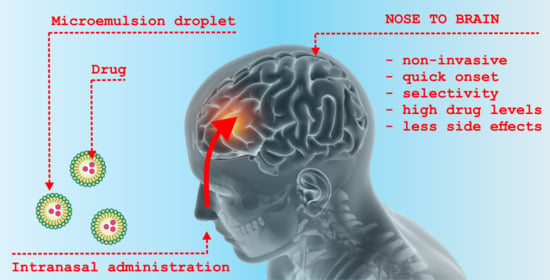
Microemulsion-Based Media in Nose-to-Brain Drug Delivery
Abstract
1. Introduction
2. Microemulsions: Structure, Properties, and Applications
2.1. Definition and Structure
2.2. Formation Process and Microemulsion Stability
2.3. Classification of Microemulsions
- Water-in-oil (W/O) with water as the dispersed phase and oil as the continuous one,
- Oil-in-water (O/W) with oil as the dispersed phase and water as the continuous one,
- Bicontinuous with water and oil forming interpenetrating three-dimensional domains without the possibility to discern internal and external phases.
2.4. Applications
3. Nasal Cavity as Drug Administration Site
3.1. Anatomy and Physiology of Nasal Cavity
3.2. Drug Delivery Pathways
3.2.1. Olfactory Pathway
3.2.2. Trigeminal Nerve Pathway
4. Transnasal Formulations in Brain Targeting
4.1. Neurodegenerative Disorders
4.2. Epilepsy
4.3. Schizophrenia
4.4. Other Applications
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alam, M.I.; Beg, S.; Samad, A.; Baboota, S.; Kohli, K.; Ali, J.; Ahuja, A.; Akbar, M. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 2010, 40, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V. Polymer nanomaterials for drug delivery across the blood brain barrier. In Neuroimmune Pharmacology; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 847–868. ISBN 9783319440224. [Google Scholar]
- Pardridge, W.M. Blood-brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier and neurotherapeutics. NeuroRx 2005, 2, 1–2. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Warnken, Z.N.; Smyth, H.D.C.; Watts, A.B.; Weitman, S.; Kuhn, J.G.; Williams, R.O. Formulation and device design to increase nose to brain drug delivery. J. Drug Deliv. Sci. Technol. 2016, 35, 213–222. [Google Scholar] [CrossRef]
- Krames, E.; Buchser, E.; Hassenbusch, S.J.; Levy, R. Future Trends in the Development of Local Drug Delivery Systems: Intraspinal, Intracerebral, and Intraparenchymal Therapies. Neuromudulation Technol. Neural Interface 2002, 2, 133–148. [Google Scholar] [CrossRef]
- Lewis, O.; Woolley, M.; Johnson, D.; Rosser, A.; Barua, N.U.; Bienemann, A.S.; Gill, S.S.; Evans, S. Chronic, intermittent convection-enhanced delivery devices. J. Neurosci. Methods 2016, 259, 47–56. [Google Scholar] [CrossRef]
- Gliadel HCP Home. Available online: https://gliadel.com/hcp/ (accessed on 1 February 2021).
- Szvalb, A.D.; Raad, I.I.; Weinberg, J.S.; Suki, D.; Mayer, R.; Viola, G.M. Ommaya reservoir-related infections: Clinical manifestations and treatment outcomes. J. Infect. 2014, 68, 216–224. [Google Scholar] [CrossRef]
- Lau, J.C.; Kosteniuk, S.E.; Walker, T.; Iansavichene, A.; Macdonald, D.R.; Megyesi, J.F. Operative complications with and without image guidance: A systematic review and meta-analysis of the Ommaya reservoir literature. World Neurosurg. 2019, 122, 404–414. [Google Scholar] [CrossRef]
- Hitt, J.M.; de Leon-Casasola, O.A. Complications of intrathecal drug delivery systems. Tech. Reg. Anesth. Pain Manag. 2011, 15, 162–166. [Google Scholar] [CrossRef]
- Gao, X.; Yue, Q.; Liu, Y.; Fan, D.; Fan, K.; Li, S.; Qian, J.; Han, L.; Fang, F.; Xu, F.; et al. Image-guided chemotherapy with specifically tuned blood brain barrier permeability in glioma margins. Theranostics 2018, 8, 3126–3137. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Wu, M.T.; Yang, F.Y. Pharmacokinetics of doxorubicin in glioblastoma multiforme following ultrasound-Induced blood-brain barrier disruption as determined by microdialysis. J. Pharm. Biomed. Anal. 2018, 149, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Georgieva, J.V.; Hoekstra, D.; Zuhorn, I.S. Smuggling drugs into the brain: An overview of ligands targeting transcytosis for drug delivery across the blood–brain barrier. Pharmaceutics 2014, 6, 557–583. [Google Scholar] [CrossRef]
- Pardridge, W.M. Delivery of Biologics Across the Blood–Brain Barrier with Molecular Trojan Horse Technology. BioDrugs 2017, 31, 503–519. [Google Scholar] [CrossRef]
- Boado, R.J.; Lu, J.Z.; Hui, E.K.W.; Pardridge, W.M. Insulin receptor antibody-sulfamidase fusion protein penetrates the primate blood-brain barrier and reduces glycosoaminoglycans in sanfilippo type a cells. Mol. Pharm. 2014, 11, 2928–2934. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody. Expert Opin. Drug Deliv. 2015, 12, 207–222. [Google Scholar] [CrossRef]
- Bertrand, Y.; Currie, J.C.; Poirier, J.; Demeule, M.; Abulrob, A.; Fatehi, D.; Stanimirovic, D.; Sartelet, H.; Castaigne, J.P.; Béliveau, R. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br. J. Cancer 2011, 105, 1697–1707. [Google Scholar] [CrossRef]
- Brasnjevic, I.; Steinbusch, H.W.M.; Schmitz, C.; Martinez-Martinez, P. Delivery of peptide and protein drugs over the blood-brain barrier. Prog. Neurobiol. 2009, 87, 212–251. [Google Scholar] [CrossRef]
- Placzek, A.T.; Ferrara, S.J.; Hartley, M.D.; Sanford-Crane, H.S.; Meinig, J.M.; Scanlan, T.S. Sobetirome prodrug esters with enhanced blood–brain barrier permeability. Bioorg. Med. Chem. 2016, 24, 5842–5854. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Jiang, J.; Wang, X.; Fu, Y.; Gong, T.; Sun, X.; Zhang, Z. Mechanism of brain targeting by dexibuprofen prodrugs modified with ethanolamine-related structures. J. Cereb. Blood Flow Metab. 2015, 35, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Xie, Y.; Huang, X.; Kadota, K.; Yao, X.S.; Yu, Y.; Chen, X.; Lu, A.; Yang, Z. Delivering Crocetin across the Blood-Brain Barrier by Using γ-Cyclodextrin to Treat Alzheimer’s Disease. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Sun, Y.; Zhao, H.; Lan, M.; Gao, F.; Song, C.; Lou, K.; Li, H.; Wang, W. A novel lactoferrin-modified β-cyclodextrin nanocarrier for brain-targeting drug delivery. Int. J. Pharm. 2013, 458, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Q.; Lv, Q.; Li, L.M.; Tang, X.J.; Li, F.Z.; Hu, Y.L.; Han, M. Glioma targeting and blood-brain barrier penetration bydual-targeting doxorubincin liposomes. Biomaterials 2013, 34, 5628–5639. [Google Scholar] [CrossRef] [PubMed]
- Rip, J.; Chen, L.; Hartman, R.; van den Heuvel, A.; Reijerkerk, A.; van Kregten, J.; van der Boom, B.; Appeldoorn, C.; de Boer, M.; Maussang, D.; et al. Glutathione PEGylated liposomes: Pharmacokinetics and delivery of cargo across the blood–brain barrier in rats. J. Drug Target. 2014, 22, 460–467. [Google Scholar] [CrossRef]
- Wohlfart, S.; Khalansky, A.S.; Gelperina, S.; Begley, D.; Kreuter, J. Kinetics of transport of doxorubicin bound to nanoparticles across the blood-brain barrier. J. Control. Release 2011, 154, 103–107. [Google Scholar] [CrossRef]
- Zhang, Y.; Walker, J.B.; Minic, Z.; Liu, F.; Goshgarian, H.; Mao, G. Transporter protein and drug-conjugated gold nanoparticles capable of bypassing the blood-brain barrier. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Nanotechnology-mediated nose to brain drug delivery for Parkinson’s disease: A mini review. J. Drug Target. 2015, 23, 775–788. [Google Scholar] [CrossRef]
- Tzeyung, A.; Md, S.; Bhattamisra, S.; Madheswaran, T.; Alhakamy, N.; Aldawsari, H.; Radhakrishnan, A. Fabrication, Optimization, and Evaluation of Rotigotine-Loaded Chitosan Nanoparticles for Nose-To-Brain Delivery. Pharmaceutics 2019, 11, 26. [Google Scholar] [CrossRef]
- Katona, G.; Balogh, G.T.; Dargó, G.; Gáspár, R.; Márki, Á.; Ducza, E.; Sztojkov-Ivanov, A.; Tömösi, F.; Kecskeméti, G.; Janáky, T.; et al. Development of Meloxicam-Human Serum Albumin Nanoparticles for Nose-to-Brain Delivery via Application of a Quality by Design Approach. Pharmaceutics 2020, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Alonso, M.J. Nose-to-brain peptide delivery—The potential of nanotechnology. Bioorg. Med. Chem. 2018, 26, 2888–2905. [Google Scholar] [CrossRef] [PubMed]
- Lalatsa, A.; Schatzlein, A.G.; Uchegbu, I.F. Strategies to deliver peptide drugs to the brain. Mol. Pharm. 2014, 11, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for “nose-to-brain” drug delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Seju, U.; Kumar, A.; Sawant, K.K. Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: In vitro and in vivo studies. Acta Biomater. 2011, 7, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.A.H.A.; Kassem, A.A.; Farid, R.M.; Ismail, F.A.; EL-Massik, M.A.E.; Boraie, N.A. A novel nasal almotriptan loaded solid lipid nanoparticles in mucoadhesive in situ gel formulation for brain targeting: Preparation, characterization and in vivo evaluation. Int. J. Pharm. 2018, 548, 609–624. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Z.; Liu, M.; Tao, Y.; Li, Z.; Wu, Z.; Gui, S. Facile nose-to-brain delivery of rotigotine-loaded polymer micelles thermosensitive hydrogels: In vitro characterization and in vivo behavior study. Int. J. Pharm. 2020, 577, 119046. [Google Scholar] [CrossRef]
- Wavikar, P.R.; Vavia, P.R. Rivastigmine-loaded in situ gelling nanostructured lipid carriers for nose to brain delivery. J. Liposome Res. 2015, 25, 141–149. [Google Scholar] [CrossRef]
- Rinaldi, F.; Oliva, A.; Sabatino, M.; Imbriano, A.; Hanieh, P.N.; Garzoli, S.; Mastroianni, C.M.; De Angelis, M.; Miele, M.C.; Arnaut, M.; et al. Antimicrobial Essential Oil Formulation: Chitosan Coated Nanoemulsions for Nose to Brain Delivery. Pharmaceutics 2020, 12, 678. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Formulation and In-vivo Pharmacokinetic Consideration of Intranasal Microemulsion and Mucoadhesive Microemulsion of Rivastigmine for Brain Targeting. Pharm. Res. 2018, 35, 1–10. [Google Scholar] [CrossRef]
- Hoar, T.P.; Schulman, J.H. Transparent water-in-oil dispersions: The oleopathic hydro-micelle. Nature 1943, 152, 102–103. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Sintov, A.C.; Botner, S. Transdermal drug delivery using microemulsion and aqueous systems: Influence of skin storage conditions on the in vitro permeability of diclofenac from aqueous vehicle systems. Int. J. Pharm. 2006, 311, 55–62. [Google Scholar] [CrossRef] [PubMed]
- He, C.X.; He, Z.G.; Gao, J.Q. Microemulsions as drug delivery systems to improve the solubility and the bioavailability of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2010, 7, 445–460. [Google Scholar] [CrossRef]
- Yin, Y.M.; Cui, F.D.; Mu, C.F.; Choi, M.K.; Kim, J.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Docetaxel microemulsion for enhanced oral bioavailability: Preparation and in vitro and in vivo evaluation. J. Control. Release 2009, 140, 86–94. [Google Scholar]
- Gannu, R.; Palem, C.R.; Yamsani, V.V.; Yamsani, S.K.; Yamsani, M.R. Enhanced bioavailability of lacidipine via microemulsion based transdermal gels: Formulation optimization, ex vivo and in vivo characterization. Int. J. Pharm. 2010, 388, 231–241. [Google Scholar] [CrossRef]
- Hu, L.; Jia, Y.; Niu, F.; Jia, Z.; Yang, X.; Jiao, K. Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. J. Agric. Food Chem. 2012, 60, 7137–7141. [Google Scholar] [CrossRef]
- Kesavan, K.; Kant, S.; Singh, P.N.; Pandit, J.K. Mucoadhesive Chitosan-Coated Cationic Microemulsion of Dexamethasone for Ocular Delivery: In Vitro and In Vivo Evaluation. Curr. Eye Res. 2013, 38, 342–352. [Google Scholar] [CrossRef]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Abrar, I.; Bhaskarwar, A.N. Microemulsion fuels for compression ignition engines: A review on engine performance and emission characteristics. Fuel 2019, 257, 115944. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal nanoemulsions for direct nose-to-brain delivery of actives for cns disorders. Pharmaceutics 2020, 12, 1230. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, F.Y.; Hung, D.K. Terpene microemulsions for transdermal curcumin delivery: Effects of terpenes and cosurfactants. Colloids Surf. B Biointerfaces 2011, 82, 63–70. [Google Scholar] [CrossRef]
- Dizaj, S.M. Preparation and study of vitamin A palmitate microemulsion drug delivery system and investigation of co-surfactant effect. J. Nanostruct. Chem. 2013, 3, 1–6. [Google Scholar]
- El Khayat, N.W.; Donia, A.A.; Mady, O.Y.; El Maghraby, G.M. Optimization of eugenol microemulsion for transdermal delivery of indomethacin. J. Drug Deliv. Sci. Technol. 2018, 48, 311–318. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Formation of flavor oil microemulsions, nanoemulsions and emulsions: Influence of composition and preparation method. J. Agric. Food Chem. 2011, 59, 5026–5035. [Google Scholar] [CrossRef]
- Eastoe, J.; Hatzopoulos, M.H.; Tabor, R. Microemulsions. In Encyclopedia of Colloid and Interface Science; Springer: Berlin/Heidelberg, Germany, 2013; pp. 688–729. [Google Scholar]
- Gautam, N.; Kesavan, K. Development of microemulsions for ocular delivery. Ther. Deliv. 2017, 8, 313–330. [Google Scholar] [CrossRef]
- Winsor, P.A. Hydrotropy, solubilisation and related Emulsification processes. Part I. Trans. Faraday Soc. 1948, 44, 376–398. [Google Scholar] [CrossRef]
- Solanki, J.N.; Sengupta, R.; Murthy, Z.V.P. Synthesis of copper sulphide and copper nanoparticles with microemulsion method. Solid State Sci. 2010, 12, 1560–1566. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, R.A.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Cabrera, C.R.; Feliu, J.M. Synthesis of Pt nanoparticles in water-in-oil microemulsion: Effect of HCl on their surface structure. J. Am. Chem. Soc. 2014, 136, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Wang, A.; Li, X.; Wang, G.; Zhao, L. Synthesis of ZnO nanoparticles from microemulsions in a flow type microreactor. Chem. Eng. J. 2014, 235, 191–197. [Google Scholar] [CrossRef]
- Mishra, S.; Chatterjee, A. Novel synthesis of polymer and copolymer nanoparticles by atomized microemulsion technique and its characterization. Polym. Adv. Technol. 2011, 22, 1593–1601. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Y.; Pan, M.; Rempel, G.L.; Pan, Q. Synthesis of poly(methyl methacrylate) nanoparticles via differential microemulsion polymerization. Eur. Polym. J. 2013, 49, 41–48. [Google Scholar] [CrossRef]
- Ovando-Medina, V.M.; Peralta, R.D.; Mendizábal, E.; Martínez-Gutiérrez, H.; Lara-Ceniceros, T.E.; Ledezma-Rodríguez, R. Synthesis of polypyrrole nanoparticles by oil-in-water microemulsion polymerization with narrow size distribution. Colloid Polym. Sci. 2011, 289, 759–765. [Google Scholar] [CrossRef]
- Asgari, S.; Saberi, A.H.; McClements, D.J.; Lin, M. Microemulsions as nanoreactors for synthesis of biopolymer nanoparticles. Trends Food Sci. Technol. 2019, 86, 118–130. [Google Scholar] [CrossRef]
- Schwarze, M.; Pogrzeba, T.; Volovych, I.; Schomäcker, R. Microemulsion systems for catalytic reactions and processes. Catal. Sci. Technol. 2015, 5, 24–33. [Google Scholar] [CrossRef]
- Watarai, H. Microemulsion Capillary Electrophoresis. Chem. Lett. 1991, 20, 391–394. [Google Scholar] [CrossRef]
- Yu, L.; Chu, K.; Ye, H.; Liu, X.; Yu, L.; Xu, X.; Chen, G. Recent advances in microemulsion electrokinetic chromatography. TrAC Trends Anal. Chem. 2012, 34, 140–151. [Google Scholar] [CrossRef]
- Ryan, R.; Altria, K.; McEvoy, E.; Donegan, S.; Power, J. A review of developments in the methodology and application of microemulsion electrokinetic chromatography. Electrophoresis 2013, 34, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Nazar, M.F.; Shah, S.S.; Khosa, M.A. Microemulsions in enhanced oil recovery: A review. Pet. Sci. Technol. 2011, 29, 1353–1365. [Google Scholar] [CrossRef]
- Klier, J.; Tucker, C.J.; Kalantar, T.H.; Green, D.P. Properties and applications of microemulsions. Adv. Mater. 2000, 12, 1751–1757. [Google Scholar] [CrossRef]
- Baglioni, M.; Jàidar Benavides, Y.; Berti, D.; Giorgi, R.; Keiderling, U.; Baglioni, P. An amine-oxide surfactant-based microemulsion for the cleaning of works of art. J. Colloid Interface Sci. 2015, 440, 204–210. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]
- Wan, T.; Xu, T.; Pan, J.; Qin, M.; Pan, W.; Zhang, G.; Wu, Z.; Wu, C.; Xu, Y. Microemulsion based gel for topical dermal delivery of pseudolaric acid B: In vitro and in vivo evaluation. Int. J. Pharm. 2015, 493, 111–120. [Google Scholar] [CrossRef]
- Pajic, N.B.; Nikolic, I.; Mitsou, E.; Papadimitriou, V.; Xenakis, A.; Randjelovic, D.; Dobricic, V.; Smitran, A.; Cekic, N.; Calija, B.; et al. Biocompatible microemulsions for improved dermal delivery of sertaconazole nitrate: Phase behavior study and microstructure influence on drug biopharamaceutical properties. J. Mol. Liq. 2018, 272, 746–758. [Google Scholar] [CrossRef]
- Lopes, L. Overcoming the Cutaneous Barrier with Microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef]
- Subongkot, T.; Sirirak, T. Development and skin penetration pathway evaluation of microemulsions for enhancing the dermal delivery of celecoxib. Colloids Surf. B Biointerfaces 2020, 193, 111103. [Google Scholar] [CrossRef]
- Talaat, S.M.; Elnaggar, Y.S.R.; Abdalla, O.Y. Lecithin Microemulsion Lipogels Versus Conventional Gels for Skin Targeting of Terconazole: In Vitro, Ex Vivo, and In Vivo Investigation. AAPS PharmSciTech 2019, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol. Physiol. 2008, 21, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Censi, R.; Martena, V.; Hoti, E.; Malaj, L.; Di Martino, P. Permeation and skin retention of quercetin from microemulsions containing Transcutol® P. Drug Dev. Ind. Pharm. 2012, 38, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Zhai, Y.; Wang, Z.; Liu, J.; Zhai, G. Ropivacaine loaded microemulsion and microemulsion-based gel for transdermal delivery: Preparation, optimization, and evaluation. Int. J. Pharm. 2014, 477, 47–56. [Google Scholar] [CrossRef]
- Shinde, U.A.; Modani, S.H.; Singh, K.H. Design and Development of Repaglinide Microemulsion Gel for Transdermal Delivery. AAPS PharmSciTech 2018, 19, 315–325. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Tomšič, M.; Podlogar, F.; Gašperlin, M.; Bešter-Rogač, M.; Jamnik, A. Water-Tween 40®/Imwitor 308®-isopropyl myristate microemulsions as delivery systems for ketoprofen: Small-angle X-ray scattering study. Int. J. Pharm. 2006, 327, 170–177. [Google Scholar] [CrossRef]
- Hu, L.; Yang, J.; Liu, W.; Li, L. Preparation and evaluation of ibuprofen-loaded microemulsion for improvement of oral bioavailability. Drug Deliv. 2011, 18, 90–95. [Google Scholar] [CrossRef]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS)—Challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar]
- Yeom, D.W.; Son, H.Y.; Kim, J.H.; Kim, S.R.; Lee, S.G.; Song, S.H.; Chae, B.R.; Choi, Y.W. Development of a solidified self-microemulsifying drug delivery system (S-SMEDDS) for atorvastatin calcium with improved dissolution and bioavailability. Int. J. Pharm. 2016, 506, 302–311. [Google Scholar] [CrossRef]
- Kollipara, S.; Gandhi, R.K. Pharmacokinetic aspects and in vitro–in vivo correlation potential for lipid-based formulations. Acta Pharm. Sin. B 2014, 4, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Nagarsenker, M.S. Parenteral microemulsions: An overview. Int. J. Pharm. 2008, 355, 19–30. [Google Scholar] [CrossRef]
- Vandamme, T.F. Microemulsions as ocular drug delivery systems: Recent developments and future challenges. Prog. Retin. Eye Res. 2002, 21, 15–34. [Google Scholar] [CrossRef]
- Üstündaǧ Okur, N.; Çaǧlar, E.Ş.; Siafaka, P.I. Novel Ocular Drug Delivery Systems: An Update on Microemulsions. J. Ocul. Pharmacol. Ther. 2020, 36, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Deng, W.; Yang, H.; Chen, X.; Jin, F. A propofol microemulsion with low free propofol in the aqueous phase: Formulation, physicochemical characterization, stability and pharmacokinetics. Int. J. Pharm. 2012, 436, 536–544. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Hu, N.; Koolivand, A.; Fan, X.; Zhu, Y.; Domszy, R.; Yang, J.; Yang, A.; Wang, N.S. Effect of a Cationic Surfactant on Microemulsion Globules and Drug Release from Hydrogel Contact Lenses. Pharmaceutics 2019, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Gizurarson, S. Anatomical and Histological Factors Affecting Intranasal Drug and Vaccine Delivery. Curr. Drug Deliv. 2012, 9, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef]
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Battaglia, L.; Panciani, P.P.; Muntoni, E.; Capucchio, M.T.; Biasibetti, E.; De Bonis, P.; Mioletti, S.; Fontanella, M.; Swaminathan, S. Lipid nanoparticles for intranasal administration: Application to nose-to-brain delivery. Expert Opin. Drug Deliv. 2018, 15, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [PubMed]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective-a review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Gänger, S.; Schindowski, K. Tailoring formulations for intranasal nose-to-brain delivery: A review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Grassin-Delyle, S.; Buenestado, A.; Naline, E.; Faisy, C.; Blouquit-Laye, S.; Couderc, L.J.; Le Guen, M.; Fischler, M.; Devillier, P. Intranasal drug delivery: An efficient and non-invasive route for systemic administration—Focus on opioids. Pharmacol. Ther. 2012, 134, 366–379. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Lötsch, J.; Walter, C.; Parnham, M.J.; Oertel, B.G.; Geisslinger, G. Pharmacokinetics of non-intravenous formulations of fentanyl. Clin. Pharmacokinet. 2013, 52, 23–36. [Google Scholar] [CrossRef]
- Panagiotou, I.; Mystakidou, K. Intranasal fentanyl: From pharmacokinetics and bioavailability to current treatment applications. Expert Rev. Anticancer Ther. 2010, 10, 1009–1021. [Google Scholar] [CrossRef]
- Haschke, M.; Suter, K.; Hofmann, S.; Witschi, R.; Fröhlich, J.; Imanidis, G.; Drewe, J.; Briellmann, T.A.; Dussy, F.E.; Krähenbühl, S.; et al. Pharmacokinetics and pharmacodynamics of nasally delivered midazolam. Br. J. Clin. Pharmacol. 2010, 69, 607–616. [Google Scholar] [CrossRef]
- Veldhorst-Janssen, N.M.L.; Fiddelers, A.A.A.; van der Kuy, P.H.M.; Theunissen, H.M.S.; de Krom, M.C.T.F.M.; Neef, C.; Marcus, M.A.E. Pharmacokinetics and Tolerability of Nasal Versus Intravenous Midazolam in Healthy Dutch Volunteers: A Single-Dose, Randomized-Sequence, Open-Label, 2-Period Crossover Pilot Study. Clin. Ther. 2011, 33, 2022–2028. [Google Scholar] [CrossRef]
- Davis, S.S.; Illum, L. Absorption Enhancers for Nasal Drug Delivery. Clin. Pharmacokinet. 2003, 42, 1107–1128. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Kher, G. Drug Delivery Systems from Nose to Brain. Curr. Pharm. Biotechnol. 2012, 13, 2355–2379. [Google Scholar] [PubMed]
- Malerba, F.; Paoletti, F.; Capsoni, S.; Cattaneo, A. Intranasal delivery of therapeutic proteins for neurological diseases. Expert Opin. Drug Deliv. 2011, 8, 1277–1296. [Google Scholar] [CrossRef] [PubMed]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, Y.; Chen, J.; Gao, X.; Feng, C.; Wang, L.; Zhang, Q.; Jiang, X. Nose-to-brain transport pathways of wheat germ agglutinin conjugated PEG-PLA nanoparticles. Pharm. Res. 2012, 29, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Landis, M.S.; Boyden, T.; Pegg, S. Nasal-to-CNS drug delivery: Where are we now and where are we heading? An industrial perspective. Ther. Deliv. 2012, 3, 195–208. [Google Scholar] [CrossRef]
- Johnson, N.J.; Hanson, L.R.; Frey, W.H. Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol. Pharm. 2010, 7, 884–893. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Kellohen, K.L.; Ronaldson, P.T.; Davis, T.P. Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- De Oliveira Junior, E.R.; Nascimento, T.L.; Salomão, M.A.; da Silva, A.C.G.; Valadares, M.C.; Lima, E.M. Increased Nose-to-Brain Delivery of Melatonin Mediated by Polycaprolactone Nanoparticles for the Treatment of Glioblastoma. Pharm. Res. 2019, 36, 1–10. [Google Scholar] [CrossRef]
- Yasir, M.; Sara, U.V.S. Solid lipid nanoparticles for nose to brain delivery of haloperidol: In vitro drug release and pharmacokinetics evaluation. Acta Pharm. Sin. B 2014, 4, 454–463. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Mahajan, M.S.; Nerkar, P.P.; Agrawal, A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014, 21, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.M.; Vyas, T.K.; Campbell, R.B.; Amiji, M.M.; Waszczak, B.L. Brain delivery of proteins by the intranasal route of administration: A comparison of cationic liposomes versus aqueous solution formulations. J. Pharm. Sci. 2010, 99, 1745–1761. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Patil, K.; Bobade, N.; Yeole, P.; Gaikwad, R. Formulation of intranasal mucoadhesive temperature-mediated in situ gel containing ropinirole and evaluation of brain targeting efficiency in rats. J. Drug Target. 2010, 18, 223–234. [Google Scholar] [CrossRef]
- Abdou, E.M.; Kandil, S.M.; Miniawy, H.M.F.E. Brain targeting efficiency of antimigrain drug loaded mucoadhesive intranasal nanoemulsion. Int. J. Pharm. 2017, 529, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Zakaria, N.F.B.; Tilang, P.A.B.; Tzeyung, A.S.; Pandey, M.; Chatterjee, B.; Alhakamy, N.A.; Bhattamishra, S.K.; Kesharwani, P.; Gorain, B.; et al. Formulation development and evaluation of rotigotine mucoadhesive nanoemulsion for intranasal delivery. J. Drug Deliv. Sci. Technol. 2019, 54, 101301. [Google Scholar] [CrossRef]
- What Are Neurological Disorders? Available online: https://www.who.int/news-room/q-a-detail/what-are-neurological-disorders (accessed on 8 December 2020).
- Dorsey, E.R.; Bloem, B.R. The Parkinson pandemic—A call to action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef]
- Schizophrenia. Available online: https://www.who.int/news-room/fact-sheets/detail/schizophrenia (accessed on 8 December 2020).
- Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 8 December 2020).
- Md, S.; Bhattmisra, S.K.; Zeeshan, F.; Shahzad, N.; Mujtaba, M.A.; Srikanth Meka, V.; Radhakrishnan, A.; Kesharwani, P.; Baboota, S.; Ali, J. Nano-carrier enabled drug delivery systems for nose to brain targeting for the treatment of neurodegenerative disorders. J. Drug Deliv. Sci. Technol. 2018, 43, 295–310. [Google Scholar] [CrossRef]
- Wen, M.M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. J. Control. Release 2017, 245, 95–107. [Google Scholar] [CrossRef]
- Alzheimer’s Disease | Exelon® Patch (Rivastigmine Transdermal System). Available online: https://www.exelonpatch.com/index.jsp (accessed on 9 December 2020).
- Shah, B.M.; Misra, M.; Shishoo, C.J.; Padh, H. Nose to brain microemulsion-based drug delivery system of rivastigmine: Formulation and ex-vivo characterization. Drug Deliv. 2015, 22, 918–930. [Google Scholar] [CrossRef]
- Khunt, D.; Polaka, S.; Shrivas, M.; Misra, M. Biodistribution and amyloid beta induced cell line toxicity study of intranasal Rivastigmine microemulsion enriched with Fish Oil and Butter oil. J. Drug Deliv. Sci. Technol. 2020, 57, 101661. [Google Scholar] [CrossRef]
- Katdare, A.; Khunt, D.; Thakkar, S.; Polaka, S.N.; Misra, M. Comparative evaluation of fish oil and butter oil in modulating delivery of galantamine hydrobromide to brain via intranasal route: Pharmacokinetic and oxidative stress studies. Drug Deliv. Transl. Res. 2020, 10, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Khunt, D.; Shrivas, M.; Polaka, S.; Gondaliya, P.; Misra, M. Role of Omega-3 Fatty Acids and Butter Oil in Targeting Delivery of Donepezil Hydrochloride Microemulsion to Brain via the Intranasal Route: A Comparative Study. AAPS PharmSciTech 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jogani, V.V.; Shah, P.J.; Mishra, P.; Mishra, A.K.; Misra, A.R. Intranasal Mucoadhesive Microemulsion of Tacrine to Improve Brain Targeting. Alzheimer Dis. Assoc. Disord. 2008, 22, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.C.; Vacacela, M.; Clares, B.; Garcia, M.L.; Fabrega, M.J.; Calpena, A.C. Development of a nasal donepezil-loaded microemulsion for treatment of Alzheimer’s disease: In vitro and ex vivo characterization. CNS Neurol. Disord. Drug Targets 2018, 17, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, G.; Hu, R.; Chen, S.; Lu, W.; Gao, S.; Xia, H.; Wang, B.; Sun, C.; Nie, X.; et al. A Nasal Temperature and pH Dual-Responsive In Situ Gel Delivery System Based on Microemulsion of Huperzine A: Formulation, Evaluation, and In Vivo Pharmacokinetic Study. AAPS PharmSciTech 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, M.; Kumar, P.; Vikram, V.; Mishra, N. Development and characterization of morin hydrate loaded microemulsion for the management of Alzheimer’s disease. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1620–1630. [Google Scholar] [CrossRef]
- Nasr, M.; Wahdan, S.A. Neuroprotective effects of novel nanosystems simultaneously loaded with vinpocetine and piracetam after intranasal administration. Life Sci. 2019, 226, 117–129. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, S.D.; Chuttani, K.; Sawant, K.K.; Subudhi, B.B. Design and evaluation of mucoadhesive microemulsion for neuroprotective effect of ibuprofen following intranasal route in the MPTP mice model. Drug Dev. Ind. Pharm. 2016, 42, 1340–1350. [Google Scholar] [CrossRef]
- Florence, K.; Manisha, L.; Kumar, B.A.; Ankur, K.; Kumar, M.A.; Ambikanandan, M. Intranasal clobazam delivery in the treatment of status epilepticus. J. Pharm. Sci. 2011, 100, 692–703. [Google Scholar] [CrossRef]
- Shringarpure, M.; Gharat, S.; Momin, M.; Omri, A. Management of epileptic disorders using nanotechnology-based strategies for nose-to-brain drug delivery. Expert Opin. Drug Deliv. 2020. [Google Scholar] [CrossRef]
- Feas, D.A.; Igartúa, D.E.; Calienni, M.N.; Martinez, C.S.; Pifano, M.; Chiaramoni, N.S.; del Valle Alonso, S.; Prieto, M.J. Nutraceutical emulsion containing valproic acid (NE-VPA): A drug delivery system for reversion of seizures in zebrafish larvae epilepsy model. J. Pharm. Investig. 2017, 47, 429–437. [Google Scholar] [CrossRef]
- Halliday, A.J.; Moulton, S.E.; Wallace, G.G.; Cook, M.J. Novel methods of antiepileptic drug delivery—Polymer-based implants. Adv. Drug Deliv. Rev. 2012, 64, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Holsti, M.; Dudley, N.; Schunk, J.; Adelgais, K.; Greenberg, R.; Olsen, C.; Healy, A.; Firth, S.; Filloux, F. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Arch. Pediatr. Adolesc. Med. 2010, 164, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Riss, J.; Cloyd, J.; Gates, J.; Collins, S. Benzodiazepines in epilepsy: Pharmacology and pharmacokinetics. Acta Neurol. Scand. 2008, 118, 69–86. [Google Scholar] [CrossRef]
- Shah, V.; Sharma, M.; Pandya, R.; Parikh, R.K.; Bharatiya, B.; Shukla, A.; Tsai, H.C. Quality by Design approach for an in situ gelling microemulsion of Lorazepam via intranasal route. Mater. Sci. Eng. C 2017, 75, 1231–1241. [Google Scholar] [CrossRef]
- Ramreddy, S.; Janapareddi, K. Brain targeting of chitosan-based diazepam mucoadhesive microemulsions via nasal route: Formulation optimization, characterization, pharmacokinetic and pharmacodynamic evaluation. Drug Dev. Ind. Pharm. 2019, 45, 147–158. [Google Scholar] [CrossRef]
- Acharya, S.P.; Pundarikakshudu, K.; Panchal, A.; Lalwani, A. Development of carbamazepine transnasal microemulsion for treatment of epilepsy. Drug Deliv. Transl. Res. 2013, 3, 252–259. [Google Scholar] [CrossRef]
- Acharya, S.P.; Pundarikakshudu, K.; Panchal, A.; Lalwani, A. Preparation and evaluation of transnasal microemulsion of carbamazepine. Asian J. Pharm. Sci. 2013, 8, 64–70. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Bhatt, K.K.; Patel, B.G. Formulation consideration and characterization of microemulsion drug delivery system for transnasal administration of carbamazepine. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 243–253. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Bhatt, K.K.; Patel, B.G.; Gaikwad, R.V. Microemulsion-based drug delivery system for transnasal delivery of Carbamazepine: Preliminary brain-targeting study. Drug Deliv. 2016, 23, 207–213. [Google Scholar] [CrossRef]
- De Oliveira, E.G.; Cardoso, A.M.; Paese, K.; Coradini, K.; de Oliveira, C.V.; Pohlmann, A.R.; Oliveira, M.S.; Guterres, S.S.; Beck, R.C.R. Reconstituted spray-dried phenytoin-loaded nanocapsules improve the in vivo phenytoin anticonvulsant effect and the survival time in mice. Int. J. Pharm. 2018, 551, 121–132. [Google Scholar] [CrossRef]
- Acharya, S.P.; Pundarikakshudu, K.; Upadhyay, P.; Shelat, P.; Lalwani, A. Development of phenytoin intranasal microemulsion for treatment of epilepsy. J. Pharm. Investig. 2015, 45, 375–384. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Non-invasive intranasal delivery of quetiapine fumarate loaded microemulsion for brain targeting: Formulation, physicochemical and pharmacokinetic consideration. Eur. J. Pharm. Sci. 2016, 91, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Bumb, J.M.; Enning, F.; Leweke, F.M. Drug repurposing and emerging adjunctive treatments for schizophrenia. Expert Opin. Pharmacother. 2015, 16, 1049–1067. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Amatya, S.; Kim, M.S.; Park, J.H.; Seol, E.; Lee, H.; Shin, Y.H.; Na, D.H. Long-acting injectable formulations of antipsychotic drugs for the treatment of schizophrenia. Arch. Pharm. Res. 2013, 36, 651–659. [Google Scholar] [CrossRef]
- Tan, M.S.A.; Parekh, H.S.; Pandey, P.; Siskind, D.J.; Falconer, J.R. Nose-to-brain delivery of antipsychotics using nanotechnology: A review. Expert Opin. Drug Deliv. 2020, 17, 839–853. [Google Scholar] [CrossRef]
- Katare, Y.K.; Piazza, J.E.; Bhandari, J.; Daya, R.P.; Akilan, K.; Simpson, M.J.; Hoare, T.; Mishra, R.K. Intranasal delivery of antipsychotic drugs. Schizophr. Res. 2017, 184, 2–13. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Bhatt, K.K.; Patel, B.G.; Gaikwad, R.V. Evaluation of brain targeting efficiency of intranasal microemulsion containing olanzapine: Pharmacodynamic and pharmacokinetic consideration. Drug Deliv. 2016, 23, 307–315. [Google Scholar] [CrossRef]
- Natarajan, J.; Baskaran, M.; Humtsoe, L.C.; Vadivelan, R.; Justin, A. Enhanced brain targeting efficacy of Olanzapine through solid lipid nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017, 45, 364–371. [Google Scholar] [CrossRef]
- Gadhave, D.; Choudhury, H.; Kokare, C. Neutropenia and leukopenia protective intranasal olanzapine-loaded lipid-based nanocarriers engineered for brain delivery. Appl. Nanosci. 2019, 9, 151–168. [Google Scholar] [CrossRef]
- Agrawal, M.B.; Patel, M.M. Optimization and in vivo evaluation of quetiapine-loaded transdermal drug delivery system for the treatment of schizophrenia. Drug Dev. Ind. Pharm. 2020, 46, 1819–1831. [Google Scholar] [CrossRef]
- Khunt, D.; Shah, B.; Misra, M. Role of butter oil in brain targeted delivery of Quetiapine fumarate microemulsion via intranasal route. J. Drug Deliv. Sci. Technol. 2017, 40, 11–20. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Bhatt, K.K.; Patel, B.G.; Gaikwad, R.V. Paliperidone microemulsion for nose-to-brain targeted drug delivery system: Pharmacodynamic and pharmacokinetic evaluation. Drug Deliv. 2016, 23, 346–354. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Bhatt, K.K.; Patel, B.G. Paliperidone-loaded mucoadhesive microemulsion in treatment of schizophrenia: Formulation consideration. J. Pharm. Innov. 2013, 8, 195–204. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Bhatt, K.K.; Patel, B.G. Risperidone-loaded mucoadhesive microemulsion for intranasal delivery: Formulation development, physicochemical characterization and ex vivo evaluation. J. Drug Deliv. Sci. Technol. 2013, 23, 561–567. [Google Scholar] [CrossRef]
- Patel, M.R.; Hirani, S.N.; Patel, R.B. Microemulsion for nasal delivery of Asenapine maleate in treatment of schizophrenia: Formulation considerations. J. Pharm. Investig. 2018, 48, 301–312. [Google Scholar]
- Ayoub, A.M.; Ibrahim, M.M.; Abdallah, M.H.; Mahdy, M.A. Sulpiride microemulsions as antipsychotic nasal drug delivery systems: In-vitro and pharmacodynamic study. J. Drug Deliv. Sci. Technol. 2016, 36, 10–22. [Google Scholar] [CrossRef]
- Lalani, J.; Baradia, D.; Lalani, R.; Misra, A. Brain targeted intranasal delivery of tramadol: Comparative study of microemulsion and nanoemulsion. Pharm. Dev. Technol. 2015, 20, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Bshara, H.; Osman, R.; Mansour, S.; El-Shamy, A.E.H.A. Chitosan and cyclodextrin in intranasal microemulsion for improved brain buspirone hydrochloride pharmacokinetics in rats. Carbohydr. Polym. 2014, 99, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Loane, C.; Politis, M. Buspirone: What is it all about? Brain Res. 2012, 1461, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tosymra (Sumatriptan Nasal Spray) 10 mg | Patient. Available online: https://www.tosymra.com/ (accessed on 21 January 2021).
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Migraine: Experimental models and novel therapeutic approaches. Int. J. Mol. Sci. 2019, 20, 2932. [Google Scholar] [CrossRef] [PubMed]
- Vyas, T.K.; Babbar, A.K.; Sharma, R.K.; Singh, S.; Misra, A. Preliminary brain-targeting studies on intranasal mucoadhesive microemulsions of sumatriptan. AAPS PharmSciTech 2006, 7, E49–E57. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Wang, A.; Ni, L.; Yan, X.; Song, Y.; Zhao, M.; Sun, K.; Mu, H.; Liu, S.; Wu, Z.; et al. Nose-to-brain delivery of temozolomide-loaded PLGA nanoparticles functionalized with anti-EPHA3 for glioblastoma targeting. Drug Deliv. 2018, 25, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.; Gorain, B.; Tagalpallewar, A.; Kokare, C. Intranasal teriflunomide microemulsion: An improved chemotherapeutic approach in glioblastoma. J. Drug Deliv. Sci. Technol. 2019, 51, 276–289. [Google Scholar] [CrossRef]
- Mena-Hernández, J.; Jung-Cook, H.; Llaguno-Munive, M.; García-López, P.; Ganem-Rondero, A.; López-Ramírez, S.; Barragán-Aroche, F.; Rivera-Huerta, M.; Mayet-Cruz, L. Preparation and Evaluation of Mebendazole Microemulsion for Intranasal Delivery: An Alternative Approach for Glioblastoma Treatment. AAPS PharmSciTech 2020, 21, 264. [Google Scholar] [CrossRef]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef]
- Van Den Berg, M.P.; Merkus, P.; Romeijn, S.G.; Verhoef, J.C.; Merkus, F.W.H.M. Uptake of melatonin into the cerebrospinal fluid after nasal and intravenous delivery: Studies in rats and comparison with a human study. Pharm. Res. 2004, 21, 799–802. [Google Scholar] [CrossRef]
- Van Den Berg, M.P.; Merkus, P.; Romeijn, S.G.; Verhoef, J.C.; Merkus, F.W.H.M. Hydroxocobalamin uptake into the cerebrospinal fluid after nasal and intravenous delivery in rats and humans. J. Drug Target. 2003, 11, 325–331. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Farid, R.M.; Helmy, M.W.; ElGamal, S.S. Pharmacological, toxicological and neuronal localization assessment of galantamine/chitosan complex nanoparticles in rats: Future potential contribution in Alzheimer’s disease management. Drug Deliv. 2016, 23, 3111–3122. [Google Scholar] [CrossRef]
- Md, S.; Mustafa, G.; Baboota, S.; Ali, J. Nanoneurotherapeutics approach intended for direct nose to brain delivery. Drug Dev. Ind. Pharm. 2015, 41, 1922–1934. [Google Scholar] [CrossRef]





| Active Component | Microemulsion Components | Drug Release/Permeation Assessment | General Conclusions | References |
|---|---|---|---|---|
| rivastigmine hydrogen tartrate | Capmul® MCM EP, Labrasol®, Transcutol® P, water, chitosan, cetyltrimethylammonium bromide | in vitro: Franz cells, cellulose acetate membrane (m.w. cut-off 12,000–14,000) ex vivo: Franz cells, goat nasal mucosa | chitosan-based microemulsion showed improved ex vivo permeation | [136] |
| rivastigmine hydrogen tartrate | Capmul® MCM EP, Labrasol®, Transcutol® P, water, chitosan | in vitro: Franz cells, cellulose acetate membrane in vivo: male Sprague-Dawley rats, blood and brain concentration, gamma scintigraphy visualization | addition of chitosan contributed to higher brain concentration of the drug | [42] |
| rivastigmine hydrogen tartrate | Capmul® MCM EP, Labrasol®, Transcutol® P, water, butter oil, fish oil | in vitro: evaluation of the protective role of ME against Amyloid Beta (1–42) oligomer induced toxicity in IMR 32 cell line | fish oil and butter oil acted as penetration enhancers through nasal mucosa, no protection in IMR 32 cell line | [137] |
| galantamine hydrochloride | Capmul® MCM EP, Labrasol®, Transcutol® P, water, butter oil, fish oil | ex vivo: Franz cells, goat nasal mucosa | enhancement of permeation by addition of fish and butter oils | [138] |
| donepezil hydrochloride | Capmul® MCM EP, Tween® 20, Transcutol® EP, water, butter oil, omega-3 fish oil | ex vivo: Franz cells, goat nasal mucosa in vitro: cell permeability studies on bEnd.3 mouse cerebral microvascular endothelial cell line | fish oil induced higher bioavailability than butter oil | [139] |
| tacrine | Labrafil® M 1944 CS, Cremophor® RH 40, Transcutol® P, water | in vivo: male C57BL/6 mice, intranasal administration, ventral mid brain and striatum drug concentration, behavioral tests | in scopolamine-induced amnesia model in mice the fastest recovery was reached for microemulsions | [140] |
| donepezil hydrochloride | castor oil, Labrasol®, Transcutol® P, propylene glycol | in vitro: Franz cells, dialysis membrane (pore size 12–14 kDa) ex vivo: Franz cells, porcine nasal mucosa | more than 32% of the drug retained in porcine nasal mucosa | [141] |
| huperzine A | 1,2-propanediol, castor oil Cremophor® RH40, water, Pluronic F68, chitosan | in vitro: Franz cells, dialysis membrane (m.w. cut-off 6000–8000 U) in vivo: male Sprague-Dawley rats, microdialysis assay | after nasal administration both the plasma and brain concentration profiles showed the evidence of sustained and prolonged release, also higher bioavailability was observed | [142] |
| morin hydrate | Capmul® MCM, Cremophor® EL, PEG-400, water | in vitro: Franz cells, cellulose membranę behavioral tests | significant memory improvement in rats with streptozotocin-induced dementia | [143] |
| vinpocetine, piracetam | Tween® 20, oleic acid, ethanol, water, soybean lecithin—Epikuron® 200 | in vivo: male Wistar rats, brain drug concentration determination, behavioral tests | increase of both pharmaceutical and pharmacological properties due to application of nanocarriers | [144] |
| ibuprofen | Capmul® MCM, Accenon® CC, Transcutol®, water, polycarbophil | in vitro: Franz cells with sheep mucosa in vivo: male C57BL/6 mice, striatal dopamine concentrations, behavioral tests, nasal cilitoxicity | increased dopamine levels and better motor activity due to application of ibuprofen-loaded microemulsion, no toxicity | [145] |
| Active Component | Microemulsion Components | Drug release/Permeation Assessment | General Conclusions | References |
|---|---|---|---|---|
| clobazam | Capmul® MCM, Acconan® C6, Tween® 20, water, Carbopol 940P | ex vivo animal mucosa, in vivo gamma-scintigraphy, pharmacodynamic tests | better efficacy of mucoadhesive formulationintranasal system | [146] |
| lorazepam | Capmul® MCM, Nikkol PBC-34, Transcutol® P, water, gellan gum, Carbopol® | ex vivo goat nasal mucosa, pharmacodynamic tests (including behavioral ones) | faster and longer duration of action than the marketed product; better results for mucoadhesive formulation | [152] |
| diazepam | oleic acid, Tween® 80, propylene glycol, water, chitosan | in vivo pharmacokinetic studies, behavioral tests | enhanced brain delivery in microemulsion systems; better performance of mucoadhesive product | [153] |
| carbamazepine | oleic acid, Tween® 80, propylene glycol or Transcutol®, water | ex vivo sheep nasal mucosa, in vivo pharmacokinetic studies, induced convulsions in mice | seizure time reduction similar to intraperitoneal drug solution; higher drug concentration in brain tissue for Transcutol®-based microemulsion | [154,155] |
| carbamazepine | Labrafil® M1944, Cremophor® RH40, Transcutol®, water, polycarbophil | ex vivo sheep nasal mucosa, pharmacokinetic studies, gamma scintigraphy | no significant differences between microemulsion-based systems and drug solution in ex vivo study; higher concentrations in brain obtained for microemulsions; selective accumulation in brain | [156,157] |
| phenytoin | Capmul® MCM, Labrasol®, Transcutol®, water | in vivo pharmacokinetic studies, gamma scintigraphy, induced convulsions in mice | better selectivity towards brain compared to intraperitoneal administration; faster recovery after epileptic seizure | [159] |
| Active Component | Microemulsion Components | Drug Release/Permeation Assessment | General Conclusions | References |
|---|---|---|---|---|
| olanzapine | oleic acid, Kolliphor® RH40, Transcutol®, water, polycarbophil | in vivo pharmacokinetic studies; pharmacodynamic tests; gamma scintigraphy | higher concentration in brain compared to intravenous microemulsion and intranasal solution; no peripheral distribution | [165] |
| olanzapine | Labrafil® M1944CS, Cremophor® RH40, ethanol, water, HPMC K4M, poloxamer 407 | ex vivo sheep nasal mucosa; in vivo studies; gamma scintigraphy | higher permeation rate compared to NLC; lower drug concentrations in brain compared to NLC; less selective drug delivery than NLC; nasal mucosa irritation | [167] |
| quetiapine | Capmul® MCM EP, Tween® 80, Transcutol® P, water, chitosan | ex vivo nasal and intestinal mucosa; in vivo pharmacokinetic studies | the highest permeation rate ex vivo and the highest drug level in brain in vivo was observed for chitosan-loaded microemulsion | [160] |
| quetiapine | Capmul® MCM EP, Tween® 80, Transcutol® P, water, butter oil | ex vivo goat nasal mucosa; in vivo pharmacokinetic studies | the highest permeation rate ex vivo and drug levels in plasma were observed for butter oil-enriched microemulsion | [169] |
| paliperidone | oleic acid, Cremophor® RH40, Transcutol®, water, polycarbophil | behavioral studies, pharmacokinetic in vivo studies, gamma scintigraphy | mucoadhesive microemulsion exhibited the best performance in behavioral studies and the better selectivity than intravenous formulation | [170] |
| paliperidone | oleic acid, Cremophor® RH40, Labrasol®, Transcutol®, water, polycarbophil | ex vivo sheep mucosa | no significant differences between microemulsion, mucoadhesive microemulsions and drug solution | [171] |
| risperidone | oleic acid, Cremophor® RH40, Labrasol®, Transcutol®, water, polycarbophil | ex vivo sheep mucosa | no significant differences between microemulsion, mucoadhesive microemulsions and drug solution | [172] |
| asenapine | Capmul MCM, Tween 80, propylene glycol, water, polycarbophil | drug release with synthetic membrane, drug permeation with excised animal mucosa | no significant differences between samples with different composition in drug release study; permeation through nasal mucosa was faster for mucoadhesive formulation | [173] |
| sulpiride | glyceryl monooleate/Labrafil, different surfactants and co-surfactants | drug release with synthetic membranes; drug permeation through sheep nasal mucosa; behavioral tests | the differences in drug release were related to drug solubility; the same results in behavioral tests obtained for microemulsions and intravenous formulation | [174] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Froelich, A.; Osmałek, T.; Jadach, B.; Puri, V.; Michniak-Kohn, B. Microemulsion-Based Media in Nose-to-Brain Drug Delivery. Pharmaceutics 2021, 13, 201. https://doi.org/10.3390/pharmaceutics13020201
Froelich A, Osmałek T, Jadach B, Puri V, Michniak-Kohn B. Microemulsion-Based Media in Nose-to-Brain Drug Delivery. Pharmaceutics. 2021; 13(2):201. https://doi.org/10.3390/pharmaceutics13020201
Chicago/Turabian StyleFroelich, Anna, Tomasz Osmałek, Barbara Jadach, Vinam Puri, and Bozena Michniak-Kohn. 2021. "Microemulsion-Based Media in Nose-to-Brain Drug Delivery" Pharmaceutics 13, no. 2: 201. https://doi.org/10.3390/pharmaceutics13020201
APA StyleFroelich, A., Osmałek, T., Jadach, B., Puri, V., & Michniak-Kohn, B. (2021). Microemulsion-Based Media in Nose-to-Brain Drug Delivery. Pharmaceutics, 13(2), 201. https://doi.org/10.3390/pharmaceutics13020201