Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People
Abstract
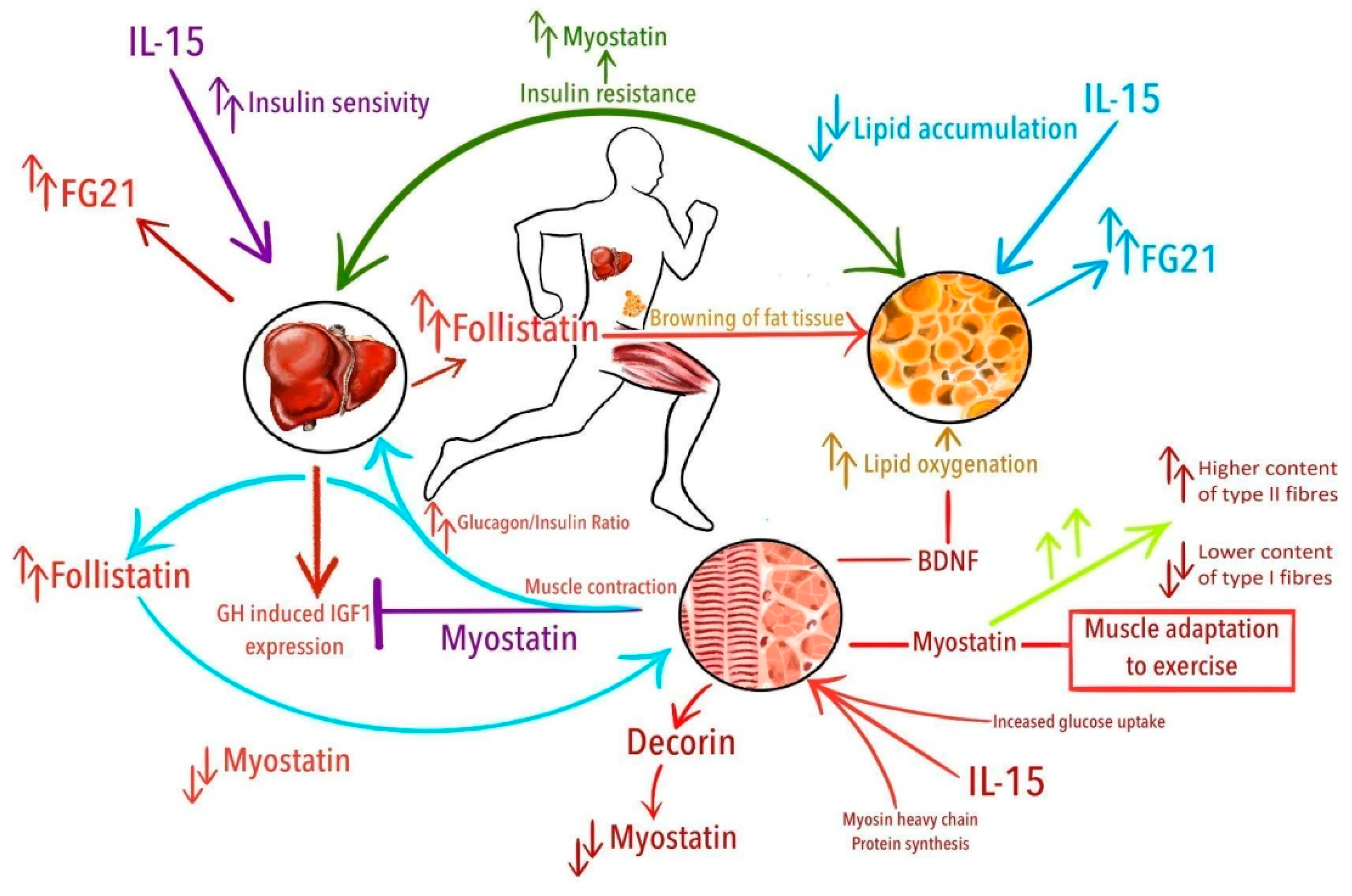
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Myostatin
3.2. Follistatin
3.3. Decorin
3.4. BDNF
3.5. Fibroblast Growth Factor 21 (FGF21)
3.6. Interleukin 15
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Febbraio, M.; Saltin, B. Searching for the exercise factor: Is IL-6 a candidate? J. Muscle Res. Cell Motil. 2003, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Peterson, J.M.; Byerly, M.S.; Wei, Z.; Wong, G.W. Myonectin (CTRP15), a Novel Myokine That Links Skeletal Muscle to Systemic Lipid Homeostasis. J. Biol. Chem. 2012, 287, 11968–11980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, T.; Yasukawa, K.; Harada, H.; Taga, T.; Watanabe, S.; Matsuda, T.; Kashiwamura, S.-I.; Nakajima, K.; Koyama, K.; Iwamatsu, A.; et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nat. Cell Biol. 1986, 324, 73–76. [Google Scholar] [CrossRef]
- Kelly, M.; Gauthier, M.-S.; Saha, A.K.; Ruderman, N.B.; Hong, E.-G.; Ko, H.J.; Cho, Y.-R.; Kim, H.-J.; Yu, T.Y.; Friedline, R.H.; et al. Activation of AMP-Activated Protein Kinase by Interleukin-6 in Rat Skeletal Muscle: Association With Changes in cAMP, Energy State, and Endogenous Fuel Mobilization. Diabetes 2009, 58, 1953–1960. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, S.-I.; Tamura, Y.; Kakehi, S.; Sanada, H.; Kawamori, R.; Watada, H. Exercise-induced increase in IL-6 level enhances GLUT4 expression and insulin sensitivity in mouse skeletal muscle. Biochem. Biophys. Res. Commun. 2016, 473, 947–952. [Google Scholar] [CrossRef]
- Van Hall, G.; Steensberg, A.; Sacchetti, M.; Fischer, C.; Keller, C.; Schjerling, P.; Hiscock, N.; Møller, K.; Saltin, B.; Febbraio, M.A.; et al. Interleukin-6 Stimulates Lipolysis and Fat Oxidation in Humans. J. Clin. Endocrinol. Metab. 2003, 88, 3005–3010. [Google Scholar] [CrossRef]
- Petersen, E.W.; Carey, A.L.; Sacchetti, M.; Steinberg, G.R.; Macaulay, S.L.; Febbraio, M.A.; Pedersen, B.K. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am. J. Physiol. Metab. 2005, 288, E155–E162. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Churchward-Venne, T.A.; Bellamy, L.; Parise, G.; Baker, S.K.; Phillips, S.M. Muscular and Systemic Correlates of Resistance Training-Induced Muscle Hypertrophy. PLoS ONE 2013, 8, e78636. [Google Scholar] [CrossRef] [Green Version]
- Begue, G.; Douillard, A.; Galbes, O.; Rossano, B.; Vernus, B.; Candau, R.; Py, G. Early Activation of Rat Skeletal Muscle IL-6/STAT1/STAT3 Dependent Gene Expression in Resistance Exercise Linked to Hypertrophy. PLoS ONE 2013, 8, e57141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northoff, H.; Berg, A. Immunologic Mediators as Parameters of the Reaction to Strenuous Exercise. Int. J. Sports Med. 1991, 12, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Rohde, T.; Zacho, M.; Asp, S.; Pedersen, B.K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 1998, 508, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Starkie, R.L.; Arkinstall, M.J.; Koukoulas, I.; Hawley, J.A.; Febbraio, M.A. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J. Physiol. 2001, 533, 585–591. [Google Scholar] [CrossRef]
- Steensberg, A.; Keller, C.; Starkie, R.L.; Osada, T.; Febbraio, M.A.; Pedersen, B.K. IL-6 and TNF-α expression in, and release from, contracting human skeletal muscle. Am. J. Physiol. Metab. 2002, 283, E1272–E1278. [Google Scholar] [CrossRef] [Green Version]
- Febbraio, M.A.; Pedersen, B.K. Contraction-Induced Myokine Production and Release: Is Skeletal Muscle an Endocrine Organ? Exerc. Sport Sci. Rev. 2005, 33, 114–119. [Google Scholar] [CrossRef]
- Leggate, M.; Nowell, M.A.; A Jones, S.; Nimmo, M.A. The response of interleukin-6 and soluble interleukin-6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress Chaperon. 2010, 15, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Zwetsloot, K.A.; John, C.S.; Lawrence, M.M.; A Battista, R.; Shanely, R.A. High-intensity interval training induces a modest systemic inflammatory response in active, young men. J. Inflamm. Res. 2014, 7, 9–17. [Google Scholar] [CrossRef]
- Steensberg, A.; Van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Exercise and interleukin-6. Curr. Opin. Hematol. 2001, 8, 137–141. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. 2002, 16, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Bostroem, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostroem, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nat. Cell Biol. 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Rioux, B.V.; Goulet, E.D.; Johanssen, N.M.; Swift, D.L.; Bouchard, D.; Loewen, H.; Sénéchal, M. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: A meta-analysis. Scand. J. Med. Sci. Sports 2017, 28, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Sun, Z.; Schumann, U.; Zügel, M.; Steinacker, J.M. Chronic Exercise Training and Circulating Irisin in Adults: A Meta-Analysis. Sports Med. 2015, 45, 1577–1588. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B.; Puszczewicz, M. A review on irisin, a new protagonist that mediates muscle-adipose-bone-neuron connectivity. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4687–4693. [Google Scholar]
- Pukajło, K.; Kolackov, K.; Laczmanski, L.; Daroszewski, J. Irisin—A new mediator of energy homeostasis. Postępy Hig. Med. Dośw. 2015, 69, 233–242. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; María, P. FNDC5/Irisin Is Not Only a Myokine but Also an Adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [Green Version]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Cakmak, T.; Albayrak, S.; Gungor, S.; Colakoglu, N.; Ozercan, I.H. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides 2014, 61, 130–136. [Google Scholar] [CrossRef]
- Kuloglu, T.; Aydin, S.; Eren, M.N.; Yilmaz, M.; Sahin, I.; Kalayci, M.; Sarman, E.; Kaya, N.; Yilmaz, O.F.; Turk, A.; et al. Irisin: A potentially candidate marker for myocardial infarction. Peptides 2014, 55, 85–91. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Eckel, J. Myokines in metabolic homeostasis and diabetes. Diabetologia 2019, 62, 1523–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, L.; You, W.; Shan, T. Myokines mediate the cross talk between skeletal muscle and other organs. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Pedersen, B.K.; Xu, G.; Lehmann, R.; Weigert, C.; Plomgaard, P. Exercise-Induced Secretion of FGF21 and Follistatin Are Blocked by Pancreatic Clamp and Impaired in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2816–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 10 December 2020).
- Rodriguez, J.; Vernus, B.; Chelh, I.; Cassar-Malek, I.; Gabillard, J.-C.; Sassi, A.H.; Seiliez, I.; Picard, B.; Bonnieu, A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell. Mol. Life Sci. 2014, 71, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Peng, Y.; Zhao, W.; Pan, J.; Ksiezak-Reding, H.; Cardozo, C.; Wu, Y.; Pajevic, P.D.; Bonewald, L.F.; Bauman, W.A.; et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J. Biol. Chem. 2017, 292, 11021–11033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-H.; Bauman, W.A.; Cardozo, C. Myostatin inhibits glucose uptake via suppression of insulin-dependent and -independent signaling pathways in myoblasts. Physiol. Rep. 2018, 6, e13837. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Huynh, T.V.; Lee, S.-J. Paracrine and endocrine modes of myostatin action. J. Appl. Physiol. 2016, 120, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Czaja, W.; Nakamura, Y.K.; Li, N.; Eldridge, J.A.; DeAvila, D.M.; Thompson, T.B.; Rodgers, B.D. Myostatin regulates pituitary development and hepatic IGF1. Am. J. Physiol. Metab. 2019, 316, E1036–E1049. [Google Scholar] [CrossRef]
- Sriram, S.; Subramanian, S.; Sathiakumar, D.; Venkatesh, R.; Salerno, M.S.; McFarlane, C.D.; Kambadur, R.; Sharma, M. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB. Aging Cell 2011, 10, 931–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, J.S.; Rutti, S.; Arous, C.; Clemmesen, J.O.; Secher, N.H.; Drescher, A.; Gonelle-Gispert, C.; Halban, P.A.; Pedersen, B.K.; Weigert, C.; et al. Circulating Follistatin Is Liver-Derived and Regulated by the Glucagon-to-Insulin Ratio. J. Clin. Endocrinol. Metab. 2016, 101, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhou, X.; Mitch, W.E.; Goldberg, A.L. Myostatin/activin pathway antagonism: Molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 2013, 45, 2333–2347. [Google Scholar] [CrossRef] [PubMed]
- Winbanks, C.E.; Weeks, K.L.; Thomson, R.E.; Sepulveda, P.V.; Beyer, C.; Qian, H.; Chen, J.L.; Allen, J.M.; Lancaster, G.I.; Febbraio, M.A.; et al. Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. J. Cell Biol. 2012, 197, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Pervin, S.; Lee, S.-J.; Kuo, A.; Grijalva, V.; David, J.; Vergnes, L.; Reddy, S.T. Metabolic profiling of follistatin overexpression: A novel therapeutic strategy for metabolic diseases. Diabetes Metab. Syndr. Obes. 2018, 11, 65–84. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, K.; Han, B.; Xu, Z.; Gao, X. The emerging role of follistatin under stresses and its implications in diseases. Gene 2018, 639, 111–116. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Lee, S.-K.; Chun, Y.-M.; Choi, Y.; Moon, S.-H.; Lee, H.-M.; Kang, H.J. Follistatin Mitigates Myofibroblast Differentiation and Collagen Synthesis of Fibroblasts from Scar Tissue around Injured Flexor Tendons. Yonsei Med. J. 2020, 61, 85–93. [Google Scholar] [CrossRef]
- Han, X.; Møller, L.L.V.; De Groote, E.; Bojsen-Møller, K.N.; Davey, J.; Henríquez-Olguin, C.; Li, Z.; Knudsen, J.R.; Jensen, T.E.; Madsbad, S.; et al. Mechanisms involved in follistatin-induced hypertrophy and increased insulin action in skeletal muscle. J. Cachex. Sarcopenia Muscle 2019, 10, 1241–1257. [Google Scholar] [CrossRef] [Green Version]
- DCN Decorin [Homo sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=1634 (accessed on 22 December 2019).
- Weishaupt, N.; Blesch, A.; Fouad, K. BDNF: The career of a multifaceted neurotrophin in spinal cord injury. Exp. Neurol. 2012, 238, 254–264. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Pedersen, M.; Krabbe, K.S.; Bruunsgaard, H.; Matthews, V.B.; Febbraio, M.A. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp. Physiol. 2009, 94, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Oulion, S.; Bertrand, S.; Escriva, H. Evolution of the FGF Gene Family. Int. J. Evol. Biol. 2012, 2012, 298147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.J.; Inagaki, T.; Satapati, S.; Ding, X.; He, T.; Goetz, R.; Mohammadi, M.; Finck, B.N.; Mangelsdorf, D.J.; Kliewer, S.A.; et al. FGF21 induces PGC-1 and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA 2009, 106, 10853–10858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1 and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Burton, J.D.; Bamford, R.N.; Peters, C.; Grant, A.J.; Kurys, G.; Goldman, C.K.; Brennan, J.; Roessler, E.; Waldmann, T.A. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA 1994, 91, 4935–4939. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, G.; Debacker, V.; De Smedt, M.; Plum, J. Differential effects of interleukin-15 and interleukin-2 on differentiation of bipotential T/natural killer progenitor cells. J. Exp. Med. 1996, 184, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Nolz, J.C.; Richer, M.J. Control of memory CD8+ T cell longevity and effector functions by IL-15. Mol. Immunol. 2020, 117, 180–188. [Google Scholar] [CrossRef]
- Furmanczyk, P.S. Interleukin-15 increases myosin accretion in human skeletal myogenic cultures. Cell Biol. Int. 2003, 27, 845–851. [Google Scholar] [CrossRef]
- Quinn, L.S.; Strait-Bodey, L.; Anderson, B.G.; Argilés, J.M.; Havel, P.J. Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: Evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol. Int. 2005, 29, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Quinn, L.S.; Anderson, B.G.; Strait-Bodey, L.; Stroud, A.M.; Argilés, J.M. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am. J. Physiol. Metab. 2009, 296, E191–E202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Liu, D. Hydrodynamic delivery of interleukin 15 gene promotes resistance to high fat diet-induced obesity, fatty liver and improves glucose homeostasis. Gene Ther. 2014, 22, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Ma, Y.; Gao, M.; Liu, D. IL-15/sIL-15Rα gene transfer induces weight loss and improves glucose homeostasis in obese mice. Gene Ther. 2016, 23, 349–356. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nat. Cell Biol. 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wrana, J.L. Signaling by the TGF Superfamily. Cold Spring Harb. Perspect. Biol. 2013, 5, a011197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, B.; Herbert, P.; Sculthorpe, N.; Grace, F.M.; Stratton, D.; Hayes, L.D. Lifelong exercise, but not short-term high-intensity interval training, increases GDF11, a marker of successful aging: A preliminary investigation. Physiol. Rep. 2017, 5, e13343. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.-J. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-J. Extracellular Regulation of Myostatin: A Molecular Rheostat for Muscle Mass. Immunol. Endocr. Metab. Agents Med. Chem. 2010, 10, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Lessard, S.J.; Rivas, D.A.; Alves-Wagner, A.B.; Hirshman, M.F.; Gallagher, I.J.; Constantin-Teodosiu, T.; Atkins, R.; Greenhaff, P.L.; Qi, N.R.; Gustafsson, T.; et al. Resistance to Aerobic Exercise Training Causes Metabolic Dysfunction and Reveals Novel Exercise-Regulated Signaling Networks. Diabetes 2013, 62, 2717–2727. [Google Scholar] [CrossRef] [Green Version]
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef]
- Covington, J.D.; Tam, C.S.; Bajpeyi, S.; Galgani, J.E.; Noland, R.C.; Smith, S.R.; Redman, L.M.; Ravussin, E. Myokine Expression in Muscle and Myotubes in Response to Exercise Stimulation. Med. Sci. Sports Exerc. 2016, 48, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hittel, D.S.; Axelson, M.; Sarna, N.; Shearer, J.; Huffman, K.M.; Kraus, W.E. Myostatin Decreases with Aerobic Exercise and Associates with Insulin Resistance. Med. Sci. Sports Exerc. 2010, 42, 2023–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabak, B.; Belviranlı, M.; Okudan, N. Irisin and myostatin responses to acute high-intensity interval exercise in humans. Horm. Mol. Biol. Clin. Investig. 2018, 35. [Google Scholar] [CrossRef] [PubMed]
- Micielska, K.; Gmiat, A.; Żychowska, M.; Kozlowska, M.; Walentukiewicz, A.; Lysak-Radomska, A.; Jaworska, J.; Rodziewicz, E.; Duda-Biernacka, B.; Ziemann, E. The beneficial effects of 15 units of high-intensity circuit training in women is modified by age, baseline insulin resistance and physical capacity. Diabetes Res. Clin. Pract. 2019, 152, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, F. The correlation of resistance exercise-induced myostatin with insulin resistance and plasma cytokines in healthy young men. J. Endocrinol. Investig. 2015, 39, 383–388. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K.; Thalmann, M.M.; Weiss, E.; Tsironi, M.; Föger-Samwald, U.; Meinhart, J.; Skenderi, K.; Pietschmann, P. Changes in Serum Levels of Myokines and Wnt-Antagonists after an Ultramarathon Race. PLoS ONE 2015, 10, e0132478. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine Response to High-Intensity Interval vs. Resistance Exercise: An Individual Approach. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine/Adipokine Response to “Aerobic” Exercise: Is It Just a Matter of Exercise Load? Front. Physiol. 2019, 10, 691. [Google Scholar] [CrossRef]
- Bagheri, R.; Rashidlamir, A.; Motevalli, M.S.; Elliott, B.T.; Mehrabani, J.; Wong, A. Effects of upper-body, lower-body, or combined resistance training on the ratio of follistatin and myostatin in middle-aged men. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 119, 1921–1931. [Google Scholar] [CrossRef]
- Hofmann, M.; Schober-Halper, B.; Oesen, S.; Franzke, B.; Tschan, H.; Bachl, N.; Strasser, E.-M.; Quittan, M.; Wagner, K.-H.; Wessner, B. Effects of elastic band resistance training and nutritional supplementation on muscle quality and circulating muscle growth and degradation factors of institutionalized elderly women: The Vienna Active Ageing Study (VAAS). Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 116, 885–897. [Google Scholar] [CrossRef] [Green Version]
- Robertson, D.; Klein, R.; De Vos, F.; McLachlan, R.; Wettenhall, R.; Hearn, M.; Burger, H.; De Kretser, D. The isolation of polypeptides with FSH suppressing activity from bovine follicular fluid which are structurally different to inhibin. Biochem. Biophys. Res. Commun. 1987, 149, 744–749. [Google Scholar] [CrossRef]
- Makanji, Y.; Zhu, J.; Mishra, R.; Holmquist, C.; Wong, W.P.S.; Schwartz, N.B.; Mayo, K.E.; Woodruff, T.K. Inhibin at 90: From Discovery to Clinical Application, a Historical Review. Endocr. Rev. 2014, 35, 747–794. [Google Scholar] [CrossRef] [PubMed]
- Schneyer, A.; Wang, Q.; Sidis, Y.; Sluss, P.M. Differential Distribution of Follistatin Isoforms: Application of a New FS315-Specific Immunoassay. J. Clin. Endocrinol. Metab. 2004, 89, 5067–5075. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Brandt, C.; Nielsen, A.R.; Hojman, P.; Whitham, M.; Febbraio, M.A.; Pedersen, B.K.; Plomgaard, P. Exercise Induces a Marked Increase in Plasma Follistatin: Evidence That Follistatin Is a Contraction-Induced Hepatokine. Endocrinology 2011, 152, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Schneyer, A.; Sidis, Y.; Gulati, A.; Sun, J.L.; Keutmann, H.; Krasney, P.A. Differential Antagonism of Activin, Myostatin and Growth and Differentiation Factor 11 by Wild-Type and Mutant Follistatin. Endocrinology 2008, 149, 4589–4595. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.S.; Plomgaard, P. Circulating follistatin in relation to energy metabolism. Mol. Cell. Endocrinol. 2016, 433, 87–93. [Google Scholar] [CrossRef]
- Perakakis, N.; Mougios, V.; Fatouros, I.; Siopi, A.; Draganidis, D.; Peradze, N.; Ghaly, W.; Mantzoros, C.S. Physiology of Activins/Follistatins: Associations With Metabolic and Anthropometric Variables and Response to Exercise. J. Clin. Endocrinol. Metab. 2018, 103, 3890–3899. [Google Scholar] [CrossRef] [Green Version]
- Hormonal responses following eccentric exercise in humans. Hormones 2018, 16, 405–413. [CrossRef] [Green Version]
- Sargeant, J.A.; Aithal, G.P.; Takamura, T.; Misu, H.; Takayama, H.; Douglas, J.A.; Turner, M.C.; Stensel, D.J.; Nimmo, M.A.; Webb, D.R.; et al. The influence of adiposity and acute exercise on circulating hepatokines in normal-weight and overweight/obese men. Appl. Physiol. Nutr. Metab. 2018, 43, 482–490. [Google Scholar] [CrossRef] [Green Version]
- Willis, S.A.; Sargeant, J.A.; Thackray, A.E.; Yates, T.; Stensel, D.J.; Aithal, G.P.; King, J.A. Effect of exercise intensity on circulating hepatokine concentrations in healthy men. Appl. Physiol. Nutr. Metab. 2019, 44, 1065–1072. [Google Scholar] [CrossRef] [Green Version]
- Kanzleiter, T.; Rath, M.; Görgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schürmann, A.; et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Knuiman, P.; Hopman, M.T.E.; Hangelbroek, R.; Mensink, M. Plasma cytokine responses to resistance exercise with different nutrient availability on a concurrent exercise day in trained healthy males. Physiol. Rep. 2018, 6, e13708. [Google Scholar] [CrossRef] [PubMed]
- Bugera, E.M.; Duhamel, T.A.; Peeler, J.D.; Cornish, S.M. The systemic myokine response of decorin, interleukin-6 (IL-6) and interleukin-15 (IL-15) to an acute bout of blood flow restricted exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 118, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, A.P.; Cooper, R.G. The role of myokines in muscle health and disease. Curr. Opin. Rheumatol. 2016, 28, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Leibrock, J.; Lottspeich, F.; Hohn, A.; Hofer, M.; Hengerer, B.; Masiakowski, P.; Thoenen, H.; Barde, Y.-A. Molecular cloning and expression of brain-derived neurotrophic factor. Nat. Cell Biol. 1989, 341, 149–152. [Google Scholar] [CrossRef]
- Acheson, A.; Conover, J.C.; Fandl, J.P.; DeChiara, T.M.; Russell, M.; Thadani, A.; Squinto, S.P.; Yancopoulos, G.D.; Lindsay, R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nat. Cell Biol. 1995, 374, 450–453. [Google Scholar] [CrossRef]
- Chacón-Fernández, P.; Säuberli, K.; Colzani, M.; Moreau, T.; Ghevaert, C.; Barde, Y.-A. Brain-derived Neurotrophic Factor in Megakaryocytes. J. Biol. Chem. 2016, 291, 9872–9881. [Google Scholar] [CrossRef] [Green Version]
- Kestin, A.S.; Ellis, P.A.; Barnard, M.R.; Errichetti, A.; Rosner, B.A.; Michelson, A.D. Effect of strenuous exercise on platelet activation state and reactivity. Circulation 1993, 88, 1502–1511. [Google Scholar] [CrossRef] [Green Version]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.W.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Håkansson, K.; Ledreux, A.; Daffner, K.; Terjestam, Y.; Bergman, P.; Carlsson, R.; Kivipelto, M.; Winblad, B.; Granholm, A.-C.; Mohammed, A.K.H. BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function. J. Alzheimer’s Dis. 2016, 55, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Hötting, K.; Schickert, N.; Kaiser, J.; Röder, B.; Schmidt-Kassow, M. The Effects of Acute Physical Exercise on Memory, Peripheral BDNF, and Cortisol in Young Adults. Neural Plast. 2016, 2016, 6860573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortunato, A.K.; Pontes, W.M.; De Souza, D.M.S.; Prazeres, J.S.F.; Marcucci-Barbosa, L.S.; Santos, J.M.M.; Veira, É.L.M.; Bearzoti, E.; Pinto, K.M.D.C.; Talvani, A.; et al. Strength Training Session Induces Important Changes on Physiological, Immunological, and Inflammatory Biomarkers. J. Immunol. Res. 2018, 2018, 9675216. [Google Scholar] [CrossRef] [PubMed]
- Murawska-Cialowicz, E.; Wojna, J.; Zuwala-Jagiello, J. Crossfit training changes brain-derived neurotrophic factor and irisin levels at rest, after wingate and progressive tests, and improves aerobic capacity and body composition of young physically active men and women. J. Physiol. Pharmacol. 2015, 66, 811–821. [Google Scholar] [PubMed]
- Tsai, C.; Pan, C.; Chen, F.; Wang, C.-H.; Chou, F. The effects of acute aerobic exercise on a task-switching paradigm and BDNF levels in young adults with different levels of cardiorespiratory fitness. Exp. Physiol. 2016, 101, 836–850. [Google Scholar] [CrossRef] [Green Version]
- Church, D.D.; Hoffman, J.R.; Mangine, G.T.; Jajtner, A.R.; Townsend, J.R.; Beyer, K.S.; Wang, R.; La Monica, M.B.; Fukuda, D.H.; Stout, J.R. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. 2016, 121, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Marquez, C.M.S.; Vanaudenaerde, B.; Troosters, T.; Wenderoth, N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J. Appl. Physiol. 2015, 119, 1363–1373. [Google Scholar] [CrossRef] [Green Version]
- Wagner, G.; Herbsleb, M.; De La Cruz, F.; Schumann, A.; Brünner, F.; Schachtzabel, C.; Gussew, A.; Puta, C.; Smesny, S.; Gabriel, H.W.; et al. Hippocampal Structure, Metabolism, and Inflammatory Response after a 6-Week Intense Aerobic Exercise in Healthy Young Adults: A Controlled Trial. Br. J. Pharmacol. 2015, 35, 1570–1578. [Google Scholar] [CrossRef]
- Antunes, B.D.M.M.; Rossi, F.E.; Teixeira, A.M.; Lira, F.S. Short-time high-intensity exercise increases peripheral BDNF in a physical fitness-dependent way in healthy men. Eur. J. Sport Sci. 2019, 20, 43–50. [Google Scholar] [CrossRef]
- Jeon, Y.K.; Ha, C.H. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ. Health Prev. Med. 2017, 22, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Roh, H.-T.; Cho, S.-Y.; So, W.-Y. Effects of Regular Taekwondo Intervention on Oxidative Stress Biomarkers and Myokines in Overweight and Obese Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyakova, M.; Schlögl, H.; Sacher, J.; Schmidt-Kassow, M.; Kaiser, J.; Stumvoll, M.; Kratzsch, J.; Schroeter, M.L. Stability of BDNF in Human Samples Stored Up to 6 Months and Correlations of Serum and EDTA-Plasma Concentrations. Int. J. Mol. Sci. 2017, 18, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, N. Hormone-like (endocrine) Fgfs: Their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res. 2010, 342, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mraz, M.; Bartlova, M.; Lacinova, Z.; Michalsky, D.; Kasalicky, M.; Haluzikova, D.; Matoulek, M.; Dostalova, I.; Humenanska, V.; Haluzik, M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin. Endocrinol. 2009, 71, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yeung, D.C.; Karpisek, M.; Stejskal, D.; Zhou, Z.-G.; Liu, F.; Wong, R.L.; Chow, W.-S.; Tso, A.W.; Lam, K.S.; et al. Serum FGF21 Levels Are Increased in Obesity and Are Independently Associated With the Metabolic Syndrome in Humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacer, K.F.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-O, M.; Mangelsdorf, D.J.; et al. Research Resource: Comprehensive Expression Atlas of the Fibroblast Growth Factor System in Adult Mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, N. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. Front. Endocrinol. 2014, 5, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, Y.; Kurosu, H.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Goetz, R.; Eliseenkova, A.V.; Mohammadi, M.; Kuro-O, M. βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl. Acad. Sci. USA 2007, 104, 7432–7437. [Google Scholar] [CrossRef] [Green Version]
- Hanks, L.J.; Gutierrez, O.M.; Bamman, M.M.; Ashraf, A.P.; McCormick, K.; Casazza, K. Circulating levels of fibroblast growth factor-21 increase with age independently of body composition indices among healthy individuals. J. Clin. Transl. Endocrinol. 2015, 2, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Mashili, F.L.; Austin, R.L.; Deshmukh, A.S.; Fritz, T.; Caidahl, K.; Bergdahl, K.; Zierath, J.R.; Chibalin, A.V.; Moller, D.E.; Kharitonenkov, A.; et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: Implications for type 2 diabetes and obesity. Diabetes Metab. Res. Rev. 2011, 27, 286–297. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, X.; Zhou, J.; Pan, X.; Hao, Y.; Zhou, M.; Lu, Z.; Gao, M.; Bao, Y.; Jia, W. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc. Diabetol. 2013, 12, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharitonenkov, A.; Wroblewski, V.J.; Koester, A.; Chen, Y.-F.; Clutinger, C.K.; Tigno, X.T.; Hansen, B.C.; Shanafelt, A.B.; Etgen, G.J. The Metabolic State of Diabetic Monkeys Is Regulated by Fibroblast Growth Factor-21. Endocrinology 2007, 148, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Stanislaus, S.; Chinookoswong, N.; Lau, Y.Y.; Hager, T.; Patel, J.; Ge, H.; Weiszmann, J.; Lu, S.-C.; Graham, M.; et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—Association with liver and adipose tissue effects. Am. J. Physiol. Metab. 2009, 297, E1105–E1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Kim, S.H.; Min, Y.-K.; Yang, H.-M.; Lee, J.-B.; Lee, M.-S. Acute Exercise Induces FGF21 Expression in Mice and in Healthy Humans. PLoS ONE 2013, 8, e63517. [Google Scholar] [CrossRef]
- Tanimura, Y.; Aoi, W.; Takanami, Y.; Kawai, Y.; Mizushima, K.; Naito, Y.; Yoshikawa, T. Acute exercise increases fibroblast growth factor 21 in metabolic organs and circulation. Physiol. Rep. 2016, 4, e12828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morville, T.; Sahl, R.E.; Trammell, S.A.; Svenningsen, J.S.; Gillum, M.P.; Helge, J.W.; Clemmensen, C. Divergent effects of resistance and endurance exercise on plasma bile acids, FGF19, and FGF21 in humans. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- JanssenDuijghuijsen, L.M.; Keijer, J.; Mensink, M.; Lenaerts, K.; Ridder, L.; Nierkens, S.; Kartaram, S.W.; Verschuren, M.C.M.; Pieters, R.H.H.; Bas, R.; et al. Adaptation of exercise-induced stress in well-trained healthy young men. Exp. Physiol. 2017, 102, 86–99. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Paloma, A.-V.; Meza-Arana, C.E.; Brito-Córdova, G.; Gómez-Pérez, F.J.; Mehta, R.; Oseguera-Moguel, J.; Aguilar-Salinas, C.A. Exercise Increases Serum Fibroblast Growth Factor 21 (FGF21) Levels. PLoS ONE 2012, 7, e38022. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Higuchi, M. Acute endurance exercise lowers serum fibroblast growth factor 21 levels in Japanese men. Clin. Endocrinol. 2016, 85, 861–867. [Google Scholar] [CrossRef]
- Khalafi, M.; Alamdari, K.A.; Symonds, M.; Nobari, H.; Carlos-Vivas, J. Impact of acute exercise on immediate and following early post-exercise FGF-21 concentration in adults: Systematic review and meta-analysis. Hormones 2020, 1–11. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Higuchi, M. Endurance exercise reduces hepatic fat content and serum fibroblast growth factor 21 levels in elderly men. J. Clin. Endocrinol. Metab. 2016, 101, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, B.; Lewis, J.E.; Samms, R.J.; Ebling, F.J.P.; Cheng, C.C.; Adams, A.C.; Mallinson, J.; Cooper, S.; Taylor, T.; Ghasemi, R.; et al. Eccentric exercise increases circulating fibroblast activation protein α but not bioactive fibroblast growth factor 21 in healthy humans. Exp. Physiol. 2018, 103, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Grabstein, K.H.; Eisenman, J.; Shanebeck, K.; Rauch, C.; Srinivasan, S.; Fung, V.; Beers, C.; Richardson, J.; A Schoenborn, M.; Ahdieh, M.; et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 1994, 264, 965–968. [Google Scholar] [CrossRef]
- Krolopp, J.E.; Thornton, S.M.; Abbott, M.J. IL-15 Activates the Jak3/STAT3 Signaling Pathway to Mediate Glucose Uptake in Skeletal Muscle Cells. Front. Physiol. 2016, 7, 626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeau, L.; Patten, D.; Caron, A.; Garneau, L.; Pinault-Masson, E.; Foretz, M.; Haddad, P.; Anderson, B.; Quinn, L.; Jardine, K.; et al. IL-15 improves skeletal muscle oxidative metabolism and glucose uptake in association with increased respiratory chain supercomplex formation and AMPK pathway activation. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 395–407. [Google Scholar] [CrossRef]
- Ostrowski, K.; Hermann, C.; Bangash, A.; Schjerling, P.; Nielsen, J.N.; Pedersen, B.K. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J. Physiol. 1998, 513, 889–894. [Google Scholar] [CrossRef]
- Nieman, D.; Davis, J.M.; Henson, D.A.; Walberg-Rankin, J.; Shute, M.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A.; et al. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J. Appl. Physiol. 2003, 94, 1917–1925. [Google Scholar] [CrossRef]
- Nieman, D.; Davis, J.M.; Brown, V.A.; Henson, D.A.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Downs, M.F.; Smith, J.C.; Carson, J.A.; et al. Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J. Appl. Physiol. 2004, 96, 1292–1298. [Google Scholar] [CrossRef]
- Riechman, S.E.; Balasekaran, G.; Roth, S.M.; Ferrell, R.E. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J. Appl. Physiol. 2004, 97, 2214–2219. [Google Scholar] [CrossRef]
- Nielsen, A.R.; Mounier, R.; Plomgaard, P.; Mortensen, O.H.; Penkowa, M.; Speerschneider, T.; Pilegaard, H.; Pedersen, B.K. Expression of interleukin-15 in human skeletal muscle—Effect of exercise and muscle fibre type composition. J. Physiol. 2007, 584, 305–312. [Google Scholar] [CrossRef]
- Nielsen, A.R.; Yfanti, C.; Nielsen, S.; Åkerström, T.C.A.; Peijs, L.; Zankari, A.; Fischer, C.P.; Pedersen, B.K. Endurance training enhances skeletal muscle interleukin-15 in human male subjects. Endocrine 2013, 45, 271–278. [Google Scholar] [CrossRef]
- Oliver, J.M.; Jenke, S.C.; Mata, J.D.; Kreutzer, A.; Jones, M.T. Acute Effect of Cluster and Traditional Set Configurations on Myokines Associated with Hypertrophy. Int. J. Sports. Med. 2016, 37, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, A.; McKendry, J.; Martin-Rincon, M.; Morales-Alamo, D.; Pérez-Köhler, B.; Valadés, D.; Buján, J.; Calbet, J.A.L.; Breen, L. Skeletal muscle IL-15/IL-15Rα and myofibrillar protein synthesis after resistance exercise. Scand. J. Med. Sci. Sports 2017, 28, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Park, S.C.; Kang, S. Effects of resistance exercise on adipokine factors and body composition in pre- and postmenopausal women. J. Exerc. Rehabil. 2019, 15, 676–682. [Google Scholar] [CrossRef]
- Bazgir, B.; Salesi, M.; Koushki, M.; Amirghofran, Z. Effects of Eccentric and Concentric Emphasized Resistance Exercise on IL-15 Serum Levels and Its Relation to Inflammatory Markers in Athletes and Non-Athletes. Asian J. Sports Med. 2015, 6, e27980. [Google Scholar] [CrossRef] [Green Version]
- Kapilevich, L.V.; Zakharova, A.N.; Kabachkova, A.V.; Kironenko, T.A.; Orlov, S.N. Dynamic and Static Exercises Differentially Affect Plasma Cytokine Content in Elite Endurance- and Strength-Trained Athletes and Untrained Volunteers. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, A.; Martin-Rincon, M.; Santana, A.; Perez-Suarez, I.; Dorado, C.; Calbet, J.A.L.; Morales-Alamo, D. Antioxidants Facilitate High-intensity Exercise IL-15 Expression in Skeletal Muscle. Int. J. Sports Med. 2018, 40, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Yargic, M.P.; Torgutalp, S.; Akin, S.; Babayeva, N.; Torgutalp, M.; Demirel, H.A.; Demirel, A.H. Acute long-distance trail running increases serum IL-6, IL-15, and Hsp72 levels. Appl. Physiol. Nutr. Metab. 2019, 44, 627–631. [Google Scholar] [CrossRef]
- Nishida, Y.; Tanaka, K.; Hara, M.; Hirao, N.; Tanaka, H.; Tobina, T.; Ikeda, M.; Yamato, H.; Ohta, M. Effects of home-based bench step exercise on inflammatory cytokines and lipid profiles in elderly Japanese females: A randomized controlled trial. Arch. Gerontol. Geriatr. 2015, 61, 443–451. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Wang, W.L.; Guo, Q.; Wen, C.; Li, Y.; Yin, Y. Interleukin-15 in obesity and metabolic dysfunction: Current understanding and future perspectives. Obes. Rev. 2017, 18, 1147–1158. [Google Scholar] [CrossRef]
| Cytokine | Potential Function | Location of Expression [36] |
|---|---|---|
| Myostatin | Muscle protein synthesis and degradation [37] Downregulation of muscle structural genes and myogenic factors [37] Inhibition of muscle cell proliferation and differentiation [37] Inhibition of activation of the AKT/mTOR pathway [37] Regulation of the number of muscle fibers in embryogenesis [37] Inhibition of osteoblastic differentiation [38] Alternating glucose metabolism [39,40] Potential regulation of the GH/IGF-1 axis [41] Promoting of ROS production [42] | Muscle tissues Adipose and soft tissue Brain Eye Endocrine tissues Gastrointestinal tract Skin Bone marrow Blood |
| Follistatin | Suppression of follicle-stimulating hormone [43] Inhibition of myostatin [44] Myostatin independent muscle growth regulation [45] Browning of white adipose tissue [46] TGF-beta family signaling inhibition [47] Inhibition of extracellular matrix turnover [48] Insulin-dependent regulation of glucose uptake [49] Reduction of ROS production [47] | Liver and gallbladder Female tissues Pancreas Muscle tissues Adipose and soft tissue Skin Bone marrow Blood |
| Decorin | Promotion of muscle hypertrophy by binding with myostatin [50] Stimulation of autophagy and inflammation [50] Inhibition of angiogenesis and tumorigenesis [50] Modulation of tumor suppression [50] | Female tissues (especially ovary, placenta, breast, endometrium) Muscle tissues Adipose and soft tissue Lung |
| Brain-derived neurotrophic factor | Development and survival of adult dorsal root ganglion neurons (motor neurons) [51] Development of neuronal and glial cells [52] Regulation of the synaptic structure and its maintenance—critical for memory and cognition [51,52] Exercise-induced neuroplasticity—may contribute to improvement in locomotor function [51] Remodeling of injured axons [52] BDNF produced in skeletal muscle enhances fat oxidation [53]. A vital component of the hypothalamic pathway that controls body mass and energy homeostasis [53] | The central and peripheral nervous system (hippocampus, cortex, and basal forebrain) Skeletal muscles Retina Kidney Saliva Prostate Megakaryocytes Others |
| Fibroblast growth factor 21 | Cell differentiation, migration and survival [54] Lipid metabolism regulation [55] Glucose metabolism regulation [55] Adaptive response to starvation [56]. Browning of white adipose tissue [57] | Liver Adipose tissue Skeletal muscle Pancreas |
| Interleukin 15 | Immune cell proliferation, differentiation and maturation [58,59] Immune response regulation [60] Muscle protein synthesis [61] Adipose tissue reduction [62,63]. Glucose metabolism regulation [64,65] | Skeletal and heart muscle Placenta Monocytes/macrophages Bone marrow Thymus Lung Liver Kidney |
| Forms of Exercise | Group Characteristics | Blood Samples | Effect of Exercise on the Cytokine Concentration | References |
|---|---|---|---|---|
| Acute exercise effects | ||||
| HIIT. Four 30-s Wingate anaerobic tests (WAnTs) with a 4-min rest on cycle ergometer between consecutive exercise bouts | Men aged 18–24 years, 10 Kick-boxers and 10 sedentary-lifestyle (n = 20) | Blood samples—before, just after, 3 and 6 h post-test | Increase of MSTN serum concentration, kick-boxers and sedentary, respectively: PRE = 2652.3 ± 960.9 pg/mL and 2501 ± 78.3 pg/mL After = 2914.5 ± 1011.6 pg/mL and 2939.1 ± 839.2 pg/mL 3 h post = 2608.9 ± 890.6 pg/mL and 2849.5 ± 1028.6 pg/mL 6 h post = 2698.2 ± 1004.2 pg/mL and 2717 ± 764 pg/mL | [75] |
| HICT with body mass, 3× per week for 5 weeks. One HICT session consisted of 3 circuits with 2-min breaks between them. Each HICT training consisted 9 exercises with one’s body as a workload performed as follow: jumping jacks, pushups, sit-ups, side plank, squats, plank, running in place, lunges, and push-ups with rotation | Women, aged 40 ± 11 years, inactive (n = 20). Age subdivision: young < 30 years (n = 11) and middle-age > 30 years (n = 9) | Blood samples—before and 1 and 24 h after the first and last session | No change | [76] |
| A single bout of resistance exercise. RE session targeted the upper and lower body, with three sets of 15 repetitions on seven machines (chest press, pull up, leg press, shoulder press, knee extension, knee flexion, and elbow flexion), each lasting 30 s. All exercises were performed at 55 % of the subjects’ 1 RM with 2-min rest intervals between sets | Men, aged 22.1 ± 2.1 years, experienced in weight training (n = 12) | Blood samples—before and 24 h after the exercise. | Decrease of MSTN serum concentration (Baseline = 9.12 ± 0.36 ng/mL; Post-exercise = 8.8 ± 0.36 ng/mL) | [77] |
| Ultramarathon—foot race of 246 km distance from Athens to Sparta; time limit <36 h | 18 men and 1 woman, aged 41–48 years, Highly trained long-distance runners (n = 19) | Blood samples—the day before a run, just after the race, and 3 days after | Increase in MSTN serum concentration (Before = 23.73 ng/mL (21.16–28.28) and just after = 26.73 ng/mL (21.22–31.68)) | [78] |
| HIIT1: two sets of six 30 s treadmill running bouts at Vmax eliciting the VO2max, with 90 s of active recovery (50% of Vmax) between bouts and 4 min of passive recovery between sets. HIIT2: five 4 min bouts at 90% Vmax, with 4 min of active recovery (50% of Vmax) between bouts | Men, aged 23 ± 2 years, sedentary (n = 17) | All blood variables were analyzed before (baseline) and immediately after each training session and 1, 3, 24, 48, and 72 h after each session. | Increase in MSTN serum concentration. HIIT1 to ~130% of the basal value. HIIT2 to ~135% of the basal value. | [79] |
| Resistance Training. Seven types of exercises targeting all the main muscle groups. Four sets of 8–10 repetitions at 70–75% of 1 RM with 60–90 s of rest between exercises and for 4 min between sets. | Men, aged 23 ± 2 years, sedentary (n = 17) | All blood variables were analyzed before (baseline) and immediately after each training session and 1, 3, 24, 48, and 72 h after each session. | Increase in MSTN serum concentration to ~145% of the basal value. | [79] |
| Session 1: 45 min of treadmill running at the intensity of the anaerobic threshold (AT; 85 ± 8% of VO2max) Session 2: 45 min of treadmill running at the intensity of the maximal fat oxidation (FATmax; 52 ± 14% of VO2max) | Men, aged 23 ± 1 years, sedentary (n = 14) | Blood variables were analyzed at baseline, immediately upon session termination (post 0 h), and 1, 3, 24, 48, and 72 h after each session, respectively. | Increase in MSTN serum concentration after AT session (Peak% = 136 ± 18). No change in FATmax. | [80] |
| Regular (chronic) exercise effects | ||||
| Supervised training three times a week, separated by at least 48 h for 8 weeks. training included three sets (50–80% 1 RM) per exercise in weeks 1–4 and four sets per exercise in weeks 5–8. | Men, aged 40–53 years, sedentary Upper-body (n = 10) Lower-body (n = 10) Upper + Lower body (n = 10) | Blood samples—48 h before the first session and 48 h after the last session | Decrease of MSTN serum concentration in LB and UP + LB LB = (−0.11 ng/mL (95% CI −0.16 to −0.06)) UB + LB = (−0.36 ng/mL (95% CI −0.48 to −0.19)) | [81] |
| HIIT. One session every 5 days for 6 weeks—nine sessions in total. Each session consisted of 6 × 30 s sprints at 40% predefined peak power output interspersed with 3 min active recovery on a cycle ergometer | Older Men. LEX = 11 active exercisers aged 62 ± 6 years, masters in sports; SED = 13 sedentary lifestyle aged 64 ± 6 years (n = 24) | Blood samples—before and after completing the program. | Trivial increase of total MSTN serum concentration in SED (PRE = 4217 ± 317 pg/mL; POST = 4163 ± 337 pg/mL). Moderate increase of total MSTN serum concentration in LEX (PRE = 3394 ± 391 pg/mL; POST = 3678 ± 438 pg/mL). Trivial increase of free MSTN in SED and LEX (PRE = 1182 ± 372 pg/mL and 1159 ± 418 pg/mL respectively; POST = 1203 ± 533 pg/mL and 1224 ± 404 pg/mL respectively) | [68] |
| HICT with body mass, 3× per week for 5 weeks. One HICT session consisted of 3 circuits with 2-min breaks between them. Each HICT training consisted 9 exercises with one’s body as a workload performed as follow: jumping jacks, pushups, sit-ups, side plank, squats, plank, running in place, lunges, and push-ups with rotation | Women, aged 40 ± 11 years, inactive (n = 20). Age subdivision: young < 30 years (n = 11) and middle-age > 30 years (n = 9) | Blood samples—before and 1 and 24 h after the first and last session | Decrease of MSTN serum concentration (more pronounced in middle-aged) | [76] |
| Supervised, resistance band training designed to train all major muscle groups based on ACSM guidelines—twice a week for 6 months | Untrained Women > 65 years. Resistance training (n = 33); Resistance training and supplementation (n = 28) | Blood samples—baseline, after 3 months and 6 months of intervention | No changes | [82] |
| Forms of Exercise | Group Characteristics | Blood Samples | Effect of Exercise on the Cytokine Concentration | References |
|---|---|---|---|---|
| Acute exercise effects | ||||
| HIIT1: two sets of six 30 s treadmill running bouts at Vmax eliciting the VO2max, with 90 s of active recovery (50% of Vmax) between bouts and 4 min of passive recovery between sets. HIIT2: five 4 min bouts at 90% Vmax, with 4 min of active recovery (50% of Vmax) between bouts | Men, aged 23 ± 2 years, sedentary (n = 17) | All blood variables were analyzed before (baseline) and immediately after each training session and 1, 3, 24, 48, and 72 h after each session. | Increase in serum FST in both sessions. HIIT1 ~200% of basal concentration and HIIT2 ~270% of the basal concentration in 3 h post-exercise | [79] |
| Resistance training: seven types of exercises targeting all the main muscle groups. Four sets of 8–10 repetitions at 70–75% of 1 RM with 60–90 s of rest between exercises and 4 min between sets. | Men, aged 23 ± 2 years, sedentary (n = 17) | All blood variables were analyzed before (baseline) and immediately after each training session and 1, 3, 24, 48, and 72 h after each session. | Increase in serum FST ~200% of the basal concentration in 3 h post-exercise | [79] |
| Session 1: 45 min of treadmill running at the intensity of the anaerobic threshold (AT; 85 ± 8% of VO2max) Session 2: 45 min of treadmill running at the intensity of the maximal fat oxidation (FATmax; 52 ± 14% of VO2max) | Men, aged 23 ± 1 years, sedentary (n = 14) | Blood variables were analyzed at baseline, immediately upon session termination (post 0 h), and 1, 3, 24, 48, and 72 h after each session, respectively. | Increase in serum FST. AT peak% = 359 ± 177 and FATmax peak% = 241 ± 86 | [80] |
| 45 min of walking/ running at 70% VO2max, followed by 90% of VO2max until exhaustion | 80 Men divided into subgroups. Young, aged 18–35 years (n = 20) and Old aged 61–79 years (n = 20); Active—VO2 max >35 mL/kg/min (n = 20) and inactive—VO2 max <35 mL/kg/min (n = 20) | Blood was obtained 1 h before the end of the exercise, immediately after, and 1 h after completing each session. | Increase in serum FST ranging 12–21% in the general population. Young active peak% = 22.8 ± 34.7 Young sedentary peak% = 14 ± 27.8 Old active peak% = 12 ± 18.9 Old sedentary peak% = 12.4 ± 17 | [89] |
| 1: High-intensity interval exercise (HIIE), consisting of 5 × 4 min walking on a treadmill at 3 km/h alternating with 4 × 4 min running at 90% of the maximum heart rate for a total of 36 min. 2: Continuous moderate-intensity exercise (MIE), defined as a 6-min walk/run on a treadmill at 65% of maximum heart rate. 3: Resistance exercise (RE), consisting of 3 sets of 8 to 12 repetitions at 75% to 80% of 1 RM | Men aged 30–56 years, sedentary (n = 14) | Blood was obtained 1 h before the end of the exercise, immediately after, and 1 h after completing each session. | Increase in serum FST ranging 5–10% in HIIE and RE group | [89] |
| Eccentric exercise bout with the knee extensors of each leg on an isokinetic dynamometer. 2 sets of 25 maximal voluntary eccentric (lengthening) muscle actions in isokinetic mode, while a 5 min break was allowed between the sets. | Men aged 25.7 ± 1.7 years; inactive for last 6 months (n = 9) | Blood samples were collected before and at 6, 48, and 120 h after the eccentric exercise, | Increase in serum FST PRE = 2080.2 pg/mL (± 200.3); 6 h = 2827.2 pg/mL (± 472.6); 48 h = 2924.4 pg/mL (± 330.2); 120 h = 2144.4 pg/mL (± 177.9) | [90] |
| 60 min of treadmill running at 60% VO2max | Men, aged 36 ± 15 years; inactive or moderately active (n = 11) | Trials were then initiated with a venous blood sample taken at ~09:00 (0 h), and additional samples were collected at 1, 1.5, 2.75, 4, and 7 h. | Increase in FST serum concentration with a peak at 2.75 h (~1000 pg/mL at the baseline to ~1400 pg/mL at peak) | [91] |
| MOD: Treadmill run at medium intensity 55% VO2max to energy expenditure 600 kcal HIGH: Treadmill run at high intensity 75% VO2max to energy expenditure 600 kcal | Men, aged 26 ± 2 years (n = 10) | Blood samples—before exercise, just after and 1, 2, 4, 7 h after | Increase in FST plasma concentration. AUC concentrations: PRE = 4518 ± 1148 pg/mL and 4566 ± 962 pg/mL in MOD and HIGH, respectively. POST = 8504 ± 2118 pg/mL and 9275 ± 1406 pg/mL in MOD and HIGH, respectively. | [92] |
| 2 h of bicycle exercise at 60% of VO2max followed by 4 h of resting recovery | Men, aged 22.9 ± 0.8 years (n = 10) | Blood samples for analysis of hormones were obtained before a test and then every hour (one extra sample 30 min post-exercise) simultaneously from the hepatic vein and the brachial artery | 5-fold increase in plasma FST in the hepatic vein and brachial artery during the first 2 h of recovery | [43] |
| Ultramarathon—foot race of 246 km distance from Athens to Sparta; time limit <36 h | 18 men and 1 woman, aged 41–48 years, highly trained long-distance runners (n = 19) | Blood samples—the day before a run, just after the race and 3 days after | Increase in serum FST PRE = 300.8 pg/mL [236.4; 831.5] POST = 1211 pg/mL [849.1; 2174] | [78] |
| Regular (chronic) exercise effects | ||||
| HIIT. One session every 5 days for 6 weeks—nine sessions in total. Each session consisted of 6x30 sec sprints at 40% predefined peak power output interspersed with 3 min active recovery on a cycle ergometer | Older Men. LEX = 11 active exercisers aged 62 ± 6 years, masters in sports; SED = 13 sedentary lifestyle aged 64 ± 6 years (n = 24) | Blood samples—before and after completing the program. | Increase in serum FST in SED while no change in LEX. PRE = 2508 ± 628 pg/mL and 2102 ± 598 pg/mL in SED and LEX, respectively. POST = 3043 ± 676 pg/mL and 2126 ± 809 pg/mL in SED and LEX, respectively. | [68] |
| Supervised resistance band training designed to train all major muscle groups based on ACSM guidelines—twice a week for 6 months | Untrained women >65 years old. RT = resistance training (n = 33); RTS = resistance training and supplementation (n = 28) | Blood samples—baseline, after 3 months and 6 months of intervention | Increase in basal serum FST in the RT group PRE: 1.92 ng/mL (1.38–2.86); POST: 2.23 ng/mL (1.34–3.61) | [82] |
| Supervised training three times a week, separated by at least 48 h for 8 weeks. training included three sets (50–80% 1 RM) per exercise in weeks 1–4 and four sets per exercise in weeks 5–8. | Men, aged 40–53 years, sedentary Upper-body (n = 10) Lower-body (n = 10) Upper + Lower body (n = 10) | Blood samples—48 h before the first session and 48 h after the last session | Increase in serum FST [UB = 0.22 ng/mL (95% CI 0.16–0.38); LB = 0.24 ng/mL (95% CI 0.20–0.28) and UB + LB = 0.55 ng/mL (95% CI 0.39–0.61)] | [81] |
| Forms of Exercise | Group Characteristics | Blood Samples | Effect of Exercise on the Cytokine Concentration | References |
|---|---|---|---|---|
| Acute exercise effects | ||||
| Strength training session of seven exercises performed in three sets at a load corresponding to 8-RM (the weight that could be lifted maximal 8 times). The exercises were leg press, leg curls, bench press, pull-down, sitting shoulder press, cable-flies, and low rowing. | Men, young, well-trained and lean (n = 10) (acute setting) | Before the start of the exercise, after the third set of leg press, immediately after the exercise session (0 min) as well as 30 min, 60 min, 90 min, and 120 min post-exercise | 1. Plasma decorin levels were significantly increased at the end of the exercise session (~30% increase) 2. Volunteers who could push more weight displayed a higher increase of decorin levels from baseline to the end of the training session | [93] |
| An endurance exercise session (90 min on a cycle ergometer at 70% VO2max) in the morning and a resistance exercise session (5 × 8 80% 1 RM—repetition max) repetitions of bilateral leg press and the leg extension with two minutes of rest in between the sets) in the afternoon with a resting period of 4 h between sessions | Men, mean age 21.2 years, recreationally active (n = 13) | At baseline, 1 h after the endurance exercise session, before the resistance exercise session, and 1, 2, and 3 h after the resistance exercise session | 1. No change of plasma decorin at 1, 2, and 3 h post resistance exercise compared to baseline. | [94] |
| A single bout of resistance exercise—bilateral knee extensions: With blood-flow restriction: 1 set of 30 repetitions, followed by 3 sets of 15 repetitions at 30% of one-repetition maximum, with 30 s rest between sets. Without blood-flow restriction: Low-intensity exercise—one set of 30 repetitions and three sets of 15 repetitions at 30% of one-repetition maximum, with 30 s rest between sets. High-intensity exercise—four sets of 7 repetitions at 80% of one-repetition maximum, with 1 min rest between sets. | Men, aged 18–35 years, physically active (1 year of resistance training experience) (n = 9) | Before, immediately after, 1 h and 24 h post-exercise | 1. Plasma concentration of decorin immediately post-exercise was 11.91% greater than immediately pre-, 1-h post-exercise, and 24-h post-exercise. 2. Low-intensity resistance exercise: pre-exercise 2016.10 ± 1250.94 pg/mL, post-exercise 2195.20 ± 1362.85 pg/mL, 1 h post-exercise 2117.57 ± 1387.60 pg/mL, 24 h post-exercise 1895.50 ± 1182.22 3. Blood-flow restriction resistance exercise: pre-exercise 1901.19 ± 1144.05 pg/mL, post-exercise 2121.24 ± 1189.63 pg/m, 1 h post-exercise 1996.01 ± 1196.53 pg/mL, 24 h post-exercise 1909.76 ± 1187.80 4. High-intensity resistance exercise: pre-exercise 1939.47 ± 1142.65, post-exercise 2237.72 ± 1446.64 pg/mL, 1 h post-exercise 1983.09 ± 1323.87 pg/mL, 24 h post-exercise 1883.66 ± 1090.47 5. No differences in decorin release between interventions | [95] |
| Regular (chronic) exercise effects | ||||
| HICT with body mass, 3× per week for 5 weeks. One HICT session consisted of 3 circuits with 2-min breaks between. Each HICT training consisted of 9 exercises with one’s body as a workload performed as follows: jumping jacks, pushups, sit-ups, side plank, squats, plank, running in place, lunges, and push-ups with rotation | Women mean age 40 years, Trained (n = 20) and control (n = 13). | Before and 1 and 24 h after the first and last session | 1. The intervention did not modify the resting plasma decorin concentration | [76] |
| Forms of Exercise | Group Characteristics | Blood Samples | Effect of Exercise on the Cytokine Concentration | References |
|---|---|---|---|---|
| Acute exercise effects | ||||
| Meta-analyses: Aerobic exercise (15 studies); strength or resistance training (5 studies); strength and endurance training (2 studies); Other (7 studies) | 1111 (29 studies) mean age 42.1 years 46.6% of women 14 studies— acute effect | Up to 60 min after exercise | 1. Increase in BDNF concentration following a single session of exercise (overall, 1.46-fold increase analyzing 14 studies) | [102] |
| 35 min sessions of physical exercise of moderate intensity compared to cognitive training or mindfulness practice | Men and women, aged 65–85 years, 11 women, 8 men (Total n = 19) | Immediately before, immediately after, and at 20 and 60 min after each exercise | 1. Only physical exercise produced a significant increase in BDNF serum concentration 2. Physical exercise: before intervention 19.21 ± 1.17 ng/mL, 0 min after 22.72 ± 1.17, 20 min after 21.73 ± 1.2 ng/mL, 60 min after 24.33 ± 1.38 ng/mL 3. Cognitive training: before intervention 20.06 ± 0.95 ng/mL, 0 min after 20.81 ± 1.35, 20 min after 19.89 ± 1.35 ng/mL, 60 min after 20.12 ± 1.13 ng/mL 4. Mindfulness practice: before intervention 21.6 ± 1.58 ng/mL, 0 min after 20.41 ± 1.48, 20 min after 21.76 ± 1.05 ng/mL, 60 min after 20.86 ± 1.74 ng/mL | [103] |
| 30 min physical exercise high or low intensity (cycle ergometer) or relaxing phase | Men and women, aged 18–29 years, 41 women, 40 men, (Total n = 81) | At the beginning of the experiment, after the learning phase and after the exercise/relaxing phase | 1. Serum BDNF concentration increased after exercising only in the high-intensity exercise group (~15% increase) | [104] |
| Single strength training session in a leg press, knee extensor, and leg curl (4 sets, training session about 35–40 min). Trained group (6 months prior training) and untrained group | Men, mean age 26.6 years, mean BMI 24.1 (n = 20) | Immediately before, immediately after, 2 and 24 h after the training | 1. The BDNF serum concentration increased only in the trained group immediately after the end of exercise (~85% increase) and not 2 or 24 h after the training | [105] |
| 12-week CrossFit program (high-intensity interval training). Sixty-minute workouts twice a week. | Men and women, mean age 25.6 years, 7 male, 5 women (Total n = 12) | At rest and 15 min after Wingate test and 15 min after progressive test—before and after the intervention (12-week CrossFit program) | 1. Before intervention—increase in serum BDNF directly after the Wingate test and progressive test (~10% in men, ~30% in women for Wingate test; ~40% in men, ~80% in women after progressive test) 2. After 12-week CrossFit program—decrease in serum BDNF directly after the Wingate test and progressive test (~15% in men after Wingate test, ~20% in men, ~10% in women after progressive test) | [106] |
| 30 min of aerobic exercise at 60% of individual VO2max. Subjects divided into higher fitness group (VO2max > 75 percentile) and lower fitness group (VO2max < 45 percentile) | Men, Aged 18–28 years, (n = 60) | Pre- and post-exercise | 1. Serum BDNF concentration was higher post-exercise than in the pre-exercise in the higher fitness group (86.09 ± 68.14 pg/mL vs. 53.06 ± 34.65 pg/mL) 2. No changes in the lower fitness group (an increase nearly reaching significance 57.07 ± 71.42 pg/mL vs. 39.78 ± 38.60 pg/mL) | [107] |
| High-intensity, low-volume strength training (at 90% 1 RM) (HI) or a high-volume, moderate-intensity strength training (at 70% 1 RM) (HV). Training program—4 times a week for 7 weeks | Men, mean age 23.5 years, Active, strength-trained (n = 20) | During the first training session of week 1 and week 7—at baseline, immediately post-exercise, 30 min post-exercise, and 60 min post-exercise. | 1. Elevations in plasma BDNF concentrations from baseline, immediately, and 60 min post-exercise in both HI and HV combined before and after the intervention (training program) 2. Before intervention: High-intensity, low-volume post-exercise (~100% increase immediately post-exercise and ~100% increase 60 min post-exercise) 3. After intervention: High-intensity, low-volume post-exercise (~50% increase immediately post-exercise and ~100% increase 60 min post-exercise) 4. Before intervention: Low-intensity, high-volume post-exercise (~180% increase immediately post-exercise and ~200% increase 60 min post-exercise) 5. After intervention: Low-intensity, high-volume post-exercise (~50% increase immediately post-exercise and ~75% increase 60 min post-exercise) | [108] |
| HIIT (High-intensity interval training) protocol—intervals of 1 min (90% maximal workload) alternating with 1 min rest at 60 W for a total of 20 min CON (continuous exercise) protocol cycle ergometer at the same intensity for 20 min (at 70% of maximal workload) | Experiment 1 7 Men (n = 7) Experiment 2 26 Men aged 22–35 years, (n = 26) | 30 min before exercise, during exercise (0, 6, 10, 14, 18, and 20 min), 20 min after exercise (experiment 1) 30 min before exercise, the start of exercise, the end of exercise (experiment 2) | 1. Serum BDNF concentration increased gradually during exercise in both protocols, reaching maximum concentrations toward the end of the exercise. (experiment 1)(CON—increase ~30% and HIT—increase ~45%) 2. BDNF serum concentration returned quickly to baseline after exercise—the measurement 20 min post-exercise was not significantly different from that at rest levels (experiment 1) 3. Both exercise protocols—an increase of serum BDNF concentration compared with a rest condition, HIT reached higher BDNF serum concentration than CON (experiment 2) | [109] |
| A 6-week supervised physical training program | Men, mean age 23.8 years, (n = 34) | Before and after the 6-week exercise intervention | 1. Decreased exercise-induced serum BDNF concentration after intervention in the exercise group; nonsignificant changes in the control group 2. Before training intervention: in control group baseline 8.1 ± 3.6 ng/mL, after exercise 12.3 ± 3.9 ng/mL and in the exercise group baseline 12.4 ± 5.8 ng/mL, after exercise 16.7 ± 7.7 ng/mL 3. After training intervention: in control group baseline 8.1 ± 2.8 ng/mL, after exercise 14.8 ± 5.9 ng/mL and in the exercise group baseline 10.1 ± 4.2 ng/mL, after exercise 11.1 ± 4.4 ng/mL | [110] |
| Three sessions performed on three separate days randomly for all participants at low (<60% VO2max—90% of VT1), moderate (60–75% VO2max—the midpoint between VT1 and VT2), and high (>90% VO2max—the midpoint between VT2 and Wmax) intensities until exhaustion or for up to 60 min | 38 Men mean age 28.8 years, (n= 38) | pre- (rest) and immediately post-exercise session | 1. Increase of BDNF serum concentration after exercise in all the intensity groups. 2. Individuals with lower physical fitness (<49.7 mL/kg/min) exhibited greater BDNF changes, mainly after high-intensity with a short-time, when compared with well-trained individuals with better physical fitness 3. Low intensity: pre 33440.85 ± 6229.58 pg/mL, post 34,900.17 ± 6908.31 pg/mL 4. Moderate intensity 28,169.05 ± 4674.63 pg/mL, post 32,793.15 ± 5198.64 pg/mL 5. High intensity 26,673.73 ± 4896.58 pg/mL, post 43,542.48 ± 6774.00 pg/mL | [111] |
| Regular (chronic) exercise effects | ||||
| Meta-analyses: Aerobic exercise (15 studies); Strength or resistance training (5 studies); Strength and endurance training (2 studies); Other (7 studies) | 1111 (29 studies) mean age 42.1 years 46.6% women | Up to 60 min after exercise | 1. Regular exercise (range 3–24 weeks) caused a more significant increase of BDNF concentration after a session of exercise (overall 1.58-fold increase analyzing 8 studies) 2. Regular exercise (range 3 weeks–2 years) caused a greater increase (but smaller than the 3–24 weeks range) increase of BDNF concentration after a session of exercise (overall 1.28-fold increase analyzing 13 studies) | [102] |
| Low, moderate, and high-intensity exercises (40%, 55%, 70% VO2max) on the treadmill four times a week for 12 weeks, 200 kcal burn in each session. Control group—stretching. | Men, mean age 15 years, (n = 40) | Before intervention and after 12 weeks | 1. Increase in serum BDNF concentration at rest compared to pre-intervention in the moderate-intensity exercise and high-intensity exercise groups. 2. No changes in the low-intensity exercise group or stretching group. 3. Low intensity exercise group: pre 24.79 ± 25.77 ng/mL, post 25.05 ± 21.47 ng/mL 4. Moderate intensity exercise group: pre 25.90 ± 26.59 ng/mL, post 27.71 ± 25.86 ng/mL 5. High intensity exercise group 25.24 ± 34.17 ng/mL, post 30.09 ± 48.00 ng/mL 6. Stretching group pre 23.96 ± 20.93 ng/mL, post 24.50 ± 22.04 ng/mL | [112] |
| 12-week CrossFit program (high-intensity interval training). Sixty-minute workouts twice a week. | Men and women, mean age 25.6 years, 7 male, 5 women (Total n = 12) | At rest and 15 min after Wingate test and 15 min after progressive test—before and after the intervention (12-week CrossFit program) | 1. Resting serum BDNF concentration increased after CrossFit training in men and women. (increase ~50% in women and ~50% in men) | [106] |
| High-intensity, low-volume strength training (at 90% 1 RM) (HI) or a high-volume, moderate-intensity strength training (at 70% 1 RM) (HV). Training program—4 times a week for 7 weeks | Men, mean age 23.5 years, Active, strength- trained (n = 20) | During the first training session of week 1 and week 7—at baseline, immediately post-exercise, 30 min post-exercise, and 60 min post-exercise. | 1. A training program of 7-week strength exercises increased the plasma BDNF response to exercise irrespective of exercise severity protocol 2. No change of resting BDNF plasma concentration was reported 3. Before intervention: High-intensity, low-volume post-exercise (~100% increase immediately post-exercise and ~100% increase 60 min post-exercise) 4. After intervention: High-intensity, low-volume post-exercise (~50% increase immediately post-exercise and ~100% increase 60 min post-exercise) 5. Before intervention: Low-intensity, high-volume post-exercise (~180% increase immediately post-exercise and ~200% increase 60 min post-exercise) 6. After intervention: Low-intensity, high-volume post-exercise (~50% increase immediately post-exercise and ~75% increase 60 min post-exercise) | [108] |
| A 6-week supervised physical training program | Men, mean age 23.8 years, (n = 34) | Before and after the 6-week exercise intervention | 1. No change in baseline serum BDNF concentration after a 6-week training program. 2. No change in baseline BDNF in the control group | [110] |
| Regular endurance exercise: 16 weeks of taekwondo training 5 × 60 min per week | Men and women, mean age 12.6 years, overweight and obese adolescents Exercising group (n = 10) Control group (n = 10) 7 men, 3 women (total n = 20) | Before and after 16 weeks of training | 1. Increase in serum BDNF concentration after a 16-week intervention 2. Exercise group pre 25.41 ± 5.36 ng/mL, post 29.52 ± 5.83 ng/mL 3. Control group pre 26.58 ± 6.10 ng/mL, post 27.68 ± 6.50 ng/mL | [113] |
| Forms of Exercise | Group Characteristics | Blood Samples | Effect of Exercise on the Cytokine Concentration | References |
|---|---|---|---|---|
| Acute exercise effects | ||||
| A single session of treadmill exercise with increasing intensity. Mean duration of one trial 14.2 min. Regular endurance training: 9 supervised trials within 14 days. | Women, aged 18–35 years, sedentary (n = 60) | Before exercise, 1 h post, 4 h post-exercise. After 2 weeks of exercise. | No change at any time-point after an acute bout of exercise. | [130] |
| Two endurance exercise sessions: 30 min treadmill running at 50% VO2max (1st trial) and 80% VO2max (2nd trial). Trials separated by 3 days. | Men, mean age 22.1 years, non-athletic (50% VO2max n = 13 80% VO2max n = 8) | Before exercise, immediately post, 1 h post-exercise. | FGF21 serum concentration increased 1 h after exercise: ~3 fold after mild-intensity (50% VO2max) and ~5 fold after high-intensity (80% VO2max) training. Concentration after high-intensity training was significantly higher than after mild-intensity. No change immediately post-exercise. | [126] |
| Three sessions: Moderate-intensity endurance exercise: treadmill run at 55% VO2max to energy expenditure 600 kcal (mean 57 min). High-intensity endurance exercise: treadmill run at 75% VO2max to energy expenditure 600 kcal (mean 42 min). Control—rest. Participants performed all training sessions with at least a 5-day interval. | Men, mean age 26 years, (n = 10) | Blood samples before exercise, immediately after, 1 h, 2 h, 4 h, 7 h after the exercise | Serum FGF21 concentrations increased up to 4 h post-exercise compared to control. More significant increases were observed at 1 h, 2 h, and 4 h after high-intensity exercise vs. moderate-intensity training. Area under FGF21 concentration versus time curve (baseline to 2 h post-exercise [pg/mL]) during control: 144 ± 124, mild intensity training: 230 ± 156 and high intensity training: 334 ± 249 | [92] |
| 60 min cycling at 75% VO2max | Men, mean age 23.7 years, sedentary (n = 19) | Before exercise, immediately post-exercise. | FGF21 serum concentration increased immediately post-exercise. | [127] |
| A single session of endurance exercise: 60 min of treadmill running at 60% VO2max | Men, mean age 36 years, sedentary or moderately active (n = 11) | Before exercise, 1 h, 1.5 h, 2.75 h, 4 h and 7 h post-exercise. | FGF21 serum concentration increased at 1 h, 1.5 h, and 4 h post-exercise compared to baseline. Peak values: ~2-fold increase at 1.5 h post-exercise compared to baseline. | [91] |
| A single endurance exercise session: 30 min cycling at 70% VO2max preceded with carbohydrate intake (180 kcal). | Men, aged 18–22 years, (n = 7) Men, aged 62–69 years, (n = 8) | Before exercise, immediately after, 30 min post, 1 h post, 3 h post, 24 h post-exercise. | No change at 30 min, 1 h, and 3 h post-exercise. A significant decrease in serum FGF21 concentration 24 h post-exercise compared to baseline, immediately post 30 min and 1 h post-exercise | [131] |
| Endurance exercise: 1 h cycling at 70% VO2max. Resistance exercise: 5 sets of high-volume exercises involving major muscle parts within 1 h. Participants completed both modes of exercise in a cross-over design. Trials separated by 6–12 days. | Men, mean age 24 years, recreationally active (n = 10) | Before exercise, immediately after, 15 min, 30 min, 1 h, 90 min, 2 h, 3 h post-exercise. | Endurance exercise: a significant increase in FGF21 serum concentration starting at 15 min post-exercise with peak at 1 h post-exercise (~ 3-fold increase) until 2 h post-exercise. Resistance exercise: no change in serum FGF21 concentration. | [128] |
| 1st day: 2 min blocks of cycling alternating between 90 and 50% of maximal workload 2nd day: 90 min cycling at 50% of maximal workload Retest- the same protocol after 1 week | Men, mean age 27 years, well-trained (n = 11) | Before exercise, immediately after exercise performed on the 2nd day, 1 h post, 24 h post Retest- the same protocol after 1 week | Test: FGF21 increased over 2-fold 1 h after the 2nd day exercise. Retest: no change in FGF21 serum concentration. | [129] |
| 3 sessions of endurance and resistance exercise separated by 7-day rest: High-intensity interval training 1: 2 sets of 6 × 30 s treadmill running at Vmax eliciting VO2max, with 90 s of active recovery (50% of Vmax) between bouts and 4 min of passive recovery between sets. High-intensity interval training 2: 5 × 4 min treadmill running at 90% Vmax, with 4 min of active recovery (50% of Vmax) between bouts. Resistance Training session: 7 types of exercises targeting main muscle groups. 4 sets of 8–10 repetitions at 70–75% of one-repetition maximum with 60–90 s of rest between exercises and for 4 min between sets. | Men, mean age 23 years, sedentary (n = 17) | Before exercise, immediately after, 1 h, 3 h, 24 h, 48 h, and 72 h after each training session. | Serum FGF21 increased after all training types. High-intensity interval training induced an increase immediately and 3 h after the exercise, whereas resistance training after 48 h. High-intensity interval training 1: less than 1.5-fold increase. High-intensity interval training 2: over 2-fold increase. Resistance Training: over 2-fold increase. | [79] |
| A single session of resistance exercise: maximal single-leg eccentric contractions—3 sets of 25 repetitions separated with 5 min rest. | Men, mean age 25.0 years, physically active (n = 8) | Before exercise, after each exercise set, every 20 min during 3 h recovery. | No significant changes in FGF21 serum concentration at all post-exercise time points compared to baseline. | [134] |
| Regular (chronic) exercise effects | ||||
| A single session of treadmill exercise with increasing intensity. Mean duration of one trial 14.2 min. Regular endurance training: 9 supervised trials within 14 days. | Women, aged 18–35 years, sedentary (n = 60) | Before exercise, 1 h post, 4 h post-exercise. After 2 weeks of exercise. | FGF21 serum concentration increased over 1.5-fold compared to baseline. Baseline: 276.8 ng/L. After 2 weeks: 460.8 ng/L. | [130] |
| 5 weeks of supervised endurance exercise. 3 sessions of cycling each week with increasing intensity (60 VO2max, 70%, and 75%) and duration (30 min, 45 min). 5 weeks without regular physical activity. Participants were randomized to the exercising or resting group, then changed in a cross-over design. | Men, mean age 69.6 years, (n = 27) | Before exercise, 5 weeks post, 10 weeks post-exercise. | Serum FGF21 concentration decreased after 5 weeks of training. Pre-exercise: 248.1 ± 88.5 pg/mL Post-exercise: 218.5 ± 94.2 pg/mL | [133] |
| Forms of Exercise | Group Characteristics | Blood Samples | Effect of Exercise on the Cytokine Concentration | References |
|---|---|---|---|---|
| Acute exercise effects | ||||
| A single session of resistance exercise: 4 sets of 10 repetitions of a back squat exercise at 70% of one-repetition maximum, using either traditional set configurations (4 × 10 with 180 s inter-set rest) or cluster sets (4 × (2 × 5) with 30 s intra-set rest and 150 s inter-set rest). Participants performed both training modes with a 7-day interval. | Men, mean age 27 years, resistance-trained (n = 10) | Blood samples before, immediately after, 30 min, 60 min, 24 h, and 48 h post-exercise | IL-15 serum concentration increased immediately post-exercise by 20–30% after both types of set configurations, then it returned to baseline values. | [144] |
| A single session of resistance exercise: 4 sets of 8–15 repetitions of bilateral leg press and knee extension at 75% of one-repetition maximum | Men, mean age 24.9 years, resistance-trained (n = 14) | Blood samples: before, mid-exercise, immediately after, 0.3 h, 1 h, 2 h, 4 h, 24 h post-exercise. | IL-15 serum concentration: increase by ~3.5-fold immediately after exercise; remained elevated until 24 h post-exercise. | [145] |
| An endurance exercise session: 90 min cycling at 70% VO2max followed by a resistance exercise session: 5 sets of 8 repetitions of bilateral leg press and leg extensions at 80% of one-repetition maximum with 2 min rest between sets and 4 h rest and meal between both exercise sessions. Participants performed the intervention twice either with carbohydrate or fat meal. | Men, mean age 21.2 years, recreationally active (n = 13) | Blood samples: before, 1 h after endurance exercise, 1 h after the meal, 1 h, 2 h and 3 h after resistance exercise. | IL-15 serum concentration increased by 15–25% 1 h after resistance exercise irrespective of meal type. No changes 1 h post endurance exercise. | [94] |
| A single session of resistance exercise—bilateral knee extensions: With blood-flow restriction: 1 set of 30 repetitions, followed by three sets of 15 repetitions at 30% of one-repetition maximum, with 30 s rest between sets. Without blood-flow restriction: Low-intensity exercise—1 set of 30 repetitions and 3 sets of 15 repetitions at 30% of one-repetition maximum, with 30 s rest between sets. High-intensity exercise—4 sets of 7 repetitions at 80% of one-repetition maximum, with 1 min rest between sets. | Men, aged 18–35 years, physically active (1 year of resistance training experience) (n = 9) | Blood samples before, immediately after, 1 h and 24 h post-exercise | No changes in serum IL-15 concentration. | [95] |
| Single resistance training session: leg press, knee extension, leg curl 4 sets of 8–10 repetitions of each exercise at 65% one-repetition maximum, completed within 35–40 min. | Men, mean age 26.6 years, Trained group (n = 10) Untrained group (n = 10) | Blood samples before, immediately after, 2 h and 24 h after the training session | No changes in serum IL-15 after a single session of resistance exercise in both groups. | [105] |
| Three sessions of endurance and resistance exercise separated by 7 days rest: High-intensity interval training 1: 2 sets of 6 × 30 s treadmill running at Vmax eliciting VO2max, with 90 s of active recovery (50% of Vmax) between bouts and 4 min of passive recovery between sets. High-intensity interval training 2: 5 × 4 min treadmill running at 90% Vmax, with 4 min of active recovery (50% of Vmax) between bouts. Resistance Training session: 7 types of exercises targeting main muscle groups. 4 sets of 8–10 repetitions at 70–75% of one-repetition maximum with 60–90 s of rest between exercises and for 4 min between sets. | Men, mean age 23 years, sedentary (n = 17) | Blood samples before, immediately after, 1 h, 3 h, 24 h, 48 h, and 72 h after each training session. | No changes in serum IL-15 after a single session of both resistance and endurance exercise. | [79] |
| Two sessions of eccentric and concentric emphasized resistance exercise with a 4-day interval. Each session comprised three sets of 8–10 repetitions of 7 exercises involving all major muscle groups (squat, chest press, seated row, leg extensions, triceps extensions, arm curl, shoulder press, and hamstring curl) at 70–80% of one-repetition maximum for concentric and 90–100% of one-repetition maximum for eccentric emphasized exercise. | Men, non-athletes, mean age 20.8 years, (n = 14) Men, athletes, mean age 24.1 years., (n = 14) | Blood samples before and immediately after (1–5 min) each training session. | An increase in IL-15 concentration after ECC (1.43 ± 0.17 vs. 1.62 ± 0.28 pg/mL) and CON (1.79 ± 0.6 vs. 2.16 ± 0.6 pg/mL) resistance exercise in non-athletes. In athletes, IL-15 serum concentration increased significantly only after ECC resistance exercise (1.72 ± 0.4 vs. 2.46 ± 1.3 pg/mL), which was noted to be the highest degree of change in IL-15 in all subjects. The increase after the CON was insignificant in this group. | [147] |
| Resistance exercise—static: holding a rod below the knees until exhaustion. Endurance exercise: 5 min cycling with the power adjusted for weight followed by 3-min rest and 5-min cycling with the power adjusted to the heart rate measured at the end of the first load. | Men, aged 18–23 years, weightlifting group (elite strength-trained athletes; n = 10) track and field group (elite endurance-trained athletes; n = 10) control group 1 and control group 2 included 10 untrained volunteers | Blood samples before, immediately after, 30 min post-exercise. | Static resistance exercise increased IL-15 concentration by ∼50% in athletes only. Endurance exercise had no impact on IL-15 in both athletes and untrained individuals. | [148] |
| Wingate test (30 s trial on a cycle ergometer with pedaling rate of 100 RPM). 4 conditions: normoxia (4 min breathing with room air before the test) after placebo or antioxidant intake and hypoxia (4 min breathing with the gas mixture with reduced oxygen concentration before the test) after placebo or antioxidant intake. | Men mean age 25.2 years, physically active (n = 9) | Blood samples and muscle biopsies before, immediately after, 30 min, and 120 min post-exercise. | IL-15 muscle protein content increased significantly after the exercise. | [149] |
| A single session of strenuous endurance exercise: 35 km trail run with total climb of 940 m completed within 6 h. | Women (n = 11) and Men (n = 26) (27 included in IL-15 concentration analysis) mean age 38.97 years | Blood samples before and after the run. | IL-15 serum concentration increased by 2.22-fold after the run compared to the baseline. | [150] |
| HICT with body mass, 3× per week for 5 weeks. One HICT session: 3 circuits of 9 exercises (jumping jacks, pushups, sit-ups, side plank, squats, plank, running in place, lunges, and push-ups) with one’s body as a workload with 2 min rest between circuits. The control group performed HICT twice at the baseline and after 5 weeks | Women Exercising group (n = 20) Control group (n = 13). Age subdivision: young (n = 11) and middle-aged (n = 9) | Blood samples. Before, 1 h and 24 h after the first and last session. | First HICT session induced a decrease in serum IL-15 by 5–25% in both groups (training and control). | [76] |
| Regular (chronic) exercise effects | ||||
| 12-week resistance training protocol: 60 min per day 3 days per week Three sets of 12 repetitions of 7 exercises (squat, lunge, chest press, vertical fly, lat pull down, long pull, crunch) at 55–65% of one-repetition maximum | Women, premenopausal, mean age 47.5 years, overweight (n = 15) Women, postmenopausal, mean age 57.8 years, normal-weight (n = 20) | Blood samples before and after complete training protocol (12 weeks). | A significant increase in IL-15 concentration after 12 weeks of regular resistance training. Premenopausal: 34.94 ± 2.76 vs. 25.90 ± 3.42 Postmenopausal: 33.54 ± 4.48 vs. 26.37 ± 3.24 | [146] |
| HICT with body mass, 3× per week for 5 weeks. One HICT session: 3 circuits of 9 exercises (jumping jacks, pushups, sit-ups, side plank, squats, plank, running in place, lunges, and push-ups) with one’s body as a workload with 2 min rest between circuits. The control group performed HICT twice at the baseline and after 5 weeks | Women, Exercising group (n = 20) Control group (n = 13). Age subdivision: young (n = 11) and middle-aged (n = 9) | Blood samples. Before, 1 h and 24 h after the first and last session. | Last HICT session: increase by~10% in serum IL-15 only in the training group. | [76] |
| 12 weeks of regular, supervised endurance exercise: bench step exercise at moderate intensity (lactate threshold) 3× per day, 10–20 min each | Women, aged 65–85 years, Exercising group (n = 31) Control group (n = 31) | Blood samples before and after 12 weeks of training. | No significant changes in serum IL-15 after 12 weeks of regular exercise. | [151] |
| Regular endurance exercise: 16 weeks of taekwondo training 5 × 60 min per week | Men and women, mean age 12.6 years, overweight and obese adolescents Exercising group (n = 10) Control group (n = 10) 7 men, 3 women (total n = 20) | Blood samples before and after 16 weeks of training | No significant IL-15 serum concentration changes after 16 weeks of regular exercise both within the exercising group and between groups. | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchała, M.; Guzik, P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. Int. J. Environ. Res. Public Health 2021, 18, 1261. https://doi.org/10.3390/ijerph18031261
Domin R, Dadej D, Pytka M, Zybek-Kocik A, Ruchała M, Guzik P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. International Journal of Environmental Research and Public Health. 2021; 18(3):1261. https://doi.org/10.3390/ijerph18031261
Chicago/Turabian StyleDomin, Remigiusz, Daniela Dadej, Michał Pytka, Ariadna Zybek-Kocik, Marek Ruchała, and Przemysław Guzik. 2021. "Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People" International Journal of Environmental Research and Public Health 18, no. 3: 1261. https://doi.org/10.3390/ijerph18031261
APA StyleDomin, R., Dadej, D., Pytka, M., Zybek-Kocik, A., Ruchała, M., & Guzik, P. (2021). Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. International Journal of Environmental Research and Public Health, 18(3), 1261. https://doi.org/10.3390/ijerph18031261






