Encapsulation of Lactoferrin for Sustained Release Using Particles from Gas-Saturated Solutions
Abstract
1. Introduction
2. Methods
2.1. Materials
2.2. Preparation of Shellac Microcapsules of Lactoferrin
2.3. Study of Microcapsule Morphology and Size Distribution
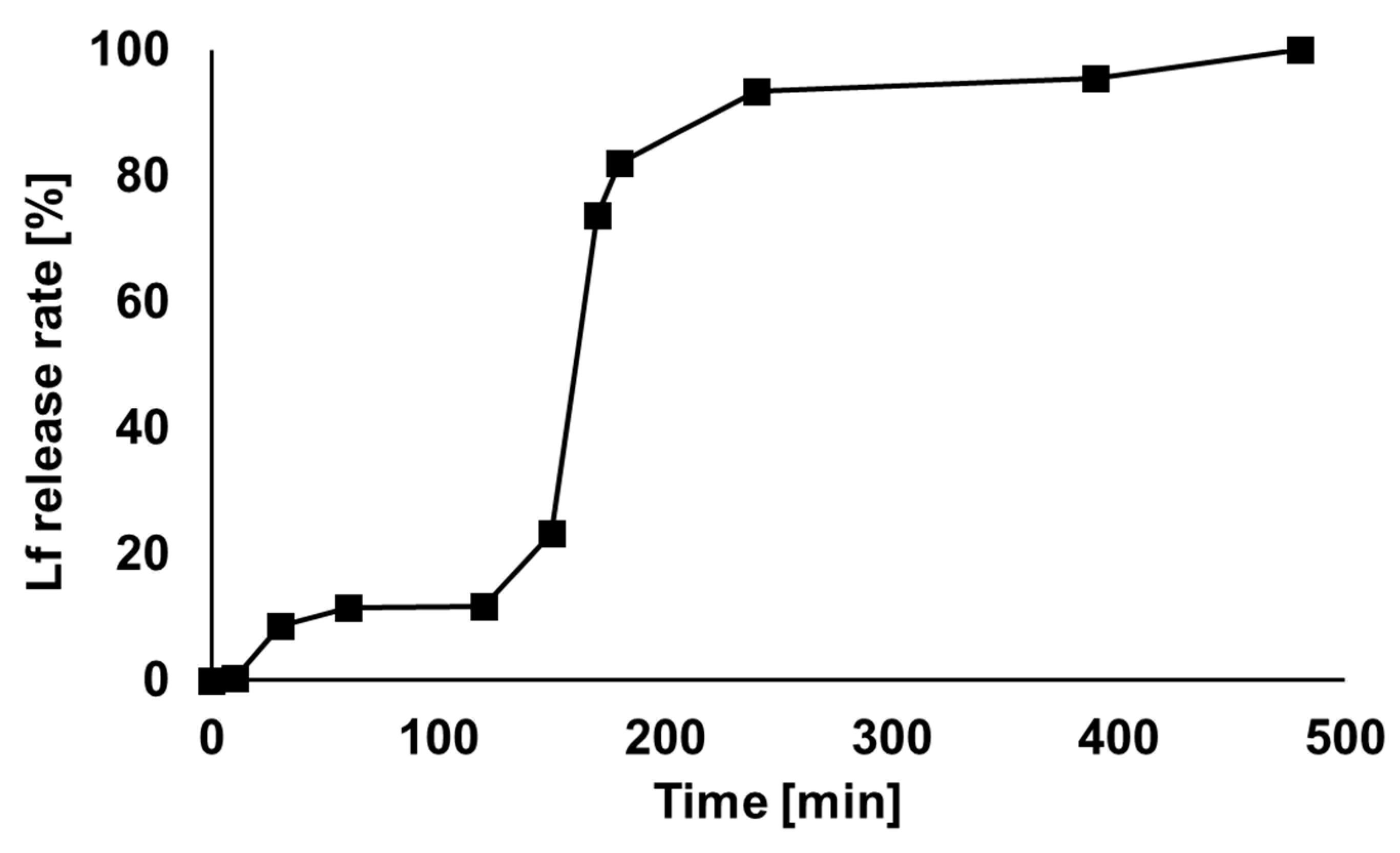
2.4. Lf microcapsule In Vitro Release
2.5. Statistical Analysis
3. Results and Discussion
3.1. Encapsulation of Lf in Shellac by PGSS
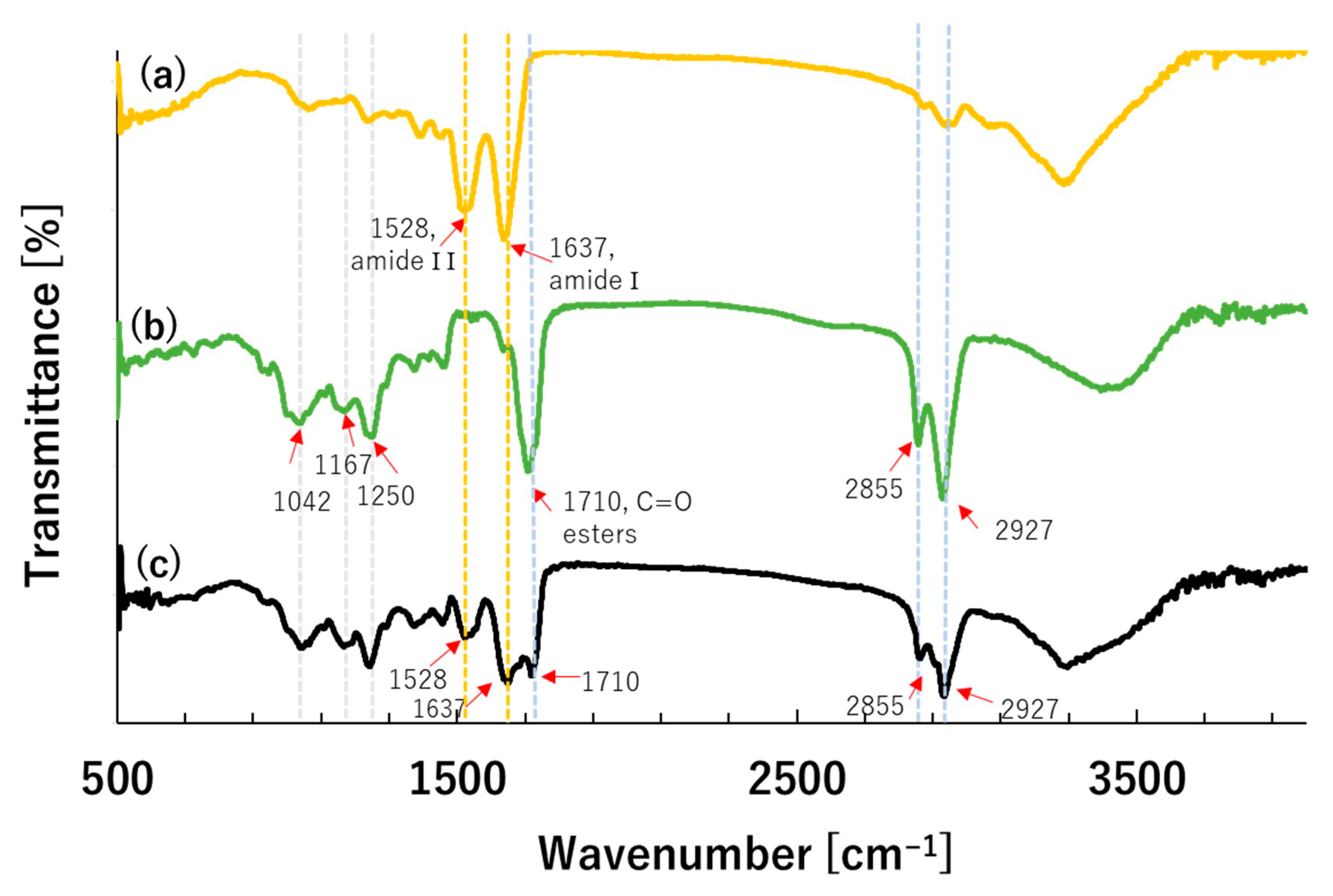
3.2. Fourier Transform Infra-Red Spectroscopy (FT-IR) Analysis
3.3. In Vitro Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez-Chavez, S.A.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Albar, A.H.; Almehdar, H.A.; Uversky, V.N.; Redwan, E.M. Structural heterogeneity and multifunctionality of lactoferrin. Curr. Protein Pept. Sci 2014, 15, 778–797. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012, 90, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Panella, G.; Leboe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.; Calvo, M.; Brock, J.H. Biological role of lactoferrin. Arch. Dis. Child. 1992, 67, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Brock, J. Lactoferrin in human milk: Its role in iron absorption and protection against enteric infection in the newborn infant. Arch. Dis. Child. 1980, 55, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Ng, T.B.; Sun, W.-Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106118. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defence against microbial and viral attack. Cell. Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Vega-Bautista, A.; Garza de la, M.; Carrero, J.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Drago-Serrano, M.E. The Impact of Lactoferrin on the Growth of Intestinal Inhabitant Bacteria. Int. J. Mol. Sci. 2019, 20, 4707. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.C.; Watanabe, R.; Koike, Y.; Mitobe, M.; Shimazaki, K.; Watanabe, S.; Azuma, I. Apoptosis in human leukemic cells induced by lactoferricin, a bovine milk protein-derived peptide: Involvement of reactive oxygen species. Biochem. Biophys. Res. Commun. 1997, 237, 624–628. [Google Scholar] [CrossRef] [PubMed]
- De Etchepare, M.A.; Barin, J.S.; Cichoski, A.J.; Jacob-Lopes, E.; Wagner, R.; Fries, L.L.M.; Menezes, C.R. Microencapsulation of probiotics using sodium alginate. Ciência Rural 2015, 45, 1319–1326. [Google Scholar] [CrossRef]
- Kilic, E.; Novoselova, M.; Lim, S.; Pyataev, N.; Pinyaev, S.; Kulikov, O.; Sindeeva, O.; Mayorova, O.; Murney, R.; Antipina, M.; et al. Formulation for Oral Delivery of Lactoferrin Based on Bovine Serum Albumin and Tannic Acid Multilayer Microcapsules. Sci. Rep. 2017, 7, 44159. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K. Biodegradable particle formation for drug and gene delivery using supercritical fluid and dense gas. Adv. Drug Deliv. Rev. 2008, 60, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Limmatvapirat, S.; Panchapornpon, D.; Limmatvapirat, C.; Nunthanid, J.; Luangtana-Anan, M.; Puttipipatkhachorn, S. Formation of shellac succinate having improved enteric film properties through dry media reaction. Eur. J. Pharm. Biopharm. 2008, 70, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, P.W.; Naicker, B.; Kalombo, L. Micronization, characterization and in-vitro dissolution of shellac from PGSS supercritical CO2 technique. Int. J. Pharm. 2016, 499, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Brisson, G.; Britten, M.; Pouliot, Y. Heat-induced aggregation of bovine lactoferrin at neutral pH: Effect of iron saturation. Int. Dairy J. 2007, 17, 617–624. [Google Scholar] [CrossRef]
- White, L.J.; Kirby, G.T.; Cox, H.C.; Qodratnama, R.; Qutachi, O.; Rose, F.R.; Shakesheff, K.M. Accelerating protein release from microparticles for regenerative medicine applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2578–2583. [Google Scholar] [CrossRef] [PubMed]



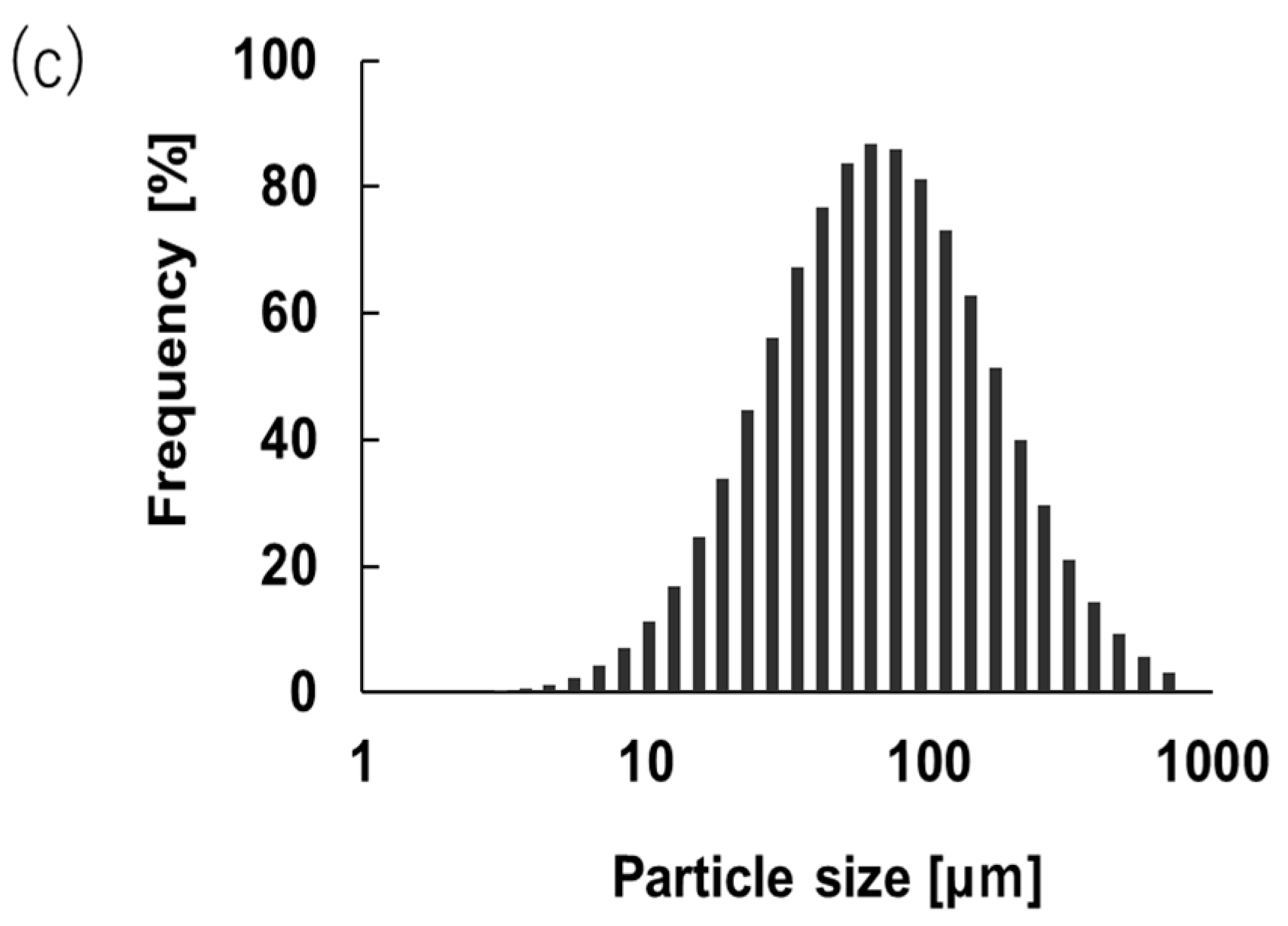


| Experimental Conditions | Avg. Particle Size [µm] | Particle Size Distribution (PSD) | EE [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P [MPa] | T [°C] | Lactoferrin [g] | Shellac EtOH Ratio | d0.1 | d0.5 | d0.9 | Span | ||
| 8 | 40 | 0.2 | 1:10 | 143.0 ± 4 b | 61.8 | 144.3 | 338.5 | 1.91 | 51 ± 5 a |
| 9 | 40 | 0.2 | 1:10 | 132.6 ± 10 b | 39.8 | 153.0 | 592.0 | 3.61 | |
| 10 | 40 | 0.2 | 1:10 | 75.4 ± 7 a | 36.8 | 77.2 | 161.3 | 1.61 | 71 ± 2 b |
| 10 | 50 | 0.2 | 1:10 | Agglomerated | - | - | - | - | - |
| 10 | 30 | 0.2 | 1:10 | 59.8 ± 2 a | 5.09 | 59.9 | 559.8 | 9.92 | - |
| 10 | 20 | 0.2 | 1:10 | 71.4 ± 3 a | 12.17 | 77.56 | 423.3 | 5.30 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, K.; Sakai, H.; Tokunaga, S.; Sharmin, T.; Aida, T.M.; Mishima, K. Encapsulation of Lactoferrin for Sustained Release Using Particles from Gas-Saturated Solutions. Processes 2021, 9, 73. https://doi.org/10.3390/pr9010073
Ono K, Sakai H, Tokunaga S, Sharmin T, Aida TM, Mishima K. Encapsulation of Lactoferrin for Sustained Release Using Particles from Gas-Saturated Solutions. Processes. 2021; 9(1):73. https://doi.org/10.3390/pr9010073
Chicago/Turabian StyleOno, Kento, Hiroki Sakai, Shinichi Tokunaga, Tanjina Sharmin, Taku Michael Aida, and Kenji Mishima. 2021. "Encapsulation of Lactoferrin for Sustained Release Using Particles from Gas-Saturated Solutions" Processes 9, no. 1: 73. https://doi.org/10.3390/pr9010073
APA StyleOno, K., Sakai, H., Tokunaga, S., Sharmin, T., Aida, T. M., & Mishima, K. (2021). Encapsulation of Lactoferrin for Sustained Release Using Particles from Gas-Saturated Solutions. Processes, 9(1), 73. https://doi.org/10.3390/pr9010073




