Red Blood Cell Membrane-Camouflaged Tedizolid Phosphate-Loaded PLGA Nanoparticles for Bacterial-Infection Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells, Bacteria and Animals
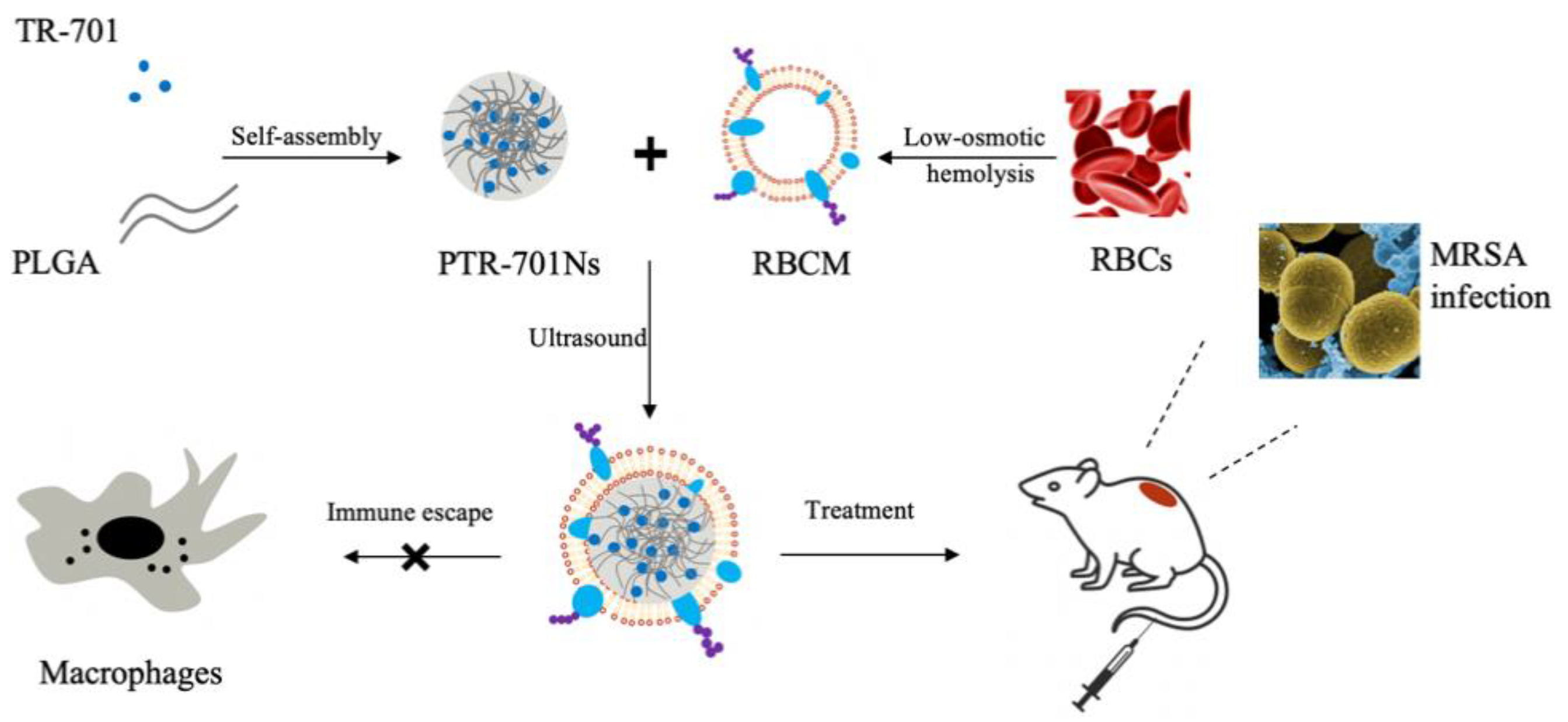
2.3. Preparation of PTR-701Ns
2.4. Preparation of RBCM
2.5. Preparation of RPTR-701Ns
2.6. Characterization and Stability Test
2.7. Morphological Observation
2.8. Drug Loading Capacity Test
2.9. In Vitro Release Study
2.10. Hemolysis Test
2.11. In Vitro Cytotoxicity Assay
2.12. Macrophage Uptake Study
2.13. In Vitro Antibacterial Efficacy Study
2.14. Bacterial Exotoxins Removing Capability Study
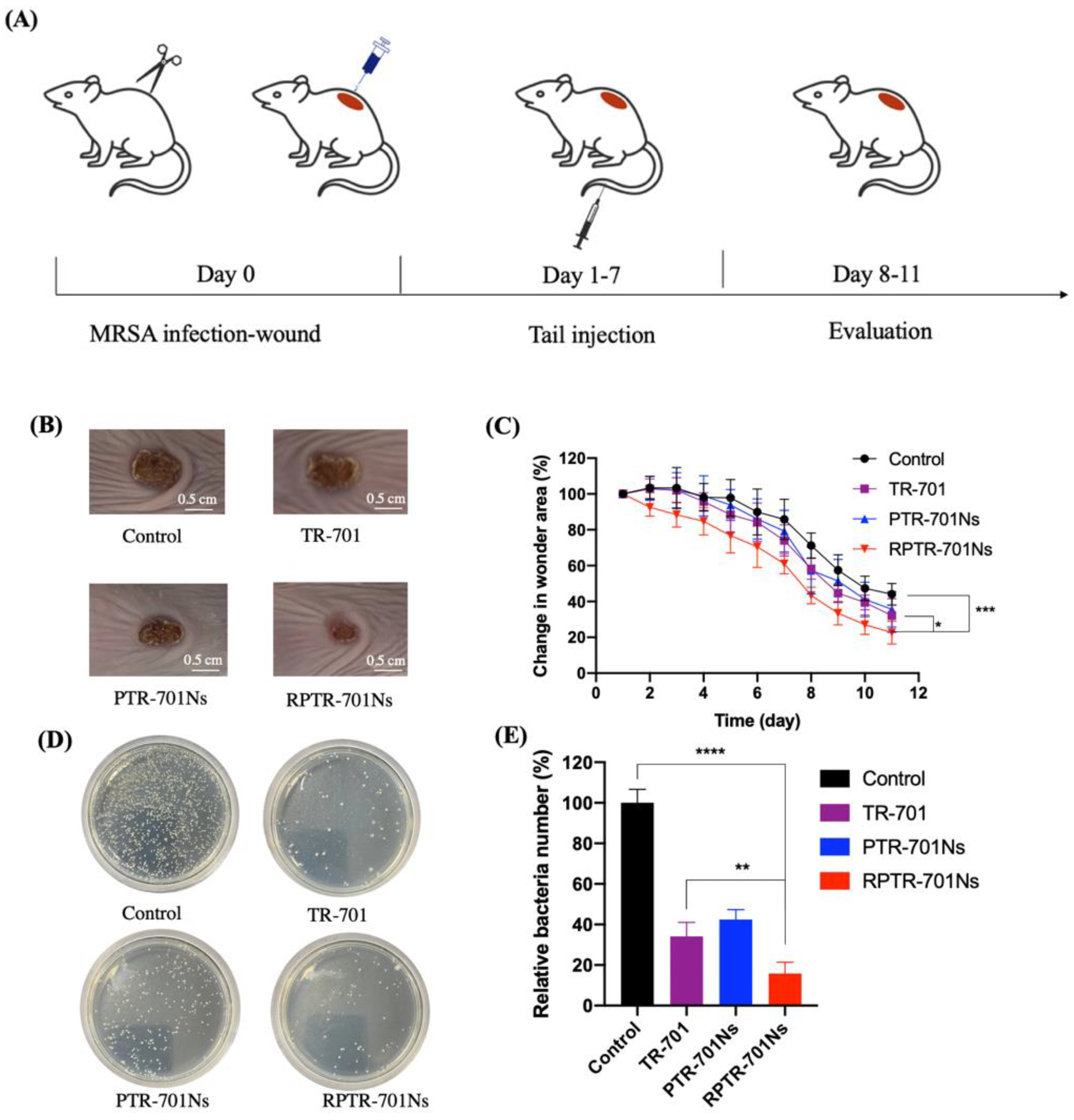
2.15. In Vivo Anti-Infection Study
2.15.1. In Vivo Anti-Bacterial Study
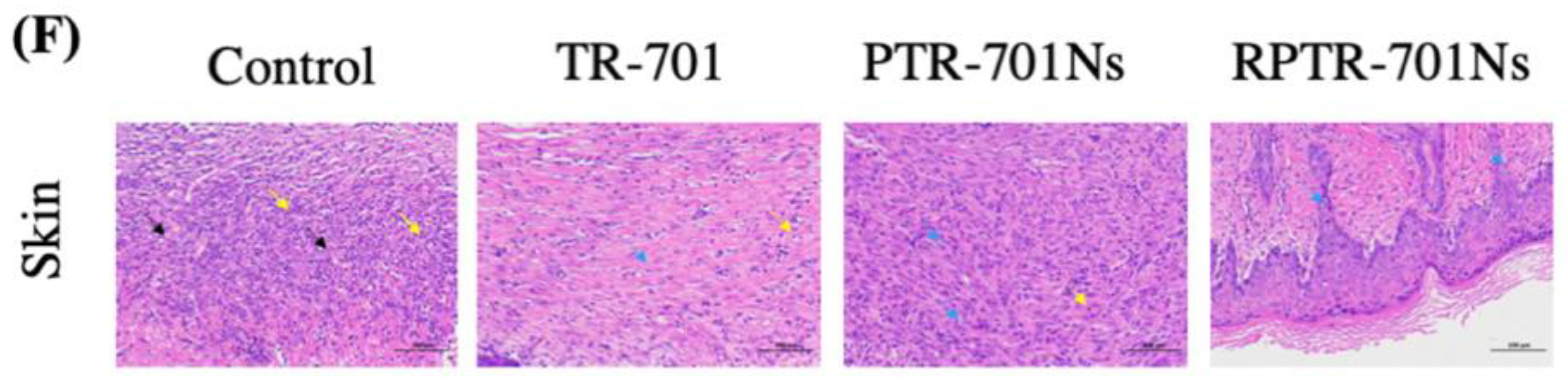
2.15.2. In Vivo Safety Study
2.16. Statistics and Data Analysis
3. Results
3.1. Preparation and Stability of PTR-701Ns and RPTR-701Ns
3.2. Morphological Observation of PTR-701Ns and RPTR-701Ns
3.3. Drug Loading Capacity and In Vitro Release Study
3.4. Hemolysis Test
3.5. In Vitro Cytotoxicity Assay
3.6. Macrophage Uptake Study
3.7. In Vitro Antibacterial Efficacy Study
3.8. Bacterial Exotoxins Removing Capability Study
3.9. In Vivo Ant-Infection Study
3.10. In Vivo Safety Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, C.A.; Murray, B.E. Antibiotic-Resistant Bugs in the 21st Century—A Clinical Super-Challenge. N. Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic Strategies to Counteract Antibiotic Resistance in MRSA Biofilm-Associated Infections. Chemmedchem 2020. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Pfaller, M.A.; Schmitz, F.J.; Smayevsky, J.; Bell, J.; Jones, R.N.; Beach, M.; SENTRY Partcipants Group. Survey of infections due to Staphylococcus species: Frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001, 32, 114–132. [Google Scholar]
- Klein, E.; Smith, D.L.; Laxminarayan, R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 2007, 13, 1840–1846. [Google Scholar] [CrossRef]
- Shaw, K.J.; Barbachyn, M.R. The oxazolidinones: Past, present, and future. Ann. N.Y. Acad. Sci. 2011, 1241, 48–70. [Google Scholar] [CrossRef]
- Douros, A.; Grabowski, K.; Stahlmann, R. Drug-drug interactions and safety of linezolid, tedizolid, and other oxazolidinones. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1849–1859. [Google Scholar] [CrossRef]
- Alcala, L.; Ruiz-Serrano, M.J.; Perez-Fernandez Turegano, C.; Garcia De Viedma, D.; Diaz-Infantes, M.; Marin-Arriaza, M.; Bouza, E. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob. Agents Chemother. 2003, 47, 416–417. [Google Scholar] [CrossRef] [Green Version]
- Vera-Cabrera, L.; Gonzalez, E.; Rendon, A.; Ocampo-Candiani, J.; Welsh, O.; Velazquez-Moreno, V.M.; Choi, S.H.; Molina-Torres, C. In vitro activities of DA-7157 and DA-7218 against Mycobacterium tuberculosis and Nocardia brasiliensis. Antimicrob. Agents Chemother. 2006, 50, 3170–3172. [Google Scholar] [CrossRef] [Green Version]
- Prokocimer, P.; Bien, P.; Deanda, C.; Pillar, C.M.; Bartizal, K. In vitro activity and microbiological efficacy of tedizolid (TR-700) against Gram-positive clinical isolates from a phase 2 study of oral tedizolid phosphate (TR-701) in patients with complicated skin and skin structure infections. Antimicrob. Agents Chemother. 2012, 56, 4608–4613. [Google Scholar] [CrossRef] [Green Version]
- Carena, A.A.; Stryjewski, M.E. Tedizolid (torezolid) for the treatment of complicated skin and skin structure infections. Expert Rev. Clin. Pharm. 2020, 13, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Corey, R.; Moran, G.; Goering, R.; Bensaci, M.; Sandison, T.; De Anda, C.; Prokocimer, P. Comparison of the microbiological efficacy of tedizolid and linezolid in acute bacterial skin and skin structure infections: Pooled data from phase 3 clinical trials. Diagn. Microbiol. Infect. Dis. 2019, 94, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.J.; Alder, J.; Li, L.; O’Riordan, W.; Rybak, M.J.; Ye, H.; Zhang, R.; Zhang, Z.; Zhu, X.; Wilcox, M.H. Efficacy and Safety of Tedizolid Phosphate versus Linezolid in a Randomized Phase 3 Trial in Patients with Acute Bacterial Skin and Skin Structure Infection. Antimicrob. Agents Chemother. 2019, 63, 15. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.L.; Tian, L.; Liu, J.J.; Huang, G.H. Construction and evaluation in vitro and in vivo of tedizolid phosphate loaded cationic liposomes. J. Liposome Res. 2018, 28, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.D.T.; Ee, P.L.R. Macromolecular Conjugate and Biological Carrier Approaches for the Targeted Delivery of Antibiotics. Antibiotics 2017, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Vanamala, K.; Tatiparti, K.; Bhise, K.; Sau, S.; Scheetz, M.H.; Rybak, M.J.; Andes, D.; Iyer, A.K. Novel approaches for the treatment of methicillin-resistant Staphylococcus aureus: Using nanoparticles to overcome multidrug resistance. Drug Discov. Today 2020. [Google Scholar] [CrossRef]
- Tsekouras, G.; Boudoire, F.; Pal, B.; Vondracek, M.; Prince, K.C.; Sarma, D.D.; Braun, A. Electronic structure origin of conductivity and oxygen reduction activity changes in low-level Cr-substituted (La,Sr)MnO3. J. Chem. Phys. 2015, 143, 114705. [Google Scholar] [CrossRef]
- Fasciani, C.; Silvero, M.J.; Anghel, M.A.; Arguello, G.A.; Becerra, M.C.; Scaiano, J.C. Aspartame-stabilized gold-silver bimetallic biocompatible nanostructures with plasmonic photothermal properties, antibacterial activity, and long-term stability. J. Am. Chem. Soc. 2014, 136, 17394–17397. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sun, P.; Zhang, L.; Wang, Z.; Wang, F.; Dong, K.; Liu, Z.; Ren, J.; Qu, X. Silver-Infused Porphyrinic Metal-Organic Framework: Surface-Adaptive, On-Demand Nanoplatform for Synergistic Bacteria Killing and Wound Disinfection. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Park, K.; Skidmore, S.; Hadar, J.; Garner, J.; Park, H.; Otte, A.; Soh, B.K.; Yoon, G.; Yu, D.; Yun, Y.; et al. Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation. J. Control. Release 2019, 304, 125–134. [Google Scholar] [CrossRef]
- Zare, E.N.; Jamaledin, R.; Naserzadeh, P.; Afjeh-Dana, E.; Ashtari, B.; Hosseinzadeh, M.; Vecchione, R.; Wu, A.; Tay, F.R.; Borzacchiello, A.; et al. Metal-Based Nanostructures/PLGA Nanocomposites: Antimicrobial Activity, Cytotoxicity, and Their Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 3279–3300. [Google Scholar] [CrossRef] [PubMed]
- Shreffler, J.W.; Pullan, J.E.; Dailey, K.M.; Mallik, S.; Brooks, A.E. Overcoming Hurdles in Nanoparticle Clinical Translation: The Influence of Experimental Design and Surface Modification. Int. J. Mol. Sci. 2019, 20, 6056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Su, J.; Liu, G.; Chen, J.; Zhang, X.; Zhang, R.; Jiang, M.; Qiu, M. Advances of blood cell-based drug delivery systems. Eur. J. Pharm. Sci. 2017, 96, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Wang, X.; Guo, P.; Li, Y.; Raza, F.; Su, J.; Qiu, M. Erythrocyte Membrane-Coated Arsenic Trioxide-Loaded Sodium Alginate Nanoparticles for Tumor Therapy. Pharmaceutics 2019, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.L.; Zou, M.Z.; Qin, S.Y.; Cheng, Y.J.; Ma, Y.H.; Sun, Y.X.; Zhang, X.-Z. Recent Advances of Cell Membrane-Coated Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 23. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, S.; Wang, F.; Fan, W.; Wu, C.X.; Mao, K.R.; Wang, H.; Hu, Z.; Yang, Y.; Sun, T. Red blood cell-derived nanovesicles for safe and efficient macrophage-targeted drug delivery in vivo. Biomater. Sci. 2019, 7, 187–195. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [Green Version]
- Rao, L.; Tian, R.; Chen, X. Cell-Membrane-Mimicking Nanodecoys against Infectious Diseases. ACS Nano 2020, 14, 2569–2574. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.M.; Fang, R.H.; Copp, J.; Luk, B.T.; Zhang, L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013, 8, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, M.; Zhang, Y.; Lee, J.H.; Escajadillo, T.; Gong, H.; Fang, R.H.; Gao, W.; Nizet, V.; Zhang, L. Broad-Spectrum Neutralization of Pore-Forming Toxins with Human Erythrocyte Membrane-Coated Nanosponges. Adv. Healthc. Mater. 2018, 7, 1701366. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Escajadillo, T.; Zhang, L.; Nizet, V.; Lawrence, S.M. Erythrocyte-Coated Nanoparticles Block Cytotoxic Effects of Group B Streptococcus beta-Hemolysin/Cytolysin. Front. Pediatr. 2019, 7, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escajadillo, T.; Olson, J.; Luk, B.T.; Zhang, L.; Nizet, V. A Red Blood Cell Membrane-Camouflaged Nanoparticle Counteracts Streptolysin O-Mediated Virulence Phenotypes of Invasive Group A Streptococcus. Front. Pharm. 2017, 8, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.L.; Xu, J.H.; Qi, G.B.; Zhao, X.Z.; Yu, F.Q.; Wang, H. Core-Shell Supramolecular Gelatin Nanoparticles for Adaptive and “On-Demand” Antibiotic Delivery. ACS Nano 2014, 8, 4975–4983. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.H.; Chen, W.S.; Angsantikul, P.; Spiekermann, K.A.; Fang, R.H.; Gao, W.; Zhang, L. Erythrocyte membrane-coated nanogel for combinatorial antivirulence and responsive antimicrobial delivery against Staphylococcus aureus infection. J. Control. Release 2017, 263, 185–191. [Google Scholar] [CrossRef]
- Lin, A.; Liu, Y.; Zhu, X.; Chen, X.; Liu, J.; Zhou, Y.; Qin, X.; Liu, J. Bacteria-Responsive Biomimetic Selenium Nanosystem for Multidrug-Resistant Bacterial Infection Detection and Inhibition. ACS Nano 2019, 13, 13965–13984. [Google Scholar] [CrossRef]
- Xie, J.; Shen, Q.; Huang, K.; Zheng, T.; Cheng, L.; Zhang, Z.; Yu, Y.; Liao, G.; Wang, X.; Li, C. Oriented Assembly of Cell-Mimicking Nanoparticles via a Molecular Affinity Strategy for Targeted Drug Delivery. ACS Nano 2019, 13, 5268–5277. [Google Scholar] [CrossRef]
- Ali, R. Preparation and characterization of dexamethasone polymeric nanoparticle by membrane emulsification method. J. Nanoparticle Res. 2020, 22, 314. [Google Scholar] [CrossRef]
- Hochmuth, R.M.; Evans, C.A.; Wiles, H.C.; McCown, J.T. Mechanical measurement of red cell membrane thickness. Science (N. Y.) 1983, 220, 101–102. [Google Scholar] [CrossRef]
- Zarrin, A.; Foroozesh, M.; Hamidi, M. Carrier erythrocytes: Recent advances, present status, current trends and future horizons. Expert Opin. Drug Deliv. 2014, 11, 433–447. [Google Scholar] [CrossRef]
- Wang, C.; Sun, C.; Li, M.; Xia, B.; Wang, Y.; Zhang, L.; Zhang, Y.; Wang, J.; Sun, F.; Lu, S.; et al. Novel fully human anti-CD47 antibodies stimulate phagocytosis and promote elimination of AML cells. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, F.; Zhu, X.; Xie, W.; Hu, X.; Zan, M.; Li, X.; Li, Q.; Guo, S.; Zhao, X.; et al. Highly biocompatible and recyclable biomimetic nanoparticles for antibiotic-resistant bacteria infection. Biomater. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]



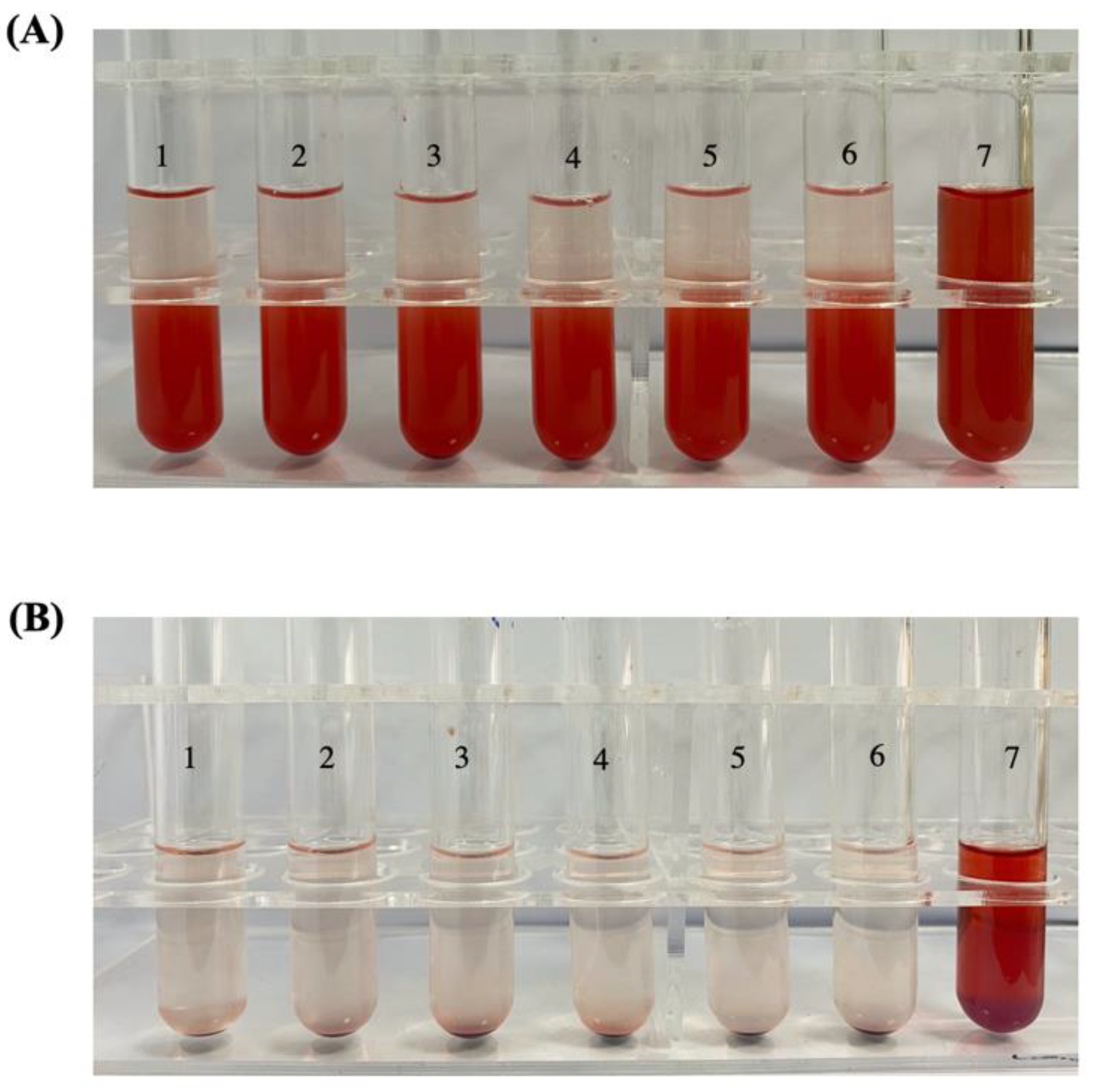





| Tube No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Normal saline (mL) | 2.4 | 2.3 | 2.2 | 2.1 | 2.0 | 2.5 | 0 |
| Ultrapure water (mL) | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 |
| 2% RBCs (mL) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| RPTR-701Ns (mL) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Li, Y.; Raza, F.; Wang, X.; Zhang, S.; Rong, R.; Qiu, M.; Su, J. Red Blood Cell Membrane-Camouflaged Tedizolid Phosphate-Loaded PLGA Nanoparticles for Bacterial-Infection Therapy. Pharmaceutics 2021, 13, 99. https://doi.org/10.3390/pharmaceutics13010099
Wu X, Li Y, Raza F, Wang X, Zhang S, Rong R, Qiu M, Su J. Red Blood Cell Membrane-Camouflaged Tedizolid Phosphate-Loaded PLGA Nanoparticles for Bacterial-Infection Therapy. Pharmaceutics. 2021; 13(1):99. https://doi.org/10.3390/pharmaceutics13010099
Chicago/Turabian StyleWu, Xinyi, Yichen Li, Faisal Raza, Xuerui Wang, Shulei Zhang, Ruonan Rong, Mingfeng Qiu, and Jing Su. 2021. "Red Blood Cell Membrane-Camouflaged Tedizolid Phosphate-Loaded PLGA Nanoparticles for Bacterial-Infection Therapy" Pharmaceutics 13, no. 1: 99. https://doi.org/10.3390/pharmaceutics13010099
APA StyleWu, X., Li, Y., Raza, F., Wang, X., Zhang, S., Rong, R., Qiu, M., & Su, J. (2021). Red Blood Cell Membrane-Camouflaged Tedizolid Phosphate-Loaded PLGA Nanoparticles for Bacterial-Infection Therapy. Pharmaceutics, 13(1), 99. https://doi.org/10.3390/pharmaceutics13010099








