Cross-Talk of Toll-Like Receptor 5 and Mu-Opioid Receptor Attenuates Chronic Constriction Injury-Induced Mechanical Hyperalgesia through a Protein Kinase C Alpha-Dependent Signaling
Abstract
:1. Introduction
2. Results
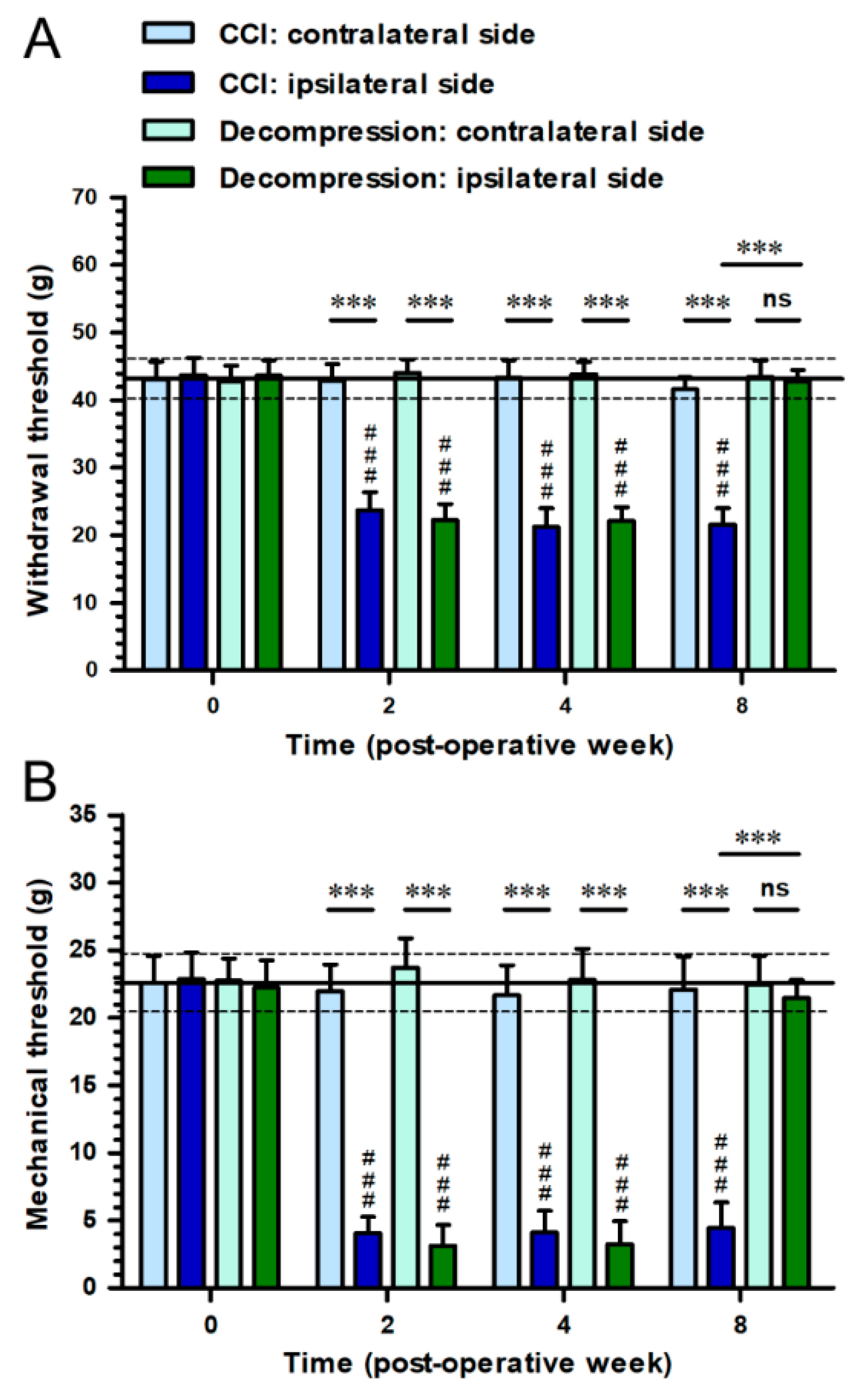
2.1. Nerve Decompression Efficiently Relieved CCI-Induced Pain Hypersensitivity
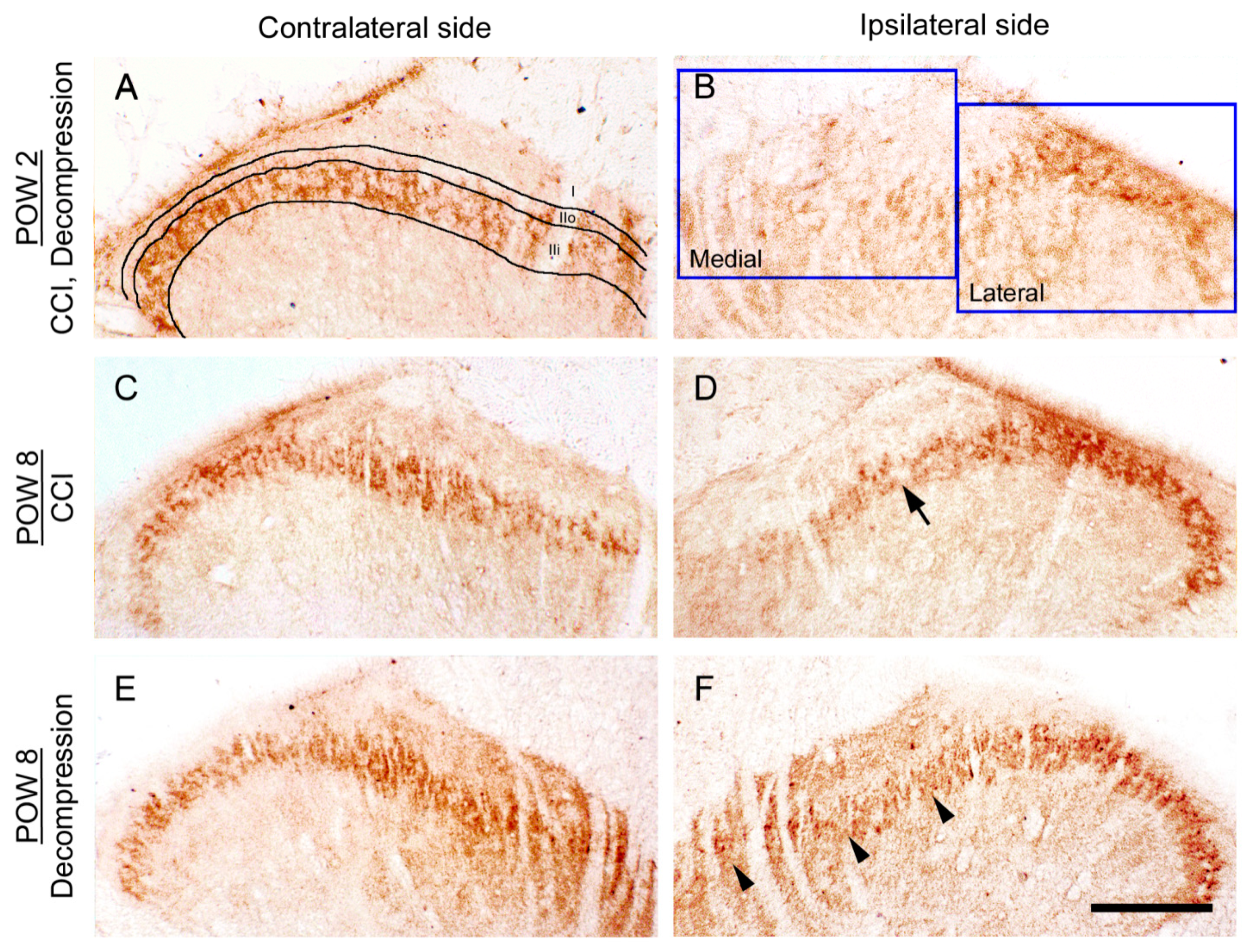
2.2. CCI-Induced Decrease of TLR5 Expression in Dorsal Horn Was Reversed by Nerve Decompression
2.3. Nerve Decompression Modulated the Reversal of Synaptic Plasticity in Dorsal Horn
2.4. Increase of TLR5 Expression Was Predominantly Observed Which Co-Expressed with IB4 Expression after Nerve Decompression
2.5. FLA-ST UtrapureAttenuated CCI-Induced Mechanical Hyperalgesia by an Intrathecal Administration in a Dose-Responsive Manner
2.6. Nerve Decompression Induced the Increase of MOR Expression, Whereas Its Co-Expression Was Mainly Detected with IB4 Expression
2.7. Increase of pPKCα Expression, Rather Than pPKA RII Expression, in Dorsal Horn, Where Its Co-Expression Was Mostly Observed with IB4 Expression after Nerve Decompression
2.8. Go 6976Re-Induced FLA-ST Ultrapure- and DAMGO-Mediated Reversal of Mechanical Hyperalgesia by an Intrathecal Administration Dose-Dependently
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgery
4.3. Behavioral Assessments
4.3.1. Mechanical Hyperalgesia
4.3.2. Mechanical Allodynia
4.4. Immunohistochemistry
4.5. Imaging Analysis
4.6. Double Immunofluorescence
4.7. Pharmacological Intervention
4.7.1. Drugs
4.7.2. Intrathecal Administration
4.7.3. Behavioral Assessments
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padua, L.; Coraci, D.; Erra, C.; Pazzaglia, C.; Paolasso, I.; Loreti, C.; Caliandro, P.; Hobson-Webb, L.D. Carpal tunnel syndrome: Clinical features, diagnosis and management. Lancet Neurol. 2016, 15, 1273–1284. [Google Scholar] [CrossRef]
- Thomson, J.G. Diagnosis and treatment of carpal tunnel syndrome. Lancet Neurol. 2017, 16, 263. [Google Scholar] [CrossRef] [Green Version]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152 (Suppl. 3), S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Dorsal horn circuitry: Complexity and implications for mechanisms of neuropathic pain. Neurology 2016, 86, 1060–1069. [Google Scholar] [CrossRef]
- Bassam, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar]
- Tseng, T.J.; Yang, M.L.; Hsieh, Y.L.; Ko, M.H.; Hsieh, S.T. Nerve decompression improves spinal synaptic plasticity of opioid receptors for pain relief. Neurotox. Res. 2018, 33, 362–376. [Google Scholar] [CrossRef]
- Handwerker, H.O. Classification of nociceptors--to what purpose? Pain 2010, 148, 355–356. [Google Scholar] [CrossRef]
- Tseng, T.J.; Chen, C.C.; Hsieh, Y.L.; Hsieh, S.T. Influences of surgical decompression on the dorsal horn after chronic constriction injury: Changes in peptidergic and delta-opioid receptor(+) nerve terminals. Neuroscience 2008, 156, 758–768. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Seltzer, Z.; Dubner, R.; Shir, Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 1990, 43, 205–218. [Google Scholar] [CrossRef]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- Lacagnina, M.J.; Watkins, L.R.; Grace, P.M. Toll-like receptors and their role in persistent pain. Pharmacol. Ther. 2018, 184, 145–158. [Google Scholar] [CrossRef]
- Liu, T.; Gao, Y.J.; Ji, R.R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 2012, 28, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, L.; Loram, L.C.; Watkins, L.R.; Hutchinson, M.R. Toll-like receptors in chronic pain. Exp. Neurol. 2012, 234, 316–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokes, J.A.; Cheung, J.; Eddinger, K.; Corr, M.; Yaksh, T.L. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J. Neuroinflammation 2013, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Vardarova, K.; Scharf, S.; Lang, F.; Schmeck, B.; Opitz, B.; Eitel, J.; Hocke, A.C.; Slevogt, H.; Flieger, A.; Hippenstiel, S.; et al. PKC(alpha) and PKC(epsilon) differentially regulate Legionella pneumophila-induced GM-CSF. Eur. Respir. J. 2009, 34, 1171–1179. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.Z.; Kim, Y.H.; Bang, S.; Zhang, Y.; Berta, T.; Wang, F.; Oh, S.B.; Ji, R.R. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med. 2015, 21, 1326–1331. [Google Scholar] [CrossRef]
- Liu, C.C.; Gao, Y.J.; Luo, H.; Berta, T.; Xu, Z.Z.; Ji, R.R.; Tan, P.H. Interferon alpha inhibits spinal cord synaptic and nociceptive transmission via neuronal-glial interactions. Sci. Rep. 2016, 6, 34356. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Liu, J.T.; Peng, Z.W.; Fan, H.; Yao, A.H.; Cheng, P.; Liu, L.; Ju, G.; Kuang, F. Different TLR4 expression and microglia/macrophage activation induced by hemorrhage in the rat spinal cord after compressive injury. J. Neuroinflammation 2013, 10, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retamal, J.S.; Ramírez-García, P.D.; Shenoy, P.A.; Poole, D.P.; Veldhuis, N.A. Internalized GPCRs as potential therapeutic targets for the management of pain. Front. Mol. Neurosci. 2019, 12, 273. [Google Scholar] [CrossRef]
- Pasternak, G.W. Opioids and their receptors: Are we there yet? Neuropharmacology 2014, 76, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, C.W.; Elballa, W.M.; Vold, S.U. Bifunctional opioid receptor ligands as novel analgesics. Neuropharmacology 2019, 151, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.T.; Hang, L.; Liu, T. Mu opioid receptor heterodimers emerge as novel therapeutic targets: Recent progress and future perspective. Front. Pharmacol. 2020, 11, 1078. [Google Scholar] [CrossRef] [PubMed]
- Melkes, B.; Markova, V.; Hejnova, L.; Novotny, J. beta-Arrestin 2 and ERK1/2 are important mediators engaged in close cooperation between TRPV1 and -opioid receptors in the plasma membrane. Int. J. Mol. Sci. 2020, 21, 4626. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Fujita, W.; Gupta, A.; Saldanha, S.A.; Negri, A.; Pinello, C.E.; Eberhart, C.; Roberts, E.; Filizola, M.; Hodder, P.; et al. Identification of a μ-δ opioid receptor heteromer-biased agonist with antinociceptive activity. Proc. Natl. Acad. Sci. USA 2013, 110, 12072–12077. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, V.; He, S.Q.; Huang, Q.; Liang, L.; Yang, F.; Chen, Z.; Tiwari, V.; Fujita, W.; Devi, L.A.; Dong, X.; et al. Activation of μ-δ opioid receptor heteromers inhibits neuropathic pain behavior in rodents. Pain 2020, 161, 842–855. [Google Scholar] [CrossRef] [PubMed]
- 29 Belcheva, M.M.; Vogel, Z.; Ignatova, E.; Avidor-Reiss, T.; Zippel, R.; Levy, R.; Young, E.C.; Barg, J.; Coscia, C.J. Opioid modulation of extracellular signal-regulated protein kinase activity is ras dependent and involves Gbetagamma subunits. J. Neurochem. 1998, 70, 635–645. [Google Scholar] [CrossRef]
- Cichewicz, D.L. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004, 74, 1317–1324. [Google Scholar] [CrossRef]
- Rios, C.; Gomes, I.; Devi, L.A. Mu opioid and CB1 cannabinoid receptor interactions: Reciprocal inhibition of receptor signaling and neuritogenesis. Br. J. Pharmacol. 2006, 148, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruscheweyh, R.; Forsthuber, L.; Schoffnegger, D.; Sandkühler, J. Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta- and C-fibers after chronic constriction injury in mice. J. Comp. Neurol. 2007, 502, 325–336. [Google Scholar] [CrossRef]
- Casals-Díaz, L.; Vivó, M.; Navarro, X. Nociceptive responses and spinal plastic changes of afferent C-fibers in three neuropathic pain models induced by sciatic nerve injury in the rat. Exp. Neurol. 2009, 217, 84–95. [Google Scholar] [CrossRef]
- Pinto, L.G.; Souza, G.R.; Kusuda, R.; Lopes, A.H.; Sant’Anna, M.B.; Cunha, F.Q.; Ferreira, S.H.; Cunha, T.M. Non-peptidergic nociceptive neurons are essential for mechanical inflammatory hypersensitivity in mice. Mol. Neurobiol. 2019, 56, 5715–5728. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, Q.; Yu, X.; Lu, T.; Zhang, P. MicroRNA-217 relieved neuropathic pain through targeting toll-like receptor 5 expression. J. Cell Biochem. 2019, 120, 3009–3017. [Google Scholar] [CrossRef]
- Jurga, A.M.; Rojewska, E.; Piotrowska, A.; Makuch, W.; Pilat, D.; Przewlocka, B.; Mika, J. Blockade of Toll-like receptors (TLR2, TLR4) attenuates pain and potentiates Buprenorphine analgesia in a rat neuropathic pain model. Neural Plast. 2016, 2016, 5238730. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Li, L.; Zhang, Y.; Zhang, Z.J.; Liu, H.L.; Bao, H.G. LncRNA X inactive specific transcript contributes to neuropathic pain development by sponging miR-154-5p via inducing toll-like receptor 5 in CCI rat models. J. Cell Biochem. 2019, 120, 1271–1281. [Google Scholar] [CrossRef]
- Das, N.; Dewan, V.; Grace, P.M.; Gunn, R.J.; Tamura, R.; Tzarum, N.; Watkins, L.R.; Wilson, I.A.; Yin, H. HMGB1 activates proinflammatory signaling via TLR5 leading to allodynia. Cell Rep. 2016, 17, 1128–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccinini, A.M.; Midwood, K.S. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Ennes, H.S.; McRoberts, J.A.; Marvizón, J.C. Mechanisms of mu-opioid receptor inhibition of NMDA receptor-induced substance P release in the rat spinal cord. Neuropharmacology 2018, 128, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Joseph, E.K.; Levine, J.D. Mu and delta opioid receptors on nociceptors attenuate mechanical hyperalgesia in rat. Neuroscience 2010, 171, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Vicario, N.; Pasquinucci, L.; Spitale, F.M.; Chiechio, S.; Turnaturi, R.; Caraci, F.; Tibullo, D.; Avola, R.; Gulino, R.; Parenti, R.; et al. Simultaneous Activation of Mu and Delta Opioid Receptors Reduces Allodynia and Astrocytic Connexin 43 in an Animal Model of Neuropathic Pain. Mol. Neurobiol. 2019, 56, 7338–7354. [Google Scholar] [CrossRef]
- Kohno, T.; Ji, R.R.; Ito, N.; Allchorne, A.J.; Befort, K.; Karchewski, L.A.; Woolf, C.J. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain 2005, 117, 77–87. [Google Scholar] [CrossRef]
- Guan, Y.; Johanek, L.M.; Hartke, T.V.; Shim, B.; Tao, Y.X.; Ringkamp, M.; Meyer, R.A.; Raja, S.N. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain 2008, 138, 318–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Q.; Shao, J.; Cao, J.; Ren, X.; Cai, W.; Su, S.; George, S.; Tan, Z.; Zang, W.; Dong, T. Protein kinase C-α upregulates sodium channel Nav1.9 in nociceptive dorsal root ganglion neurons in an inflammatory arthritis pain model of rat. J. Cell Biochem. 2020, 121, 768–778. [Google Scholar] [CrossRef]
- Kopach, O.; Krotov, V.; Shysh, A.; Sotnic, A.; Viatchenko-Karpinski, V.; Dosenko, V.; Voitenko, N. Spinal PKCα inhibition and gene-silencing for pain relief: AMPAR trafficking at the synapses between primary afferents and sensory interneurons. Sci. Rep. 2018, 8, 10285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Leitges, M.; Gereau, R.W., 4th. Isozyme-specific effects of protein kinase C in pain modulation. Anesthesiology 2011, 115, 1261–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velázquez, K.T.; Mohammad, H.; Sweitzer, S.M. Protein kinase C in pain: Involvement of multiple isoforms. Pharmacol. Res. 2007, 55, 578–589. [Google Scholar] [CrossRef] [Green Version]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and exogenous opioids in pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Narita, M.; Mizoguchi, H.; Tseng, L.F. Inhibition of protein kinase C but not of protein kinase A, blocks the development of acute antinociceptive tolerance to an intrathecally administered μ-opioid receptor agonist in the mouse. Eur. J. Pharmacol. 1995, 280, R1–R3. [Google Scholar] [CrossRef]
- Madera-Salcedo, I.K.; Cruz, S.L.; Gonzalez-Espinosa, C. Morphine prevents lipopolysaccharide-induced TNF secretion in mast cells blocking IκB kinase activation and SNAP-23 phosphorylation: Correlation with the formation of a β-arrestin/TRAF6 complex. J. Immunol. 2013, 191, 3400–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araldi, D.; Bogen, O.; Green, P.G.; Levine, J.D. Role of nociceptor Toll-like receptor 4 (TLR4) in opioid-induced hyperalgesia and hyperalgesic priming. Proc. Natl. Acad. Sci. USA 2012, 109, 6325–6330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Loram, L.C.; Ramos, K.; de Jesus, A.J.; Thomas, J.; Cheng, K.; Reddy, A.; Somogyi, A.A.; Hutchinson, M.R.; Watkins, L.R.; et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl. Acad. Sci. USA 2012, 109, 6325–6330. [Google Scholar] [CrossRef] [Green Version]
- N’Guessan, P.D.; Etouem, M.O.; Schmeck, B.; Hocke, A.C.; Scharf, S.; Vardarova, K.; Opitz, B.; Flieger, A.; Suttorp, N.; Hippenstiel, S. Legionella pneumophila-induced PKCalpha-, MAPK- and NF-kappaB-dependent COX-2 expression in human lung epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L267–L277. [Google Scholar]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Samur, D.N.; Arslan, R.; Aydın, S.; Bektas, N. Valnoctamide: The effect on relieving of neuropathic pain and possible mechanisms. Eur. J. Pharmacol. 2018, 827, 208–214. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; She, Y.; Smith, G.M.; Xu, X.M. Cortical PKC inhibition promotes axonal regeneration of the corticospinal tract and forelimb functional recovery after cervical dorsal spinal hemisection in adult rats Cereb. Cortex 2014, 24, 3069–3079. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.J.; Walla, B.C.; Diaz, M.F.; Fuller, G.N.; Gutstein, H.B. Intermittent lumbar puncture in rats: A novel method for the experimental study of opioid tolerance. Anesth. Analg. 2006, 103, 714–720. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.; Liu, H.-K.; Yeh, C.-B.; Yang, M.-L.; Liao, W.-C.; Liu, C.-H.; Tseng, T.-J. Cross-Talk of Toll-Like Receptor 5 and Mu-Opioid Receptor Attenuates Chronic Constriction Injury-Induced Mechanical Hyperalgesia through a Protein Kinase C Alpha-Dependent Signaling. Int. J. Mol. Sci. 2021, 22, 1891. https://doi.org/10.3390/ijms22041891
Chang C, Liu H-K, Yeh C-B, Yang M-L, Liao W-C, Liu C-H, Tseng T-J. Cross-Talk of Toll-Like Receptor 5 and Mu-Opioid Receptor Attenuates Chronic Constriction Injury-Induced Mechanical Hyperalgesia through a Protein Kinase C Alpha-Dependent Signaling. International Journal of Molecular Sciences. 2021; 22(4):1891. https://doi.org/10.3390/ijms22041891
Chicago/Turabian StyleChang, Ching, Hung-Kai Liu, Chao-Bin Yeh, Ming-Lin Yang, Wen-Chieh Liao, Chiung-Hui Liu, and To-Jung Tseng. 2021. "Cross-Talk of Toll-Like Receptor 5 and Mu-Opioid Receptor Attenuates Chronic Constriction Injury-Induced Mechanical Hyperalgesia through a Protein Kinase C Alpha-Dependent Signaling" International Journal of Molecular Sciences 22, no. 4: 1891. https://doi.org/10.3390/ijms22041891
APA StyleChang, C., Liu, H.-K., Yeh, C.-B., Yang, M.-L., Liao, W.-C., Liu, C.-H., & Tseng, T.-J. (2021). Cross-Talk of Toll-Like Receptor 5 and Mu-Opioid Receptor Attenuates Chronic Constriction Injury-Induced Mechanical Hyperalgesia through a Protein Kinase C Alpha-Dependent Signaling. International Journal of Molecular Sciences, 22(4), 1891. https://doi.org/10.3390/ijms22041891





