Feature Importance Analysis for Postural Deformity Detection System Using Explainable Predictive Modeling Technique
Abstract
:1. Introduction
2. Materials and Methods
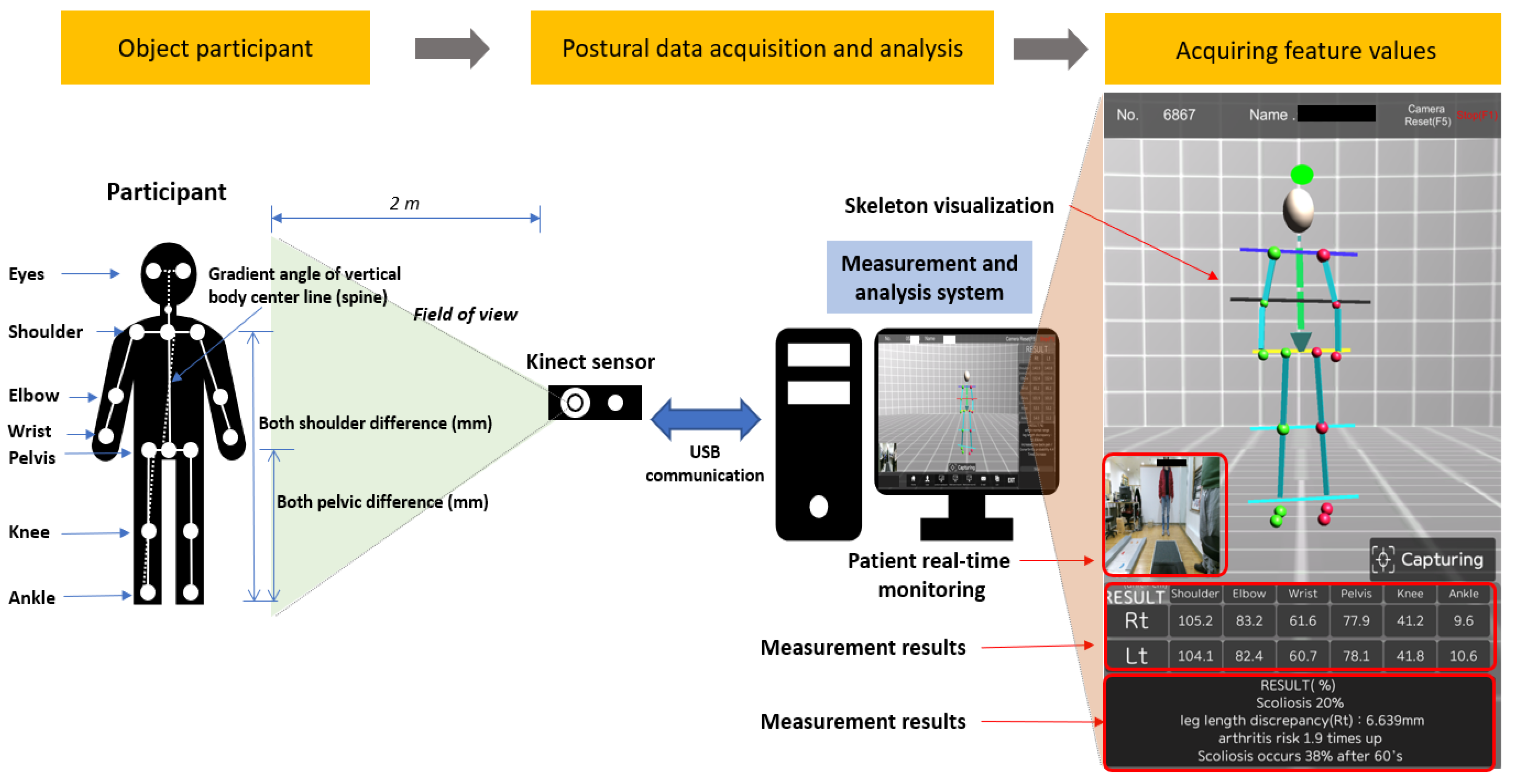
2.1. Data Acquisition and Participant Characteristics
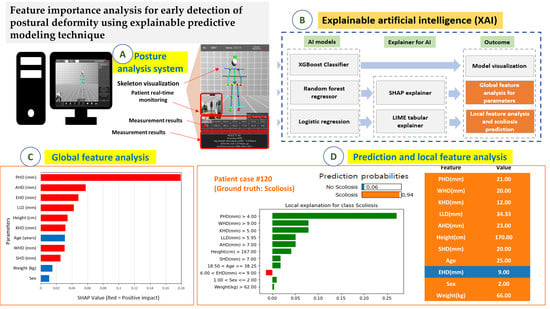
2.2. Predictive Models and Model Explainers
2.3. Research Process for Predictive Modeling
3. Results
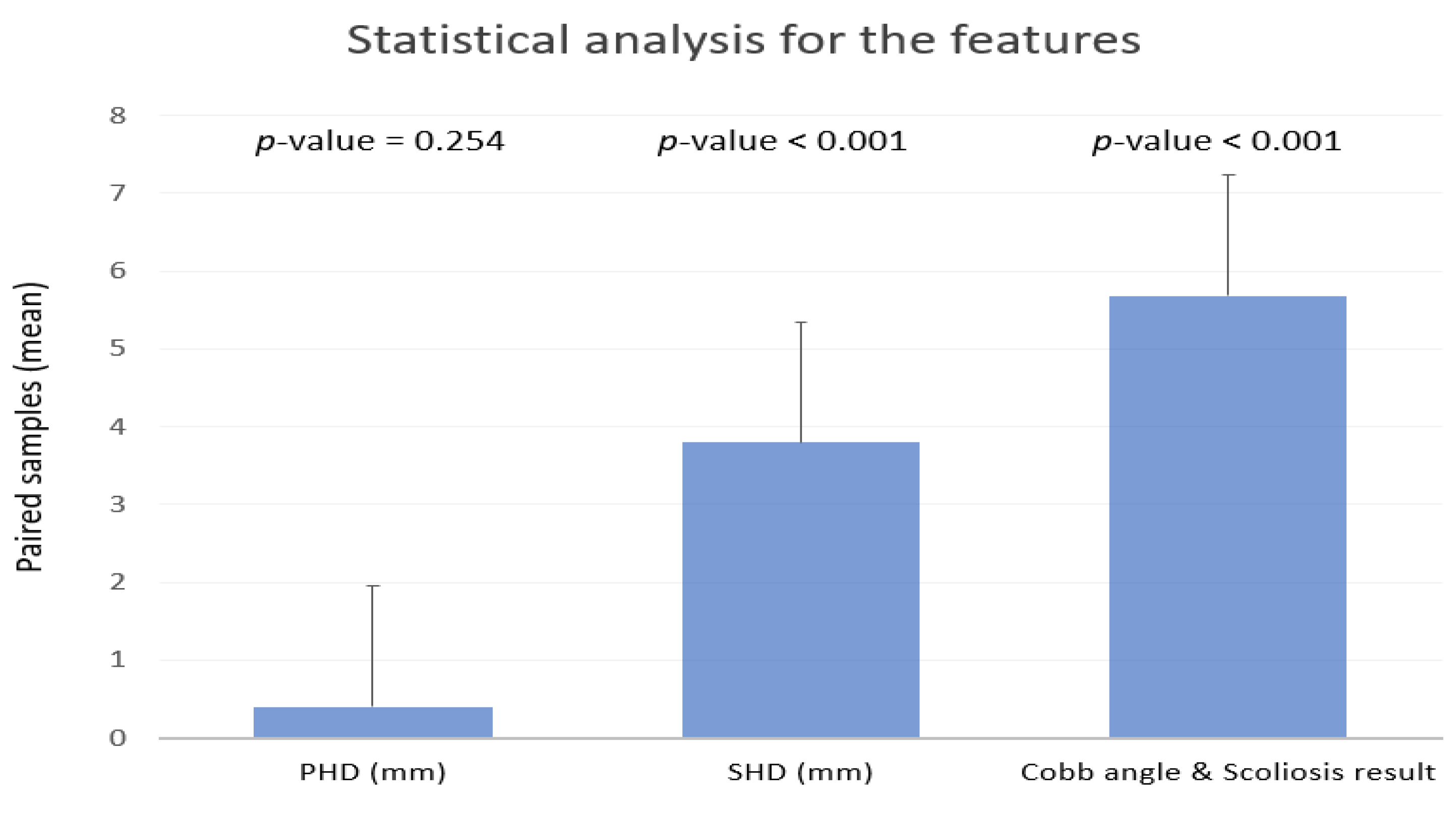
3.1. Radiographic Assessment and Statistical Analysis
3.2. Predictive Modeling Performance
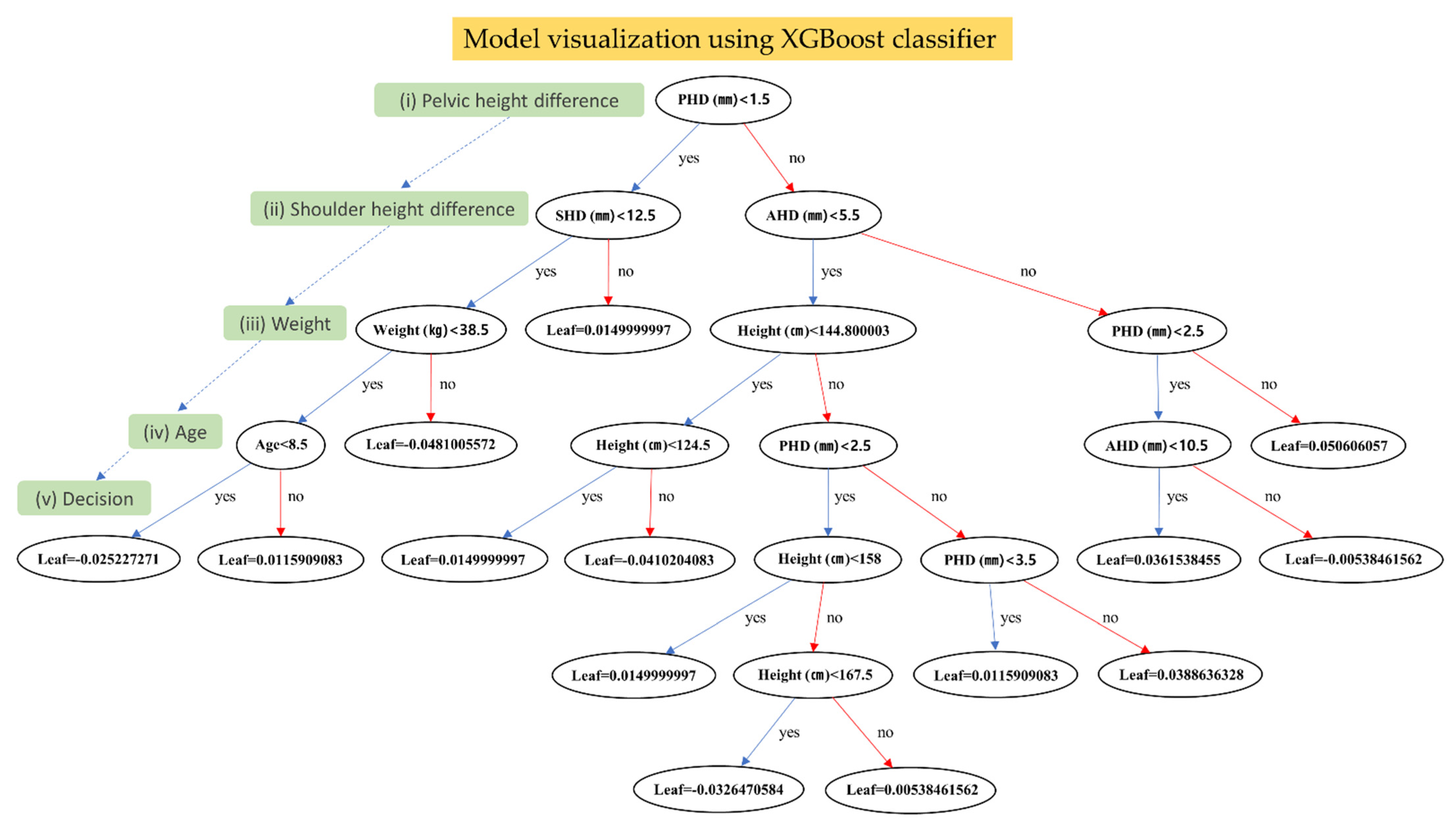
3.3. Model Visualization for the Predictive Model
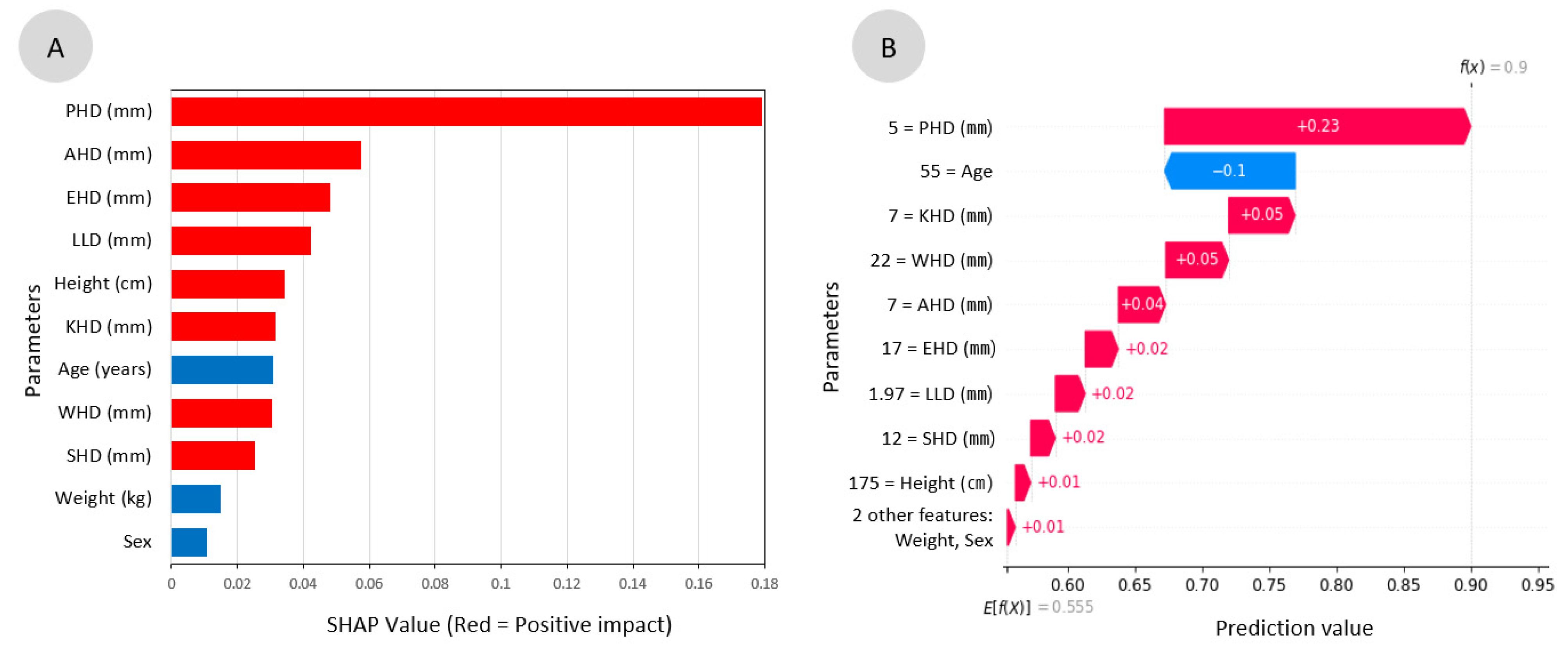
3.4. Feature Analysis for the Parameters and Global Interpretation
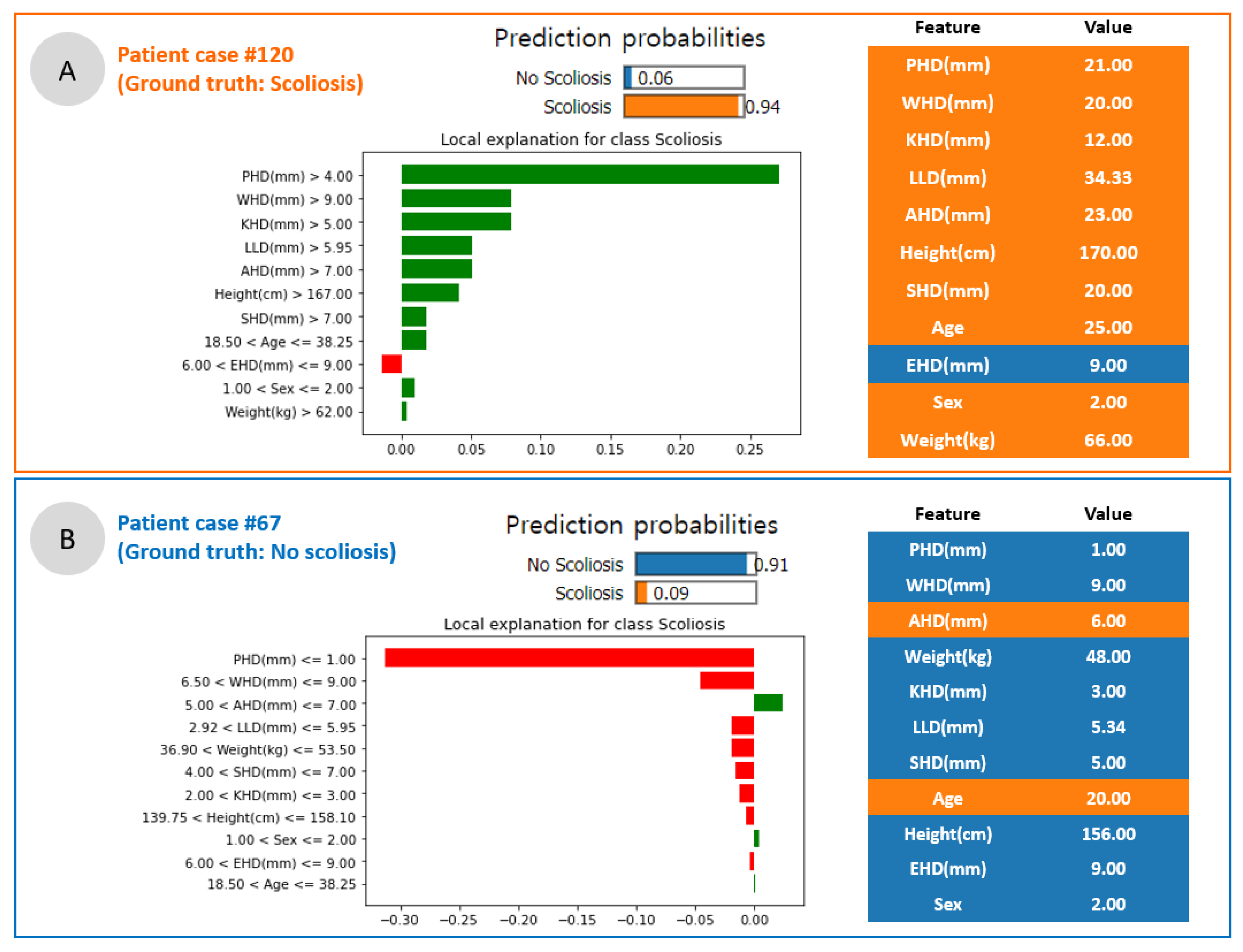
3.5. Scoliosis Prediction and Local Interpretation
4. Discussion
4.1. Scoliosis Screening System Combined with AI
4.2. Use of Explainable Artificial Intelligence for In-Depth Predictive Modeling
4.3. Global vs. Local Interpretation for the Parameters
4.4. Limitations of Dataset
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hawes, M.C.; O’brien, J.P. The transformation of spinal curvature into spinal deformity: Pathological processes and implications for treatment. Scoliosis 2006, 1, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balagué, F.; Pellisé, F. Adolescent idiopathic scoliosis and back pain. Scoliosis Spinal Disord. 2016, 11, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Improvement of back pain with operative and nonoperative treatment in adults with scoliosis. Neurosurgery 2009, 65, 86–94. [CrossRef]
- Akel, I.; Pekmezci, M.; Hayran, M.; Genc, Y.; Kocak, O.; Derman, O.; Erdoğan, I.; Yazici, M. Evaluation of shoulder balance in the normal adolescent population and its correlation with radiological parameters. Eur. Spine J. 2008, 17, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Malfair, D.; Flemming, A.K.; Dvorak, M.F.; Munk, P.L.; Vertinsky, A.T.; Heran, M.K.; Graeb, D.A. Radiographic evaluation of scoliosis. Am. J. Roentgenol. 2010, 194, S8–S22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soucacos, P.N.; Zacharis, K.; Soultanis, K.; Gelalis, J.; Xenakis, T.; Beris, A.E. Risk factors for idiopathic scoliosis: Review of a 6-year prospective study. Orthopedics 2000, 23, 833–838. [Google Scholar] [CrossRef]
- Walker, A.; Dickson, R. School screening and pelvic tilt scoliosis. Lancet 1984, 324, 152–154. [Google Scholar] [CrossRef]
- Alrehily, F.; Hogg, P.; Twiste, M.; Johansen, S.; Tootell, A. Scoliosis imaging: An analysis of radiation risk in the CT scan projection radiograph and a comparison with projection radiography and EOS. Radiography 2019, 25, e68–e74. [Google Scholar] [CrossRef]
- Mahaudens, P.; Banse, X.; Mousny, M.; Detrembleur, C. Gait in adolescent idiopathic scoliosis: Kinematics and electromyographic analysis. Eur. Spine J. 2009, 18, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.-s.; Cho, Y.-S.; Moon, S.-B.; Kim, M.-J.; Lee, H.D.; Lee, S.Y.; Ji, Y.-H.; Park, Y.-S.; Han, C.-S.; Jang, S.-H. Scoliosis screening through a machine learning based gait analysis test. Int. J. Precis. Eng. Manuf. 2018, 19, 1861–1872. [Google Scholar] [CrossRef]
- Alharbi, R.H.; Alshaye, M.B.; Alkanhal, M.M.; Alharbi, N.M.; Alzahrani, M.A.; Alrehaili, O.A. Deep Learning Based Algorithm For Automatic Scoliosis Angle Measurement. In Proceedings of the 2020 3rd International Conference on Computer Applications & Information Security (ICCAIS), Riyadh, Saudi Arabia, 19–21 March 2020; pp. 1–5. [Google Scholar]
- Pasha, S.; Shah, S.; Newton, P.; Group, H.S. Machine Learning Predicts the 3D Outcomes of Adolescent Idiopathic Scoliosis Surgery Using Patient–Surgeon Specific Parameters. Spine 2021, 46, 579–587. [Google Scholar] [CrossRef]
- Tajdari, M.; Pawar, A.; Li, H.; Tajdari, F.; Maqsood, A.; Cleary, E.; Saha, S.; Zhang, Y.J.; Sarwark, J.F.; Liu, W.K. Image-based modelling for Adolescent Idiopathic Scoliosis: Mechanistic machine learning analysis and prediction. Comput. Methods Appl. Mech. Eng. 2021, 374, 113590. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, K.; Fan, H.; Huang, Z.; Xiang, Y.; Yang, J.; He, L.; Zhang, L.; Yang, Y.; Li, R. Development and validation of deep learning algorithms for scoliosis screening using back images. Commun. Biol. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Cheng, J.C.; Castelein, R.M.; Chu, W.C.; Danielsson, A.J.; Dobbs, M.B.; Grivas, T.B.; Gurnett, C.A.; Luk, K.D.; Moreau, A.; Newton, P.O. Adolescent idiopathic scoliosis. Nat. Rev. Dis. Primers 2015, 1, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hresko, M.T.; Talwalkar, V.; Schwend, R. Early detection of idiopathic scoliosis in adolescents. JBJS 2016, 98, e67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunning, D.; Stefik, M.; Choi, J.; Miller, T.; Stumpf, S.; Yang, G.-Z. XAI—Explainable artificial intelligence. Sci. Robot. 2019, 4, eaay7120. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H. Xgboost: Extreme gradient boosting. R Package Version 0.4-2. Microsoft. 2015, 1, 1–4. [Google Scholar]
- Segal, M.R. Machine Learning Benchmarks and Random Forest Regression; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Christodoulou, E.; Ma, J.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; Van Calster, B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar] [CrossRef]
- Van den Broeck, G.; Lykov, A.; Schleich, M.; Suciu, D. On the tractability of SHAP explanations. arXiv 2020, arXiv:2009.08634. [Google Scholar]
- Ribeiro, M.T.; Singh, S.; Guestrin, C. Model-agnostic interpretability of machine learning. arXiv 2016, arXiv:1606.05386. [Google Scholar]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An overview of clinical decision support, systems: Benefits, risks, and strategies for success. NPJ Digit. Med. 2020, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhai, X.; Sun, K.; Wang, H.; Yang, C.; Li, M. A narrative review of machine learning as promising revolution in clinical practice of scoliosis. Ann. Transl. Med. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Yip, J.; To, K.-T.M.; Fan, Y. Machine Learning Approaches to Predict Scoliosis. In International Conference on Applied Human Factors and Ergonomics; Springer: Cham, Switzerland, 2021; pp. 116–121. [Google Scholar]
- Arrieta, A.B.; Díaz-Rodríguez, N.; Del Ser, J.; Bennetot, A.; Tabik, S.; Barbado, A.; García, S.; Gil-López, S.; Molina, D.; Benjamins, R. Explainable Artificial Intelligence (XAI): Concepts, taxonomies, opportunities and challenges toward responsible AI. Inf. Fusion 2020, 58, 82–115. [Google Scholar] [CrossRef] [Green Version]
- Visani, G.; Bagli, E.; Chesani, F.; Poluzzi, A.; Capuzzo, D. Statistical stability indices for LIME: Obtaining reliable explanations for machine learning models. J. Oper. Res. Soc. 2020, 1–11. [Google Scholar] [CrossRef]
- Deshpande, G.; Ruhe, G.; Saunders, C. How Much Data Analytics is Enough? The ROI of Machine Learning Classification and its Application to Requirements Dependency Classification. arXiv 2021, arXiv:2109.14097. [Google Scholar]
- Staartjes, V.E.; Stumpo, V.; Kernbach, J.M.; Klukowska, A.M.; Gadjradj, P.S.; Schröder, M.L.; Veeravagu, A.; Stienen, M.N.; van Niftrik, C.H.; Serra, C. Machine learning in neurosurgery: A global survey. Acta Neurochir. 2020, 162, 3081–3091. [Google Scholar] [CrossRef]
- Abdollahi, B.; Tomita, N.; Hassanpour, S. Data augmentation in training deep learning models for medical image analysis. In Deep Learners and Deep Learner Descriptors for Medical Applications; Springer: Cham, Switzerland, 2020; pp. 167–180. [Google Scholar]


| Authors | Feature Importance | Dataset | Modeling | Outcome |
|---|---|---|---|---|
| Alharbi et al. [11] | None | Radiographic images | Deep learning | Scoliosis angle measurement |
| Pasha et al. [12] | None | Radiographic images | Machine learning | Surgical outcome |
| Tajdari et al. [13] | None | Radiographic images | Machine learning | Biomechanic prediction |
| Yang et al. [14] | None | Radiographic images | Deep learning | Scoliotic area prediction |
| This study | Shoulder height difference, wrist height difference, pelvic height difference, etc. | Tabular data | Machine learning | Feature importance analysis and postural deformity prediction |
| Category | Characteristics | Mean | p-Value | Data Type | Values | |
|---|---|---|---|---|---|---|
| Parameters from computer vision-based posture analysis system | Age (years) | 24.94 ± 17.36 | 0.245 | Float | 3 to 69 | |
| Sex | Male | 59 (42.14%) | 0.549 | Binary | 1: Male, | |
| Female | 81 (57.86%) | 2: Female | ||||
| Height (cm) | 153.43±18.57 | 0.671 | Float | 94 to 186 | ||
| Weight (kg) | 51.51±18.13 | 0.966 | Float | 14.6 to 98 | ||
| SHD (mm) | 4.91±4.78 | 0.142 | Float | 0 to 31 | ||
| EHD (mm) | 6.41±5.30 | 0.041 | Float | 0 to 36 | ||
| WHD (mm) | 7.29±7.26 | 0.017 | Float | 0 to 58 | ||
| PHD (mm) | 2.49±2.73 | <0.01 | Float | 0 to 21 | ||
| KHD (mm) | 3.94±3.73 | <0.01 | Float | 0 to 27 | ||
| AHD (mm) | 6.03±6.16 | <0.01 | Float | 0 to 39 | ||
| LLD (mm) | 4.54±4.73 | 0.005 | Float | 0 to 34.33 | ||
| Radiographic assessment | Cobb angle | 6.16°±8.50 | NA | |||
| SHD (mm) | 1.12±3.27 | |||||
| PHD (mm) | 2.89±4.22 | |||||
| Curve type | Normal range | 61 (43.57%) | ||||
| Thoracic | 39 (27.86%) | |||||
| Thoraco-lumbar | 22 (15.71%) | |||||
| lumbar | 18 (12.86%) | |||||
| Models | Accuracy | Sensitivity | Specificity | AUC |
|---|---|---|---|---|
| Logistic regression | 0.78 | 0.72 | 0.83 | 0.63 |
| Random forest regression | 0.79 | 0.83 | 0.77 | 0.89 |
| XGBoost classifier | 0.79 | 0.79 | 0.79 | 0.79 |
| mean | 0.79 ± 0.00 | 0.78 ± 0.05 | 0.80 ± 0.02 | 0.77 ± 0.11 |
| Parameters | MI Scores |
|---|---|
| PHD (mm) | 0.22 |
| KHD (mm) | 0.09 |
| Age | 0.08 |
| LLD (mm) | 0.06 |
| Weight (kg) | 0.05 |
| EHD (mm) | 0.02 |
| WHD (mm) | 0.00 |
| Sex | 0.00 |
| Height (cm) | 0.00 |
| SHD (mm) | 0.00 |
| AHD (mm) | 0.00 |
| Authors | Aim | Dataset (n) | AI models | Evaluation | Results | Year | Reference |
|---|---|---|---|---|---|---|---|
| Pasha et al. | To predict the 3D radiographic outcomes of the spinal surgery in AIS | 371 | Random forest | Accuracy and AUC | The surgical factors, upper and lower instrumented vertebrae, and the operating surgeon were important surgical predictors (Accuracy = 75% and Max AUC = 0.86) | 2021 | [12] |
| Tajdari et al. | To propose a mechanistic machine learning algorithm in order to study patient-specific AIS curve progression | 353 | Mechanistic neural network | RAE | Age identification using X-ray images (RAE for 68 months (1.37%), 84 months (2.55%) and 127 months (0.87%)) | 2021 | [13] |
| Alharbi et al. | Scoliosis prediction by using deep learning | 800 | Convolutional neural network | Accuracy | Absolute Cobb angledifference < 5° in 69 images, 5°–10° in 50 images, and 10°–15° in 45 images (Accuracy = 90%) | 2020 | [11] |
| Yang et al. | To develop screening system using non-ionizing radiation in order to identify cases with a curve ≥20° | 3640 | Faster-RCNN and Resnet | AUC, sensitivity, specificity, and PPV | The level of trunk asymmetry revealed in the heat maps (AUC (0.946), sensitivity (87.5%), specificity (83.5%), and PPV (86.2%)) | 2019 | [14] |
| Cho et al. | Automatic cognition of gaitchanges due to scoliosis using gait measures | 42 | Support vector machine | Accuracy, sensitivity, and specificity | Analysis of the lower limb joint angle based on gait phase segmentation and clustering of scoliosis patients (Accuracy (90.5%), specificity (88.8%), and sensitivity (91.6%)) | 2018 | [10] |
| This study | To analyze feature importance to postural deformity parameters extracted from a CVPAS | 140 | Logistic regression, random forest, and XGBoost with SHAP and LIME (XAI) | Accuracy, AUC, sensitivity, and specificity | PHD was a major parameter with a difference of 3 mm threshold (Mean accuracy (0.79), sensitivity (0.78), specificity (0.80), and AUC (0.77)) | Present | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.H.; Choi, W.-J.; Sohn, M.-J. Feature Importance Analysis for Postural Deformity Detection System Using Explainable Predictive Modeling Technique. Appl. Sci. 2022, 12, 925. https://doi.org/10.3390/app12020925
Kim KH, Choi W-J, Sohn M-J. Feature Importance Analysis for Postural Deformity Detection System Using Explainable Predictive Modeling Technique. Applied Sciences. 2022; 12(2):925. https://doi.org/10.3390/app12020925
Chicago/Turabian StyleKim, Kwang Hyeon, Woo-Jin Choi, and Moon-Jun Sohn. 2022. "Feature Importance Analysis for Postural Deformity Detection System Using Explainable Predictive Modeling Technique" Applied Sciences 12, no. 2: 925. https://doi.org/10.3390/app12020925
APA StyleKim, K. H., Choi, W.-J., & Sohn, M.-J. (2022). Feature Importance Analysis for Postural Deformity Detection System Using Explainable Predictive Modeling Technique. Applied Sciences, 12(2), 925. https://doi.org/10.3390/app12020925