Clinical Experiences and Mechanism of Action with the Use of Oxytocin Injection at Parturition in Domestic Animals: Effect on the Myometrium and Fetuses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Oxytocin: Pharmacology and Clinical Application at Parturition in Domestic Animals
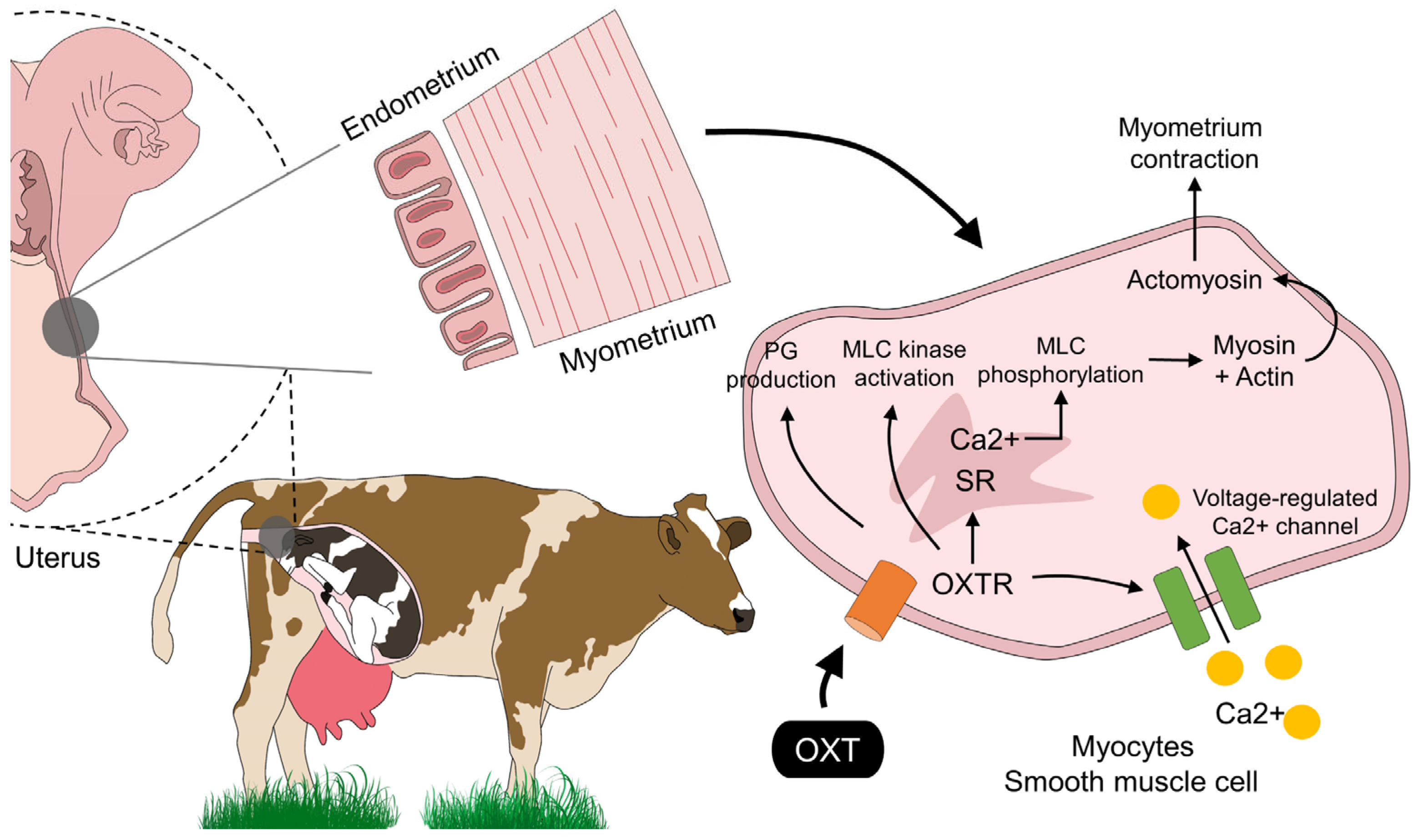
3. OXT: Its Mechanism of Action and Receptor Signaling in the Myometrium in Domestic Animals
4. Favorable Effects of the Use of Oxytocin during Parturition and Recommendations for Its Use in Veterinary Obstetrics
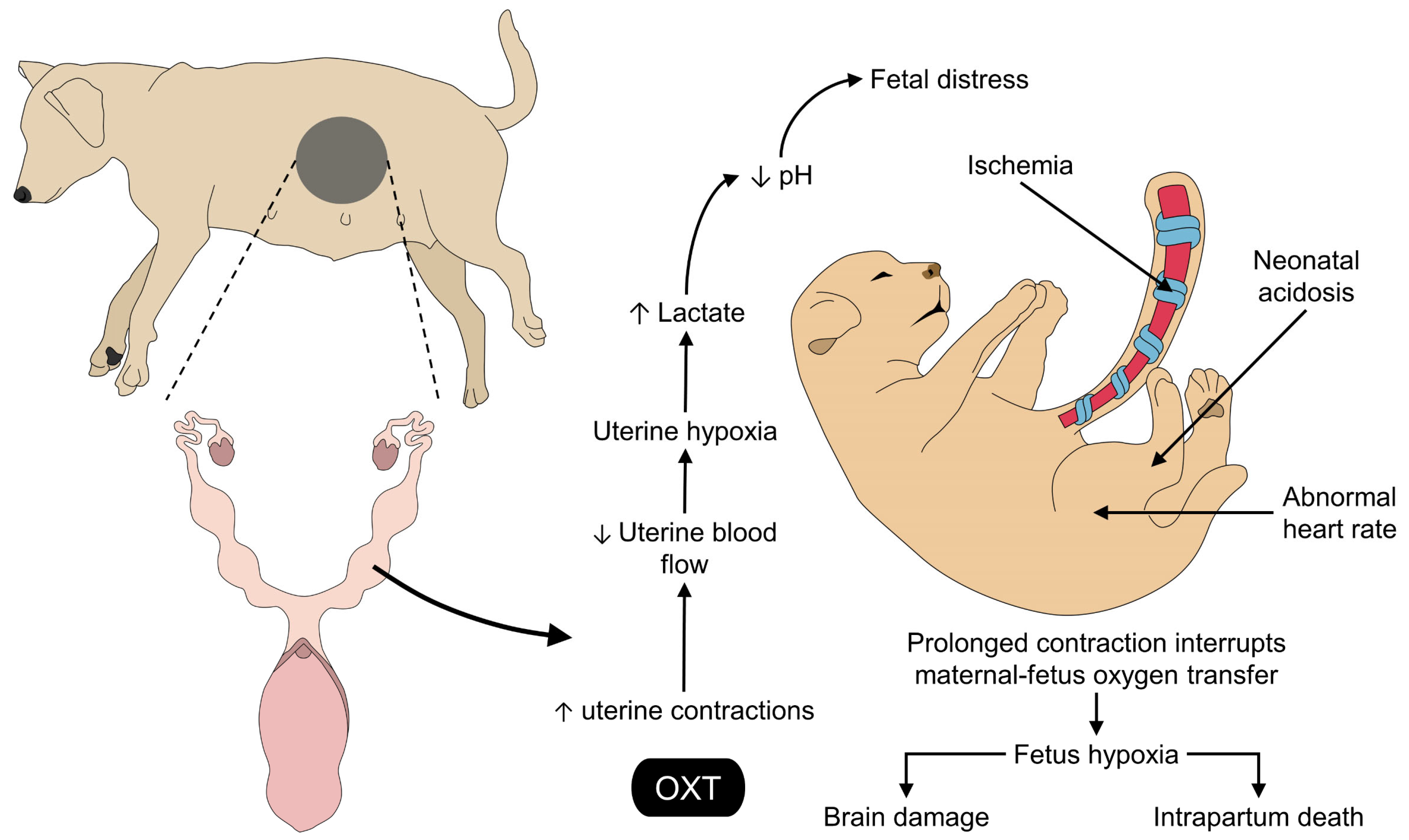
5. Effects of the Exogenous Application of Oxytocin on the Fetus and the Umbilical Cord
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lévy, F. Neuroendocrine Control of Maternal Behavior in Non-Human and Human Mammals. Ann. D’endocrinologie 2016, 77, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.H.A.; Kia, H.D.; Ramin, F. Physiology of Parturition. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 214–221. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saller, S.; Kunz, L.; Dissen, G.A.; Stouffer, R.; Ojeda, S.R.; Berg, D.; Berg, U.; Mayerhofer, A. Oxytocin Receptors in the Primate Ovary: Molecular Identity and Link to Apoptosis in Human Granulosa Cells. Hum. Reprod. 2010, 25, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Stadler, B.; Whittaker, M.R.; Exintaris, B.; Middendorff, R. Oxytocin in the Male Reproductive Tract; The Therapeutic Potential of Oxytocin-Agonists and-Antagonists. Front. Endocrinol. 2020, 11, 565731. [Google Scholar] [CrossRef]
- Assinder, S.J.; Carey, M.; Parkinson, T.; Nicholson, H.D. Oxytocin and Vasopressin Expression in the Ovine Testis and Epididymis: Changes with the Onset of Spermatogenesis1. Biol. Reprod. 2000, 63, 448–456. [Google Scholar] [CrossRef]
- Chen, S.; Sato, S. Role of Oxytocin in Improving the Welfare of Farm Animals—A Review. Asian-Australas. J. Anim. Sci. 2016, 30, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Young, L.J.; Zingg, H.H. Oxytocin. In Hormones, Brain and Behavior, 3rd ed.; Pfaff, D.W., Joels, M., Eds.; Elsevier Science & Technology: Amsterdam, The Netherlands, 2016; Volume 3, p. 2474. [Google Scholar]
- Mota-Rojas, D.; Bienboire-Frosini, C.; Marcet-Rius, M.; Domínguez-Oliva, A.; Mora-Medina, P.; Lezama-García, K.; Orihuela, A. Mother-Young Bond in Non-Human Mammals: Neonatal Communication Pathways and Neurobiological Basis. Front. Psychol. 2022, 13, 1064444. [Google Scholar] [CrossRef]
- Orihuela, A.; Mota-Rojas, D.; Strappini, A.; Serrapica, F.; Braghieri, A.; Mora-Medina, P.; Napolitano, F. Neurophysiological Mechanisms of Cow- Calf Bonding in Buffalo and Other Farm Animals. Animals 2021, 11, 1968. [Google Scholar] [CrossRef]
- Bienboire-Frosini, C.; Marcet-Rius, M.; Orihuela, A.; Domínguez-Oliva, A.; Mora-Medina, P.; Olmos-Hernández, A.; Casas-Alvarado, A.; Mota-Rojas, D. Mother–Young Bonding: Neurobiological Aspects and Maternal Biochemical Signaling in Altricial Domesticated Mammals. Animals 2023, 13, 532. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Velarde, A.; Marcet-Rius, M.; Orihuela, A.; Bragaglio, A.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Domínguez-Oliva, A.; Whittaker, A.L. Analgesia during Parturition in Domestic Animals: Perspectives and Controversies on Its Use. Animals 2022, 12, 2686. [Google Scholar] [CrossRef]
- Lezama-García, K.; Mariti, C.; Mota-Rojas, D.; Martínez-Burnes, J.; Barrios-García, H.; Gazzano, A. Maternal Behaviour in domestic dogs. Int. J. Vet. Sci. Med. 2019, 7, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Bragaglio, A.; Braghieri, A.; Napolitano, F.; Domínguez-Oliva, A.; Mora-Medina, P.; Álvarez-Macías, A.; De Rosa, G.; Pacelli, C.; José, N.; et al. Dairy Buffalo Behavior: Calving, Imprinting and Allosuckling. Animals 2022, 12, 2899. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Burnes, J.; Muns, R.; Barrios-García, H.; Villanueva-García, D.; Domínguez-Oliva, A.; Mota-Rojas, D. Parturition in Mammals: Animal Models, Pain and Distress. Animals 2021, 11, 2960. [Google Scholar] [CrossRef] [PubMed]
- Phillippe, R.; Francoise, M.; Marie-José, F.-M. Central Effects of Oxytocin. Am. Physiol. Soc. 1991, 71, 331–355. [Google Scholar] [CrossRef]
- Nagel, C.; Aurich, C.; Aurich, J. Stress Effects on the Regulation of Parturition in Different Domestic Animal Species. Anim. Reprod. Sci. 2019, 207, 153–161. [Google Scholar] [CrossRef]
- Lee, H.-J.; Macbeth, A.H.; Pagani, J.; Young, W.S. Oxytocin: The Great Facilitator of Life. Prog. Neurobiol. 2009, 88, 127–151. [Google Scholar] [CrossRef] [Green Version]
- Dale, H.H. On Some Physiological Actions of Ergot. J. Physiol. 1906, 34, 163–206. [Google Scholar] [CrossRef]
- Goodwin, M.; Zograbyan, A. Antagonistas de Receptores de Oxitocina. In Clínicas De Perinatología; Strauss, J.M., Ed.; McGrawHill Interamericana: Mexico City, Mexico, 1998; pp. 917–928. [Google Scholar]
- Phaneuf, S.; Linares, B.R.; TambyRaja, R.; MacKenzie, I.; Bernal, A.L. Loss of Myometrial Oxytocin Receptors during Oxytocin-Induced and Oxytocin-Augmented Labour. Reproduction 2000, 120, 91–97. [Google Scholar] [CrossRef]
- Robinson, C.; Schumann, R.; Zhang, P.; Young, R.C. Oxytocin-Induced Desensitization of the Oxytocin Receptor. Am. J. Obstet. Gynecol. 2003, 188, 497–502. [Google Scholar] [CrossRef]
- Kustritz, M.V.R. Reproductive Behavior of Small Animals. Theriogenology 2005, 64, 734–746. [Google Scholar] [CrossRef]
- Pageat, P.; Gaultier, E. Current Research in Canine and Feline Pheromones. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 187–211. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and Vasopressin in the Human Brain: Social Neuropeptides for Translational Medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef]
- Numan, M.; Stolzenberg, D.S. Medial Preoptic Area Interactions with Dopamine Neural Systems in the Control of the Onset and Maintenance of Maternal Behavior in Rats. Front. Neuroendocrinol. 2009, 30, 46–64. [Google Scholar] [CrossRef]
- Bridges, R.S. Neuroendocrine Regulation of Maternal Behavior. Front. Neuroendocrinol. 2015, 36, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Kendrick, K.M.; Keverne, E.B.; Baldwin, B.A. Intracerebroventricular Oxytocin Stimulates Maternal Behaviour in the Sheep. Neuroendocrinology 1987, 46, 56–61. [Google Scholar] [CrossRef]
- Gilbert, C.; Burne, T.; Goode, J.; Murfitt, P.; Walton, S. Indomethacin Blocks Pre-Partum Nest Building Behaviour in the Pig (Sus Scrofa): Effects on Plasma Prostaglandin F Metabolite, Oxytocin, Cortisol and Progesterone. J. Endocrinol. 2002, 172, 507–517. [Google Scholar] [CrossRef]
- Castrén, H.; Algers, B.; de Passillé, A.-M.; Rushen, J.; Uvnäs-Moberg, K. Preparturient Variation in Progesterone, Prolactin, Oxytocin and Somatostatin in Relation to Nest Building in Sows. Appl. Anim. Behav. Sci. 1993, 38, 91–102. [Google Scholar] [CrossRef]
- Oliviero, C.; Heinonen, M.; Valros, A.; Peltoniemi, O. Environmental and Sow-Related Factors Affecting the Duration of Farrowing. Anim. Reprod. Sci. 2010, 119, 85–91. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Atkinson, C.; Wähälä, K.; Lampe, J.W. Obesity Prevalence in Relation to Gut Microbial Environments Capable of Producing Equol or O-Desmethylangolensin from the Isoflavone Daidzein. Eur. J. Clin. Nutr. 2014, 68, 526–530. [Google Scholar] [CrossRef] [Green Version]
- González-Lozano, M.; Trujillo-Ortega, M.E.; Becerril-Herrera, M.; Alonso-Spilsbury, M.; Rosales-Torres, A.M.; Mota-Rojas, D. Uterine Activity and Fetal Electronic Monitoring in Parturient Sows Treated with Vetrabutin Chlorhydrate. J. Vet. Pharmacol. Ther. 2010, 33, 28–34. [Google Scholar] [CrossRef]
- Herpin, P.; Le Dividich, J.; Hulin, J.C.; Fillaut, M.; De Marco, F.; Bertin, R. Effects of the Level of Asphyxia during Delivery on Viability at Birth and Early Postnatal Vitality of Newborn Pigs. J. Anim. Sci. 1996, 74, 2067–2075. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Chen, J.; Li, H.; Song, Z.; Chang, L.; He, X.; Fan, Z. Isomaltooligosaccharide and Bacillus Regulate the Duration of Farrowing and Weaning-Estrous Interval in Sows during the Perinatal Period by Changing the Gut Microbiota of Sows. Anim. Nutr. 2021, 7, 72–83. [Google Scholar] [CrossRef]
- Hydbring, E.; Madej, A.; MacDonald, E.; Drugge-Boholm, G.; Berglund, B.; Olsson, K. Hormonal Changes during Parturition in Heifers and Goats Are Related to the Phases and Severity of Labour. J. Endocrinol. 1999, 160, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Straw, B.E.; Bush, E.J.; Dewey, C.E. Types and Doses of Injectable Medications given to Periparturient Sows. J. Am. Vet. Med. Assoc. 2000, 216, 510–515. [Google Scholar] [CrossRef] [Green Version]
- Wilker, C.E.; Ellington, J.E. Reproductive Toxicology of the Female Companion Animal. In Small Animal Toxicology, 2nd ed.; Peterson, M.E., Talcott, P.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 475–499. [Google Scholar]
- Plumb, D.C. Oxytocin. In Plumb´s Veterinary Drugs; John Wiley & Sons: Tulsa, OK, USA, 2015. [Google Scholar]
- Linneen, S.K.; Benz, J.M.; DeRouchey, J.M.; Goodband, R.D.; Tokach, M.D.; Dritz, S.S. A Review of Oxytocin Use for Sows and Gilts. Kans. Agric. Exp. Stn. Res. Rep. 2005, 0, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Assad, N.I.; Pandey, A.K.; Sharma, L.M. Oxytocin, Functions, Uses and Abuses: A Brief Review. Insight Int. J. Reprod. All Anim. 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Ahmad, M. Oxytocin; Effects on Milk Production. Pure Appl. Biol. 2021, 10, 318–324. [Google Scholar] [CrossRef]
- Münnich, A.; Küchenmeister, U. Dystocia in numbers evidence based parameters for intervention in the dog: Causes for dystocia and treatment recommendations. Reprod. Domest. Anim. 2009, 44, 141–147. [Google Scholar] [CrossRef]
- Gutkowska, J.; Jankowski, M. Oxytocin Revisited: Its Role in Cardiovascular Regulation. J. Neuroendocrinol. 2012, 24, 599–608. [Google Scholar] [CrossRef]
- González-Lozano, M.; Trujillo-Ortega, M.E.; Alonso-Spilsbury, M.; Rosales, A.M.; Ramírez-Necoechea, R.; González-Maciel, A.; Martínez-Rodríguez, R.; Becerril-Herrera, M.; Mota-Rojas, D. Vetrabutine Clorhydrate Use in Dystocic Farrowings Minimizes Hemodynamic Sequels in Piglets. Theriogenology 2012, 78, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Villanueva-García, D.; Mota-Reyes, A.; Orihuela, A.; Hernández-Ávalos, I.; Domínguez-Oliva, A.; Casas-Alvarado, A.; Flores-Padilla, K.; Jacome-Romero, J.; Martínez-Burnes, J. Meconium Aspiration Syndrome in Animal Models: Inflammatory Process, Apoptosis, and Surfactant Inactivation. Animals 2022, 12, 3310. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Spilsbury, M.; Mota-Rojas, D.; Martínez-Burnes, J.; Arch, E.; López Mayagoitia, A.; Ramírez-Necoechea, R.; Olmos, A.; Trujillo, M.E. Use of Oxytocin in Penned Sows and Its Effect on Fetal Intra-Partum Asphyxia. Anim. Reprod. Sci. 2004, 84, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Villanueva-García, D.; Velazquez-Armenta, E.Y.; Nava-Ocampo, A.A.; Ramírez-Necoechea, R.; Alonso-Spilsbury, M.; Trujillo, M.E. Influence of Time at Which Oxytocin Is Administered during Labor on Uterine Activity and Perinatal Death in Pigs. Biol. Res. 2007, 40, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Stenning, F.J.; Polglase, G.R.; te Pas, A.B.; Crossley, K.J.; Kluckow, M.; Gill, A.W.; Wallace, E.M.; McGillick, E.V.; Binder, C.; Blank, D.A.; et al. Effect of Maternal Oxytocin on Umbilical Venous and Arterial Blood Flows during Physiological-Based Cord Clamping in Preterm Lambs. PLoS ONE 2021, 16, e0253306. [Google Scholar] [CrossRef]
- Trujillo, O.M.; Mota-Rojas, D.; Juárez, O.; Villanueva-García, D.; Roldan-Santiago, P.; Becerril-Herrera, R.; Hernández-Gónzalez, R.; Mora-Medina, P.; Alonso-Spilsbury, M.; Rosales, A.; et al. Porcine Neonates Failing Vitality Score: Physio-Metabolicprofile and Latency to the First Teat Contact. Czech J. Anim. Sci. 2011, 56, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Mota-Rojas, D.; Villanueva-García, D.; Solimano, A.; Muns, R.; Ibarra-Ríos, D.; Mota-Reyes, A. Pathophysiology of Perinatal Asphyxia in Humans and Animal Models. Biomedicines 2022, 10, 347. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; López, A.; Martínez-Burnes, J.; Muns, R.; Villanueva-García, D.; Mora-Medina, P.; González-Lozano, M.; Olmos-Hernández, A.; Ramírez-Necoechea, R. Is Vitality Assessment Important in Neonatal Animals? CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Rosales, A.M.; Trujillo, M.E.; Orozco, H.; Ramírez, R.; Alonso-Spilsbury, M. The Effects of Vetrabutin Chlorhydrate and Oxytocin on Stillbirth Rate and Asphyxia in Swine. Theriogenology 2005, 64, 1889–1897. [Google Scholar] [CrossRef]
- González-Lozano, M.; Mota-Rojas, D.; Orihuela, A.; Martínez-Burnes, J.; Di Francia, A.; Braghieri, A.; Berdugo-Gutiérrez, J.; Mora-Medina, P.; Ramírez-Necoechea, R.; Napolitano, F. Review: Behavioral, Physiological, and Reproductive Performance of Buffalo Cows during Eutocic and Dystocic Parturitions. Appl. Anim. Sci. 2020, 36, 407–422. [Google Scholar] [CrossRef]
- Homeida, A.M.; Cooke, R.G. Biological Half-Life of Oxytocin in the Goat. Res. Vet. Sci. 1984, 37, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, R.J. The Estimation of Small Amounts of Oxytocin in Blood. In Oxytocin; Caldeyro-Bracia, R., Heller, H., Eds.; Pergamon Press: Oxford, UK, 1961; p. 14. [Google Scholar]
- Steckler, D.; Naidoo, V.; Gerber, D.; Kähn, W. Ex Vivo Influence of Carbetocin on Equine Myometrial Muscles and Comparison with Oxytocin. Theriogenology 2012, 78, 502–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, K.W.; Smith, B.R. A Comprehensive Quantitative and Qualitative Evaluation of Extrapolation of Intravenous Pharmacokinetic Parameters from Rat, Dog, and Monkey to Humans. II. Volume of Distribution and Mean Residence Time. Drug Metab. Dispos. 2004, 32, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.-R.; Dawoodf, Y.M. Oxytocin Release and Uterine Activation during Parturition in Rabbits. Endocrinology 1980, 107, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Al-Eknah, M.M.; Homeida, A.M. A Review of Some Aspects of the Pharmacology of Oxytocin in Domestic Animals. Vet. Res. Commun. 1991, 15, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W.B.; Parsons, M.T.; Pak, S.C.; Wilson, L. Morphine Inhibits Nocturnal Oxytocin Secretion and Uterine Contractions in the Pregnant Baboon1. Biol. Reprod. 1998, 58, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Troncy, E.; Morin, V.; Del Castillo, J.R.E.; Authier, S.; Ybarra, N.; Otis, C.; Gauvin, D.; Gutkowska, J. Evidence for Non-Linear Pharmacokinetics of Oxytocin in Anesthetizetized Rat. J. Pharm. Pharm. Sci. 2008, 11, 12–24. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Lal, M. Preclinical Safety and Pharmacokinetics of Heat Stable Oxytocin in Sublingual Fast-Dissolving Tablet Formulation. Pharmaceutics 2022, 14, 953. [Google Scholar] [CrossRef]
- Wagner, B.K.; Relling, A.E.; Kieffer, J.D.; Moraes, L.E.; Parker, A.J. Short communication: Pharmacokinetics of oxytocin administered intranasally to beef cattle. Domest. Anim. Endocrinol. 2020, 71, 106387. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Nava-Ocampo, A.A.; Trujillo, M.E.; Velázquez-Armenta, Y.; Ramírez-Necoechea, R.; Martínez-Burnes, J.; Alonso-Spilsbury, Y.M. Dose minimization study of oxytocin in early labor in sows: Uterine activity and fetal outcome. Reprod. Toxicol. 2005, 20, 255–259. [Google Scholar] [CrossRef]
- Hill, S.V.; Amezcua, M.D.R.; Ribeiro, E.S.; O’Sullivan, T.L.; Friendship, R.M. Defining the Effect of Oxytocin Use in Farrowing Sows on Stillbirth Rate: A Systematic Review with a Meta-Analysis. Animals 2022, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Trujillo, M.E.; Martínez, J.; Rosales, A.M.; Orozco, H.; Ramírez, R.; Sumano, H.; Alonso-Spilsbury, M. Comparative routes of oxytocin administration in crated farrowing sows and its effects on fetal and postnatal asphyxia. Anim. Reprod. Sci. 2006, 92, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Ybarra-Navarro, N.T. Evaluation of Oxytocin Pharmacokinetic: Pharmacodynamic Profile and Establishment of Its Cardiomyogenic Potential in Swine. Ph.D. Thesis, Université of Montréal, Montréal, QC, Canada, 2010. [Google Scholar]
- Wiebe, V.J.; Howard, J.P. Pharmacologic Advances in Canine and Feline Reproduction. Top. Companion Anim. Med. 2009, 24, 71–99. [Google Scholar] [CrossRef]
- Brander, G.C.; Pugh, D.M.; Bywater, R.J. Veterinary Applied Pharmacology and Therapeutics; Bailliere Tindall Ltd: London, UK, 1982. [Google Scholar]
- Bajcsy, Á.; Kindahl, H.; Szenci, O.; van der Weijden, G.; Bartyik, J.; Taverne, M. The Effect of Two Different Routes of Administration of Oxytocin on Peripheral Plasma Prostaglandin F2α Metabolite Levels in Early Post-partum Dairy Cows. Reprod. Domest. Anim. 2012, 47, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Nickles, K.R.; Relling, A.E.; Parker, A.J. Intranasal oxytocin treatment on the day of weaning does not decrease walking behavior or improve plasma metabolites in beef calves placed on pasture. Transl. Anim. Sci. 2021, 5, txab191. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.-L.; Dunshea, F.; Pluske, J. Effects of Oxytocin Administration on the Response of Piglets to Weaning. Animals 2015, 5, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Villanueva, D.; Alonso-Spilsbury, M.; Becerril, H.M.; Ramirez-Necoechea, R.; Gonzalez-Lozano, M.; Trujillo-Ortega, M.E. Effect of Different Doses of Oxytocin at Delivery on Suffering and Survival of Newborn Pigs. J. Med. Sci. 2007, 7, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Villani, M.; Romano, G. Induction of Parturition with Daily Low-dose Oxytocin Injections in Pregnant Mares at Term: Clinical Applications and Limitations. Reprod. Domest. Anim. 2008, 43, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, M.L.; Chaffin, M.K.; Carroll, G.L.; Jorgensen, J.; Arrott, C.; Varner, D.D.; Blanchard, T.L. Three methods of oxytocin-induced parturition and their effects of foals. J. Am. Vet. Med. Assoc. 1997, 210, 799–803. [Google Scholar] [PubMed]
- Brunton, P.J. Endogenous opioid signalling in the brain during pregnancy and lactation. Cell Tissue Res. 2019, 375, 69–83. [Google Scholar] [CrossRef]
- Taverne, M.; de Schwartz, N.; Kankofer, M.; Bevers, M.; van Oord, H.; Schams, D.; Gutjahr, S.; van der Weijden, G. Uterine Responses to Exogenous Oxytocin Before and After Pre-partum Luteolysis in the Cow. Reprod. Domest. Anim. 2001, 36, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, B.M.; Dodge, K.; Monga, M.; Qian, A.; Wang, W.; Yue, C. Molecular Mechanisms Regulating the Effects of Oxytocin on Myometrial Intracellular Calcium. In Vasopressin and Oxytocin; Springer: Boston, MA, USA, 1998; Volume 449, pp. 277–286. [Google Scholar] [CrossRef]
- Ku, C.Y.; Qian, A.; Wen, Y.; Anwer, K.; Sanborn, B.M. Oxytocin stimulates myometrial guanosine triphosphatase and phospholipase-C activities via coupling to G alpha q/11. Endocrinology 1995, 136, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Riemer, R.K.; Heymann, M.A. Regulation of Uterine Smooth Muscle Function during Gestation. Pediatr. Res. 1998, 44, 615–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidder, G.M.; Winterhager, E. Physiological roles of connexins in labour and lactation. Reproduction 2015, 150, R129–R136. [Google Scholar] [CrossRef] [Green Version]
- Shynlova, O.; Tsui, P.; Jaffer, S.; Lye, S.J. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, S2–S10. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.C.; Li, D.; Li, T.; Yang, H.-P.; Wang, L.; Wang, Y.-F.; Parpura, V. Role of Connexin 36 in Autoregulation of Oxytocin Neuronal Activity in Rat Supraoptic Nucleus. ASN Neuro 2019, 11, 175909141984376. [Google Scholar] [CrossRef]
- Petersson, M. Petersson, M. Cardiovascular effects of oxytocin. Prog. Brain. Res. 2002, 131, 281–288. [Google Scholar]
- Tuğtepe, H.; Şener, G.; Bıyıklı, N.K.; Yüksel, M.; Çetinel, Ş.; Gedik, N.; Yeğen, B.Ç. The protective effect of oxytocin on renal ischemia/reperfusion injury in rats. Regul. Pept. 2007, 140, 101–108. [Google Scholar] [CrossRef]
- Lundeberg, T.; Uvnäs-Moberg, K.; Ågren, G.; Bruzelius, G. Anti-nociceptive effects of oxytocin in rats and mice. Neurosci. Lett. 1994, 170, 153–157. [Google Scholar] [CrossRef]
- Manresa, J.A.B.; Schliessbach, J.; Vuilleumier, P.H.; Müller, M.; Musshoff, F.; Stamer, U.; Stüber, F.; Arendt-Nielsen, L.; Curatolo, M. Anti-nociceptive effects of oxytocin receptor modulation in healthy volunteers–A randomized, double-blinded, placebo-controlled study. Eur. J. Pain 2021, 25, 1723–1738. [Google Scholar] [CrossRef]
- Ramagnoli, S. Practical use of hormones in small animal reproduction. Rev. Bras. Reprod. Anim. 2017, 41, 59–67. [Google Scholar]
- Bazer, F.W.; First, N.L. Pregnancy and parturition. J. Anim. Sci. 1983, 57 (Suppl. 2), 425–460. [Google Scholar] [PubMed]
- Erickson, E.N.; Krol, K.M.; Perkeybile, A.M.; Connelly, J.J.; Myatt, L. Oxytocin receptor single nucleotide polymorphism predicts atony-related postpartum hemorrhage. BMC Pregnancy Childbirth 2022, 22, 884. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Martínez-Burnes, J.; Napolitano, F.; Domínguez-Muñoz, M.; Guerrero-Legarreta, I.; Mora-Medina, P.; Ramírez-Necoechea, R.; Lezama-García, K.; González-Lozano, M. Dystocia: Factors affecting parturition in domestic animals. CABI Rev. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Feldman, E.C.; Nelson, R.W. Canine and Feline Endocrinology and Reproduction; W.B. Saunders: Philadelphia, PA, USA, 2004. [Google Scholar]
- Davidson, A.P. Uterine and Fetal Monitoring in the Bitch. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.P. Primary Uterine Inertia in Four Labrador Bitches. J. Am. Anim. Hosp. Assoc. 2011, 47, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Martinez-Burnes, J.; Trujillo-Ortega, M.E.; Alonso-Spilsbury, M.L.; Ramirez-Necoechea, R.; Lopez, A. Effect of oxytocin treatment in sows on umbilical cord morphology, meconium staining, and neonatal mortality of piglets. Am. J. Vet. Res. 2002, 63, 1571–1574. [Google Scholar] [CrossRef]
- González-Lozano, M.; Mota-Rojas, D.; Velázquez-Armenta, E.Y.; Nava-Ocampo, A.A.; Hernández-González, R.; Becerril-Herrera, M.; Trujillo-Ortega, M.E.; Alonso-Spilsbury, M. Obstetric and fetal outcomes in dystocic and eutocic sows to an injection of exogenous oxytocin during farrowing. Am. Jew. Hist. 2009, 50, 1273–1277. [Google Scholar]
- Paccamonti, D. Milk electrolytes and induction of parturition. Pferdeheilkd. Equine Med. 2001, 17, 616–618. [Google Scholar] [CrossRef]
- Olmos-Hernández, A. Caracterización del patrón de comportamiento uterino y fetal a través del monitoreo electrónico en cerdas peri-parturientas en dos sistemas de alojamiento y su efecto sobre la viabilidad y grado de asfixia del neonato. MSc Thesis, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, 2006. [Google Scholar]
- Valros, A.; Rundgren, M.; Špinka, M.; Saloniemi, H.; Hultén, F.; Uvnäs-Moberg, K.; Tománek, M.; Krejcı, P.; Algers, B.; Krejcı, P.; et al. Oxytocin, prolactin and somatostatin in lactating sows: Associations with mobilisation of body resources and maternal behaviour. Livest. Prod. Sci. 2004, 85, 3–13. [Google Scholar] [CrossRef]
- Francis, D.D.; Young, L.J.; Meaney, M.J.; Insel, T.R. Naturally Occurring Differences in Maternal Care are Associated with the Expression of Oxytocin and Vasopressin (V1a) Receptors: Gender Differences. J. Neuroendocrinol. 2002, 14, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, S.; Stewart, R.A.; Demas, G.E.; Alberts, J.R. Maternal Contact Differentially Modulates Central and Peripheral Oxytocin in Rat Pups During a Brief Regime of Mother-Pup Interaction that Induces a Filial Huddling Preference. J. Neuroendocrinol. 2012, 24, 831–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, R.; Lévy, F.; Chaillou, E.; Cornilleau, F.; Cognié, J.; Marnet, P.-G.; Williams, P.D.; Keller, M. Neonatal Suckling, Oxytocin, and Early Infant Attachment to the Mother. Front. Endocrinol. 2021, 11, 612651. [Google Scholar] [CrossRef] [PubMed]
- Kockaya, M.; Ercan, N.; Demirbas, Y.S.; Pereira, G.D.G. Serum oxytocin and lipid levels of dogs with maternal cannibalism. J. Vet. Behav. 2018, 27, 23–26. [Google Scholar] [CrossRef]
- Feldman, R.; Gordon, I.; Zagoory-Sharon, O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: Considering stress and affiliation components of human bonding. Dev. Sci. 2011, 14, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Ogi, A.; Mariti, C.; Pirrone, F.; Baragli, P.; Gazzano, A. The Influence of Oxytocin on Maternal Care in Lactating Dogs. Animals 2021, 11, 1130. [Google Scholar] [CrossRef]
- Bienboire-Frosini, C.; Chabaud, C.; Cozzi, A.; Codecasa, E.; Pageat, P. Validation of a Commercially Available Enzyme ImmunoAssay for the Determination of Oxytocin in Plasma Samples from Seven Domestic Animal Species. Front. Neurosci. 2017, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- MacLean, E.L.; Wilson, S.R.; Martin, W.L.; Davis, J.M.; Nazarloo, H.P.; Carter, C.S. Challenges for measuring oxytocin: The blind men and the elephant? Psychoneuroendocrinology 2019, 107, 225–231. [Google Scholar] [CrossRef]
- Faraz, A.; Waheed, A.; Nazir, M.M.; Hameed, A.; Tauqir, N.A.; Mirza, R.H.; Ishaq, H.M.; Bilal, R.M. Impact of Oxytocin Administration on Milk Quality, Reproductive Performance and Residual Effects in Dairy Animals-A Review. Punjab Univ. J. Zool. 2020, 35, 61–67. [Google Scholar] [CrossRef]
- Simpson, K.R.; James, D.C. Effects of oxytocin-induced uterine hyperstimulation during labor on fetal oxygen status and fetal heart rate patterns. Am. J. Obstet. Gynecol. 2008, 199, 34.e1–34.e5. [Google Scholar] [CrossRef]
- Singhi, S.; Singh, M. Pathogenesis of oxytocin-induced neonatal hyperbilirubinaemia. Arch. Dis. Child. 1979, 54, 400–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Ávalos, I.; Mota-Rojas, D.; Mora-Medina, P.; Martínez-Burnes, J.; Casas-Alvarado, A.; Verduzco-Mendoza, A.; Lezama-García, K.; Olmos-Hernández, A. Review of different methods used for clinical recognition and assessment of pain in dogs and cats. Int. J. Vet. Sci. Med. 2019, 7, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnanadesikan, G.E.; Hammock, E.A.D.; Tecot, S.R.; Lewis, R.J.; Hart, R.; Carter, C.S.; MacLean, E.L. What are oxytocin assays measuring? Epitope mapping, metabolites, and comparisons of wildtype & knockout mouse urine. Psychoneuroendocrinology 2022, 143, 105827. [Google Scholar] [CrossRef]
- Carter, A.M.; Mess, A.M. The evolution of fetal membranes and placentation in carnivores and ungulates (Ferungulata). Anim. Reprod. 2017, 14, 124–135. [Google Scholar] [CrossRef]
- Rapacz-Leonard, A.; Leonard, M.; Chmielewska-Krzesińska, M.; Siemieniuch, M.; Janowski, T.E. The oxytocin-prostaglandins pathways in the horse (Equus caballus) placenta during pregnancy, physiological parturition, and parturition with fetal membrane retention. Sci. Rep. 2020, 10, 2089. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, A.R.; Helmer, H.; Chang, S.M.; Fields, J.M. Concentration of oxytocin receptors in the palcenta and fetal membranes of cows during pregancy and labour. J. Reprod. Fert. 1992, 96, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Giri, T.; Jiang, J.; Xu, Z.; McCarthy, R.; Halabi, C.M.; Tycksen, E.; Cahill, A.G.; England, S.K.; Palanisamy, A. Labor induction with oxytocin in pregnant rats is not associated with oxidative stress in the fetal brain. Sci. Rep. 2022, 12, 3143. [Google Scholar] [CrossRef]
- Vannucchi, C.I.; Rodrigues, J.A.; Silva, L.C.G.; Lúcio, C.F.; Veiga, G.A.L. Effect of dystocia and treatment with oxytocin on neonatal calf vitality and acid-base, electrolyte and haematological status. Vet. J. 2015, 203, 228–232. [Google Scholar] [CrossRef]
- Muro, B.B.D.; Carnevale, R.F.; Andretta, I.; Leal, D.F.; Monteiro, M.S.; Poor, A.P.; Almond, G.W.; Garbossa, C.A.P. Effects of uterotonics on farrowing traits and piglet vitality: A systematic review and meta-analysis. Theriogenology 2021, 161, 151–160. [Google Scholar] [CrossRef]
- Panaitescu, A.; Isac, S.; Pavel, B.; Illie, A.S.; Ceanga, M.; Totan, A.; Zagrean, L.; Peltecu, G.; Zagrean, A.M. Oxytocin reduces seizure burden and hippocampal injury in a rat model of perinatal asphyxia. Acta Endocrinol. 2018, 14, 315–319. [Google Scholar] [CrossRef]
- Nagel, C.; Aurich, C. Induction of parturition in horses-from physiological pathways to clinical applications. Domest. Anim. Endocrinol. 2022, 78, 106670. [Google Scholar] [CrossRef] [PubMed]
- Hermes, R.; Saragusty, J.; Schaftenaar, W.; Göritz, F.; Schmitt, D.L.; Hildebrandt, T.B. Obstetrics in elephants. Theriogenology 2008, 70, 131–144. [Google Scholar] [CrossRef]
- Thitaram, C.; Pongsopawijit, P.; Thongtip, N.; Angkavanich, T.; Chansittivej, S.; Wongkalasin, W.; Somgird, C.; Suwankong, N.; Prachsilpchai, W.; Suchit, K.; et al. Dystocia following prolonged retention of a dead fetus in an Asian elephant (Elephas maximus). Theriogenology 2006, 66, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D. Reproductive System. In Biology, Medicine, and Surgery of Elephants; Fowler, M.E., Mikota, S.K., Eds.; Blackwell Publishing Ltd: Oxford, UK, 2006; pp. 347–355. [Google Scholar]
- Leung, A.S.; Leung, E.K.; Paul, R.H. Uterine rupture after previous cesarean delivery: Maternal and fetal consequences. Am. J. Obstet. Gynecol. 1993, 169, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Humm, K.R.; Adamantos, S.E.; Benigni, L.; Armitage-Chan, E.A.; Brockman, D.J.; Chan, D.L. Uterine Rupture and Septic Peritonitis Following Dystocia and Assisted Delivery in a Great Dane Bitch. J. Am. Anim. Hosp. Assoc. 2010, 46, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Peltoniemi, O.; Björkman, S.; Oliviero, C. Parturition effects on reproductive health in the gilt and sow. Reprod. Domest. Anim. 2016, 51, 36–47. [Google Scholar] [CrossRef] [Green Version]
- van Wettere, W.H.E.J.; Toplis, P.; Miller, H.M. Effect of oral progesterone and caffeine at the end of gestation on farrowing duration and piglet growth and survival. Animal 2018, 12, 1638–1641. [Google Scholar] [CrossRef]
- Ishii, M.; Kobayashi, S.; Acosta, T.J.; Miki, W.; Matsui, M.; Yamanoi, T.; Miyake, Y.; Miyamoto, A. Effective Oxytocin Treatment on placenta Expulsion after Foaling in Heavy Draft Mares. J. Vet. Med. Sci. 2009, 71, 293–297. [Google Scholar] [CrossRef] [Green Version]
- Sgorbini, M.; Freccero, F.; Castagnetti, C.; Mariella, J.; Lanci, A.; Marmorini, P.; Camillo, F. Peripartum findings and blood gas analysis in newborn foals born after spontaneous or induced parturition. Theriogenology 2020, 158, 18–23. [Google Scholar] [CrossRef]
- Duggan, V.E.; Holyoak, G.R.; MacAllister, C.G.; Confer, A.W. Influence of induction of parturition on the neonatal acute phase response in foals. Theriogenology 2007, 67, 372–381. [Google Scholar] [CrossRef]
- Glasper, E.R.; Kenkel, W.M.; Bick, J.; Rilling, J.K. More than just mothers: The neurobiological and neuroendocrine underpinnings of allomaternal caregiving. Front. Neuroendocrinol. 2019, 53, 100741. [Google Scholar] [CrossRef] [PubMed]
- Rørvang, M.V.; Nielsen, B.L.; Herskin, M.S.; Jensen, M.B. Prepartum Maternal Behavior of Domesticated Cattle: A Comparison with Managed, Feral, and Wild Ungulates. Front. Vet. Sci. 2018, 5, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uvnäs-Moberg, K.; Johansson, B.; Lupoli, B.; Svennersten-Sjaunja, K. Oxytocin facilitates behavioural, metabolic and physiological adaptations during lactation. Appl. Anim. Behav. Sci. 2001, 72, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Nevard, R.P.; Pant, S.D.; Broster, J.C.; Norman, S.T.; Stephen, C.P. Maternal Behavior in Beef Cattle: The Physiology, Assessment and Future Directions—A Review. Vet. Sci. 2022, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Holtfrerich, S.K.C.; Pfister, R.; El Gammal, A.T.; Bellon, E.; Diekhof, E.K. Endogenous testosterone and exogenous oxytocin influence the response to baby schema in the female brain. Sci. Rep. 2018, 8, 7672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witteveen, A.B.; Stramrood, C.A.I.; Henrichs, J.; Flanagan, J.C.; van Pampus, M.G.; Olff, M. The oxytocinergic system in PTSD following traumatic childbirth: Endogenous and exogenous oxytocin in the peripartum period. Arch. Womens. Ment. Health 2020, 23, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Quintana, D.S.; Lischke, A.; Grace, S.; Scheele, D.; Ma, Y.; Becker, B. Advances in the field of intranasal oxytocin research: Lessons learned and future directions for clinical research. Mol. Psychiatry 2021, 26, 80–91. [Google Scholar] [CrossRef]
- Lee, M.R.; Shnitko, T.A.; Blue, S.W.; Kaucher, A.V.; Winchell, A.J.; Erikson, D.W.; Grant, K.A.; Leggio, L. Labeled oxytocin administered via the intranasal route reaches the brain in rhesus macaques. Nat. Commun. 2020, 11, 2783. [Google Scholar] [CrossRef]
- Munn, M. Comparison of two oxytocin regimens to prevent uterine atony at cesarean delivery: A randomized controlled trial. Obstet. Gynecol. 2001, 98, 386–390. [Google Scholar] [CrossRef]
- Carvalho, J.C.A.; Balki, M.; Kingdom, J.; Windrim, R. Oxytocin Requirements at Elective Cesarean Delivery: A Dose-Finding Study. Obstet. Gynecol. 2004, 104, 1005–1010. [Google Scholar] [CrossRef] [Green Version]
- Grotegut, C.A.; Paglia, M.J.; Johnson, L.N.C.; Thames, B.; James, A.H. Oxytocin exposure during labor among women with postpartum hemorrhage secondary to uterine atony. Am. J. Obstet. Gynecol. 2011, 204, 56.e1–56.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehan, S.R.; Montgomery, A.A.; Carey, M.; McAuliffe, F.M.; Eogan, M.; Gleeson, R.; Geary, M.; Murphy, D.J. Oxytocin bolus versus oxytocin bolus and infusion for control of blood loss at elective caesarean section: Double blind, placebo controlled, randomised trial. BMJ 2011, 343, d4661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, A.P. Obstetrical monitoring in dogs. Vet. Med. 2003, 85, 508–516. [Google Scholar]
| Dose | Absorption Kinetics | Pharmacokinetics | Species | Reference | |
|---|---|---|---|---|---|
| Administration Route | Evaluated Parameters | ||||
| 25 IU | IV | T1/2: 5.89 min., clearance rate of 11.67 L/min., and mean residence time of 7.78 min. | Maximum effective concentration of 0.45 ng/mL and a plasma concentration of 0.25 ng/mL | Mare | Steckler et al. [57] |
| 10 IU | IM | Minimal plasma concentration at 20 min. of administration 4822 pg/h/mL. | Tmax = 1.13 ± 0.91 h, Cmax = 2662 ± 567 pg/mL, AUC = 4822 ± 728 pg/h/mL, T1/2 = 1.02 ± 0.33 h. | Rabbit | Zhu and Lal [63] |
| 400 IU | Sublingual | Plasmatic concentration at 20 min. of administration 1234 pg/h/mL. | Tmax = 0.93 ± 0.64 h, Cmax = 1164 ± 1179 pg/mL, AUC = 1234 ± 1001 pg/h/mL, T1/2 = 0.90 ± 0.33 h. | ||
| 0.33–1.32 IU | Intranasal | T1/2 = 12.2 min., Tmax = 12.2 min., Mean plasma concentration = 18.9 pg/mL. | Cmax = 77.3 pg/mL, AUC = 1726 pg/mL/min., mean residence time = 20 min. | Cow | Wagner et al. [64] |
| 0.083, 0.11 and 0.17 IU | IM | T1/2 = 1–6 min. Bioavailability: 70–100%, Vss: 40 min. | Clearance rate 7.87 mL. | Sow | Mota-Rojas et al. [65], Hill [66] |
| 40 IU 20 IU | IM IV Intravulvar | T1/2 = 1.94 ± 0.21 min. | Vd = 0.46 ± 0.02 L/kg, T1/2 of elimination = 22.3 ± 0.3 min. | Sow | Mota-Rojas et al. [67], Ybarra Navarro [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcet-Rius, M.; Bienboire-Frosini, C.; Lezama-García, K.; Domínguez-Oliva, A.; Olmos-Hernández, A.; Mora-Medina, P.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Gazzano, A. Clinical Experiences and Mechanism of Action with the Use of Oxytocin Injection at Parturition in Domestic Animals: Effect on the Myometrium and Fetuses. Animals 2023, 13, 768. https://doi.org/10.3390/ani13040768
Marcet-Rius M, Bienboire-Frosini C, Lezama-García K, Domínguez-Oliva A, Olmos-Hernández A, Mora-Medina P, Hernández-Ávalos I, Casas-Alvarado A, Gazzano A. Clinical Experiences and Mechanism of Action with the Use of Oxytocin Injection at Parturition in Domestic Animals: Effect on the Myometrium and Fetuses. Animals. 2023; 13(4):768. https://doi.org/10.3390/ani13040768
Chicago/Turabian StyleMarcet-Rius, Míriam, Cécile Bienboire-Frosini, Karina Lezama-García, Adriana Domínguez-Oliva, Adriana Olmos-Hernández, Patricia Mora-Medina, Ismael Hernández-Ávalos, Alejandro Casas-Alvarado, and Angelo Gazzano. 2023. "Clinical Experiences and Mechanism of Action with the Use of Oxytocin Injection at Parturition in Domestic Animals: Effect on the Myometrium and Fetuses" Animals 13, no. 4: 768. https://doi.org/10.3390/ani13040768
APA StyleMarcet-Rius, M., Bienboire-Frosini, C., Lezama-García, K., Domínguez-Oliva, A., Olmos-Hernández, A., Mora-Medina, P., Hernández-Ávalos, I., Casas-Alvarado, A., & Gazzano, A. (2023). Clinical Experiences and Mechanism of Action with the Use of Oxytocin Injection at Parturition in Domestic Animals: Effect on the Myometrium and Fetuses. Animals, 13(4), 768. https://doi.org/10.3390/ani13040768









