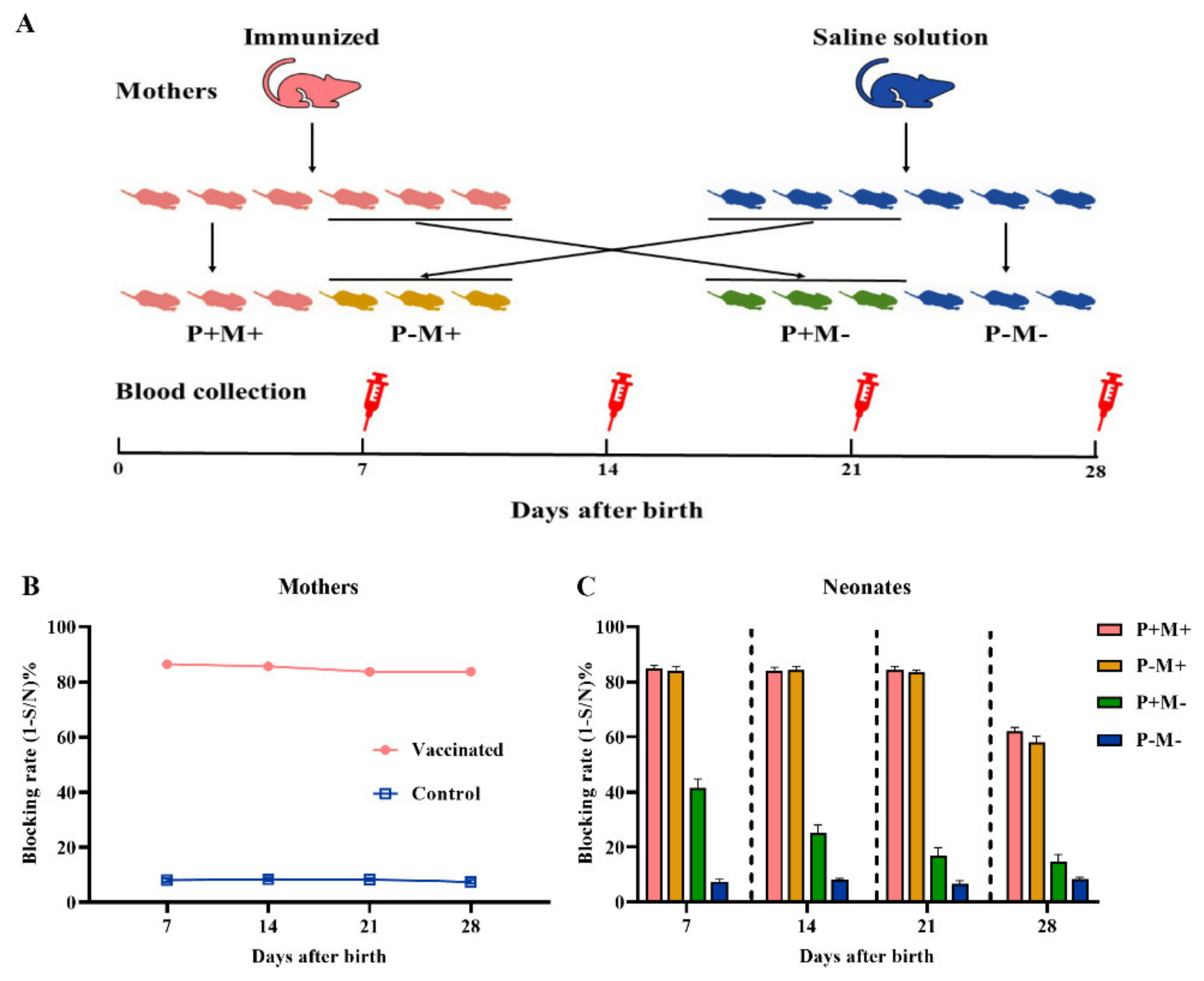
2.4. Experimental Design for Transfer of Maternal Antibody from Mother to Neonate
2.5. Experimental Design for Effect of GSe on the Immune Responses to aPrV Vaccine in Neonates with MatAb about 50% of Blocking Rate
2.6. Experimental Design for Effect of GSe on the Immune Responses to aPrV Vaccine in Neonates with MatAb about 90% of Blocking Rate
2.7. Experimental Design for Effect of GSe on Cytokine Production by Neonatal Splenocytes
2.8. Experimental Design for the Effect of GSe on the Primary and Secondary Immunizations
2.9. Assay for PrV gB Specific Antibody
2.10. Experimental Design for Transcriptome Analysis
2.11. Validation of Gene Expression by RT-qPCR
2.12. Cytokine Assay
2.13. Statistical Analysis
3. Results
3.1. Transfer of Maternal Antibody from Mother to Neonate
3.2. Effect of GSe on the Immune Responses to aPrV Vaccine in Neonates with MatAb about 50% of Blocking Rate
3.3. Effect of GSe on the Immune Responses to aPrV Vaccine in Neonates with MatAb about 90% of Blocking Rate
3.4. Effect of GSe on Cytokine Production in Neonates with MatAb
3.5. Effect of GSe on the Primary and Secondary Immunizations in Neonates
3.6. DEGs between Neonates Immunized with aP-GSe and aPrV
3.7. GO Enrichment Analysis of DEGs
3.8. KEGG Pathway Enrichment Analysis of DEGs
3.9. Validation of DEGs by RT-qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chase, C.C.L.; Hurley, D.J.; Reber, A.J. Neonatal immune development in the calf and its impact on vaccine response. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, E.; Blomqvist, G.; Bellman, A.; Holmdahl, R.; Mattsson, A.; Mattsson, R. Maternal antibodies protect immunoglobulin deficient neonatal mice from mouse hepatitis virus (MHV)-associated wasting syndrome. Am. J. Reprod. Immunol. 1996, 36, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.M. Maternal antibodies and infant immune responses to vaccines. Vaccine 2015, 33, 6469–6472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgins, D.C.; Shewen, P.E. Vaccination of neonates: Problem and issues. Vaccine 2012, 30, 1541–1559. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Latimer, L.; Wang, Y.; Simko, E.; Gerdts, V.; Potter, A. Maternal immunization with respiratory syncytial virus fusion protein formulated with a novel combination adjuvant provides protection from RSV in newborn lambs. Vaccine 2016, 34, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, E.M.A.; Moonen-Leusen, H.W.; De Visser, Y.E.; Middel, W.G.J.; Boersma, W.J.A.; Bianchi, A.T.J. A DNA vaccine coding for gB and gD of pseudorabies virus (suid herpes type 1) primes the immune system in the presence of maternal immunity more efficiently than conventional vaccines. Vaccine 2006, 24, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Luo, Y.; Wang, C.-H.; Yuan, J.; Li, N.; Song, K.; Qiu, H.J. Control of swine pseudorabies in China: Opportunities and limitations. Vet. Microbiol. 2016, 183, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biololgy of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Zhou, Z.; Hu, D.; Zhang, Q.; Han, T.; Li, X.; Gu, X.; Yuan, L.; Zhang, S.; Wang, B.; et al. Pathogenic pseudorabies virus, China, 2012. Emerg. Infect. Dis. 2014, 20, 102–104. [Google Scholar] [CrossRef] [Green Version]
- Freuling, C.M.; Mueller, T.F.; Mettenleiter, T.C. Vaccines against pseudorabies virus (PrV). Vet. Microbiol. 2017, 206, 3–9. [Google Scholar] [CrossRef]
- Brockmeier, S.L.; Lager, K.M.; Mengeling, W.L. Successful pseudorabies vaccination in maternally immune piglets using recombinant vaccinia virus vaccines. Res. Vet. Sci. 1997, 62, 281–285. [Google Scholar] [CrossRef]
- Fischer, L.; Barzu, S.; Andreoni, C.; Buisson, N.; Brun, A.; Audonnet, J.C. DNA vaccination of neonate piglets in the face of maternal immunity induces humoral memory and protection against a virulent pseudorabies virus challenge. Vaccine 2003, 21, 1732–1741. [Google Scholar] [CrossRef]
- Zou, M.; Yang, X.; Zheng, H.; Wang, H.; Li, L.; Sun, J.; Wang, F.; Wu, F.; Fan, G. Epidemiological survey of pseudorabies in some areas in China from 2012 to 2013. China Anim. Health Inspect. 2015, 32, 1–5. [Google Scholar]
- Scheid, A.; Borriello, F.; Pietrasanta, C.; Christou, H.; Diray-Arce, J.; Pettengill, M.A.; Joshi, S.; Li, N.; Bergelson, I.; Kollmann, T.; et al. Adjuvant effect of bacille calmette-guerin on hepatitis B vaccine immunogenicity in the preterm and term newborn. Front. Immunol. 2018, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Pomorska-Mol, M.; Markowska-Daniel, I. Interferon-gamma secretion and proliferative responses of peripheral blood mononuclear cells after vaccination of pigs against Aujeszky’s disease in the presence of maternal immunity. FEMS Immunol. Med. Microbiol. 2010, 58, 405–411. [Google Scholar] [CrossRef]
- Pomorska-Mol, M.; Markowska-Daniel, I.; Pejsak, Z. Evaluation of humoral and antigen-specific T-cell responses after vaccination of pigs against pseudorabies in the presence of maternal antibodies. Vet. Microbiol. 2010, 144, 450–454. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Yuan, L.; Maqbool, B.; Xu, W.; He, S.; Guan, R.; Hu, S. A solution with ginseng saponins and selenium as vaccine diluent to increase Th1/Th2 immune responses in mice. J. Immunol. Res. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Luo, G.; Zeng, Y.; Li, Y.; Yu, S.; Zhao, B.; Wang, Y.; Li, T.; Ge, S.; Xia, N. The distinct impact of maternal antibodies on the immunogenicity of live and recombinant rotavirus vaccines. Vaccine 2019, 37, 4061–4067. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Maqbool, B.; Yuan, L.; He, S.; Zhang, C.; Xu, W.; Hu, S. Early IgG response to foot and mouth disease vaccine formulated with a vegetable oil adjuvant. Vaccines 2019, 7, 143. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Wang, Y.; Ma, X.; Cui, X.; Lu, M.; Guan, R.; Chi, X.; Xu, W.; Hu, S. Sunflower seed oil combined with ginseng stem-leaf saponins as an adjuvant to enhance the immune response elicited by Newcastle disease vaccine in chickens. Vaccine 2020, 38, 5343–5354. [Google Scholar] [CrossRef]
- Xu, W.; Fang, S.; Wang, Y.; Zhang, T.; Hu, S. Molecular mechanisms associated with macrophage activation by Rhizoma Atractylodis Macrocephalae polysaccharides. Int. J. Biol. Macromol. 2020, 147, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fang, S.; Cui, X.; Guan, R.; Wang, Y.; Shi, F.; Hu, S. Signaling pathway underlying splenocytes activation by polysaccharides from Atractylodis macrocephalae Koidz. Mol. Immunol. 2019, 111, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.S. Evolution of the mammary gland defense system and the ontogeny of the immune system. J. Mammary Gland Biol. Neoplasia 2002, 7, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Yorty, J.L.; Schultz, S.A.; Bonneau, R.H. Postpartum maternal corticosterone decreases maternal and neonatal antibody levels and increases the susceptibility of newborn mice to herpes simplex virus-associated mortality. J. NeuroImmunol. 2004, 150, 48–58. [Google Scholar] [CrossRef]
- Morein, B.; Abusugra, I.; Blomqvist, G. Immunity in neonates. Vet. Immunol. Immunopathol. 2002, 87, 207–213. [Google Scholar] [CrossRef]
- Crowe, J.E., Jr.; Firestone, C.Y.; Murphy, B.R. Passively acquired antibodies suppress humoral but not cell-mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J. Immunol. 2001, 167, 3910–3918. [Google Scholar] [CrossRef] [Green Version]
- Siegrist, C.A. Mechanisms by which maternal antibodies influence infant vaccine responses: Review of hypotheses and definition of main determinants. Vaccine 2003, 21, 3406–3412. [Google Scholar] [CrossRef]
- Bjorkholm, B.; Granstrom, M.; Taranger, J.; Wahl, M.; Hagberg, L. Influence of high titers of maternal antibody on the serologic response of infants to diphtheria vaccination at 3.; 5 and 12 months of age. Pediatr. Infect. Dis. J. 1995, 14, 846–850. [Google Scholar] [CrossRef]
- Dagan, R.; Amir, J.; Mijalovsky, A.; Kalmanovitch, I.; Bar-Yochai, A.; Thoelen, S.; Safary, A.; Ashkenazi, S. Immunization against hepatitis A in the first year of life: Priming despite the presence of maternal antibody. Pediatr. Infect. Dis. J. 2000, 19, 1045–1052. [Google Scholar] [CrossRef]
- Johansson, E.; Istrate, C.; Charpilienne, A.; Cohen, J.; Hinkula, J.; Poncet, D.; Svensson, L.; Johansen, K. Amount of maternal rotavirus-specific antibodies influence the outcome of rotavirus vaccination of newborn mice with virus-like particles. Vaccine 2008, 26, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Niewiesk, S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2015, 5, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Huey, D.; Oglesbee, M.; Niewiesk, S. Insights into the regulatory mechanism controlling the inhibition of vaccine-induced seroconversion by maternal antibodies. Blood 2011, 117, 6143–6151. [Google Scholar] [CrossRef] [PubMed]
- Vono, M.; Eberhardt, C.S.; Auderset, F.; Mastelic-Gavillet, B.; Lemeille, S.; Christensen, D.; Andersen, P.; Lambert, P.-H.; Siegrist, C.-A. Maternal antibodies inhibit neonatal and infant responses to vaccination by shaping the early-life B cell repertoire within germinal centers. Cell Rep. 2019, 28, 1773–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Ihara, T.; Nunoya, T.; Kuwahara, H.; Ishihama, A.; Ueda, S. Role of pseudorabies virus glycoprotein II in protection from lethal infection. Vet. Microbiol. 1993, 36, 83–90. [Google Scholar] [CrossRef]
- Grabowska, A.K.; Lipinska, A.D.; Rohde, J.; Szewczyk, B.; Bienkowska-Szewczyk, K.; Rziha, H.-J. New baculovirus recombinants expressing pseudorabies virus (PRV) glycoproteins protect mice against lethal challenge infection. Vaccine 2009, 27, 3584–3591. [Google Scholar] [CrossRef]
- Van Rooij, E.M.A.; De Bruin, M.G.M.; De Visser, Y.E.; Middel, W.G.J.; Boersma, W.J.A.; Bianchi, A.T.J. Vaccine-induced T cell-mediated immunity plays a critical role in early protection against pseudorabies virus (suid herpes virus type 1) infection in pigs. Vet. Immunol. Immunopathol. 2004, 99, 113–125. [Google Scholar] [CrossRef]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef]
- Millan, C.L.B.; Weeratna, R.; Krieg, A.M.; Siegrist, C.A.; Davis, H.L. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc. Natl. Acad. Sci. USA 1998, 95, 15553–15558. [Google Scholar] [CrossRef] [Green Version]
- Honda-Okubo, Y.; Ong, C.H.; Petrovsky, N. Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection. Vaccine 2015, 33, 4892–4900. [Google Scholar] [CrossRef]
- Song, X.; Bao, S.; Wu, L.; Hu, S. Ginseng stem-leaf saponins (GSLS) and mineral oil act synergistically to enhance the immune responses to vaccination against foot-and-mouth disease in mice. Vaccine 2009, 27, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Bi, S.; Xu, W.; Zhang, C.; Lu, Y.; Zhai, L.; Hu, S. Improved immune response to an attenuated pseudorabies virus vaccine by ginseng stem-leaf saponins (GSLS) in combination with thimerosal. Antivir. Res. 2016, 132, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ma, Y.; Zhai, L.; Lu, Y.; Chi, X.; Wu, J.; Hu, S. Enhanced immune response to foot-and-mouth disease vaccine by oral administration of ginseng stem-leaf saponins. Immunopharmacol. Immunotoxicol. 2016, 38, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Gavillet, B.M.; Eberhardt, C.S.; Auderset, F.; Castellino, F.; Seubert, A.; Tregoning, J.S.; Lambert, P.H.; De Gregorio, E.; Del Giudice, G.; Siegrist, C.A. MF59 mediates its b cell adjuvanticity by promoting T follicular helper cells and thus germinal center responses in adult and early life. J. Immunol. 2015, 194, 4836–4845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHeyzer-Williams, M.; Okitsu, S.; Wang, N.; McHeyzer-Williams, L. Molecular programming of B cell memory. Nat. Rev. Immunol. 2012, 12, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crotty, S. Follicular Helper CD4 T Cells (T-FH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Pettini, E.; Fiorino, F.; Lucchesi, S.; Pastore, G.; Brunetti, J.; Brunetti, J.; Santoro, F.; Andersen, P.; Bracci, L.; et al. Heterologous prime-boost combinations highlight the crucial role of adjuvant in priming the immune system. Front. Immunol. 2018, 9, 380. [Google Scholar] [CrossRef]
- Ciabattini, A.; Pettini, E.; Fiorino, F.; Pastore, G.; Andersen, P.; Pozzi, G.; Medaglini, D. Modulation of primary immune response by different vaccine adjuvants. Front. Immunol. 2016, 7, 427. [Google Scholar] [CrossRef] [Green Version]
- Siegrist, C.A. Neonatal and early life vaccinology. Vaccine 2001, 19, 3331–3346. [Google Scholar] [CrossRef]
- Basha, S.; Surendran, N.; Pichichero, M. Immune responses in neonates. Expert Rev. Clin. Immunol. 2014, 10, 1171–1184. [Google Scholar] [CrossRef]
- Siegrist, C.A.; Barrios, C.; Martinez, X.; Brandt, C.; Berney, M.; Cordova, M.; Kovarik, J.; Lambert, P.H. Influence of maternal antibodies on vaccine responses: Inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur. J. Immunol. 1998, 28, 4138–4148. [Google Scholar] [CrossRef]
- Blomqvist, G.A.M.; Lovgren-Bengtsson, K.; Morein, B. Influence of maternal immunity on antibody and T-cell response in mice. Vaccine 2003, 21, 2022–2031. [Google Scholar] [CrossRef]
- Crowe, J.E., Jr. Influence of maternal antibodies on neonatal immunization against respiratory viruses. Clin. Infect. Dis. 2001, 33, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Van Binnendijk, R.S.; Poelen, M.C.; Van Amerongen, G.; De Vries, P.; Osterhaus, A.D. Protective immunity in macaques vaccinated with live attenuated, recombinant, and subunit measles vaccines in the presence of passively acquired antibodies. J. Infect. Dis. 1997, 175, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Premenko-Lanier, M.; Hodge, G.; Rota, P.; Tamin, A.; Bellini, W.; McChesney, M. Maternal antibody inhibits both cellular and humoral immunity in response to measles vaccination at birth. Virology 2006, 350, 429–432. [Google Scholar] [CrossRef] [Green Version]
- Bouma, A.; De Jong, M.D.; Kimman, T.G. The influence of maternal immunity on the development of the in vitro lymphocyte proliferation response against pseudorabies virus in pigs. Res. Vet. Sci. 1998, 64, 167–171. [Google Scholar] [CrossRef]
- Kolev, M.; Dimeloe, S.; Le Friec, G.; Navarini, A.; Arbore, G.; Povoleri, G.A.; Povoleri, G.A.; Fischer, M.; Belle, R.; Loeliger, J.; et al. Complement regulates nutrient influx and metabolic reprogramming during Th1 cell responses. Immunity 2015, 42, 1033–1047. [Google Scholar] [CrossRef] [Green Version]
- Marchant, A.; Goetghebuer, T.; Ota, M.O.; Wolfe, I.; Ceesay, S.J.; De Groote, D.; Corrah, T.; Bennett, S.; Wheeler, J.; Huygen, K.; et al. Newborns develop a Th1-type immune response to mycobacterium bovis bacillus calmette-guerin vaccination. J. Immunol. 1999, 163, 2249–2255. [Google Scholar]
- Demirjian, A.; Levy, O. Safety and efficacy of neonatal vaccination. Eur. J. Immunol. 2009, 39, 36–46. [Google Scholar] [CrossRef]
- Heldwein, K.A.; Liang, M.D.; Andresen, T.K.; Thomas, K.E.; Marty, A.M.; Cuesta, N.; Vogel, S.N.; Fenton, M. J. TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. J. Leukoc. Biol. 2003, 74, 277–286. [Google Scholar] [CrossRef]
- Adkins, B.; Leclerc, C.; Marshall-Clarke, S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004, 4, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Firth, M.A.; Shewen, P.E.; Hodgins, D.C. Passive and active components of neonatal innate immune defenses. Anim. Health Res. Rev. 2005, 6, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Pasare, C. Innate control of adaptive immunity: Beyond the three-signal paradigm. J. Immunol. 2017, 198, 3791–3800. [Google Scholar] [CrossRef] [Green Version]
- Pihlgren, M.; Tougne, C.; Bozzotti, P.; Fulurija, A.; Duchosal, M.A.; Lambert, P.-H.; Siegrist, C.A. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J. Immunol. 2003, 170, 2824–2832. [Google Scholar] [CrossRef] [Green Version]
- Bjarnarson, S.P.; Adarna, B.C.; Benonisson, H.; Del Giudice, G.; Jonsdottir, I. The adjuvant LT-K63 can restore delayed maturation of follicular dendritic cells and poor persistence of both protein- and polysaccharide-specific antibody-secreting cells in neonatal mice. J. Immunol. 2012, 189, 1265–1273. [Google Scholar] [CrossRef] [Green Version]
- Vono, M.; Eberhardt, C.; Mohr, E.; Auderset, F.; Christensen, D.; Schmolke, M.; Coler, R.; Meinke, A.; Andersen, P.; Lambert, P.H.; et al. Overcoming the neonatal limitations of inducing germinal centers through liposome-based adjuvants including c-type lectin agonists trehalose dibehenate or curdlan. Front. Immunol. 2018, 9, 381. [Google Scholar] [CrossRef] [Green Version]
- Pind, A.A.A.; Dubik, M.; Thorsdottir, S.; Meinke, A.; Harandi, A.M.; Holmgren, J.; Del Giudice, G.; Ingileif, J.; Stefania, P.B. Adjuvants enhance the induction of germinal center and antibody secreting cells in spleen and their persistence in bone marrow of neonatal mice. Front. Immunol. 2019, 10, 2214. [Google Scholar] [CrossRef] [Green Version]









| Gene | Primer Sequence |
|---|---|
| Zfp979 (ENSMUSG00000066000) | Forward: 5-GCTGGCCTCCTAGGACATTC-3 |
| Reverse: 5-GGAGCAAACATTCAAGTTCTGGAT-3 | |
| C2cd4a (ENSMUSG00000047990) | Forward: 5-TCTGACTCTGAATACCAGGCAGC-3 |
| Reverse: 5-GGTCTGGAGTGAGCACGTTT-3 | |
| Ces1f (ENSMUSG00000031725) | Forward: 5-TGTAAGACCACCACGTCTGC-3 |
| Reverse: 5-TGGTCGCTATTTTTGGTATCTCCT-3 | |
| Inmt (ENSMUSG00000003477) | Forward: 5-CCTTTCTGGCCATGGAGTGT-3 |
| Reverse: 5-TGGAAGCGCAGAGTAACCAG-3 | |
| Lilrb4 (ENSMUSG00000112148) | Forward: 5-GGACCTGCCCTCAAGATGAC-3 |
| Reverse: 5-GGTTCCAGAATAAGACCTCACCA-3 | |
| Gm5771 (ENSMUSG00000058119) | Forward: 5-TCCCTGTGGATGATGATAAGATCG-3 |
| Reverse: 5-CACTTGGATGCGGGTTTTGT-3 | |
| Gys2 (ENSMUSG00000030244) | Forward: 5-ATCACCACCAACGACGGA-3 |
| Reverse: 5-GCCTCCTCTTCCTCATCATACC-3 | |
| Tmprss13 (ENSMUSG00000037129) | Forward: 5-CCAGGTCTCAGTTTCCCCAA-3 |
| Reverse: 5-CTCTCCAGAAGTAGAAGAGAAGG-3 | |
| Ighv5-9-1 (ENSMUSG00000095210) | Forward: 5-GAGATGGTGAATCGGCCCTT-3 |
| Reverse: 5-GTGCAGCCTCTGGATTCACT-3 | |
| Olfr56 (ENSMUSG00000040328) | Forward: 5-TCCTATGCTCAACCCCCTCA-3 |
| Reverse: 5-GGCAAACATCAGGCAACACA-3 | |
| Ctrl (ENSMUSG00000031896) | Forward: 5-ATCAGTGGTGTGGGCAATGT-3 |
| Reverse: 5-CATGGCATCGGTAATGCGTG-3 | |
| Tmed11 (ENSMUSG00000004821) | Forward: 5-ATCTGCTCCTGGCTTAGGAATG-3 |
| Reverse: 5-ATATCTAAGTGGATGCGCAGCTT-3 | |
| GAPDH (ENSMUSG00000057666) | Forward: 5-TCG TCC GGT AGA CAA AAT GG-3 |
| Reverse: 5-GAG GTC AAT GAA GGG GTC GT-3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yuan, L.; Cui, X.; Xu, W.; Fang, S.; Li, Z.; Lu, M.; Wu, Y.; Ma, X.; Chi, X.; et al. Ginseng Stem-Leaf Saponins in Combination with Selenium Promote the Immune Response in Neonatal Mice with Maternal Antibody. Vaccines 2020, 8, 755. https://doi.org/10.3390/vaccines8040755
Wang Y, Yuan L, Cui X, Xu W, Fang S, Li Z, Lu M, Wu Y, Ma X, Chi X, et al. Ginseng Stem-Leaf Saponins in Combination with Selenium Promote the Immune Response in Neonatal Mice with Maternal Antibody. Vaccines. 2020; 8(4):755. https://doi.org/10.3390/vaccines8040755
Chicago/Turabian StyleWang, Yong, Lijia Yuan, Xuemei Cui, Wei Xu, Sijia Fang, Zoushuyi Li, Meiqian Lu, Ye Wu, Xiaodan Ma, Xiaoqing Chi, and et al. 2020. "Ginseng Stem-Leaf Saponins in Combination with Selenium Promote the Immune Response in Neonatal Mice with Maternal Antibody" Vaccines 8, no. 4: 755. https://doi.org/10.3390/vaccines8040755
APA StyleWang, Y., Yuan, L., Cui, X., Xu, W., Fang, S., Li, Z., Lu, M., Wu, Y., Ma, X., Chi, X., & Hu, S. (2020). Ginseng Stem-Leaf Saponins in Combination with Selenium Promote the Immune Response in Neonatal Mice with Maternal Antibody. Vaccines, 8(4), 755. https://doi.org/10.3390/vaccines8040755





