Vincristine in Combination Therapy of Cancer: Emerging Trends in Clinics
Abstract
:Simple Summary
Abstract
1. Introduction
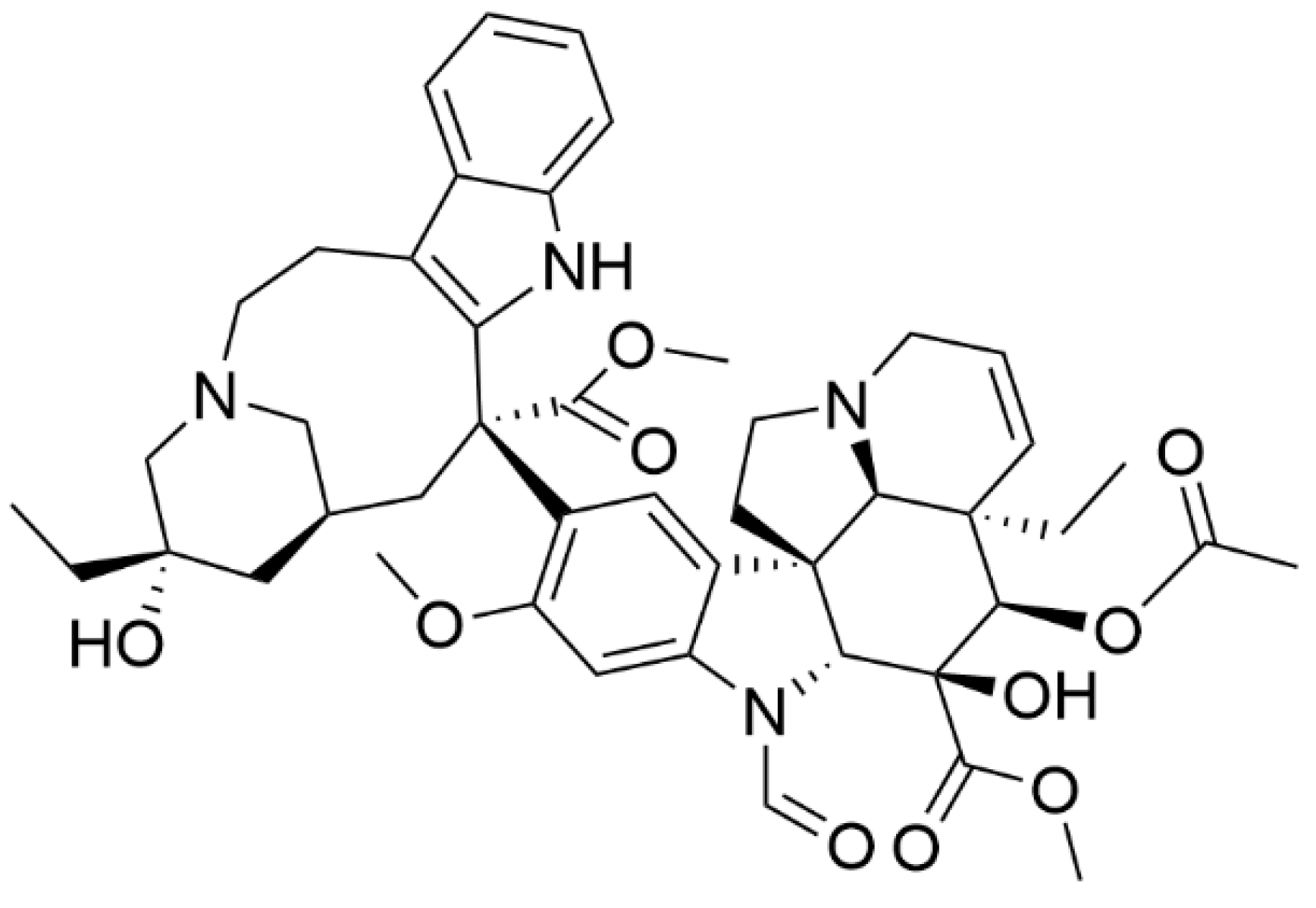
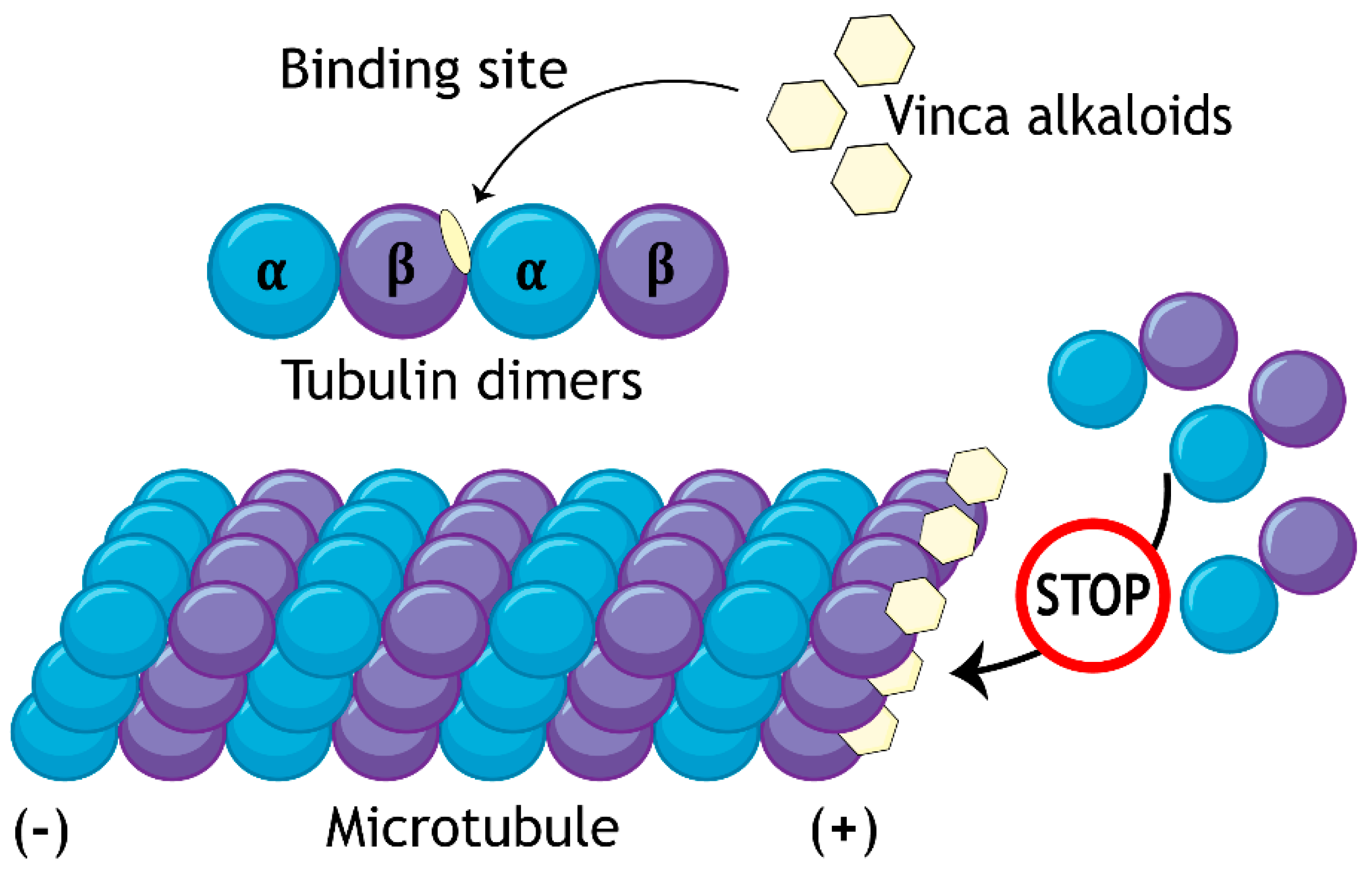
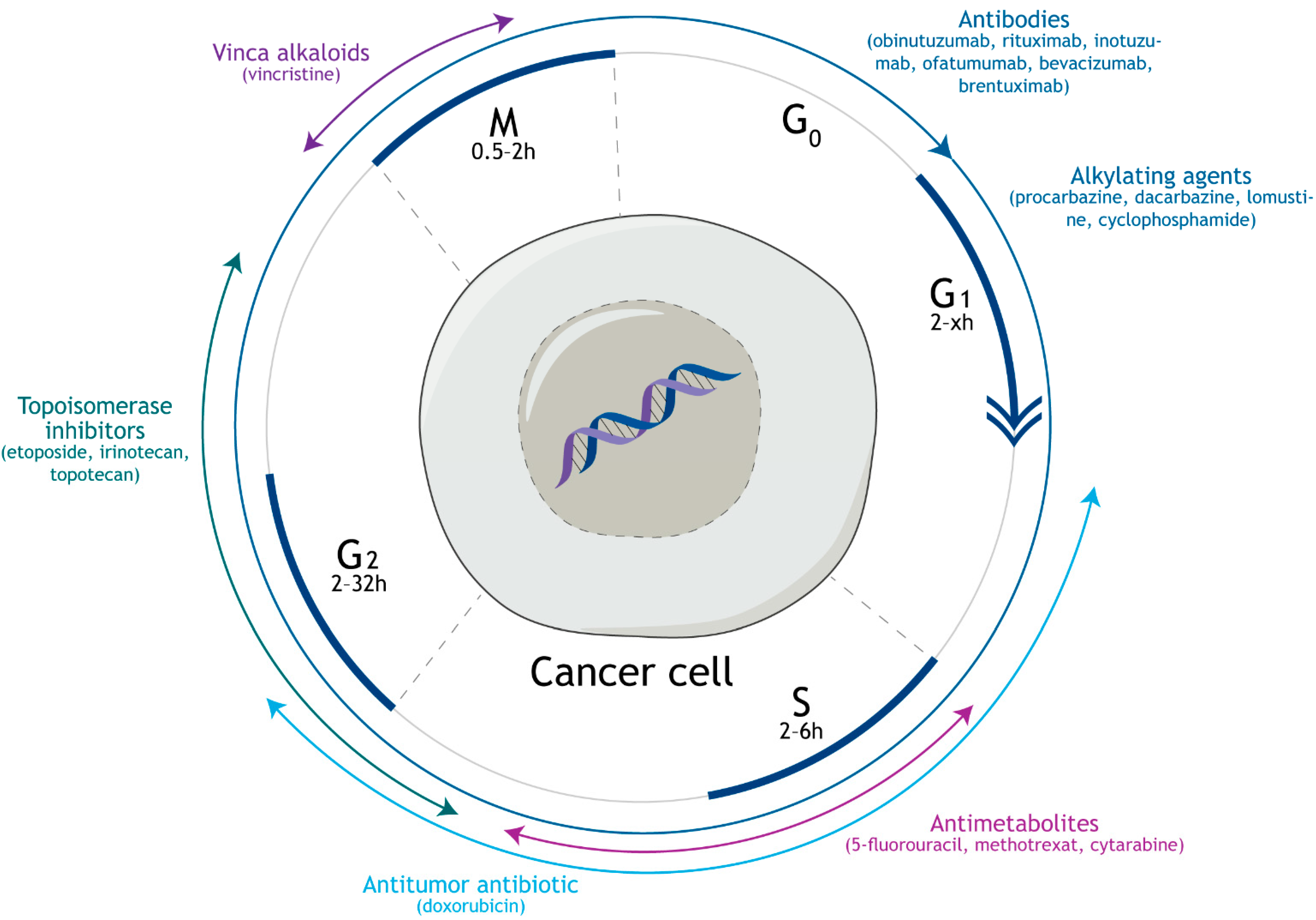
2. Mechanism of Action and Side Effects of Vincristine
3. Combinations of Vincristine with Cyclophosphamide, Doxorubicin, and Prednisone
4. Combination of Vincristine with Methotrexate
5. Combination of Vincristine with Procarbazine or Dacarbazine
6. Other Vincristine Combination Therapies
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A549 | Adenocarcinomic human alveolar basal epithelial cells |
| ABL | Tyrosine-protein kinase transforming protein Abl |
| ABCB1/Flp-In™-293 | Fetal kidney cell line stably expressing P-gp |
| ABVD | Chemotherapy with doxorubicin, bleomycin, vinblastine, and dacarbazine |
| BCR | Breakpoint cluster region protein (renal carcinoma antigen NY-REN-26) |
| BEACOPP | Chemotherapy combination of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone |
| CHOP | Chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone |
| CNS | Central nervous system |
| CVD | Chemotherapy with cyclophosphamide, vincristine, and dacarbazine |
| DNA | Deoxyribonucleic acid |
| Eca109/VCR | Human cells from esophageal cancer |
| EPOCH-(R) | Combination therapy with cyclophosphamide, doxorubicin, vincristine, prednisone, rituximab, and etoposide |
| EMA | Chemotherapy combination of methotrexate, etoposide, and dactinomy-cin |
| EMA/CO | Chemotherapy combination of etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine |
| FDA | US Food and Drug Administration |
| G401 | Human cells from a rhabdoid tumor of the kidney |
| IC50 | Half-maximal inhibitory concentration |
| IDH1/2 | Gene encoding nicotinamide adenine dinucleotide phosphate-dependent isocitrate dehydrogenases |
| IL-1 | Interleukin 1 receptor |
| KB-V | Cervical adenocarcinoma cell line |
| LGG | Low-grade gliomas |
| LC3-I | Microtubule-associated proteins 1A/1B light chain 3B |
| lncRNA | Long non-coding RNA |
| MCF-7/ADR | Multidrug-resistant breast cancer cell line |
| MEG3 | Maternally expressed gene 3 |
| MOPP | Combination of vincristine, procarbazine, prednisone, and mechlorethamine |
| NHP2L1 | Non-histone protein 2-like protein 1 |
| NOXA | Gene coding phorbol-12-myristate-13-acetate-induced protein 1 |
| PCNSL | Primary central nervous system lymphoma |
| PCV | Chemotherapy combination of procarbazine, lomustine, and vincristine |
| P-gp | P-glycoprotein |
| PMAIP1 | Gene encoding phorbol-12-myristate-13-acetate-induced protein 1 |
| R-CHOP | Combination therapy with cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab |
| R-CHOPE | Combination therapy with cyclophosphamide, doxorubicin, vincristine, prednisone, rituximab, and etoposide |
| RNA | Ribonucleic acid |
| VIPN | Vincristine-induced peripheral neuropathy |
| WERI-Rb-1 | Human cells from retinoblastoma |
| XIST | X-inactive specific transcript |
| Y79 | Human cells from retinoblastoma |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Neuss, N.; Gorman, M.; Boaz, H.E.; Cone, N.J. Vinca alkaloids. XI.1Structures of leurocristine (LCR) and vincaleukoblastine (VLB)2. J. Am. Chem. Soc. 1962, 84, 1509–1510. [Google Scholar] [CrossRef]
- Freireich, E.J.; Wiernik, P.H.; Steensma, D.P. The leukemias: A half-century of discovery. J. Clin. Oncol. 2014, 32, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Škubník, J.; Jurášek, M.; Ruml, T.; Rimpelová, S. Mitotic poisons in research and medicine. Molecules 2020, 25, 4632. [Google Scholar] [CrossRef] [PubMed]
- Field, J.J.; Waight, A.B.; Senter, P.D. A previously undescribed tubulin binder. Proc. Natl. Acad. Sci. USA 2014, 111, 13684–13685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himes, R.H. Interactions of the catharanthus (Vinca) alkaloids with tubulin and microtubules. Pharmacol. Ther. 1991, 51, 257–267. [Google Scholar] [CrossRef]
- Jordan, M.A.; Thrower, D.; Wilson, L. Mechanism of inhibition of cell proliferation by vinca alkaloids. Cancer Res. 1991, 51, 2212–2222. [Google Scholar]
- Boussios, S.; Pentheroudakis, G.; Katsanos, K.; Pavlidis, N. Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Ann. Gastroenterol. 2012, 25, 106–118. [Google Scholar]
- Botchkarev, V.A. Molecular mechanisms of chemotherapy-induced hair loss. J. Investig. Dermatol. Symp. Proc. 2003, 8, 72–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, M.B.; Maher, K.E. Chemotherapy-induced myelosuppression. Semin. Oncol. Nurs. 1992, 8, 113–123. [Google Scholar] [CrossRef]
- Kaufman, I.A.; Kung, F.H.; Koenig, H.M.; Giammona, S.T. Overdosage with vincristine. J. Pediatr. 1976, 89, 671–674. [Google Scholar] [CrossRef]
- Cantwell, B.M.J.; Begent, R.H.J.; Rubens, R.D. Augmentation of vincristine-induced thrombocytosis by norethisterone. Eur. J. Cancer 1965, 15, 1065–1069. [Google Scholar] [CrossRef]
- Buchanan, G.R.; Buchsbaum, H.J.; O’Banion, K.; Gojer, B. Extravasation of dactinomycin, vincristine, and cisplatin: Studies in an animal model. Med. Pediatr. Oncol. 1985, 13, 375–380. [Google Scholar] [CrossRef]
- Gidding, C.E.; Kellie, S.J.; Kamps, W.A.; de Graaf, S.S. Vincristine revisited. Crit. Rev. Oncol. Hematol. 1999, 29, 267–287. [Google Scholar] [CrossRef]
- Verma, P.; Devaraj, J.; Skiles, J.L.; Sajdyk, T.; Ho, R.H.; Hutchinson, R.; Wells, E.; Li, L.; Renbarger, J.; Cooper, B.; et al. A metabolomics approach for early prediction of vincristine-induced peripheral neuropathy. Sci. Rep. 2020, 10, 9659. [Google Scholar] [CrossRef] [PubMed]
- Stockstill, K.; Doyle, T.M.; Yan, X.; Chen, Z.; Janes, K.; Little, J.W.; Braden, K.; Lauro, F.; Giancotti, L.A.; Harada, C.M.; et al. Dysregulation of sphingolipid metabolism contributes to bortezomib-induced neuropathic pain. J. Exp. Med. 2018, 215, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Bielawski, J.; Kistner-Griffin, E.; Othman, A.; Alecu, I.; Ernst, D.; Kornhauser, D.; Hornemann, T.; Spassieva, S. Neurotoxic 1-deoxysphingolipids and paclitaxel-induced peripheral neuropathy. FASEB J. 2015, 29, 4461–4472. [Google Scholar] [CrossRef] [Green Version]
- Janes, K.; Little, J.W.; Li, C.; Bryant, L.; Chen, C.; Chen, Z.; Kamocki, K.; Doyle, T.; Snider, A.; Esposito, E.; et al. The development and maintenance of paclitaxel-induced neuropathic pain require activation of the sphingosine 1-phosphate receptor subtype 1. J. Biol. Chem. 2014, 289, 21082–21097. [Google Scholar] [CrossRef] [Green Version]
- Starobova, H.; Monteleone, M.; Adolphe, C.; Batoon, L.; Sandrock, C.J.; Tay, B.; Deuis, J.R.; Smith, A.V.; Mueller, A.; Nadar, E.I.; et al. Vincristine-induced peripheral neuropathy is driven by canonical NLRP3 activation and IL-1β release. J. Exp. Med. 2021, 218, e20201452. [Google Scholar] [CrossRef]
- Linschoten, M.; Kamphuis, J.A.M.; Van Rhenen, A.; Bosman, L.P.; Cramer, M.J.; Doevendans, P.A.; Teske, A.J.; Asselbergs, F.W. Cardiovascular adverse events in patients with non-Hodgkin lymphoma treated with first-line cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP with rituximab (R-CHOP): A systematic review and meta-analysis. Lancet Haematol. 2020, 7, E295–E308. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Barone, A.; Chi, D.-C.; Theoret, M.R.; Chen, H.; He, K.; Kufrin, D.; Helms, W.S.; Subramaniam, S.; Zhao, H.; Patel, A.; et al. FDA approval summary: Trabectedin for unresectable or metastatic liposarcoma or leiomyosarcoma following an anthracycline-containing regimen. Clin. Cancer Res. 2017, 23, 7448–7453. [Google Scholar] [CrossRef] [Green Version]
- Lossignol, D. A little help from steroids in oncology. J. Transl. Int. Med. 2016, 4, 52–54. [Google Scholar] [CrossRef] [Green Version]
- Coiffier, B.; Lepage, E.; Briere, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef]
- Mohammed, R.; Milne, A.; Kayani, K.; Ojha, U. How the discovery of rituximab impacted the treatment of B-cell non-Hodgkin’s lymphomas. J. Blood Med. 2019, 10, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Mondello, P.; Mian, M. Frontline treatment of diffuse large B-cell lymphoma: Beyond R-CHOP. Hematol. Oncol. 2019, 37, 333–344. [Google Scholar] [CrossRef]
- Baldwin, E.L.; Osheroff, N. Etoposide, topoisomerase II and cancer. Curr. Med. Chem. Anticancer Agents 2005, 5, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, K.; Fanale, M.A.; Abramson, J.S.; Noy, A.; Caimi, P.F.; Pittaluga, S.; Parekh, S.; Lacasce, A.; Hayslip, J.W.; Jagadeesh, D.; et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: A prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018, 5, E609–E617. [Google Scholar] [CrossRef]
- Magrath, I.; Adde, M.; Shad, A.; Venzon, D.; Seibel, N.; Gootenberg, J.; Neely, J.; Arndt, C.; Nieder, M.; Jaffe, E.; et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J. Clin. Oncol. 1996, 14, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Roschewski, M.; Dunleavy, K.; Abramson, J.S.; Powell, B.L.; Link, B.K.; Patel, P.; Bierman, P.J.; Jagadeesh, D.; Mitsuyasu, R.T.; Peace, D.; et al. Multicenter study of risk-adapted therapy with dose-adjusted EPOCH-R in adults with untreated burkitt lymphoma. J. Clin. Oncol. 2020, 38, 2519–2529. [Google Scholar] [CrossRef]
- Shah, N.N.; Szabo, A.; Huntington, S.F.; Epperla, N.; Reddy, N.; Ganguly, S.; Vose, J.; Obiozor, C.; Faruqi, F.; Kovach, A.E.; et al. R-CHOP versus dose-adjusted R-EPOCH in frontline management of primary mediastinal B-cell lymphoma: A multi-centre analysis. Br. J. Haematol. 2018, 180, 534–544. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=etoposide+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=lenalidomide+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=ibrutinib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=chidamide+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=nelarabine+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=azacitidine+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=decitabine+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=venetoclax+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=dasatinib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=ponatinib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=romidepsin+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=vorinostat+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=bortezomib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=ixazomib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=acalabrutinib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=zanubrutinib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=umbralisib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=parsaclisib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=pralatrexate+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=duvelisib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=copanlisib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03147885 (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=iberdomide+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=TAK-659+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=carfilzomib+vincristine+cyclophosphamide+hydroxydaunorubicin+prednisone&cntry=&state=&city=&dist=&Search=Search&recrs=a&recrs=b&recrs=d&recrs=f (accessed on 28 May 2021).
- Graf, S.A.; Lynch, R.C.; Coffey, D.G.; Shadman, M.; Kanan, S.; Libby, E.N., III; Warren, E.H.; Godwin, C.; Gooley, T.; Fromm, J.R.; et al. Ixazomib in previously untreated indolent B-cell non-hodgkin lymphoma. Blood 2018, 132, 5326. [Google Scholar] [CrossRef]
- Leonard, J.P.; Kolibaba, K.S.; Reeves, J.A.; Tulpule, A.; Flinn, I.W.; Kolevska, T.; Robles, R.; Flowers, C.R.; Collins, R.; DiBella, N.J.; et al. Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell-like diffuse large B-cell lymphoma. J. Clin. Oncol. 2017, 35, 3538–3546. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Jin, J.; Pylypenko, H.; Verhoef, G.; Siritanaratkul, N.; Drach, J.; Raderer, M.; Mayer, J.; Pereira, J.; Tumyan, G.; et al. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: Final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol. 2018, 19, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=cancer&term=vincristine&cntry=&state=&city=&dist=&Search=Search&recrs=b (accessed on 23 June 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=cancer&term=vincristine&cntry=&state=&city=&dist=&Search=Search&recrs=a (accessed on 23 June 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=cancer&term=vincristine&cntry=&state=&city=&dist=&Search=Search&recrs=f (accessed on 23 June 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=cancer&term=vincristine&cntry=&state=&city=&dist=&Search=Search&recrs=d (accessed on 23 June 2021).
- Goodsell, D.S. The molecular perspective: Methotrexate. Stem Cells 1999, 17, 314–315. [Google Scholar] [CrossRef]
- Costanzi, J.J.; Coltman, C.A., Jr.; Col, L. Combination chemotherapy using cyclophosphamide, vincristine, methotrexate and 5-fluorouracil in solid tumors. Cancer 1969, 23, 589–596. [Google Scholar] [CrossRef]
- Bearden, J.D., 3rd; Coltman, C.A., Jr.; Moon, T.E.; Costanzi, J.J.; Saiki, J.H.; Balcerzak, S.P.; Rivkin, S.E.; Morrison, F.S.; Lane, M.; Spigel, S.C. Combination chemotherapy using cyclophosphamide, vincristine, methotrexate, 5-fluorouracil, and prednisone in solid tumors. Cancer 1977, 39, 21–26. [Google Scholar] [CrossRef]
- Fyfe, M.J.; Goldman, I.D. Characteristics of vincristine-induced augmentation of methotrexate uptake in ehrlich ascites tumor-cells. J. Biol. Chem. 1973, 248, 5067–5073. [Google Scholar] [CrossRef]
- Mulder, J.H.; van Putten, L.M. Vincristine-methotrexate combination chemotherapy and the influence of weight loss on experimental tumour growth. Cancer Chemother. Pharmacol. 1979, 3, 111–116. [Google Scholar] [CrossRef]
- Chello, P.L.; Sirotnak, F.M.; Dorick, D.M.; Moccio, D.M. Schedule-dependent synergism of methotrexate and vincristine against murine L1210 leukemia. Cancer Treat Rep. 1979, 63, 1889–1894. [Google Scholar] [PubMed]
- Kano, Y.; Ohnuma, T.; Okano, T.; Holland, J.F. Effects of vincristine in combination with methotrexate and other antitumor agents in human acute lymphoblastic leukemia cells in culture. Cancer Res. 1988, 48, 351–356. [Google Scholar] [PubMed]
- Freeman, T.; Legasto, C.S.; Schickli, M.A.; McLaughlin, E.M.; Giglio, P.; Puduvalli, V.; Gonzalez, J. High-dose methotrexate-based regimens with or without vincristine for the treatment of primary central nervous system lymphoma. Neurooncol. Adv. 2020, 2, vdaa077. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Yamaguchi, M.; Atsuta, Y.; Matsue, K.; Sato, K.; Kusumoto, S.; Nagai, H.; Takizawa, J.; Fukuhara, N.; Nagafuji, K.; et al. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone combined with high-dose methotrexate plus intrathecal chemotherapy for newly diagnosed intravascular large B-cell lymphoma (PRIMEUR-IVL): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 593–602. [Google Scholar] [CrossRef]
- Alifrangis, C.; Agarwal, R.; Short, D.; Fisher, R.A.; Sebire, N.J.; Harvey, R.; Savage, P.M.; Seckl, M.J. EMA/CO for high-risk gestational trophoblastic neoplasia: Good outcomes with induction low-dose etoposide-cisplatin and genetic analysis. J. Clin. Oncol. 2013, 31, 280–286. [Google Scholar] [CrossRef]
- Bower, M.; Newlands, E.S.; Holden, L.; Short, D.; Brock, C.; Rustin, G.J.; Begent, R.H.; Bagshawe, K.D. EMA/CO for high-risk gestational trophoblastic tumors: Results from a cohort of 272 patients. J. Clin. Oncol. 1997, 15, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Newlands, E.S.; Bagshawe, K.D.; Begent, R.H.; Rustin, G.J.; Holden, L. Results with the EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) regimen in high risk gestational trophoblastic tumours, 1979 to 1989. Br. J. Obstet. Gynaecol. 1991, 98, 550–557. [Google Scholar] [CrossRef]
- Singh, K.; Gillett, S.; Ireson, J.; Hills, A.; Tidy, J.A.; Coleman, R.E.; Hancock, B.W.; Winter, M.C. M-EA (methotrexate, etoposide, dactinomycin) and EMA-CO (methotrexate, etoposide, dactinomycin/cyclophosphamide, vincristine) regimens as first-line treatment of high-risk gestational trophoblastic neoplasia. Int. J. Cancer 2021, 148, 2335–2344. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avendaño, C.; Menéndez, J.C. (Eds.) DNA alkylating agents. In Medicinal Chemistry of Anticancer Drugs, 2nd ed.; Elsevier: Boston, MA, USA, 2015; pp. 197–241. [Google Scholar] [CrossRef]
- Solimando, D.A., Jr.; Waddell, J.A. Procarbazine, lomustine, and vincristine (PCV) regimen for central nervous system tumors. Hosp. Pharm. 2017, 52, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassman, A.B. Procarbazine, lomustine and vincristine or temozolomide: Which is the better regimen? CNS Oncol. 2015, 4, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Parasramka, S.; Talari, G.; Rosenfeld, M.; Guo, J.; Villano, J.L. Procarbazine, lomustine and vincristine for recurrent highgrade glioma. Cochrane Database Syst. Rev. 2017, 7, CD011773. [Google Scholar] [CrossRef] [PubMed]
- Esteyrie, V.; Dehais, C.; Martin, E.; Carpentier, C.; Uro-Coste, E.; Figarella-Branger, D.; Bronniman, C.; Pouessel, D.; Ciron, D.L.; Ducray, F.; et al. Radiotherapy plus procarbazine, lomustine, and vincristine versus radiotherapy plus temozolomide for IDH-mutant anaplastic astrocytoma: A retrospective multicenter analysis of the French POLA cohort. Oncologist 2021, 26, e838–e846. [Google Scholar] [CrossRef]
- Bell, E.H.; Zhang, P.X.; Shaw, E.G.; Buckner, J.C.; Barger, G.R.; Bullard, D.E.; Mehta, M.P.; Gilbert, M.R.; Brown, P.D.; Stelzer, K.J.; et al. Comprehensive genomic analysis in NRG oncology/RTOG 9802: A phase III trial of radiation versus dadiation plus procarbazine, lomustine (CCNU), and vincristine in high-risk low-grade glioma. J. Clin. Oncol. 2020, 38, 3407–3417. [Google Scholar] [CrossRef]
- Cairncross, J.G.; Wang, M.H.; Jenkins, R.B.; Shaw, E.G.; Giannini, C.; Brachman, D.G.; Buckner, J.C.; Fink, K.L.; Souhami, L.; Laperriere, N.J.; et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J. Clin. Oncol. 2014, 32, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Canellos, G.P.; Arseneau, J.C.; DeVita, V.T.; Whang-Peng, J.; Johnson, R.E. Second malignancies complicating Hodgkin’s disease in remission. Lancet 1975, 1, 47–49. [Google Scholar] [CrossRef]
- Henry-Amar, M. Second cancer after the treatment for Hodgkin’s disease: A report from the International Database on Hodgkin’s Disease. Ann Oncol. 1992, 3, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Rheingold, S.R.; Neugut, A.I.; Meadows, A.T. Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Bast, R.C., Gansler, T.S., Holland, J.F., Frei, E., Eds.; Therapy-Related Secondary Cancers; BC Decker: Hamilton, ON, Canada, 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK13999/ (accessed on 27 May 2021).
- Averbuch, S.D.; Steakley, C.S.; Young, R.C.; Gelmann, E.P.; Goldstein, D.S.; Stull, R.; Keiser, H.R. Malignant pheochromocytoma: Effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine. Ann. Intern. Med. 1988, 109, 267–273. [Google Scholar] [CrossRef]
- Deutschbein, T.; Fassnacht, M.; Weismann, D.; Reincke, M.; Mann, K.; Petersenn, S. Treatment of malignant phaeochromocytoma with a combination of cyclophosphamide, vincristine and dacarbazine: Own experience and overview of the contemporary literature. Clin. Endocrinol. 2015, 82, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, N.D.; Alblas, G.; van Hulsteijn, L.T.; Dekkers, O.M.; Corssmit, E.P. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: Systematic review and meta-analysis. Clin. Endocrinol. (Oxf.) 2014, 81, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Katabami, T.; Tsuiki, M.; Tanaka, Y.; Naruse, M. Controlling tumor progression with cyclophosphamide, vincristine, and dacarbazine treatment improves survival in patients with metastatic and unresectable malignant pheochromocytomas/paragangliomas. Horm Cancer 2017, 8, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Deutschbein, T.; Matuszczyk, A.; Moeller, L.C.; Unger, N.; Yuece, A.; Lahner, H.; Mann, K.; Petersenn, S. Treatment of advanced medullary thyroid carcinoma with a combination of cyclophosphamide, vincristine, and dacarbazine: A single-center experience. Exp. Clin. Endocrinol. Diabet. 2011, 119, 540–543. [Google Scholar] [CrossRef]
- Andre, M.P.E.; Carde, P.; Viviani, S.; Bellei, M.; Fortpied, C.; Hutchings, M.; Gianni, A.M.; Brice, P.; Casasnovas, O.; Gobbi, P.G.; et al. Long-term overall survival and toxicities of ABVD vs BEACOPP in advanced Hodgkin lymphoma: A pooled analysis of four randomized trials. Cancer Med. 2020, 9, 6565–6575. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.; Collins, A.; Fowler, A.; Karanth, M.; Saha, C.; Docherty, S.; Padayatty, J.; Maw, K.; Lentell, I.; Cooke, L.; et al. Advanced Hodgkin lymphoma in the East of England: A 10-year comparative analysis of outcomes for real-world patients treated with ABVD or escalated-BEACOPP, aged less than 60 years, compared with 5-year extended follow-up from the RATHL trial. Ann. Hematol. 2021, 100, 1049–1058. [Google Scholar] [CrossRef]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illés, Á.; Picardi, M.; et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N. Engl. J. Med. 2017, 378, 331–344. [Google Scholar] [CrossRef]
- Eichenauer, D.A.; Plütschow, A.; Kreissl, S.; Sökler, M.; Hellmuth, J.C.; Meissner, J.; Mathas, S.; Topp, M.S.; Behringer, K.; Klapper, W.; et al. Incorporation of brentuximab vedotin into first-line treatment of advanced classical Hodgkin’s lymphoma: Final analysis of a phase 2 randomised trial by the German Hodgkin Study Group. Lancet Oncol. 2017, 18, 1680–1687. [Google Scholar] [CrossRef]
- Metzger, M.L.; Link, M.P.; Billett, A.L.; Flerlage, J.; Lucas, J.T.; Mandrell, B.N.; Ehrhardt, M.J.; Bhakta, N.; Yock, T.I.; Friedmann, A.M.; et al. Excellent outcome for pediatric patients with high-risk Hodgkin lymphoma treated with brentuximab vedotin and risk-adapted residual node radiation. J. Clin. Oncol. 2021, 39, 2276–2283. [Google Scholar] [CrossRef]
- Raymakers, A.J.N.; Costa, S.; Cameron, D.; Regier, D.A. Cost-effectiveness of brentuximab vedotin in advanced stage Hodgkin’s lymphoma: A probabilistic analysis. BMC Cancer 2020, 20, 992. [Google Scholar] [CrossRef]
- Amin, M.S.A.; Brunckhorst, O.; Scott, C.; Wrench, D.; Gleeson, M.; Kazmi, M.; Ahmed, K. ABVD and BEACOPP regimens’ effects on fertility in young males with Hodgkin lymphoma. Clin. Transl. Oncol. 2021, 23, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Policiano, C.; Subira, J.; Aguilar, A.; Monzo, S.; Iniesta, I.; Rubio, J.M.R. Impact of ABVD chemotherapy on ovarian reserve after fertility preservation in reproductive-aged women with Hodgkin lymphoma. J. Assist. Reprod. Genet. 2020, 37, 1755–1761. [Google Scholar] [CrossRef]
- Ramos, S.; Navarrete-Meneses, P.; Molina, B.; Cervantes-Barragán, D.E.; Lozano, V.; Gallardo, E.; Marchetti, F.; Frias, S. Genomic chaos in peripheral blood lymphocytes of Hodgkin’s lymphoma patients one year after ABVD chemotherapy/radiotherapy. Environ. Mol. Mutagen. 2018, 59, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Gnekow, A.K.; Walker, D.A.; Kandels, D.; Picton, S.; Perilongo, G.; Grill, J.; Stokland, T.; Sandstrom, P.E.; Warmuth-Metz, M.; Pietsch, T.; et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤ 16 years) low grade glioma—A final report. Eur. J. Cancer 2017, 81, 206–225. [Google Scholar] [CrossRef] [Green Version]
- Chintagumpala, M.; Eckel, S.P.; Krailo, M.; Morris, M.; Adesina, A.; Packer, R.; Lau, C.; Gajjar, A. A pilot study using carboplatin, vincristine, and temozolomide in children with progressive/symptomatic low-grade glioma: A Children’s Oncology Group study. Neuro-Oncology 2015, 17, 1132–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qaddoumi, I.; Billups, C.A.; Tagen, M.; Stewart, C.F.; Wu, J.; Helton, K.; McCarville, M.B.; Merchant, T.E.; Brennan, R.; Free, T.M.; et al. Topotecan and vincristine combination is effective against advanced bilateral intraocular retinoblastoma and has manageable toxicity. Cancer 2012, 118, 5663–5670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, B.A.; Sahr, N.; Sykes, A.; Wilson, M.W.; Brennan, R.C. Chemoreduction with topotecan and vincristine: Quantifying tumor response in bilateral retinoblastoma patients. Pediatr. Blood Cancer 2021, 68, e28882. [Google Scholar] [CrossRef]
- Amoroso, L.; Erminio, G.; Makin, G.; Pearson, A.D.J.; Brock, P.; Valteau-Couanet, D.; Castel, V.; Pasquet, M.; Laureys, G.; Thomas, C.; et al. Topotecan-vincristine-doxorubicin in stage 4 high-risk neuroblastoma patients failing to achieve a complete metastatic response to rapid COJEC: A SIOPEN study. Cancer Res. Treat. 2018, 50, 148–155. [Google Scholar] [CrossRef]
- Mascarenhas, L.; Felgenhauer, J.L.; Bond, M.C.; Villaluna, D.; Femino, J.D.; Laack, N.N.; Ranganathan, S.; Meyer, J.; Womer, R.B.; Gorlick, R.; et al. Pilot study of adding vincristine, topotecan, and cyclophosphamide to interval-compressed chemotherapy in newly diagnosed patients with localized Ewing sarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2016, 63, 493–498. [Google Scholar] [CrossRef]
- Papageorgiou, G.I.; Tsakatikas, S.A.; Fioretzaki, R.G.; Kosmas, C. Notable response of a young adult with recurrent glioblastoma multiforme to vincristine-irinotecan-temozolomide and bevacizumab. Anti-Cancer Drugs 2021, 32, 330–336. [Google Scholar] [CrossRef]
- Venkatramani, R.; Malogolowkin, M.; Davidson, T.B.; May, W.; Sposto, R.; Mascarenhas, L. A phase I study of vincristine, irinotecan, temozolomide and bevacizumab (Vitb) in pediatric patients with relapsed solid tumors. PLoS ONE 2013, 8, e68416. [Google Scholar] [CrossRef]
- Venkatramani, R.; Malogolowkin, M.H.; Mascarenhas, L. Treatment of multiply relapsed Wilms tumor with vincristine, irinotecan, temozolomide and bevacizumab. Pediatr. Blood Cancer 2014, 61, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.; Turpin, B.; Nagarajan, R.; Weiss, B.; Cripe, T.; Geller, J. Pilot study of vincristine, oral irinotecan, and temozolomide (VOIT regimen) combined with bevacizumab in pediatric patients with recurrent solid tumors or brain tumors. Pediatr. Blood Cancer 2013, 60, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Schiavetti, A.; Varrasso, G.; Collini, P.; Clerico, A. Vincristine, irinotecan, and bevacizumab in relapsed Wilms tumor with diffuse anaplasia. J. Pediatr. Hematol. Oncol. 2018, 40, 331–333. [Google Scholar] [CrossRef]
- Ganjoo, K.; Hong, F.X.; Horning, S.J.; Gascoyne, R.D.; Natkunam, Y.; Swinnen, L.J.; Habermann, T.M.; Kahl, B.S.; Advani, R.H. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: An Eastern Cooperative Oncology Group study (E2404). Leuk. Lymphoma 2014, 55, 768–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehn, L.H.; Martelli, M.; Trněný, M.; Liu, W.; Bolen, C.R.; Knapp, A.; Sahin, D.; Sellam, G.; Vitolo, U. A randomized, open-label, Phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-Cell lymphoma: Final analysis of GOYA. J. Hematol. Oncol. 2020, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- NCT04692155. Available online: https://clinicaltrials.gov/ct2/show/NCT04692155 (accessed on 27 May 2021).
- NCT03677141. Available online: https://clinicaltrials.gov/ct2/show/NCT03677141 (accessed on 27 May 2021).
- NCT03467373. Available online: https://clinicaltrials.gov/ct2/show/NCT03467373 (accessed on 27 May 2021).
- NCT01527149. Available online: https://clinicaltrials.gov/ct2/show/NCT01527149 (accessed on 27 May 2021).
- Peyrade, F.; Bologna, S.; Delwail, V.; Emile, J.F.; Pascal, L.; Fermé, C.; Schiano, J.M.; Coiffier, B.; Corront, B.; Farhat, H.; et al. Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: An open-label, multicentre, single-arm, phase 2 trial from the LYSA group. Lancet Haematol. 2017, 4, e46–e55. [Google Scholar] [CrossRef]
- NCT01445535. Available online: https://clinicaltrials.gov/ct2/show/NCT01445535 (accessed on 27 May 2021).
- Roswarski, J.; Roschewski, M.; Lucas, A.; Melani, C.; Pittaluga, S.; Jaffe, E.S.; Steinberg, S.M.; Waldmann, T.A.; Wilson, W.H. Phase I dose escalation study of the anti-CD2 monoclonal antibody, siplizumab, with DA-EPOCH-R in aggressive peripheral T-cell lymphomas. Leuk. Lymphoma 2018, 59, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Ravandi, F.; Kebriaei, P.; Huang, X.; Short, N.J.; Thomas, D.; Sasaki, K.; Rytting, M.; Jain, N.; Konopleva, M.; et al. Salvage Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD for patients with relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia: A phase 2 clinical trial. JAMA Oncol. 2018, 4, 230–234. [Google Scholar] [CrossRef]
- Kantarjian, H.; Ravandi, F.; Short, N.J.; Huang, X.; Jain, N.; Sasaki, K.; Daver, N.; Pemmaraju, N.; Khoury, J.D.; Jorgensen, J.; et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: A single-arm, phase 2 study. Lancet Oncol. 2018, 19, 240–248. [Google Scholar] [CrossRef]
- NCT04824092. Available online: https://clinicaltrials.gov/ct2/show/NCT04824092 (accessed on 27 May 2021).
- NCT04661007. Available online: https://clinicaltrials.gov/ct2/show/NCT04661007 (accessed on 27 May 2021).
- NCT04134936. Available online: https://clinicaltrials.gov/ct2/show/NCT04134936 (accessed on 27 May 2021).
- NCT03518112. Available online: https://clinicaltrials.gov/ct2/show/NCT03518112 (accessed on 27 May 2021).
- NCT04448834. Available online: https://clinicaltrials.gov/ct2/show/NCT04448834 (accessed on 27 May 2021).
- NCT03914625. Available online: https://clinicaltrials.gov/show/NCT03914625 (accessed on 27 May 2021).
- NCT03147612. Available online: https://clinicaltrials.gov/ct2/show/NCT03147612 (accessed on 27 May 2021).
- NCT02877303. Available online: https://clinicaltrials.gov/ct2/show/NCT02877303 (accessed on 27 May 2021).
- NCT03643276. Available online: https://clinicaltrials.gov/ct2/show/NCT03643276 (accessed on 27 May 2021).
- NCT02003222. Available online: https://clinicaltrials.gov/ct2/show/NCT02003222 (accessed on 27 May 2021).
- NCT02734771. Available online: https://clinicaltrials.gov/ct2/show/NCT02734771 (accessed on 27 May 2021).
- NCT02398240. Available online: https://clinicaltrials.gov/ct2/show/NCT02398240 (accessed on 27 May 2021).
- NCT02166463. Available online: https://clinicaltrials.gov/ct2/show/NCT02166463 (accessed on 27 May 2021).
- NCT01920932. Available online: https://clinicaltrials.gov/ct2/show/NCT01920932 (accessed on 27 May 2021).
- NCT04139304. Available online: https://clinicaltrials.gov/ct2/show/NCT04139304 (accessed on 27 May 2021).
- NCT03384654. Available online: https://clinicaltrials.gov/ct2/show/NCT03384654 (accessed on 27 May 2021).
- NCT03860844. Available online: https://clinicaltrials.gov/ct2/show/NCT03860844 (accessed on 27 May 2021).
- NCT01030900. Available online: https://clinicaltrials.gov/ct2/show/NCT01030900 (accessed on 27 May 2021).
- NCT00069238. Available online: https://clinicaltrials.gov/ct2/show/NCT00069238 (accessed on 27 May 2021).
- NCT01256398. Available online: https://clinicaltrials.gov/ct2/show/NCT01256398 (accessed on 27 May 2021).
- NCT04231877. Available online: https://clinicaltrials.gov/ct2/show/NCT04231877 (accessed on 27 May 2021).
- NCT03274492. Available online: https://clinicaltrials.gov/ct2/show/NCT03274492 (accessed on 27 May 2021).
- Cheah, C.Y.; Fowler, N.H.; Neelapu, S.S. Targeting the programmed death-1/programmed death-ligand 1 axis in lymphoma. Curr. Opin. Oncol. 2015, 27, 384–391. [Google Scholar] [CrossRef]
- NCT04796012. Available online: https://clinicaltrials.gov/ct2/show/NCT04796012 (accessed on 27 May 2021).
- NCT04759586. Available online: https://clinicaltrials.gov/ct2/show/NCT04759586 (accessed on 27 May 2021).
- NCT03586999. Available online: https://clinicaltrials.gov/ct2/show/NCT03586999 (accessed on 27 May 2021).
- NCT03749018. Available online: https://clinicaltrials.gov/ct2/show/NCT03749018 (accessed on 27 May 2021).
- NCT03704714. Available online: https://clinicaltrials.gov/show/NCT03704714 (accessed on 27 May 2021).
- NCT04058470. Available online: https://clinicaltrials.gov/ct2/show/NCT04058470 (accessed on 27 May 2021).
- NCT03407144. Available online: https://clinicaltrials.gov/ct2/show/NCT03407144 (accessed on 27 May 2021).
- NCT04113226. Available online: https://clinicaltrials.gov/ct2/show/NCT04113226 (accessed on 27 May 2021).
- NCT03003520. Available online: https://clinicaltrials.gov/ct2/show/NCT03003520 (accessed on 27 May 2021).
- NCT04181489. Available online: https://clinicaltrials.gov/ct2/show/NCT04181489 (accessed on 27 May 2021).
- NCT04023916. Available online: https://clinicaltrials.gov/ct2/show/NCT04023916 (accessed on 27 May 2021).
- NCT03244176. Available online: https://clinicaltrials.gov/ct2/show/NCT03244176 (accessed on 27 May 2021).
- NCT03786783. Available online: https://clinicaltrials.gov/ct2/show/NCT03786783 (accessed on 27 May 2021).
- NCT02306161. Available online: https://clinicaltrials.gov/ct2/show/NCT02306161 (accessed on 27 May 2021).
- NCT03991884. Available online: https://clinicaltrials.gov/ct2/show/NCT03991884 (accessed on 27 May 2021).
- NCT03851081. Available online: https://clinicaltrials.gov/ct2/show/NCT03851081 (accessed on 27 May 2021).
- NCT02981628. Available online: https://clinicaltrials.gov/ct2/show/NCT02981628 (accessed on 27 May 2021).
- NCT03249870. Available online: https://clinicaltrials.gov/ct2/show/NCT03249870 (accessed on 27 May 2021).
- NCT01925131. Available online: https://clinicaltrials.gov/show/NCT01925131 (accessed on 27 May 2021).
- NCT04747912. Available online: https://clinicaltrials.gov/ct2/show/NCT04747912 (accessed on 27 May 2021).
- NCT04307576. Available online: https://clinicaltrials.gov/ct2/show/NCT04307576 (accessed on 27 May 2021).
- NCT03150693. Available online: https://clinicaltrials.gov/ct2/show/NCT03150693 (accessed on 27 May 2021).
- NCT01371630. Available online: https://clinicaltrials.gov/ct2/show/NCT01371630 (accessed on 27 May 2021).
- NCT03817853. Available online: https://clinicaltrials.gov/ct2/show/NCT03817853 (accessed on 27 May 2021).
- NCT03269669. Available online: https://clinicaltrials.gov/ct2/show/NCT03269669 (accessed on 27 May 2021).
- NCT02529852. Available online: https://clinicaltrials.gov/ct2/show/NCT02529852 (accessed on 27 May 2021).
- NCT01332968. Available online: https://clinicaltrials.gov/ct2/show/NCT01332968 (accessed on 27 May 2021).
- Syed, S.B.; Lin, S.Y.; Arya, H.; Fu, I.H.; Yeh, T.K.; Charles, M.R.C.; Periyasamy, L.; Hsieh, H.P.; Coumar, M.S. Overcoming vincristine resistance in cancer: Computational design and discovery of piperine-inspired P-glycoprotein inhibitors. Chem. Biol. Drug Des. 2021, 97, 51–66. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, K.; Chen, X.; Li, H.; Wan, Q.; Morris-Natschke, S.; Lee, K.H.; Chen, Y. Seco-4-methyl-DCK derivatives as potent chemosensitizers. Bioorg. Med. Chem. Lett. 2019, 29, 28–31. [Google Scholar] [CrossRef]
- Wan, Q.; Jin, X.; Guo, Y.; Yu, Z.; Guo, S.; Morris-Natschke, S.; Lee, K.-H.; Liu, H.; Chen, Y. New Seco-DSP derivatives as potent chemosensitizers. Eur. J. Med. Chem. 2020, 204, 112555. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Yang, X.; Zhao, D.S.; Cai, Y.; Huang, Z.; Wu, R.; Wang, S.J.; Liu, G.J.; Wang, J.; Bao, X.Z.; et al. Design, synthesis and bioactivity study on 5-phenylfuran derivatives as potent reversal agents against P-glycoprotein-mediated multidrug resistance in MCF-7/ADR cell. Eur. J. Med. Chem. 2021, 216, 113336. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.N.; Lin, K.I.; Lin, Y.C.; Thang, T.D.; Lan, Y.H.; Hung, C.C. A novel flavonoid from Fissistigma cupreonitens, 5-hydroxy-7,8-dimethoxyflavanone, competitively inhibited the efflux function of human P-glycoprotein and reversed cancer multi-drug resistance. Phytomedicine 2021, 85, 153528. [Google Scholar] [CrossRef] [PubMed]
- Diouf, B.; Wing, C.; Panetta, J.C.; Eddins, D.; Lin, W.W.; Yang, W.J.; Fan, Y.P.; Pei, D.Q.; Cheng, C.; Delaney, S.M.; et al. Identification of small molecules that mitigate vincristine-induced neurotoxicity while sensitizing leukemia cells to vincristine. Clin. Transl. Sci. 2021, 14, 1490–1504. [Google Scholar] [CrossRef]
- Buzun, K.; Gornowicz, A.; Lesyk, R.; Bielawski, K.; Bielawska, A. Autophagy Modulators in Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 5804. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Qu, X.L.; Liu, L.Y.; Qian, D.H.; Jing, H.Y. LncRNA MEG3 promotes the sensitivity of vincristine by inhibiting autophagy in lung cancer chemotherapy. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1020–1027. [Google Scholar] [CrossRef]
- Yao, L.; Yang, L.; Song, H.; Liu, T.G.; Yan, H. Silencing of lncRNA XIST suppresses proliferation and autophagy and enhances vincristine sensitivity in retinoblastoma cells by sponging miR-204-5p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3526–3537. [Google Scholar] [CrossRef]
- Li, L.J.; Wang, Y.L.; Yuan, L.Q.; Gu, W.Z.; Zhu, K.; Yang, M.; Zhou, D.; Lv, Y.; Li, M.J.; Zhao, Z.Y.; et al. Autophagy inhibition in childhood nephroblastoma and the therapeutic significance. Curr. Cancer Drug Targets. 2018, 18, 295–303. [Google Scholar] [CrossRef]
- Shan, C.; Hui, W.; Li, H.; Wang, Z.; Guo, C.; Peng, R.; Gu, J.; Chen, Y.; Ouyang, Q. Discovery of novel autophagy inhibitors and their sensitization abilities for vincristine-resistant esophageal cancer cell line Eca109/VCR. ChemMedChem 2020, 15, 970–981. [Google Scholar] [CrossRef]
- American Childhood Cancer Organization. Available online: https://www.acco.org/blog/the-vincristine-drug-shortage-update/ (accessed on 18 August 2021).
- Food and Drug Administration. Available online: https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Vincristine%20Sulfate%20Injection,%20USP%20(Preservative-Free)&st=c (accessed on 18 August 2021).

| Compound | Trade Name | Manufacturer | Molecular Target | Number of Clinical Trials | Reference |
|---|---|---|---|---|---|
| Etoposide | Vepesid® | Bristol-Myers Squibb, New York, NY, USA | Topoisomerase II | 40 | [33] |
| Lenalidomide | Revlimid® | Celgene corporation, Summit, CO, USA | Cereblon, Ikaros transcription factors | 18 | [34] |
| Ibrutinib | Imbruvica® | Pharmacyclics, Synnyvale, CA, USA | Bruton’s tyrosine kinase | 4 | [35] |
| Chidamide | Epidaza® | Schenzen Chipscreen, Shenzhen, China | Histone deacetylases | 4 | [36] |
| Nelarabine | Arranon® | GlaxoSmithKline, London, Great Britain | DNA synthesis | 4 | [37] |
| Azacitidine | Vidaza® | Bristol-Myers Squibb, New York, NY, USA | 3 | [38] | |
| Decitabine | Dacogen® | Janssen Pharmaceuticals, Beerse, Belgium | 3 | [39] | |
| Venetoclax | Venclexta® | Genentech, South San Francisco, CA, USA | B-cell lymphoma-2 protein | 3 | [40] |
| Dasatinib | Sprycel® | Bristol-Myers Squibb, New York, NY, USA | BCR-ABL 1 tyrosine kinase | 3 | [41] |
| Ponatinib | Iclusig® | ARIAD Pharmaceuticals, Cambridge, MA, USA | 2 | [42] | |
| Romidepsin | Istodax® | Gloucester Pharmaceuticals, Cambridge, MA, USA | Histone deacetylases | 2 | [43] |
| Vorinostat | Zolinza® | Merck & Co., Kenilworth, IL, USA | 2 | [44] | |
| Bortezomib | Velcade® | Takeda Pharmaceutical Company Limited, Tokyo, Japan | 26S proteasome | 2 | [45] |
| Ixazomib | Ninlaro® | Proteasome subunit beta type-5 | 2 | [46] | |
| Acalabrutinib | Calquence® | AstraZeneca, Cambridge, Great Britain | Bruton’s tyrosine kinase | 2 | [47] |
| Zanubrutinib | Brukinsa® | BeiGene, Beijing, China | 2 | [48] | |
| Umbralisib | Ukoniq® | Rhizen Pharmaceuticals AG, Basel, Switzerland | Phosphatidylinositol 3-kinase delta | 2 | [49] |
| Parsaclisib | n.a. 2 | Innovent Biologics, Suzhou, China | 1 | [50] | |
| Pralatrexate | Folotyn® | Allos Therapeutics, Westminster, CO, USA | Dihydrofolate reductase | 1 | [51] |
| Duvelisib | Copiktra® | Verastem Oncology, Needham, MA, USA | Phosphatidylinositol 3-kinase | 1 | [52] |
| Copansilib | Aliqopa® | Bayer, Leverkusen, Germany | 1 | [53] | |
| Selinexor | n.a. | Karyopharm Therapeutics, Newton, MA, USA | Exportin-1 | 1 | [54] |
| Iberdomide | n.a. | Celgene Corporation, Summit, CO, USA | Cereblon | 1 | [55] |
| TAK-659 | n.a. | Takeda Pharmaceutical Company Limited, Tokyo, Japan | Spleen tyrosine kinase | 1 | [56] |
| Carfilzomib | Kyprolis® | Amgen, Thousand Oaks, CA, USA | 20S proteasome | 1 | [57] |
| Status | Number of Clinical Trials | Reference |
|---|---|---|
| Not yet recruiting | 30 | [61] |
| Recruiting | 178 | [62] |
| Enrolling by invitation | 1 | [63] |
| Active, not recruiting | 150 | [64] |
| Monoclonal Antibody | Trade Name | Manufacturer | Molecular Target | Condition | Trial Identifier | Ref. |
|---|---|---|---|---|---|---|
| Tafasitamab | Monjuvi® | MorphoSys, Planegg, Germany | CD19 1 | Diffuse large B-cell lymphoma | NCT04824092 | [125] |
| NCT04661007 | [126] | |||||
| NCT04134936 | [127] | |||||
| Atezolizumab | Tecentriq® | Roche, Basel, Switzerland | Programmed cell death-ligand 1 | Solid tumors | NCT04796012 | [148] |
| Nivolumab | Opdiva® | Bristol-Myers Squibb, New York, NY, USA | Primary mediastinal (thymic) large B-cell lymphoma | NCT04759586 | [149] | |
| Peripheral T-cell lymphoma | NCT03586999 | [150] | ||||
| Various lymphoma types | NCT03749018 | [151] | ||||
| NCT03704714 | [152] | |||||
| Toripalimab | Tuoyi® | Junshi Biosciences, Shanghai, China | NCT04058470 | [153] | ||
| Pembrolizumab | Keytruda® | Merck & Co., Kenilworth, IL, USA | NCT04058470 | [153] | ||
| Classical Hodgkin lymphoma | NCT03407144 | [154] | ||||
| Camrelizumab | AiRuiKa® | Jiangsu Hengrui Medicine, Lianyungang, China | NCT04113226 | [155] | ||
| Durvalumab | Imfinzi® | MedImmune, Gaithersburg, MD, USA | Large B-cell lymphoma | NCT03003520 | [156] | |
| Sintilimab | Tyvyt® | Eli Lilly and Company, Indianapolis, IN, USA | Epstein-Barr virus-positive diffuse large B-cell lymphoma | NCT04181489 | [157] | |
| Diffuse large B-cell lymphoma | NCT04023916 | [158] | ||||
| Avelumab | Bavencio® | EMD Serono, Rockland, USA; Pfizer, New York, NY, USA | Non-Hodgkin B-cell lymphoma | NCT03244176 | [159] | |
| Ublituximab | n.a. 2 | LFB Group, Alès, France | CD20 | Mantle cell lymphoma | NCT04692155 | [116] |
| Blinatumomab | Blincyto® | Amgen, Thousand Oaks, CA, USA | CD19, CD3 | B-cell acute lymphoblastic leukemia | NCT03518112 | [128] |
| NCT04448834 | [129] | |||||
| NCT03914625 | [130] | |||||
| Various leukemia types | NCT03147612 | [131] | ||||
| B-cell acute lymphoblastic leukemia and lymphoma | NCT02877303 | [132] | ||||
| Acute lymphoblastic leukemia | NCT03643276 | [133] | ||||
| NCT02003222 | [134] | |||||
| Daratumumab | Darzalex® | Janssen Biotech, Horsham, PA, USA | CD38 | Various types of lymphoma | NCT04139304 | [139] |
| Precursor cell lymphoblastic leukemia and lymphoma | NCT03384654 | [140] | ||||
| Isatuximab | Sarclisa® | Sanofi, Paris, France | Acute lymphoblastic leukemia, acute myeloid leukemia | NCT03860844 | [141] | |
| Inotuzumab | Besponza® | Pfizer, New York, NY, USA | CD22 | Recurrent and refractory B-cell lymphoma and leukemia | NCT03991884 | [162] |
| NCT03851081 | [163] | |||||
| NCT02981628 | [164] | |||||
| Acute lymphoblastic leukemia | NCT03249870 | [165] | ||||
| B-cell acute lymphoblastic leukemia and lymphoma | NCT02877303 | [132] | ||||
| Various leukemia types | NCT01925131 | [166] | ||||
| Lymphoblastic leukemia | NCT04747912 | [167] | ||||
| NCT04307576 | [168] | |||||
| B-acute lymphoblastic leukemia | NCT03150693 | [169] | ||||
| NCT01371630 | [170] | |||||
| Obinutuzumab | Gazyva® | Genentech, South San Francisco, CA, USA | CD20 | Advanced follicular lymphoma | NCT03817853 | [171] |
| B-cell lymphoma, non-Hodgkin lymphoma | NCT03467373 | [118] | ||||
| Follicular lymphoma | NCT03269669 | [172] | ||||
| Lymphoma | NCT02529852 | [173] | ||||
| Non-Hodgkin lymphoma | NCT01332968 | [174] | ||||
| Dinutuximab | Unituxin® | United Therapeutics, Silver Spring, MD, USA | Ganglioside G2 | Ganglioneuroblastoma, high risk neuroblastoma | NCT03786783 | [160] |
| Mosunetuzumab | - | Genentech, South San Francisco, CA, USA | CD20, CD3 | B-cell non-Hodgkin lymphoma | NCT03677141 | [117] |
| Polatuzumab | Polivy® | CD79B | Various lymphoma types | NCT04231877 | [145] | |
| Diffuse large B-cell lymphoma | NCT03274492 | [146] | ||||
| Brentuximab | Adcetris® | Takeda Oncology, Cambridge, CA, USA | CD30 | NCT02734771 | [135] | |
| Hodgkin lymphoma | NCT02398240 | [136] | ||||
| NCT02166463 | [137] | |||||
| NCT01920932 | [138] | |||||
| Ganitumab | - | Amgen, Thousand Oaks, CA, USA | Type 1 insulin-like growth factor receptor | Solid tumors | NCT02306161 | [161] |
| Ofatumumab | Arzerra® | Novartis, Basel, Switzerland | CD20 | Mantle cell lymphoma | NCT01527149 | [119] |
| Glofitamab | - | Genentech, South San Francisco, CA, USA | Diffuse large B-cell lymphoma | NCT03467373 | [118] | |
| Siplizumab | - | BioInvent, Lund, Sweden | CD2 | T-cell lymphoma | NCT01445535 | [121] |
| Alemtuzumab | Campath® | Sanofi, Paris, France | CD52 | Hodgkin lymphoma, diffuse large B-cell lymphoma | NCT01030900 | [142] |
| T-cell lymphoma | NCT00069238 | [143] | ||||
| Acute lymphoblastic leukemia | NCT01256398 | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škubník, J.; Pavlíčková, V.S.; Ruml, T.; Rimpelová, S. Vincristine in Combination Therapy of Cancer: Emerging Trends in Clinics. Biology 2021, 10, 849. https://doi.org/10.3390/biology10090849
Škubník J, Pavlíčková VS, Ruml T, Rimpelová S. Vincristine in Combination Therapy of Cancer: Emerging Trends in Clinics. Biology. 2021; 10(9):849. https://doi.org/10.3390/biology10090849
Chicago/Turabian StyleŠkubník, Jan, Vladimíra Svobodová Pavlíčková, Tomáš Ruml, and Silvie Rimpelová. 2021. "Vincristine in Combination Therapy of Cancer: Emerging Trends in Clinics" Biology 10, no. 9: 849. https://doi.org/10.3390/biology10090849
APA StyleŠkubník, J., Pavlíčková, V. S., Ruml, T., & Rimpelová, S. (2021). Vincristine in Combination Therapy of Cancer: Emerging Trends in Clinics. Biology, 10(9), 849. https://doi.org/10.3390/biology10090849









