The Special Developmental Biology of Craniofacial Tissues Enables the Understanding of Oral and Maxillofacial Physiology and Diseases
Abstract
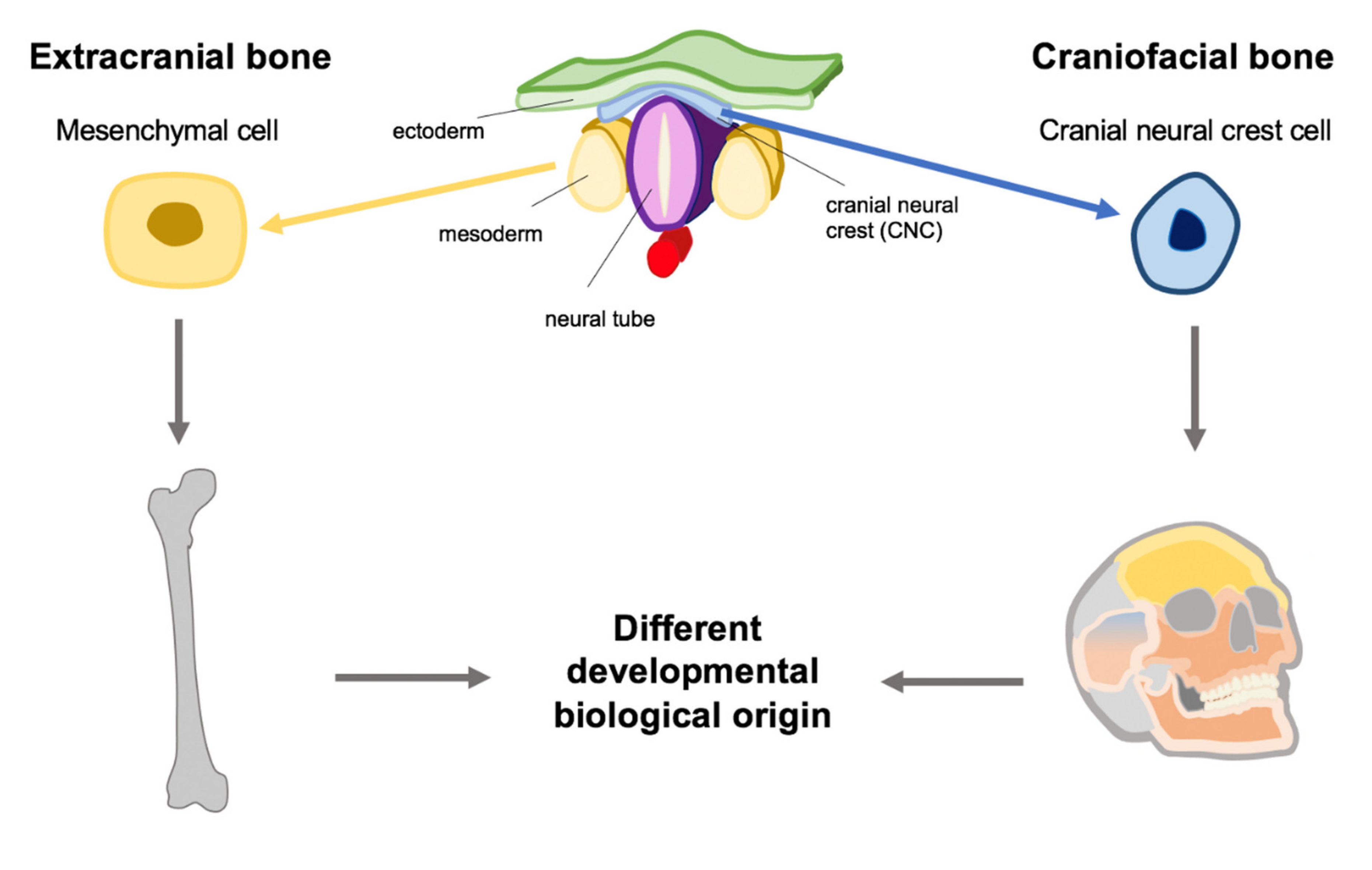
:1. Introduction
1.1. The Body Plan
1.2. Early Embryonic Development
1.3. Development of the Cotyledons
1.4. Development of the Neural Tube
1.5. Development of the Head
1.5.1. Development of the Pharyngeal Arches
1.5.2. Development of the Face
1.5.3. Development of the Tongue
1.5.4. Development of the Nervous System in the Head Area
2. Methods
- Cranial neural crest;
- Head and neck development;
- Craniofacial Abnormalities;
- Branchial arch;
- Jaw development;
- Tongue development;
- Mandibular osteogenesis;
- Cleft palate.
3. Special Features of Craniofacial Development
3.1. The Role of the CNC
3.1.1. Creation of CNC Cells
3.1.2. Migration of the CNC Cells
3.1.3. Contribution of the CNC Cells to the Development of Facial Prominences
3.1.4. Cellular Characteristics of CNC Cells
3.1.5. The Role of CNC Cells in Tooth Development
3.2. Determination of the Body Axes by Hox and Dlx Genes
3.3. Development of the Jawbone
3.4. Development of the Tongue
4. Clinical Impact of the Craniofacial Development
4.1. Significance for Specific Diseases of Craniofacial Tissue
4.2. Significance for Orthodontic Treatment
4.3. Impact for Oral Implant Osseointegration and Mesoderm Derived Bone Transplants
4.4. Impact for Syndromes and Malformations
4.4.1. Fetal Alcohol Syndrome
4.4.2. Treacher Collins Syndrome
4.4.3. Cleft Malformations
4.4.4. Pierre Robin Sequence
4.4.5. Hemifacial Microsomia
4.4.6. Goldenhar Syndrome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Alx | ALX Home Box |
| BMP | Bone Morphogenic Protein |
| Barx | BARX homeobox |
| CNC | Cranial Neural Crest |
| Dlx | Distal-less homeobox |
| EMT | Epithelial-Mesenchymal Translation |
| Ets | E26 transformation-specific factor |
| FAS | Fetal alcohol syndrome |
| FGF | Fibroblast growth factor |
| FNP | Frontonasal celebrities |
| Foxf | Forkhead Box F |
| HOX | Home box |
| IHH | Indian Hedgehog |
| KFO | Orthodontics |
| MRONJ | Medication related osteonecrosis of the jaw |
| Msx | Msh homeobox |
| NC | Neural Crest |
| Pax | Paired Box Protein |
| PRS | Pierre Robin sequence |
| Runx | Runt Related Transcription Factor |
| T-Box | T-Box Transcription Factor |
| TCOF | Treacle Ribosome Biogenesis Factor |
| TCS | Treacher Collins Syndrome |
| TGF | Transforming Growth Factor |
| Wnt | WNT, Wingless-related interrogation site |
References
- Rohen, J.W.; Lütjen-Drecoll, E. Funktionelle Embryologie: Die Entwicklung der Funktionssysteme des Menschlichen Organismus; Mit 9 Tabellen; Schattauer: Stuttgart, Germany, 2006. [Google Scholar]
- Moore, K.L.; TPersaud, V.N.; Viebahn, C. Embryologie: Entwicklungsstadien, Frühentwicklung, Organogenese, Klinik; Elsevier: Amsterdam, The Netherlands; Urban & Fischer: Munich, Germany, 2007. [Google Scholar]
- Ulfig, N.; Brand-Saberi, B. Kurzlehrbuch Embryologie; Thieme: New York, NY, USA, 2017. [Google Scholar]
- Betancur, P.; Bronner-Fraser, M.; Sauka-Spengler, T. Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proc. Natl. Acad. Sci. USA 2010, 107, 3570–3575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero, R.D.; Brugmann, S.; Chu, Y.; Bajpai, R.; Jame, M.; Helms, J.A. Cranial neural crest cells on the move: Their roles in craniofacial development. Am. J. Med. Genet. A 2011, 155, 270–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, S.G. Cranial neural crest: Migratory cell behavior and regulatory networks. Exp. Cell Res. 2014, 325, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Adameyko, I.; Fried, K. The Nervous System Orchestrates and Integrates Craniofacial Development: A Review. Front. Physiol. 2016, 7, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Parada, C.; Chai, Y. Cellular and molecular mechanisms of tooth root development. Development 2017, 144, 374–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, F.D.; Prendergast, A.; Swalla, B.J. Man is but a worm: Chordate origins. Genesis 2008, 46, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Lappin, R.T.; Grier, D.G.; Thompson, A.; Halliday, H.L. HOX genes: Seductive science, mysterious mechanisms. Ulst. Med. J. 2006, 75, 23–31. [Google Scholar]
- Parada, C.; Chai, Y. Mandible and Tongue Development. Curr. Top. Dev. Biol. 2015, 115, 31–58. [Google Scholar]
- Couly, G.; Creuzet, S.; Bennaceur, S.; Vincent, C.; Le Douarin, N.M. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development 2002, 129, 1061–1073. [Google Scholar]
- Noce, L.M.; Mele, L.; Tirino, V.; Paino, F.; de Rosa, A.; Naddeo, P.; Papagerakis, P.; Papaccio, G.; Desiderio, V. Neural crest stem cell population in craniomaxillofacial development and tissue repair. Eur. Cell Mater. 2014, 28, 348–357. [Google Scholar] [CrossRef]
- Mao, J.J.; Nah, H.D. Growth and development: Hereditary and mechanical modulations. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.S. Toward a modern synthesis for craniofacial biology: A genomic-epigenomic basis for dentofacial orthopedic treatment. In The 40th Moyers Symposium: Looking back. Looking forward. Ann Arbor: Center for Human Growth and Development; De Koster, K.Y., McNamara, J.A., Eds.; Department of Orthodontics and Pediatric Dentistry, School of Dentistry and Center for Human Growth and Development, University of Michigan: Ann Arbor, MI, USA, 2014. [Google Scholar]
- Mendenhall, M.W.; Fernandes, R.; Werning, J.W.; Vaysberg, M.; Malyapa, R.S.; Mendenhall, N.P. Head and neck osteosarcoma. Am. J. Otolaryngol. 2011, 32, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Guadagnolo, A.B.; Zagars, G.K.; Raymond, A.K.; Benjamin, R.S.; Sturgis, E.M. Osteosarcoma of the jaw/craniofacial region: Outcomes after multimodality treatment. Cancer 2009, 115, 3262–3270. [Google Scholar] [CrossRef] [PubMed]
- Thariat, J.; Julieron, M.; Brouchet, A.; Italiano, A.; Schouman, T.; Marcy, P.Y.; Odin, G.; Lacout, A.; Dassonville, O.; Peyrottes-Birstwisles, I.; et al. Osteosarcomas of the mandible: Are they different from other tumor sites? Crit. Rev. Oncol. Hematol. 2012, 82, 280–295. [Google Scholar] [CrossRef]
- Weber, M.; Soder, S.; Sander, J.; Ries, J.; Geppert, C.; Kesting, M.; Wehrhan, F. Craniofacial Osteosarcoma-Pilot Study on the Expression of Osteobiologic Characteristics and Hypothesis on Metastasis. Front. Oncol. 2020, 10, 745. [Google Scholar] [CrossRef]
- Finkelman, R.D.; Eason, A.L.; Rakijian, D.R.; Tutundzhyan, Y.; Hardesty, R.A. Elevated IGF-II and TGF-beta concentrations in human calvarial bone: Potential mechanism for increased graft survival and resistance to osteoporosis. Plast. Reconstr. Surg. 1994, 93, 732–738. [Google Scholar] [CrossRef]
- Wehrhan, F.; Amann, K.; Mobius, P.; Weber, M.; Preidl, R.; Ries, J.; Stockmann, P. BRONJ-related jaw bone is associated with increased Dlx-5 and suppressed osteopontin-implication in the site-specific alteration of angiogenesis and bone turnover by bisphosphonates. Clin. Oral. Investig. 2015, 19, 1289–1298. [Google Scholar] [CrossRef]
- Groetz, K.A.; Piesold, J.U.; Al-Nawas, B. Bisphosphonat-assoziierte Kiefernekrose (BP-ONJ) und andere Medikamenten-assoziierte Kiefernekrosen. AWMF Online 2012, 4, Nr. 007/0912012. [Google Scholar]
- Paolone, M.G.; Kaitsas, R. Orthodontic-periodontal interactions: Orthodontic extrusion in interdisciplinary regenerative treatments. Int. Orthod. 2018, 16, 217–245. [Google Scholar] [CrossRef]
- Meikle, M.C. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur. J. Orthod. 2006, 28, 221–240. [Google Scholar] [CrossRef]
- Golz, L.; Vestewig, E.; Blankart, M.; Kraus, D.; Appel, T.; Frede, S.; Jager, A. Differences in human gingival and dermal fibroblasts may contribute to oral-induced tolerance against nickel. J. Allergy Clin. Immunol. 2016, 138, 1202–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, N.; Gros, E.; Bieber, T.; Allam, J.P. Human skin and oral mucosal dendritic cells as ‘good guys’ and ‘bad guys’ in allergic immune responses. Clin. Exp. Immunol. 2010, 161, 28–33. [Google Scholar] [CrossRef]
- Sun, L.; Wong, H.M.; McGrath, C.P. Relationship between the Severity of Malocclusion and Oral Health Related Quality of Life: A Systematic Review and Meta-analysis. Oral. Health Prev. Dent. 2017, 15, 503–517. [Google Scholar] [PubMed]
- Diedrich, P. Praxis der Zahnheilkunde. Kieferorthopädie. Studienausgabe. Paket: Kieferorthopädie 1, 2, 3; Elsevier: Amsterdam, The Netherlands; Urban & Fischer: Munich, Germany, 2005. [Google Scholar]
- Jayesh, R.S.; Dhinakarsamy, V. Osseointegration. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. 1), S226–S229. [Google Scholar] [PubMed]
- Mouraret, S.; Hunter, D.J.; Bardet, C.; Brunski, J.B.; Bouchard, P.; Helms, J.A. A pre-clinical murine model of oral implant osseointegration. Bone 2014, 58, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Soares, P.B.; Moura, C.C.; Claudino, M.; Carvalho, V.F.; Rocha, F.S.; Zanetta-Barbosa, D. Influence of Implant Surfaces on Osseointegration: A Histomorphometric and Implant Stability Study in Rabbits. Br. Dent. J. 2015, 26, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Wijbenga, J.G.; Schepers, R.H.; Werker, P.M.; Witjes, M.J.; Dijkstra, P.U. A systematic review of functional outcome and quality of life following reconstruction of maxillofacial defects using vascularized free fibula flaps and dental rehabilitation reveals poor data quality. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 1024–1036. [Google Scholar] [CrossRef]
- Sozzi, D.; Novelli, G.; Silva, R.; Connelly, S.T.; Tartaglia, G.M. Implant rehabilitation in fibula-free flap reconstruction: A retrospective study of cases at 1–18 years following surgery. J. Craniomaxillofac. Surg. 2017, 45, 1655–1661. [Google Scholar] [CrossRef]
- Howe, M.S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef]
- Schlegel, K.A.; Rupprecht, S.; Petrovic, L.; Honert, C.; Srour, S.; von Wilmowsky, C.; Felszegy, E.; Nkenke, E.; Lutz, R. Preclinical animal model for de novo bone formation in human maxillary sinus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, e37–e44. [Google Scholar] [CrossRef]
- Helms, J.A.; Amasha, R.R.; Leucht, P. Bone voyage: An expedition into the molecular and cellular parameters affecting bone graft fate. Bone 2007, 41, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lutz, R.; Prechtl, C.; Nonhoff, J.; Weisel, T.; Damien, C.J.; Schlegel, K.A. Biofunctionalization of the implant surface with different concentrations of a synthetic peptide (P-15). Clin. Oral Implant. Res. 2013, 24, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Lutz, R.; Srour, S.; Nonhoff, J.; Weisel, T.; Damien, C.J.; Schlegel, K.A. Biofunctionalization of titanium implants with a biomimetic active peptide (P-15) promotes early osseointegration. Clin. Oral Implant. Res. 2010, 21, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Lutz, R.; Park, J.; Felszeghy, E.; Wiltfang, J.; Nkenke, E.; Schlegel, K.A. Bone regeneration after topical BMP-2-gene delivery in circumferential peri-implant bone defects. Clin. Oral Implant. Res. 2008, 19, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lutz, R.; Felszeghy, E.; Wiltfang, J.; Nkenke, E.; Neukam, F.W.; Schlegel, K.A. The effect on bone regeneration of a liposomal vector to deliver BMP-2 gene to bone grafts in peri-implant bone defects. Biomaterials 2007, 28, 2772–2782. [Google Scholar] [CrossRef] [PubMed]
- Lutz, R.; Sendlbeck, C.; Wahabzada, H.; Tudor, C.; Prechtl, C.; Schlegel, K.A. Periosteal elevation induces supracortical peri-implant bone formation. J. Craniomaxillofac. Surg. 2017, 45, 1170–1178. [Google Scholar] [CrossRef]
- Shegarfi, H.; Reikeras, O. Review article: Bone transplantation and immune response. J. Orthop. Surg. (Hong Kong) 2009, 17, 206–211. [Google Scholar] [CrossRef]
- Smith, S.M.; Garic, A.; Flentke, G.R.; Berres, M.E. Neural crest development in fetal alcohol syndrome. Birth Defects Res. C Embryo Today 2014, 102, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Trainor, P.A. Craniofacial birth defects: The role of neural crest cells in the etiology and pathogenesis of Treacher Collins syndrome and the potential for prevention. Am. J. Med. Genet. A 2010, 152, 2984–2994. [Google Scholar] [CrossRef] [Green Version]
- Everson, J.L.; Fink, D.M.; Yoon, J.W.; Leslie, E.J.; Kietzman, H.W.; Ansen-Wilson, L.J.; Chung, H.M.; Walterhouse, D.O.; Marazita, M.L.; Lipinski, R.J. Sonic hedgehog regulation of Foxf2 promotes cranial neural crest mesenchyme proliferation and is disrupted in cleft lip morphogenesis. Development 2017, 144, 2082–2091. [Google Scholar] [CrossRef] [Green Version]
- Leslie, E.J.; Liu, H.; Carlson, J.C.; Shaffer, J.R.; Feingold, E.; Wehby, G.; Laurie, C.A.; Jain, D.; Laurie, C.C.; Doheny, K.F.; et al. A Genome-wide Association Study of Nonsyndromic Cleft Palate Identifies an Etiologic Missense Variant in GRHL3. Am. J. Hum. Genet. 2016, 98, 744–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangold, E.; Bohmer, A.C.; Ishorst, N.; Hoebel, A.K.; Gultepe, P.; Schuenke, H.; Klamt, J.; Hofmann, A.; Golz, L.; Raff, R.; et al. Sequencing the GRHL3 Coding Region Reveals Rare Truncating Mutations and a Common Susceptibility Variant for Nonsyndromic Cleft Palate. Am. J. Hum. Genet. 2016, 98, 755–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyrard-Janvid, M.; Leslie, E.J.; Kousa, Y.A.; Smith, T.L.; Dunnwald, M.; Magnusson, M.; Lentz, B.A.; Unneberg, P.; Fransson, I.; Koillinen, H.K.; et al. Dominant mutations in GRHL3 cause Van der Woude Syndrome and disrupt oral periderm development. Am. J. Hum. Genet. 2014, 94, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.M.; Moonis, G.; Green, G.E.; Carmody, R.; Burbank, H.N. Syndromes of the first and second branchial arches, part 2: Syndromes. Am. J. Neuroradiol. 2011, 32, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parada, C.; Han, D.; Grimaldi, A.; Sarrion, P.; Park, S.S.; Pelikan, R.; Sanchez-Lara, P.A.; Chai, Y. Disruption of the ERK/MAPK pathway in neural crest cells as a potential cause of Pierre Robin sequence. Development 2015, 142, 3734–3745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Yuan, J.; Yao, X.; Zhang, R.; Yang, H.; Zhao, R.; Guo, J.; Jin, K.; Mei, H.; Luo, Y.; et al. BMPR1B mutation causes Pierre Robin sequence. Oncotarget 2017, 8, 25864–25871. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.B.; Hu, J.; Zhang, J.; Zhou, X.; Li, X.; Gu, C.; Liu, T.; Xie, Y.; Liu, J.; Gu, M.; et al. Genome-wide association study identifies multiple susceptibility loci for craniofacial microsomia. Nat. Commun. 2016, 7, 10605. [Google Scholar] [CrossRef] [Green Version]
- Bogusiak, K.; Puch, A.; Arkuszewski, P. Goldenhar syndrome: Current perspectives. World J. Pediatrics 2017, 13, 405–415. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, M.; Wehrhan, F.; Deschner, J.; Sander, J.; Ries, J.; Möst, T.; Bozec, A.; Gölz, L.; Kesting, M.; Lutz, R. The Special Developmental Biology of Craniofacial Tissues Enables the Understanding of Oral and Maxillofacial Physiology and Diseases. Int. J. Mol. Sci. 2021, 22, 1315. https://doi.org/10.3390/ijms22031315
Weber M, Wehrhan F, Deschner J, Sander J, Ries J, Möst T, Bozec A, Gölz L, Kesting M, Lutz R. The Special Developmental Biology of Craniofacial Tissues Enables the Understanding of Oral and Maxillofacial Physiology and Diseases. International Journal of Molecular Sciences. 2021; 22(3):1315. https://doi.org/10.3390/ijms22031315
Chicago/Turabian StyleWeber, Manuel, Falk Wehrhan, James Deschner, Janina Sander, Jutta Ries, Tobias Möst, Aline Bozec, Lina Gölz, Marco Kesting, and Rainer Lutz. 2021. "The Special Developmental Biology of Craniofacial Tissues Enables the Understanding of Oral and Maxillofacial Physiology and Diseases" International Journal of Molecular Sciences 22, no. 3: 1315. https://doi.org/10.3390/ijms22031315
APA StyleWeber, M., Wehrhan, F., Deschner, J., Sander, J., Ries, J., Möst, T., Bozec, A., Gölz, L., Kesting, M., & Lutz, R. (2021). The Special Developmental Biology of Craniofacial Tissues Enables the Understanding of Oral and Maxillofacial Physiology and Diseases. International Journal of Molecular Sciences, 22(3), 1315. https://doi.org/10.3390/ijms22031315





