Study on the Dissolution and Precipitation Behavior of Self-Designed (NbTi)C Nanoparticles Addition in 1045 Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. (NbTi)C Particles Preparations and Smelting Test
2.2. Microstructure Characterization and Mechanical Property Test
2.3. Mechanical Properties Test
3. Results
4. Discussion
5. Conclusions
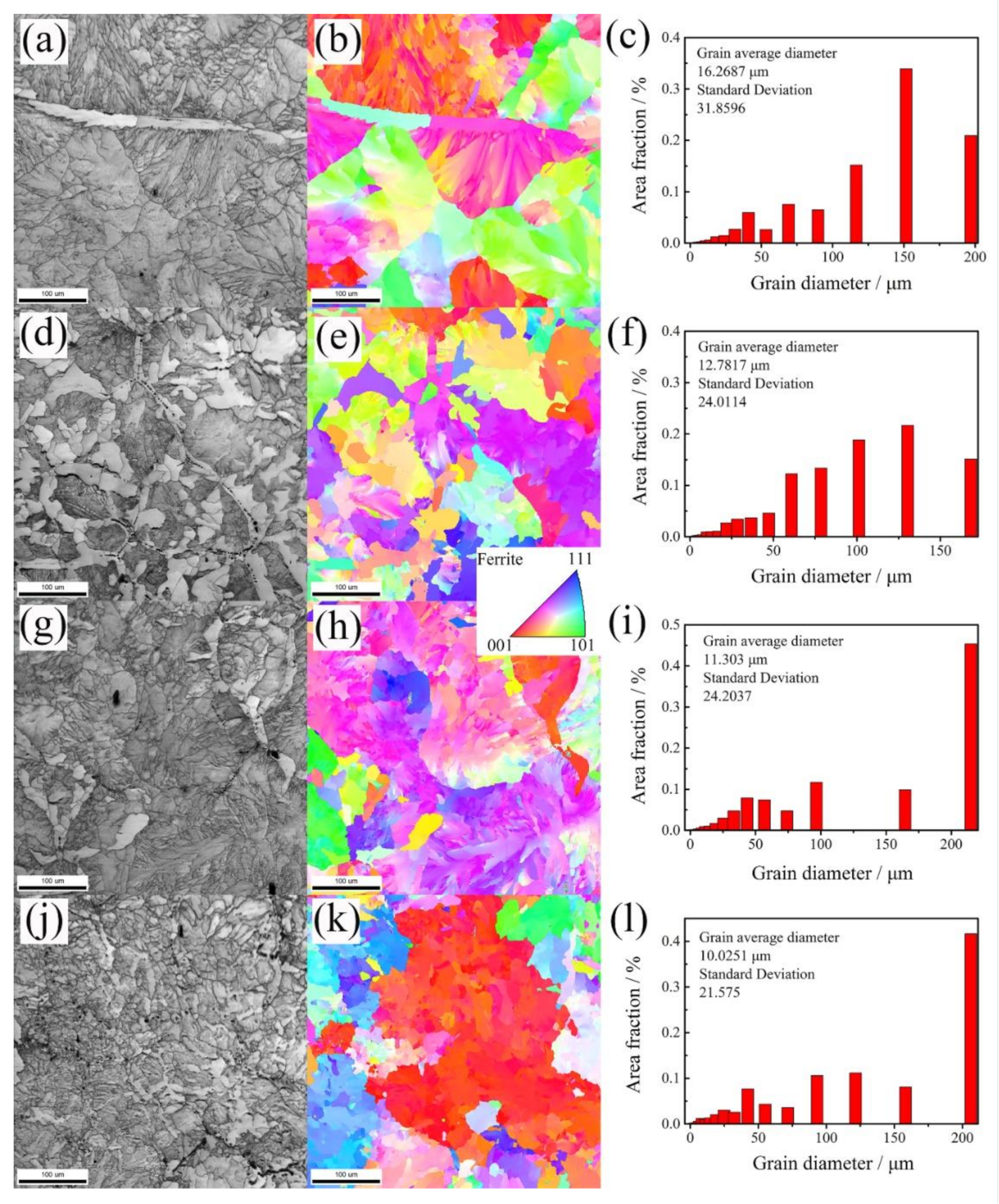
- The presence of the second phases in the cast steel, which are different from the added (NbTi)C nanoparticles, is due to the dissolution and precipitation behavior of (NbTi)C nanoparticles during the smelting process. Among them, the square nano-(NbTi)C with a size of about 100 nm are formed by intragranular precipitation, and the micro-(NbTi)C is formed by the eutectic precipitation at the grain boundaries.
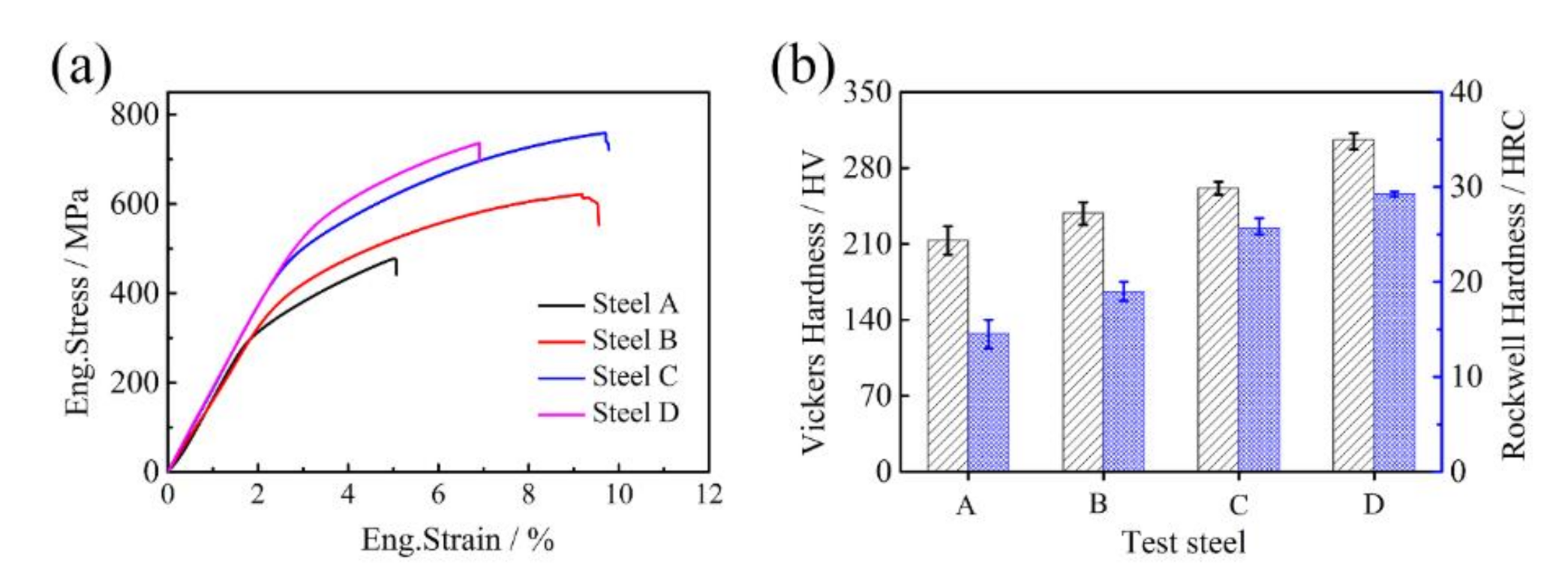
- The addition of (NbTi)C nanoparticles can refine the microstructure and improve the hardness and strength. The average grain diameter decreased from 16.3 μm in steel A to 10.0 μm in steel D; the tensile strength increased from 498.9 MPa in steel A to 759.0 MPa in steel D, an increase of 52.1%. A continued increase in (NbTi)C will lead to a decrease in strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, Z.; Misra, R.D.K.; O’Malley, R.; Jansto, S.J. Fine-scal precipitation and mechanical properties of thin slab processed titanium-niobium bearing high strength steels. Mater. Sci. Eng. A 2011, 528, 7077–7083. [Google Scholar] [CrossRef]
- Olalla, V.C.; Bliznuk, V.; Sanchez, N.; Thibaux, P.; Kestens, L.A.I.; Petrov, R.H. Analysis of the strengthening mechanisms in pipeline steels as a function of the hot rolling parameters. Mater. Sci. Eng. A 2014, 604, 46–56. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, W.; Mishra, R.S.; Charit, I. Microstructure and mechanical properties of friction stir welded oxide dispersion strengthened alloy. J. Nucl. Mater. 2013, 432, 274–280. [Google Scholar] [CrossRef]
- El-Faramawy, H.S.; Ghali, S.N.; Eissa, M.M. Effect of Titanium addition on the behavior of medium carbon steel. J. Miner. Mater. Charact. Eng. 2012, 11, 1108–1112. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Z.; Lee, C.S. Enhancing impact fracture toughness and tensile properties of a microalloyed cast steel by hot forging and post-forging heat treatment process. Mater. Des. 2013, 47, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Shi, J.; Xu, L.; Cao, W.Q.; Dong, H. TiC precipitation induced effect on microstructure and mechanical properties in low carbon medium manganese steel. Mater. Sci. Eng. A 2011, 530, 643–651. [Google Scholar] [CrossRef]
- Luo, Z.C.; Ning, J.P.; Wang, J.; Zheng, K.H. Microstructure and wear properties of TiC-strengthened high-manganese steel matrix composites fabricated by hypereutectic solidification. Wear 2019, 432, 202970. [Google Scholar] [CrossRef]
- Lee, Y.H.; Ko, S.; Park, H.; Lee, D.; Shin, S.; Jo, I.; Lee, S.-B.; Lee, S.-K.; Kim, Y.; Cho, S. Effect of TiC particle size on high temperature oxidation behavior of TiC reinforced stainless steel. Appl. Surf. Sci. 2019, 480, 951–955. [Google Scholar] [CrossRef]
- Gomes, U.U.; Oliveira, L.A.; Soares, S.R.S.; Furukava, M.; Souza, C.P. Effect of the dispersion of nanosized carbides (NbC-TaC) in the sintered microstructure of the stainless steel 316L. Mater. Sci. Forum 2008, 591–593, 294–298. [Google Scholar] [CrossRef]
- Gülsoy, H.O. Production of injection moulded 316L stainless steels reinforced with TiC(N) particles. Mater. Sci. Technol. 2008, 24, 1484–1491. [Google Scholar] [CrossRef]
- Qin, S.; Liao, B.; Mao, L.; Xiao, F. A novel method for preparing nano-NbC/Fe powder and nano-NbC particle reinforced cast low-carbon steel. Mater. Lett. 2014, 121, 162–165. [Google Scholar] [CrossRef]
- Lazarova, R.; Petrov, R.H.; Gaydarova, V.; Davidkov, A.; Alexeev, A.; Manchev, M.; Manolov, V. Microstructure and mechanical properties of P265GH cast steel after modification with TiCN particles. Mater. Des. 2011, 32, 2734–2741. [Google Scholar] [CrossRef]
- Qin, S.; Liao, B.; Xiao, F.; Mao, L. Fabrication of NbC reinforced low carbon steel by immersing Nb(C)-Fe powders in steel melt. Mater. Manuf. Process. 2015, 30, 116–121. [Google Scholar] [CrossRef]
- Lazarova, R.; Bojanova, N.; Dimitrova, R.; Panov, I.; Manolov, V. Influence of nanoparticles introducing in the melt of aluminum alloys on castings microstructure and properties. Int. J. Metalcast. 2016, 10, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Sang-Hoon, L.E.E.; Jin-Ju, P.A.R.K.; Sung-Mo, H.O.N.G.; Byoung-Sun, H.A.N.; Min-Ku, L.E.E.; Chang-Kyu, R.H.E.E. Fabrication of cast carbon steel with ultrafine TiC particles. Trans. Nonferr. Metals Soc. China 2011, 21, 54–57. [Google Scholar]
- Park, J.J.; Hong, S.M.; Park, E.K.; Kim, K.Y.; Lee, M.K.; Rhee, C.K. Microstructure and properties of SA 106B carbon steel after treatment of the melt with nano-sized TiC particles. Mater. Sci. Eng. A 2014, 613, 217–223. [Google Scholar] [CrossRef]
- Hong, S.M.; Park, E.K.; Park, J.J.; Lee, M.K.; Lee, J.G. Effect of nano-sized TiC particle addition on microstructure and mechanical properties of SA-106B carbon steel. Mater. Sci. Eng. A 2015, 643, 37–46. [Google Scholar] [CrossRef]
- Hong, S.M.; Park, J.J.; Park, E.K.; Lee, M.K.; Rhee, C.K.; Lee, J.K. Cavitation erosion behavior of SA 106B carbon steel after treatment of the melt with nano-sized TiC particles. Tribol. Int. 2015, 92, 585–594. [Google Scholar] [CrossRef]
- Park, J.J.; Park, E.K.; Lee, G.J.; Rhee, C.K.; Lee, M.K. Effect of a nano-sized TiC particle addition on the flow-assisted corrosion resistance of SA 106B carbon steel. Appl. Surf. Sci. 2017, 415, 143–148. [Google Scholar] [CrossRef]
- Podgornik, B.; Torkar, M.; Burja, J.; Godec, M.; Senčič, B. Improving properties of spring steel though nano-particles alloying. Mater. Sci. Eng. A 2015, 638, 183–189. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, J.S.; Yun, H.S. Microstructure and mechanical properties of TiC nanoparticle-reinforced iron-matrix composites. Strength Mater. 2014, 46, 177–182. [Google Scholar] [CrossRef]
- Zhu, H.W.; Ke, W.J.; Zhao, Z.P.; Qin, S.; Xiao, F.R.; Liao, B. Refinement effectiveness of self-prepared (NbTi)C nanoparticles on as-cast 1045 steel. Mater. Des. 2018, 139, 531–540. [Google Scholar] [CrossRef]
- Zhu, H.W.; Xu, K.; Qin, S.; Xiao, F.R.; Liao, B. Effect of heat treatment on microstructure and properties of 1045 steel modified with (NbTi)C nanoparticles. Mater. Sci. Eng. A 2018, 728, 175–182. [Google Scholar] [CrossRef]
- Rajabi, A.; Ghazali, M.J.; Daud, A.R. Chemical composition, microstructure and sintering temperature modifications on mechanical properties of TiC-based cermet—A review. Mater. Des. 2015, 67, 95–106. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, L.; Tang, H.; Luo, X.; Zuo, L.; Wang, Z.; Wang, Y. Effect of nanoparticles formed in liquid melt on microstructure and mechanical property of high strength naval steel. J. Mater. Process. Technol. 2015, 222, 224–233. [Google Scholar] [CrossRef]
- Nordberg, H.; Aronsson, B. Solubility of Niobium Carbide in Austenite. Available online: https://www.researchgate.net/publication/284059865_Solubility_of_niobium_carbide_in_austenite (accessed on 29 November 2020).
- Irvine, K.J.; Pickering, F.B.; Gladman, T. Grain Refined C-Mn Steels. Available online: https://www.researchgate.net/publication/301327997_Grain-refined_C-Mn_steels (accessed on 29 November 2020).
- Chen, J.X. Manual of Chart and Data in Common Use of Steel Making; Metallurgical Industry Press: Beijing, China, 1984. [Google Scholar] [CrossRef]





| Grade | C | Si | Mn | Nb | Ti | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| A | 0.40 | 0.27 | 0.50 | – | – | 0.01 | 0.01 | Bal. |
| B | 0.40 | 0.27 | 0.51 | 0.08 | 0.02 | 0.01 | 0.01 | Bal. |
| C | 0.40 | 0.27 | 0.51 | 0.11 | 0.03 | 0.01 | 0.01 | Bal. |
| D | 0.40 | 0.27 | 0.50 | 0.47 | 0.12 | 0.01 | 0.01 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Li, H.; Xiao, F.; Gao, Z. Study on the Dissolution and Precipitation Behavior of Self-Designed (NbTi)C Nanoparticles Addition in 1045 Steel. Metals 2021, 11, 184. https://doi.org/10.3390/met11020184
Zhu H, Li H, Xiao F, Gao Z. Study on the Dissolution and Precipitation Behavior of Self-Designed (NbTi)C Nanoparticles Addition in 1045 Steel. Metals. 2021; 11(2):184. https://doi.org/10.3390/met11020184
Chicago/Turabian StyleZhu, Hongwei, Haonan Li, Furen Xiao, and Zhixiang Gao. 2021. "Study on the Dissolution and Precipitation Behavior of Self-Designed (NbTi)C Nanoparticles Addition in 1045 Steel" Metals 11, no. 2: 184. https://doi.org/10.3390/met11020184






