Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways
Abstract
:1. Introduction
2. Curcumin as an Antiviral Agent with Proven Health Benefits
3. Anti-Herpesvirus Drugs Used in Clinics
4. Role of Curcumin in Inhibition of Herpes Simplex Virus Infections
5. Curcumin Targets Thymidine Kinase Encoded by Herpes Simplex Virus
6. Role of Curcumin in Inhibition of Various Herpesviruses Infections
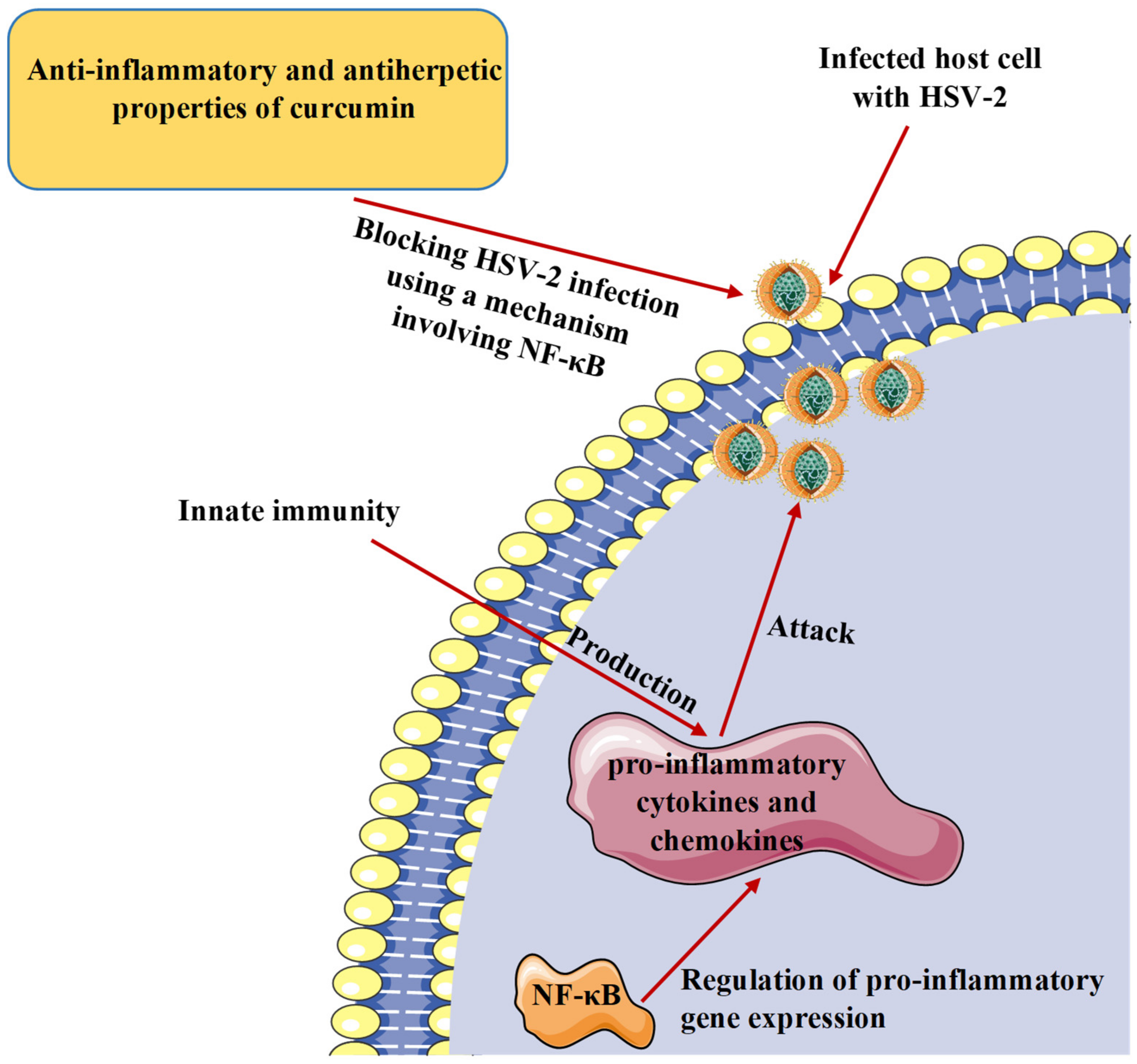
7. Curcumin and Inflammatory Response to Herpesvirus Infections
8. Safety Profile and Reported Undesirable Effects in Clinical Studies
9. Challenges with Bioavailability and Developed Strategies
10. New Directions for Herpesviruses Treatment
11. Conclusion and Future Visions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brezáni, V.; Leláková, V.; Hassan, S.T.S.; Berchová-Bímová, K.; Nový, P.; Klouček, P.; Maršík, P.; Dall’Acqua, S.; Hošek, J.; Šmejkal, K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.T.S.; Šudomová, M.; Berchová-Bímová, K.; Šmejkal, K.; Echeverría, J. Psoromic Acid, a Lichen-Derived Molecule, Inhibits the Replication of HSV-1 and HSV-2, and Inactivates HSV-1 DNA Polymerase: Shedding Light on Antiherpetic Properties. Molecules 2019, 24, 2912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.T.S.; Šudomová, M.; Masarčíková, R. Herpes simplex virus infection: An overview of the problem, pharmacologic therapy and dietary measures. Ceska Slov. Farm. 2017, 66, 95–102. [Google Scholar] [PubMed]
- Ho, D.Y.; Enriquez, K.; Multani, A. Herpesvirus Infections Potentiated by Biologics. Infect. Dis. Clin. N. Am. 2020, 34, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Roizman, B.; Pellett, P.E. The family Herpesviridae: A brief introduction. In Fields—Virology, 4th ed.; Knipe, D.M., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 2381–2397. [Google Scholar]
- Johnston, B.P.; McCormick, C. Herpesviruses and the Unfolded Protein Response. Viruses 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.I. Herpesvirus latency. J Clin Investig. 2020, 130, 3361–3369. [Google Scholar] [CrossRef]
- Pellett, P.; Roizman, B. Herpesviridae. In Fields Virology; Knipe, D., Howley, P., Eds.; Lippincott, Wil-liams and Wilkins: Philadelphia, PA, USA, 2013; pp. 1802–1822. [Google Scholar]
- Savva, R. The Essential Co-Option of Uracil-DNA Glycosylases by Herpesviruses Invites Novel Antiviral Design. Microorganisms 2020, 8, 461. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.T.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef]
- Čulenová, M.; Sychrová, A.; Hassan, S.T.S.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P.; et al. Multiple In vitro biological effects of phenolic compounds from Morus alba root bark. J. Ethnopharmacol. 2020, 248, 112296. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Q.; Zhang, Y.; Xiaofei, E.; Gao, W.; Zhang, M.; Zhai, W.; Rajkumar, R.S.; Liu, Z. Toll-like receptor-mediated innate immunity against herpesviridae infection: A current perspective on viral infection signaling pathways. Virol. J. 2020, 17, 192. [Google Scholar] [CrossRef]
- Treml, J.; Gazdová, M.; Šmejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T.S. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J.; Hassan, K.T.S. Cucurbitacin B interacts synergistically with antibiotics against Staphylococcus aureus clinical isolates and exhibits antiviral activity against HSV-1. S. Afr. J. Bot. 2017, 108, 90–94. [Google Scholar] [CrossRef]
- Andreu, S.; Ripa, I.; Bello-Morales, R.; López-Guerrero, J.A. Valproic Acid and Its Amidic Derivatives as New Antivirals against Alphaherpesviruses. Viruses 2020, 12, 1356. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S. Shedding Light on the Effect of Natural Anti-Herpesvirus Alkaloids on SARS-CoV-2: A Treatment Option for COVID-19. Viruses 2020, 12, 476. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Milobedeska, J.; Kostanecki, S.; Lampe, V. Zur kenntnis des curcumins. Ber. Deut. Chem. Ges. 1910, 43, 2163–2170. [Google Scholar] [CrossRef] [Green Version]
- Lampe, V.; Milobedeska, J. Studien über curcumin. Eur. J. Pharm. Biopharm. 1913, 46, 2235–2240. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, M.; Song, Y.; Wang, W.; Zhao, H.; Tian, Y.; Wang, Y.; Bai, S.; Zhao, Y.; Chen, X.; et al. Two traditional Chinese medicines Curcumae radix and Curcumae Rhizoma: An ethnopharmacology, phytochemistry, and pharmacology review. Evid. Based Complement. Altern. Med. 2016, 2016, 4973128. [Google Scholar] [CrossRef] [Green Version]
- Seidi Damyeh, M.; Mereddy, R.; Netzel, M.E.; Sultanbawa, Y. An insight into curcumin-based photosensitization as a promising and green food preservation technology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1727–1759. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective Properties of the Golden Spice Curcumin. Front Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef] [Green Version]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014, 2014, 186864. [Google Scholar] [PubMed]
- Jha, A.; Mohapatra, P.P.; AlHarbi, S.A.; Jahan, N. Curcumin: Not So Spicy After All. Mini Rev. Med. Chem. 2017, 17, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef] [Green Version]
- Birkmann, A.; Zimmermann, H. HSV antivirals—Current and future treatment options. Curr. Opin. Virol. 2016, 18, 9–13. [Google Scholar] [CrossRef]
- Kenny, K.; Leung, W.; Stephanson, K.; Ross, S. Clinical practice in prevention of neonatal HSV infection: A survey of obstetrical care providers in Alberta. J. Obstet. Gynaecol. Can. 2013, 35, 131–137. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Antiviral resistance in herpes simplex virus and varicella-zoster virus infections: Diagnosis and management. Curr. Opin. Infect Dis. 2016, 29, 654–662. [Google Scholar] [CrossRef]
- Rechenchoski, D.Z.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Herpesvirus: An underestimated virus. Folia Microbiol. 2017, 62, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Britt, W.J.; Prichard, M.N. New therapies for human cytomegalovirus infections. Antivir. Res. 2018, 159, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Maple, P.A.C. Cytomegalovirus and Epstein-Barr Virus Associations with Neurological Diseases and the Need for Vaccine Development. Vaccines 2020, 8, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonizzoli, M.; Arvia, R.; di Valvasone, S.; Liotta, F.; Zakrzewska, K.; Azzi, A.; Peris, A. Human herpesviruses respiratory infections in patients with acute respiratory distress (ARDS). Med. Microbiol. Immunol. 2016, 205, 371–379. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a Novel Anti-Herpes Simplex Virus Nutraceutical Agent: An Overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef] [Green Version]
- Zinser, E.; Krawczyk, A.; Mühl-Zürbes, P.; Aufderhorst, U.; Draßner, C.; Stich, L.; Zaja, M.; Strobl, S.; Steinkasserer, A.; Heilingloh, C.S. A new promising candidate to overcome drug resistant herpes simplex virus infections. Antivir. Res. 2018, 149, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.; Sattler, C.; Adler, H. Herpesviruses and Their Host Cells: A Successful Liaison. Trends Microbiol. 2017, 25, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.T.S. Brassicasterol with Dual Anti-Infective Properties against HSV-1 and Mycobacterium tuberculosis, and Cardiovascular Protective Effect: Nonclinical in Vitro and In Silico Assessments. Biomedicines 2020, 8, 132. [Google Scholar] [CrossRef]
- Memish, Z.A.; Almasri, M.; Chentoufi, A.A.; Al-Tawfiq, J.A.; Al-Shangiti, A.M.; Al-Kabbani, K.M.; Otaibi, B.; Assirri, A.; Yezli, S. Seroprevalence of Herpes Simplex Virus Type 1 and Type 2 and Coinfection with HIV and Syphilis: The First National Seroprevalence Survey in Saudi Arabia. Sex. Trans. Dis. 2015, 42, 526–532. [Google Scholar] [CrossRef]
- Ma, W.; He, H.; Wang, H. Oncolytic herpes simplex virus and immunotherapy. BMC Immunol. 2018, 19, 40. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.T.S.; Švajdlenka, E.; Berchová-Bímová, K. Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules 2017, 22, 722. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.; Corey, L. Current Concepts for Genital Herpes Simplex Virus Infection: Diagnostics and Pathogenesis of Genital Tract Shedding. Clin. Microbiol. Rev. 2016, 29, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widener, R.W.; Whitley, R.J. Herpes simplex virus. Handb. Clin. Neurol. 2014, 123, 251–263. [Google Scholar]
- Goins, W.F.; Hall, B.; Cohen, J.B.; Glorioso, J.C. Retargeting of herpes simplex virus (HSV) vectors. Curr. Opin. Virol. 2016, 21, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Dai, J.; Gu, L.; Su, Y.; Wang, Q.; Zhao, Y.; Chen, X.; Deng, H.; Li, W.; Wang, G.; Li, K. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int. Immunopharmacol. 2018, 54, 177–187. [Google Scholar] [CrossRef]
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 2008, 373, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Bourne, K.Z.; Bourne, N.; Reising, S.F.; Stanberry, L.R. Plant products as topical microbicide candidates: Assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antivir. Res. 1999, 42, 219–226. [Google Scholar] [CrossRef]
- Ferreira, V.H.; Nazli, A.; Dizzell, S.E.; Mueller, K.; Kaushic, C. The anti-inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. PLoS ONE 2015, 10, e0124903. [Google Scholar] [CrossRef] [Green Version]
- Flores, D.J.; Lee, L.H.; Adams, S.D. Inhibition of Curcumin-Treated Herpes Simplex Virus 1 and 2 in Vero Cells. Adv. Microbiol. 2016, 6, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Vitali, D.; Bagri, P.; Wessels, J.M.; Arora, M.; Ganugula, R.; Parikh, A.; Mandur, T.; Felker, A.; Garg, S.; Kumar, M.R.; et al. Curcumin Can Decrease Tissue Inflammation and the Severity of HSV-2 Infection in the Female Reproductive Mucosa. Int. J. Mol. Sci. 2020, 21, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandi, K.; Ramedani, E.; Mohammadi, K.; Tajbakhsh, S.; Deilami, I.; Rastian, Z.; Fouladvand, M.; Yousefi, F.; Farshadpour, F. Evaluation of antiviral activities of curcumin derivatives against HSV-1 in Vero cell line. Nat. Prod. Commun. 2010, 5, 1935–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, C.L.; James, S.H. Antiviral Therapies for Herpesviruses: Current Agents and New Directions. Clin. Ther. 2018, 40, 1282–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannigan, B.M.; Barnett, Y.A.; Armstrong, D.B.; McKelvey-Martin, V.J.; McKenna, P.G. Thymidine kinases: The enzymes and their clinical usefulness. Cancer Biother. 1993, 8, 189–197. [Google Scholar] [CrossRef]
- Fujii, H.; Harada, S.; Yoshikawa, T.; Yamada, S.; Omura, N.; Shibamura, M.; Inagaki, T.; Kato, H.; Fukushi, S.; Saijo, M. Differences in the Likelihood of Acyclovir Resistance-Associated Mutations in the Thymidine Kinase Genes of Herpes Simplex Virus 1 and Varicella-Zoster Virus. Antimicrob. Agents Chemother. 2019, 63, e00017–e00019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coen, N.; Duraffour, S.; Haraguchi, K.; Balzarini, J.; van den Oord, J.J.; Snoeck, R.; Andrei, G. Antiherpesvirus activities of two novel 4′-thiothymidine derivatives, KAY-2-41 and KAH-39-149, are dependent on viral and cellular thymidine kinases. Antimicrob. Agents Chemother. 2014, 58, 4328–4340. [Google Scholar] [CrossRef] [Green Version]
- Topalis, D.; Gillemot, S.; Snoeck, R.; Andrei, G. Thymidine kinase and protein kinase in drug-resistant herpesviruses: Heads of a Lernaean Hydra. Drug Resist. Updat. 2018, 37, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; et al. Alpha-Herpesvirus Thymidine Kinase Genes Mediate Viral Virulence and Are Potential Therapeutic Targets. Front. Microbiol. 2019, 10, 941. [Google Scholar] [CrossRef] [Green Version]
- El-Halim, S.M.A.; Mamdouh, M.A.; El-Haddad, A.E.; Soliman, S.M. Fabrication of Anti-HSV-1 Curcumin Stabilized Nanostructured Proniosomal Gel: Molecular Docking Studies on Thymidine Kinase Proteins. Sci. Pharm. 2020, 88, 9. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Du, H.; Zhang, G.; Wu, Y.; Qiu, P.; Liu, J.; Guo, J.; Liu, X.; Sun, L.; Du, B.; et al. Curcumin plays a synergistic role in combination with HSV-TK/GCV in inhibiting growth of murine B16 melanoma cells and melanoma xenografts. PeerJ 2019, 7, e7760. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.; Baraniak, I.; Reeves, M. The pathogenesis of human cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Dooley, A.L.; O’Connor, C.M. Regulation of the MIE Locus during HCMV Latency and Reactivation. Pathogens 2020, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; An, Z.; Chen, H.; Wang, Z.; and Liu, L. Mechanism of curcumin resistance to human cytomegalovirus in HELF cells. BMC Complement. Altern. Med. 2014, 14, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Y.; Gong, L.; Wang, Z.; Han, F.; Liu, H.; Lu, X.; Liu, L. Curcumin inhibits human cytomegalovirus by downregulating heat shock protein 90. Mol. Med. Rep. 2015, 12, 4789–4793. [Google Scholar] [CrossRef]
- Lv, Y.; Lei, N.; Wang, D.; An, Z.; Li, G.; Han, F.; Liu, H.; Liu, L. Protective effect of curcumin against cytomegalovirus infection in Balb/c mice. Environ. Toxicol. Pharmacol. 2014, 37, 140–147. [Google Scholar] [CrossRef]
- Guito, J.; Lukac, D.M. KSHV Rta Promoter Specification and Viral Reactivation. Front. Microbiol. 2012, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.A.M.; Alfhili, M.A.; Pakala, P.; Simon, S.; Hussain, J.; McCubrey, J.A.; Akula, S.M. miRNAs and their roles in KSHV pathogenesis. Virus Res. 2019, 266, 15–24. [Google Scholar] [CrossRef]
- Zhong, C.; Xu, M.; Wang, Y.; Xu, J.; Yuan, Y. An APE1 inhibitor reveals critical roles of the redox function of APE1 in KSHV replication and pathogenic phenotypes. PLoS Pathog. 2017, 13, e1006289. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Wang, Y.; Yuan, Y. Inhibitors of APE1 redox function effectively inhibit γ-herpesvirus replication in vitro and in vivo. Antivir. Res. 2020, 185, 104985. [Google Scholar] [CrossRef]
- Li, H.; Zhong, C.; Wang, Q.; Chen, W.; Yuan, Y. Curcumin is an APE1 redox inhibitor and exhibits an antiviral activity against KSHV replication and pathogenesis. Antivir. Res. 2019, 167, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T. EBV-Encoded Latent Genes. Adv. Exp. Med. Biol. 2018, 1045, 377–394. [Google Scholar] [PubMed]
- Farina, A.; Cirone, M.; York, M.; Lenna, S.; Padilla, C.; Mclaughlin, S.; Faggioni, A.; Lafyatis, R.; Trojanowska, M.; Farina, G.A. Epstein-Barr virus infection induces aberrant TLR activation pathway and fibroblast-myofibroblast conversion in scleroderma. J. Investig. Dermatol. 2014, 134, 954–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascherio, A.; Munger, K.L. EBV and Autoimmunity. Curr. Top. Microbiol. Immunol. 2015, 390, 365–385. [Google Scholar] [PubMed]
- Hergenhahn, M.; Soto, U.; Weninger, A.; Polack, A.; Hsu, C.H.; Cheng, A.L.; Rosl, F. The chemopreventive compound curcumin is an efficient inhibitor of Epstein-Barr virus BZLF1 transcription in Raji DR-LUC cells. Mol. Carcinog. 2002, 33, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ding, X.; Tao, J.; Wang, J.; Zhao, X.; Zhu, G. Critical role of cholesterol in bovine herpesvirus type 1infection of MDBK cells. Vet. Microbiol. 2010, 144, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.V.; Oliveira, T.M.A.; Bezerra, F.C.; Alonso, L.; Alonso, A.; Borissevitch, I.E.; Gonçalves, P.J.; Souza, G.R.L. Photodynamic inactivation of Bovine herpesvirus type 1 (BoHV-1) by porphyrins. J. Gen. Virol. 2018, 99, 1301–1306. [Google Scholar] [CrossRef]
- Zhu, L.; Ding, X.; Zhang, D.; Yuan, C.; Wang, J.; Ndegwa, E.; Zhu, G. Curcumin inhibits bovine herpesvirus type 1 entry into MDBK cells. Acta Virol. 2015, 59, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Reolon, J.B.; Brustolin, M.; Accarini, T.; Viçozzi, G.P.; Sari, M.H.M.; Bender, E.A.; Haas, S.E.; Brum, M.C.S.; Gündel, A.; Colomé, L.M. Co-encapsulation of acyclovir and curcumin into microparticles improves the physicochemical characteristics and potentiates in vitro antiviral action: Influence of the polymeric composition. Eur. J. Pharm. Sci. 2019, 131, 167–176. [Google Scholar] [CrossRef]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef] [Green Version]
- Freuling, C.M.; Müller, T.F.; Mettenleiter, T.C. Vaccines against pseudorabies virus (PrV). Vet. Microbiol. 2017, 206, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Chen, D.Y.; Wen, H.W.; Ou, J.L.; Chiou, S.S.; Chen, J.M.; Wong, M.L.; Hsu, W.L. Inhibition of enveloped viruses infectivity by curcumin. PLoS ONE 2013, 8, e62482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carty, M.; Guy, C.; Bowie, A.G. Detection of viral infections by innate immunity. Biochem. Pharmacol. 2020, 183, 114316. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- Boyanapalli, S.S.S.; Huang, Y.; Su, Z.; Cheng, D.; Zhang, C.; Guo, Y.; Rao, R.; Androulakis, I.P.; Kong, A.N. Pharmacokinetics and Pharmacodynamics of Curcumin in regulating anti-inflammatory and epigenetic gene expression. Biopharm. Drug Dispos. 2018, 39, 289–297. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 5, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Liu, W.; Zhou, X.; Zhu, X.; Suo, F.; Yao, S. The effectiveness and safety of curcumin as a complementary therapy in inflammatory bowel disease: A protocol of systematic review and meta-analysis. Medicine 2020, 99, e22916. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocaadam, B.; Sanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [Green Version]
- Jennings, M.R.; Parks, R.J. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef]
- Yang, B.; Luo, G.; Zhang, C.; Feng, L.; Luo, X.; Gan, L. Curcumin protects rat hippocampal neurons against pseudorabies virus by regulating the BDNF/TrkB pathway. Sci. Rep. 2020, 10, 22204. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennertz, K.; Joyal, S. A pilot crossover study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J. Pharm. Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Allegri, P.; Mastromarino, A.; Neri, P. Management of chronic anterior uveitis relapses: Efficacy of oral phospholipidic curcumin treatment. Long-term follow-up. Clin. Ophthalmol. 2010, 4, 1201–1206. [Google Scholar]
- Kurita, T.; Makino, Y. Novel curcumin oral delivery systems. Anticancer Res. 2013, 33, 2807–2821. [Google Scholar]
- McFarlin, B.K.; Venable, A.S.; Henning, A.L.; Sampson, J.N.; Pennel, K.; Vingren, J.L.; Hill, D.W. Reduced inflammatory and muscle damage biomarkers following oral supplementation with bioavailable curcumin. BBA Clin. 2016, 5, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Mathew, D.; Hsu, W.L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Li, J.; Xu, Y.; Wang, X.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef]
- Misra, R.; Sahoo, S.K. Coformulation of doxorubicin and curcumin in poly(D, Llactide- co-glycolide) nanoparticles suppresses the development of multidrug resistance in K562 cells. Mol. Pharm. 2011, 8, 852–866. [Google Scholar] [CrossRef]
- Sun, M.; Gao, Y.; Guo, C.Y.; Cao, F.L.; Song, Z.M.; Xi, Y.W.; Yu, A.; Li, A.; Zhai, G. Enhancement of transport of curcumin to brain in mice by poly(n-butylcyanoacrylate) nanoparticle. J. Nanopart. Res. 2010, 12, 3111–3122. [Google Scholar] [CrossRef]
- Onoue, S.; Takahashi, H.; Kawabata, Y.; Seto, Y.; Hatanaka, J.; Timmermann, B.; Yamada, S. Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J. Pharm. Sci. 2010, 99, 1871–1881. [Google Scholar] [CrossRef]
- Gou, M.; Men, K.; Shi, H.; Xiang, M.; Zhang, J.; Song, J.; Long, J.; Wan, Y.; Luo, F.; Zhao, X.; et al. Curcumin loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale 2011, 3, 1558–1567. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Puri, A.; Tele, S.; Blumenthal, R.; Maheshwari, R.K. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int. J. Oncol. 2008, 32, 1119–1123. [Google Scholar] [CrossRef] [Green Version]
- Aboali, F.A.; Habib, D.A.; Elbedaiwy, H.M.; Farid, R.M. Curcumin-loaded proniosomal gel as a biofreindly alternative for treatment of ocular inflammation: In-vitro and in-vivo assessment. Int. J. Pharm. 2020, 589, 119835. [Google Scholar] [CrossRef]
- Van Diemen, F.R.; Kruse, E.M.; Hooykaas, M.J.; Bruggeling, C.E.; Schürch, A.C.; van Ham, P.M.; Imhof, S.M.; Nijhuis, M.; Wiertz, E.J.; Lebbink, R.J. CRISPR/Cas9-Mediated Genome Editing of Herpesviruses Limits Productive and Latent Infections. PLoS Pathog. 2016, 12, e1005701. [Google Scholar] [CrossRef]
- Van Diemen, F.R.; Lebbink, R.J. CRISPR/Cas9, a powerful tool to target human herpesviruses. Cell Microbiol. 2017, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Sheng, J.; Trang, P.; Liu, F. Potential Application of the CRISPR/Cas9 System against Herpesvirus Infections. Viruses 2018, 10, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Type of Study, Test Performed, Virus, and Cells/Animal Model | Results | Mechanism of Action and Pathway | Reference |
|---|---|---|---|
| In vitro. Plaque assays, ChIP assay, and western blot analysis. HSV-1. HeLa and Vero cells. | Inhibition of HSV-1 replication and suppression of IE gene expression. | Curcumin was observed to utilize the mechanism independent of the transcriptional coactivator proteins p300/CBP histone acetyltransferase activity to affect the viral transactivator protein VP16-mediated enlistment of RNA polymerase II to IE gene promoters, leading to suppressing gene expression and blocking viral infection. | [50] |
| In vitro and in vivo. Plaque reduction assay. HSV-2. Primary rabbit kidney cells (in vitro). Guinea pig model (in vivo). | In an in vitro plaque reduction assay, curcumin suppressed the replication of HSV-2 with an ED50 value of 0.32 mg/mL, while at a concentration of 100 mg/mL, the in vivo inhibitory activity was confirmed using a mouse model of genital HSV-2 infection. | The mechanism is unknown. | [51] |
| In vitro. Plaque assay. HSV-2. Human genital epithelial cells. | In primary human genital epithelial cells, pre-treatment of cells with curcumin (5 µM) decreased HSV-2 shedding by 1000-fold and at a concentration of 50 µM, entirely blocked HSV-2 production. | Investigation of the cellular pathways known to be regulated by curcumin involving the transcription factor NF-κB. | [52] |
| In vitro. Plaque assay and virus adsorption assay. HSV-1 and HSV-2. Vero cells. | At a concentration of 30 µM, curcumin inhibited the replication of HSV-1 and HSV-2. | Inhibition of adsorption and replication of HSV-1 and HSV-2. | [53] |
| In vivo. Plaque assay. HSV-2. Genital epithelial cells of female C57BL/6 mice. | Nanoparticle-containing curcumin (0.5 mg) reduced tissue inflammation and the severity of HSV-2 infection in an animal model. | The mechanism of action was detected to be correlated with the anti-inflammatory properties of curcumin. | [54] |
| In vitro. Cytopathic inhibition assay. HSV-1. Vero cells. | Curcumin, gallium-curcumin, and copper-curcumin inhibited the replication of HSV-1 with IC50 values of 33.0, 13.9, and 23.1 µg/mL, respectively. | The mechanisms of action of both gallium-curcumin and copper-curcumin have been suggested to be investigated in further studies. | [55] |
| Herpesvirus and Type of Study | Results | Mechanism of Action and Pathway | Reference |
|---|---|---|---|
| HCMV (in vitro, in vivo, and in silico). | At various concentrations in micromolar ranges, curcumin was detected with anti-HCMV properties. | Inhibition of IEA and UL83A expressions and downregulation of Hsp90. Determination of anti-inflammatory and antioxidant effects as possible mechanisms underlying the anti-HCMV activity. | [66,67,68] |
| KSHV (in vitro). | At various concentrations (in µM), curcumin efficiently inhibited KSHV replication and virus-associated pathogenic properties. | Blocking APE1-mediated redox function. | [73] |
| EBV (in vitro). | Inhibition of EBV reactivation in Raji DR-CAT cells with curcumin treatment (15 µM). | Inhibition of BZLF1 gene transcription. | [77] |
| BoHV-1 (in vitro). | At a concentration of 10 µM, curcumin reduced BoHV-1 titer, leading to inhibiting viral replication. Co-encapsulation of acyclovir and curcumin into three microparticle formulations noticeably reduced the BoVH-1 plaque formation at a concentration of 75 µg/mL. | Inhibition of virus post-binding entry process by upregulating the lipid raft formation. | [80,81] |
| PRV (in vitro). | Treatment with curcumin (30 µM) blocked PRV infectivity in PK-15 cells by decreasing the viral plaque formation. | No mechanism of action was revealed. | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šudomová, M.; Hassan, S.T.S. Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways. Microorganisms 2021, 9, 292. https://doi.org/10.3390/microorganisms9020292
Šudomová M, Hassan STS. Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways. Microorganisms. 2021; 9(2):292. https://doi.org/10.3390/microorganisms9020292
Chicago/Turabian StyleŠudomová, Miroslava, and Sherif T. S. Hassan. 2021. "Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways" Microorganisms 9, no. 2: 292. https://doi.org/10.3390/microorganisms9020292
APA StyleŠudomová, M., & Hassan, S. T. S. (2021). Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways. Microorganisms, 9(2), 292. https://doi.org/10.3390/microorganisms9020292







