Lipid Nanoparticles as Delivery Vehicles for Inhaled Therapeutics
Abstract
1. Introduction
1.1. Lung Diseases
1.2. Nanotechnology, Nanomedicine and Lipid Nanoparticles
2. History and Development of LNP Therapeutics
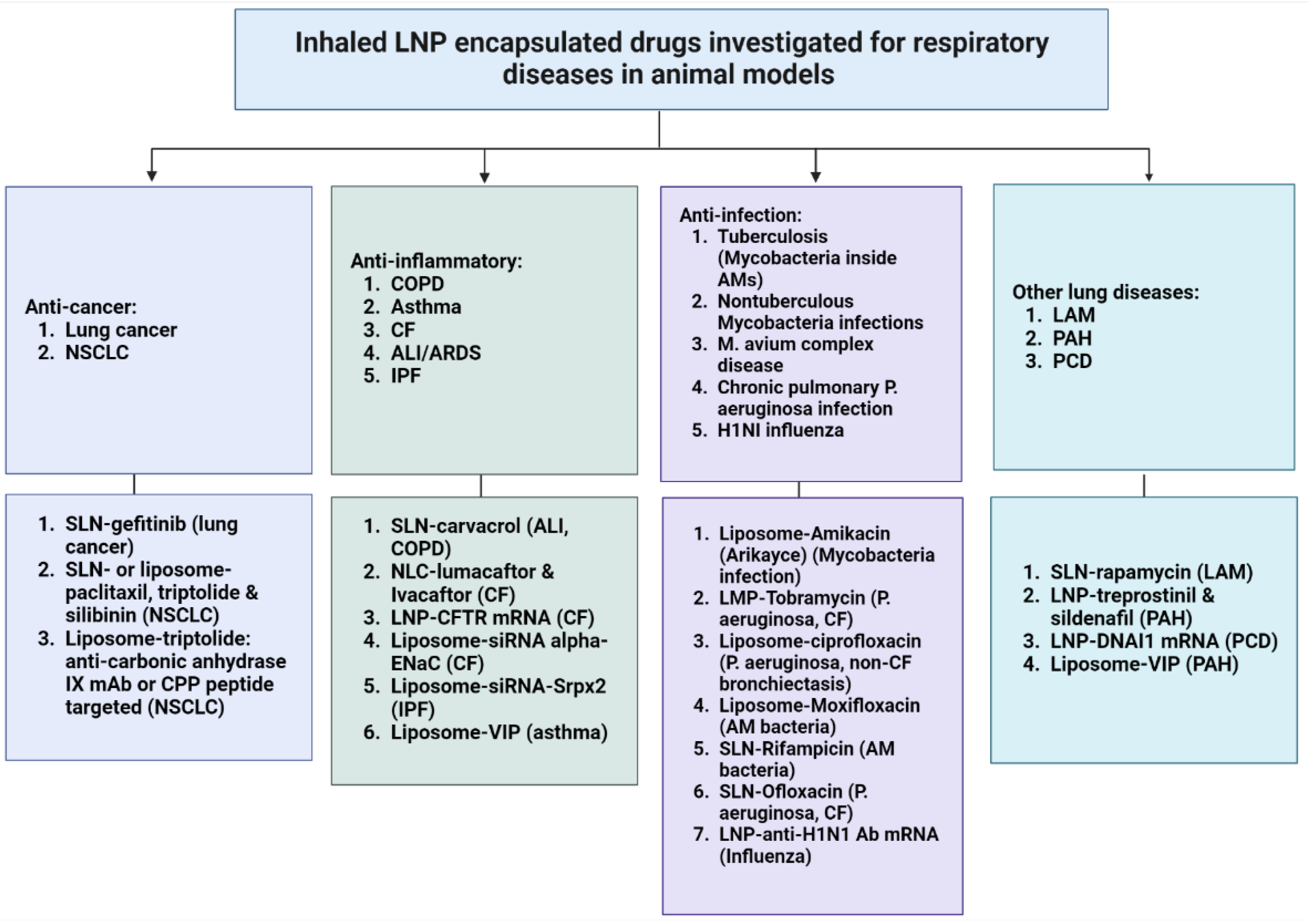
3. Developments in Inhaled LNP Therapeutics for Respiratory Diseases
3.1. Small Molecule Drugs
3.2. Nucleic Acids Drugs
3.3. Protein and Peptide Drugs
4. Advantages of Inhaled LNP Therapeutics
4.1. Diverse Drug Compounds Delivered
4.2. Protection from Degradation and Improved Drug Stability
4.3. Enhanced Drug Retention and Reduced Systemic Toxicity
4.4. Reduced Immunogenicity
5. Challenges of Inhaled LNP Therapeutics and Design Considerations
5.1. Overcoming Physiological Barriers in the Lungs
5.1.1. Airway Structure
5.1.2. Airway Clearance Mechanisms
5.2. Design Considerations for LNP Aerosols for Airway Delivery
5.2.1. Formulating the Appropriate Particle Size
5.2.2. Increasing Mucus Adhesion and Penetration
5.3. Selection of Inhaler Devices and Improving Their Compatibility
6. Inhalation LNP Drugs Approved and in Clinical Trials
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease, 2nd ed.; European Respiratory Society: Sheffield, UK, 2017. [Google Scholar]
- WHO. Asthma. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 29 August 2022).
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 24 May 2022).
- WHO. Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 25 December 2020).
- World Health Organization. Chronic Respiratory Diseases; World Health Organization: Geneva, Switzerland; Available online: https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1 (accessed on 9 June 2021).
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020, 213, 54–56.e1. [Google Scholar] [CrossRef] [PubMed]
- Galiatsatos, P. COVID-19 Lung Damage. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/what-coronavirus-does-to-the-lungs (accessed on 19 August 2022).
- Gallelli, L.; Zhang, L.; Wang, T.; Fu, F. Severe Acute Lung Injury Related to COVID-19 Infection: A Review and the Possible Role for Escin. J. Clin. Pharmacol. 2020, 60, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2020, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- WHO. COVID-19 Weekly Epidemiological Update; World Health Organisation: Geneva, Switzerland, 2022. [Google Scholar]
- Rossi, A. Long-acting β2-agonists (LABA) in chronic obstructive pulmonary disease: Efficacy and safety. Int. J. Chronic Obstr. Pulm. Dis. 2008, 3, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Matera, M.G.; Page, C.P.; Calzetta, L.; Rogliani, P.; Cazzola, M. Pharmacology and Therapeutics of Bronchodilators Revisited. Pharmacol. Rev. 2019, 72, 218–252. [Google Scholar] [CrossRef]
- Karakiulakis, G.; Roth, M. Muscarinic Receptors and Their Antagonists in COPD: Anti-Inflammatory and Antiremodeling Effects. Mediat. Inflamm. 2012, 2012, 409580. [Google Scholar] [CrossRef]
- Newman, S.P. Drug delivery to the lungs: Challenges and opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef]
- Allen, D.B. Inhaled Corticosteroids and Endocrine Effects in Childhood. Endocrinol. Metab. Clin. North Am. 2020, 49, 651–665. [Google Scholar] [CrossRef]
- Agusti, A.; Fabbri, L.M.; Singh, D.; Vestbo, J.; Celli, B.; Franssen, F.M.; Rabe, K.F.; Papi, A. Inhaled corticosteroids in COPD: Friend or foe? Eur. Respir. J. 2018, 52, 1801219. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Strange, C. Inhaled corticosteroids for chronic obstructive pulmonary disease: What is their role in therapy? Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2587–2601. [Google Scholar] [CrossRef]
- Ferguson, G.T.; Hickey, A.J.; Dwivedi, S. Co-suspension delivery technology in pressurized metered-dose inhalers for multi-drug dosing in the treatment of respiratory diseases. Respir. Med. 2018, 134, 16–23. [Google Scholar] [CrossRef] [PubMed]
- GOLD. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obsructive Pulmonary Diseases (2022 Report); Global Initiative for Chronic Obstructive Pulmonary Disease: Barcelona, Spain, 2021. [Google Scholar]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Chen, D.; Liu, J.; Wu, J.; Suk, J.S. Enhancing nanoparticle penetration through airway mucus to improve drug delivery efficacy in the lung. Expert Opin. Drug Deliv. 2020, 18, 595–606. [Google Scholar] [CrossRef]
- Barnes, P.J. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat. Rev. Drug Discov. 2013, 12, 543–559. [Google Scholar] [CrossRef]
- Praphawatvet, T.; Peters, J.I.; Williams, R.O. Inhaled nanoparticles—An updated review. Int. J. Pharm. 2020, 587, 119671. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 3, 655–663. [Google Scholar] [CrossRef]
- Omlor, A.J.; Nguyen, J.; Bals, R.; Dinh, Q.T. Nanotechnology in respiratory medicine. Respir. Res. 2015, 16, 64. [Google Scholar] [CrossRef]
- Montoto, S.S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Ball, R.L.; Bajaj, P.; Whitehead, K.A. Oral delivery of siRNA lipid nanoparticles: Fate in the GI tract. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- O’Driscoll, C.M.; Bernkop-Schnürch, A.; Friedl, J.D.; Préat, V.; Jannin, V. Oral delivery of non-viral nucleic acid-based therapeutics - do we have the guts for this? Eur. J. Pharm. Sci. 2019, 133, 190–204. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef]
- Thi, T.; Suys, E.; Lee, J.; Nguyen, D.; Park, K.; Truong, N. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2021, 2, 2100109. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Guo, Y.; Bera, H.; Shi, C.; Zhang, L.; Cun, D.; Yang, M. Pharmaceutical strategies to extend pulmonary exposure of inhaled medicines. Acta Pharm. Sin. B 2021, 11, 2565–2584. [Google Scholar] [CrossRef] [PubMed]
- MHRA. Public Assessment Report Authorisation for Temporary Supply COVID-19 mRNA Vaccine BNT162b2 (BNT162b2 RNA) Concentrate for Solution for Injection; Medicines and Healthcare Products Regulatory Agency: London, UK, 2021.
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release Off. J. Control Release Soc. 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Batist, G.; Ramakrishnan, G.; Rao, C.S.; Chandrasekharan, A.; Gutheil, J.; Guthrie, T.; Shah, P.; Khojasteh, A.; Nair, M.K.; Hoelzer, K.; et al. Reduced Cardiotoxicity and Preserved Antitumor Efficacy of Liposome-Encapsulated Doxorubicin and Cyclophosphamide Compared With Conventional Doxorubicin and Cyclophosphamide in a Randomized, Multicenter Trial of Metastatic Breast Cancer. J. Clin. Oncol. 2001, 19, 1444–1454. [Google Scholar] [CrossRef]
- Soundararajan, A.; Bao, A.; Phillips, W.T.; Perez, R.; Goins, B.A. [186Re]Liposomal doxorubicin (Doxil): In vitro stability, pharmacokinetics, imaging and biodistribution in a head and neck squamous cell carcinoma xenograft model. Nucl. Med. Biol. 2009, 36, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.A.; Waterhouse, D.N.; Mayer, L.D.; Cullis, P.R.; Madden, T.D.; Bally, M.B. The Liposomal Formulation of Doxorubicin. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2005; Volume 391, pp. 71–97. [Google Scholar]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.S.; Lee, A.C.H.; Akinc, A.; Bramlage, B.; Bumcrot, D.; Fedoruk, M.N.; Harborth, J.; Heyes, J.A.; Jeffs, L.B.; John, M.; et al. RNAi-mediated gene silencing in non-human primates. Nature 2006, 441, 111–114. [Google Scholar] [CrossRef]
- Rungta, R.; Choi, H.B.; Lin, P.J.; Ko, R.W.; Ashby, D.; Nair, J.; Manoharan, M.; Cullis, P.R.; MacVicar, B.A. Lipid Nanoparticle Delivery of siRNA to Silence Neuronal Gene Expression in the Brain. Mol. Ther. Nucleic Acids 2013, 2, e136. [Google Scholar] [CrossRef]
- Basha, G.; Novobrantseva, T.I.; Rosin, N.; Tam, Y.Y.C.; Hafez, I.M.; Wong, M.K.; Sugo, T.; Ruda, V.M.; Qin, J.; Klebanov, B.; et al. Influence of Cationic Lipid Composition on Gene Silencing Properties of Lipid Nanoparticle Formulations of siRNA in Antigen-Presenting Cells. Mol. Ther. 2011, 19, 2186–2200. [Google Scholar] [CrossRef] [PubMed]
- Basha, G.; Ordobadi, M.; Scott, W.R.; Cottle, A.; Liu, Y.; Wang, H.; Cullis, P.R. Lipid Nanoparticle Delivery of siRNA to Osteocytes Leads to Effective Silencing of SOST and Inhibition of Sclerostin In Vivo. Mol. Ther. Nucleic Acids 2016, 5, e363. [Google Scholar] [CrossRef]
- Feldman, R.A.; Fuhr, R.; Smolenov, I.; Ribeiro, A.; Panther, L.; Watson, M.; Senn, J.J.; Smith, M.; Almarsson, Ö.; Pujar, H.S.; et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 2019, 37, 3326–3334. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078. [Google Scholar] [CrossRef]
- Alamer, E.; Alhazmi, A.; Qasir, N.A.; Alamer, R.; Areeshi, H.; Gohal, G.; Qadri, M.; Hashem, A.M.; Algaissi, A. Side Effects of COVID-19 Pfizer-BioNTech mRNA Vaccine in Children Aged 12–18 Years in Saudi Arabia. Vaccines 2021, 9, 1297. [Google Scholar] [CrossRef]
- Canonico, A.E.; Plitman, J.D.; Conary, J.T.; Meyrick, B.O.; Brigham, K.L. No lung toxicity after repeated aerosol or intravenous delivery of plasmid-cationic liposome complexes. J. Appl. Physiol. 1994, 77, 415–419. [Google Scholar] [CrossRef]
- Gilbert, B.E.; Knight, C.; Alvarez, F.G.; Waldrep, J.C.; Rodarte, J.R.; Knight, V.; Eschenbacher, W.L. Tolerance of Volunteers to Cyclosporine A-dilauroylphosphatidylcholine Liposome Aerosol. Am. J. Respir. Crit. Care Med. 1997, 156, 1789–1793. [Google Scholar] [CrossRef]
- Myers, M.A.; Thomas, D.A.; Straub, L.; Soucy, D.W.; Niven, R.W.; Kaltenbach, M.; Hood, C.I.; Schreier, H.; Gonzalez-Rothi, R.J. Pulmonary Effects of Chronic Exposure to Liposome Aerosols in Mice. Exp. Lung Res. 1993, 19, 1–19. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Gilbert, B.; Mehta, K. Aerosol delivery of liposomal all- trans -retinoic acid to the lungs. Cancer Chemother. Pharmacol. 1999, 43, 277–283. [Google Scholar] [CrossRef]
- Saari, M.; Vidgren, M.T.; Koskinen, M.O.; Turjanmaa, V.M.; Nieminen, M.M. Pulmonary distribution and clearance of two beclomethasone liposome formulations in healthy volunteers. Int. J. Pharm. 1999, 181, 1–9. [Google Scholar] [CrossRef]
- Taylor, K.M.G.; Taylor, G.; Kellaway, I.W.; Stevens, J. The Influence of Liposomal Encapsulation on Sodium Cromoglycate Pharmacokinetics in Man. Pharm. Res. 1989, 6, 633–636. [Google Scholar] [CrossRef]
- Ten, R.; Anderson, P.; Zein, N.; Temesgen, Z.; Clawson, M.L.; Weiss, W. Interleukin-2 liposomes for primary immune deficiency using the aerosol route. Int. Immunopharmacol. 2001, 2, 333–344. [Google Scholar] [CrossRef]
- Khanna, C.; Anderson, P.M.; Hasz, D.E.; Katsanis, E.; Neville, M.; Klausner, J.S. Interleukin-2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastases. Cancer 1997, 79, 1409–1421. [Google Scholar] [CrossRef]
- Anderson, C.F.; Anderson, C.F.; Grimmett, M.E.; Grimmett, M.E.; Domalewski, C.J.; Domalewski, C.J.; Cui, H.; Cui, H. Inhalable nanotherapeutics to improve treatment efficacy for common lung diseases. WIREs Nanomed. Nanobiotechnol. 2019, 12, e1586. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.; MacDonald, K.D.; Slaughter, K.; McKinney, M.; Patel, S.; Sun, C.; Sahay, G. Lipid Nanoparticle-Delivered Chemically Modified mRNA Restores Chloride Secretion in Cystic Fibrosis. Mol. Ther. 2018, 26, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Rudokas, M.; Najlah, M.; Alhnan, M.A.; Elhissi, A. Liposome Delivery Systems for Inhalation: A Critical Review Highlighting Formulation Issues and Anticancer Applications. Med. Princ. Pract. 2016, 25, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Gao, W.; Li, T.; Zhou, Q. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: Challenges and opportunities. Acta Pharmacol. Sin. 2017, 38, 782–797. [Google Scholar] [CrossRef]
- Truzzi, E.; Nascimento, T.L.; Iannuccelli, V.; Costantino, L.; Lima, E.M.; Leo, E.; Siligardi, C.; Gualtieri, M.L.; Maretti, E. In Vivo Biodistribution of Respirable Solid Lipid Nanoparticles Surface-Decorated with a Mannose-Based Surfactant: A Promising Tool for Pulmonary Tuberculosis Treatment? Nanomaterials 2020, 10, 568. [Google Scholar] [CrossRef]
- Hamedinasab, H.; Rezayan, A.H.; Mellat, M.; Mashreghi, M.; Jaafari, M.R. Development of chitosan-coated liposome for pulmonary delivery of N-acetylcysteine. Int. J. Biol. Macromol. 2019, 156, 1455–1463. [Google Scholar] [CrossRef]
- Zhang, J.; Leifer, F.; Rose, S.; Chun, D.Y.; Thaisz, J.; Herr, T.; Nashed, M.; Joseph, J.; Perkins, W.R.; DiPetrillo, K. Amikacin Liposome Inhalation Suspension (ALIS) Penetrates Non-tuberculous Mycobacterial Biofilms and Enhances Amikacin Uptake Into Macrophages. Front. Microbiol. 2018, 9, 915. [Google Scholar] [CrossRef]
- FDA. FDA Approves a New Antibacterial Drug to Treat a Serious Lung Disease using a Novel Pathway to Spur Innovation. 2018. Available online: https://www.amr-insights.eu/fda-approves-a-new-antibacterial-drug-to-treat-a-serious-lung-disease-using-a-novel-pathway-to-spur-innovation/ (accessed on 29 August 2022).
- Insmed. Insmed Initiates Frontline Clinical Trial Program for ARIKAYCE® (amikacin liposome inhalation suspension) in Patients with MAC Lung Disease. 2021. Available online: https://investor.insmed.com/2021-01-04-Insmed-Initiates-Frontline-Clinical-Trial-Program-for-ARIKAYCE-R-amikacin-liposome-inhalation-suspension-in-Patients-with-MAC-Lung-Disease (accessed on 29 August 2022).
- Moreton, C. Poor Solubility—Where Do We Stand 25 Years after the ‘Rule of Five’? | American Pharmaceutical Review—The Review of American Pharmaceutical Business & Technology. American Pharmaceutical Review. 2021. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/573402-Poor-Solubility-Where-Do-We-Stand-25-Years-after-the-Rule-of-Five/ (accessed on 29 August 2022).
- Narvekar, M.; Xue, H.Y.; Eoh, J.Y.; Wong, H.L. Nanocarrier for Poorly Water-Soluble Anticancer Drugs—Barriers of Translation and Solutions. AAPS PharmSciTech 2014, 15, 822–833. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, X.; Chen, H.; Bian, Z.; Zhang, G.; Riaz, M.K.; Tyagi, D.; Lin, G.; Zhang, Y.; Wang, J.; et al. Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineage-homing cell-penetrating peptide. Drug Deliv. 2018, 25, 256–266. [Google Scholar] [CrossRef]
- Rosière, R.; Van Woensel, M.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Van Antwerpen, P.; Amighi, K.; Wauthoz, N. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.O.; Silva, R.; Nunes, P.S.; Felipe, F.A.; Ramos, K.P.P.; Ferreira, L.A.S.; Lima, V.N.B.; Shanmugam, S.; Oliveira, A.S.; Guterres, S.S.; et al. Effects of the solid lipid nanoparticle of carvacrol on rodents with lung injury from smoke inhalation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 393, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Osman, R.; Al-Jamal, K.T.; Holayel, S.M.; Geneidi, A.-S. Enhanced antitubercular activity, alveolar deposition and macrophages uptake of mannosylated stable nanoliposomes. J. Drug Deliv. Sci. Technol. 2019, 51, 513–523. [Google Scholar] [CrossRef]
- Maretti, E.; Costantino, L.; Rustichelli, C.; Leo, E.; Croce, M.A.; Buttini, F.; Truzzi, E.; Iannuccelli, V. Surface engineering of Solid Lipid Nanoparticle assemblies by methyl α- d -mannopyranoside for the active targeting to macrophages in anti-tuberculosis inhalation therapy. Int. J. Pharm. 2017, 528, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Montoto, S.S.; Sbaraglini, M.; Di Ianni, M.; Ruiz, M.; Talevi, A.; Alvarez, V.; Durán, N.; Castro, G.; Islan, G. Hybrid Ofloxacin/eugenol co-loaded solid lipid nanoparticles with enhanced and targetable antimicrobial properties. Int. J. Pharm. 2019, 569, 118575. [Google Scholar] [CrossRef]
- Garbuzenko, O.; Kbah, N.; Kuzmov, A.; Pogrebnyak, N.; Pozharov, V.; Minko, T. Inhalation treatment of cystic fibrosis with lumacaftor and ivacaftor co-delivered by nanostructured lipid carriers. J. Control. Release 2019, 296, 225–231. [Google Scholar] [CrossRef]
- Patel, P.; Raval, M.; Airao, V.; Bhatt, V.; Shah, P. Silibinin loaded inhalable solid lipid nanoparticles for lung targeting. J. Microencapsul. 2021, 39, 2002448. [Google Scholar] [CrossRef]
- Satari, N.; Taymouri, S.; Varshosaz, J.; Rostami, M.; Mirian, M. Preparation and evaluation of inhalable dry powder containing glucosamine-conjugated gefitinib SLNs for lung cancer therapy. Drug Dev. Ind. Pharm. 2020, 46, 1265–1277. [Google Scholar] [CrossRef]
- Chapman, R.W.; Li, Z.; Corboz, M.R.; Gauani, H.; Plaunt, A.J.; Konicek, D.M.; Leifer, F.G.; Laurent, C.E.; Yin, H.; Salvail, D.; et al. Inhaled hexadecyl-treprostinil provides pulmonary vasodilator activity at significantly lower plasma concentrations than infused treprostinil. Pulm. Pharmacol. Ther. 2018, 49, 104–111. [Google Scholar] [CrossRef]
- Landh, E.; Moir, L.M.; Dos Reis, L.G.; Traini, D.; Young, P.M.; Ong, H.X. Inhaled rapamycin solid lipid nano particles for the treatment of Lymphangioleiomyomatosis. Eur. J. Pharm. Sci. 2019, 142, 105098. [Google Scholar] [CrossRef]
- Leifer, F.G.; Konicek, D.M.; Chen, K.-J.; Plaunt, A.J.; Salvail, D.; Laurent, C.E.; Corboz, M.R.; Li, Z.; Chapman, R.W.; Perkins, W.R.; et al. Inhaled Treprostinil-Prodrug Lipid Nanoparticle Formulations Provide Long-Acting Pulmonary Vasodilation. Drug Res. 2018, 68, 605–614. [Google Scholar] [CrossRef]
- Li, Z.; Qiao, W.; Wang, C.; Wang, H.; Ma, M.; Han, X.; Tang, J. DPPC-coated lipid nanoparticles as an inhalable carrier for accumulation of resveratrol in the pulmonary vasculature, a new strategy for pulmonary arterial hypertension treatment. Drug Deliv. 2020, 27, 736–744. [Google Scholar] [CrossRef]
- Nafee, N.; Makled, S.; Boraie, N. Nanostructured lipid carriers versus solid lipid nanoparticles for the potential treatment of pulmonary hypertension via nebulization. Eur. J. Pharm. Sci. 2018, 125, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Gairola, A.; Benjamin, A.; Weatherston, J.D.; Cirillo, J.D.; Wu, H. Recent Developments in Drug Delivery for Treatment of Tuberculosis by Targeting Macrophages. Adv. Ther. 2022, 5, 202100193. [Google Scholar] [CrossRef]
- Jain, S.; Kaur, J.; Prasad, S.; Roy, I. Nucleic acid therapeutics: A focus on the development of aptamers. Expert Opin. Drug Discov. 2020, 16, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Mukalel, A.J.; Riley, R.S.; Zhang, R.; Mitchell, M.J. Nanoparticles for nucleic acid delivery: Applications in cancer immunotherapy. Cancer Lett. 2019, 458, 102–112. [Google Scholar] [CrossRef]
- Zhang, H.; Leal, J.; Soto, M.; Smyth, H.; Ghosh, D. Aerosolizable Lipid Nanoparticles for Pulmonary Delivery of mRNA through Design of Experiments. Pharmaceutics 2020, 12, 1042. [Google Scholar] [CrossRef]
- NIH. ONPATTRO-Patisiran Injection, Lipid Complex. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e87ec36f-b4b4-49d4-aea4-d4ffb09b0970 (accessed on 27 February 2022).
- CDC. Moderna COVID-19 Vaccine Overview and Safety. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html#ingredients (accessed on 29 August 2022).
- Pfizer-BioNTech COVID-19 Vaccine (Also Known as COMIRNATY) Overview and Safety. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html (accessed on 11 January 2022).
- Cross, R. Without these lipid shells, there would be no mRNA vaccines for COVID. Chem. Eng. News 2021, 99, 16–19. [Google Scholar] [CrossRef]
- Semple, S.C.; Leone, R.; Barbosa, C.J.; Tam, Y.K.; Lin, P.J.C. Lipid Nanoparticle Delivery Systems to Enable mRNA-Based Therapeutics. Pharmaceutics 2022, 14, 398. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Chan, H.-K. Lipid nanoparticles for the inhalation of mRNA. Nat. Biomed. Eng. 2021, 5, 949–950. [Google Scholar] [CrossRef]
- Lokugamage, M.P.; Vanover, D.; Beyersdorf, J.; Hatit, M.Z.C.; Rotolo, L.; Echeverri, E.S.; Peck, H.E.; Ni, H.; Yoon, J.-K.; Kim, Y.; et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 2021, 5, 1059–1068. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study to Evaluate the Safety & Tolerability of MRT5005 Administered by Nebulization in Adults With Cystic Fibrosis. Available online: https://clinicaltrials.gov/ct2/show/NCT03375047 (accessed on 29 August 2022).
- Woo, C.J.; Allawzi, A.; Clark, N.; Kaushal, N.; Efthymiou, T.; Thamsen, M.; Nguyen, J.; Wooster, R.; Sullivan, J.C. Inhaled delivery of a lipid nanoparticle encapsulated messenger RNA encoding a ciliary protein for the treatment of primary ciliary dyskinesia. Pulm. Pharmacol. Ther. 2022, 75, 102134. [Google Scholar] [CrossRef] [PubMed]
- Tagalakis, A.D.; Munye, M.M.; Ivanova, R.; Chen, H.; Smith, C.M.; Aldossary, A.; Rosa, L.Z.; Moulding, D.; Barnes, J.L.; Kafetzis, K.N.; et al. Effective silencing of ENaC by siRNA delivered with epithelial-targeted nanocomplexes in human cystic fibrosis cells and in mouse lung. Thorax 2018, 73, 847–856. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Hu, Y.; Pan, T.; Xu, Y.; Yu, J.; Xiong, W.; Zhou, Q.; Wang, Y. Local administration of liposomal-based Srpx2 gene therapy reverses pulmonary fibrosis by blockading fibroblast-to-myofibroblast transition. Theranostics 2021, 11, 7110–7125. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 1–27. [Google Scholar] [CrossRef]
- Matthews, A.A.; Ee, P.L.R.; Ge, R. Developing inhaled protein therapeutics for lung diseases. Mol. Biomed. 2020, 1, 1–14. [Google Scholar] [CrossRef]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef]
- Fröhlich, E.; Salar-Behzadi, S. Oral inhalation for delivery of proteins and peptides to the lungs. Eur. J. Pharm. Biopharm. 2021, 163, 198–211. [Google Scholar] [CrossRef]
- Respaud, R.; Vecellio, L.; Diot, P.; Heuzé-Vourc’h, N. Nebulization as a delivery method for mAbs in respiratory diseases. Expert Opin. Drug Deliv. 2015, 12, 1027–1039. [Google Scholar] [CrossRef]
- Fellner, R.C.; Terryah, S.T.; Tarran, R. Inhaled protein/peptide-based therapies for respiratory disease. Mol. Cell. Pediatr. 2016, 3, 1–5. [Google Scholar] [CrossRef]
- Enlo-Scott, Z.; Bäckström, E.; Mudway, I.; Forbes, B. Drug metabolism in the lungs: Opportunities for optimising inhaled medicines. Expert Opin. Drug Metab. Toxicol. 2021, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Sécher, T.; Mayor, A.; Heuzé-Vourc’h, N. Inhalation of Immuno-Therapeutics/-Prophylactics to Fight Respiratory Tract Infections: An Appropriate Drug at the Right Place. Front. Immunol. 2019, 10, 2760. [Google Scholar] [CrossRef]
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Murata, M.; Nakano, K.; Tahara, K.; Tozuka, Y.; Takeuchi, H. Pulmonary delivery of elcatonin using surface-modified liposomes to improve systemic absorption: Polyvinyl alcohol with a hydrophobic anchor and chitosan oligosaccharide as effective surface modifiers. Eur. J. Pharm. Biopharm. 2012, 80, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Sugihara, H. Absorption of Calcitonin in Oral and Pulmonary Administration with Polymer-coated Liposomes. Yakugaku Zasshi 2010, 130, 1135–1142. [Google Scholar] [CrossRef]
- Bi, R.; Shao, W.; Wang, Q.; Zhang, N. Solid lipid nanoparticles as insulin inhalation carriers for enhanced pulmonary delivery. J. Biomed. Nanotechnol. 2009, 5, 84–92. [Google Scholar] [CrossRef]
- Liu, J.; Gong, T.; Fu, H.; Wang, C.; Wang, X.; Chen, Q.; Zhang, Q.; He, Q.; Zhang, Z. Solid lipid nanoparticles for pulmonary delivery of insulin. Int. J. Pharm. 2008, 356, 333–344. [Google Scholar] [CrossRef]
- Oleck, J.; Kassam, S.; Goldman, J.D. Commentary: Why Was Inhaled Insulin a Failure in the Market? Diabetes Spectr. 2016, 29, 180–184. [Google Scholar] [CrossRef]
- Hajos, F.; Stark, B.; Hensler, S.; Prassl, R.; Mosgoeller, W. Inhalable liposomal formulation for vasoactive intestinal peptide. Int. J. Pharm. 2008, 357, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Laube, B.L. Aerosolized Medications for Gene and Peptide Therapy. Respir. Care 2015, 60, 806–824. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Hukkanen, J.; Pelkonen, O.; Raunio, H. Expression of xenobiotic-metabolizing enzymes in human pulmonary tissue: Possible role in susceptibility for ILD. Eur. Respir. J. Suppl. 2001, 18, 122. [Google Scholar]
- Anttila, S.; Hukkanen, J.; Hakkola, J.; Stjernvall, T.; Beaune, P.; Edwards, R.J.; Boobis, A.R.; Pelkonen, O.; Raunio, H. Expression and localization of CYP3A4 and CYP3A5 in human lung. Am. J. Respir. Cell Mol. Biol. 1997, 16, 242–249. [Google Scholar] [CrossRef]
- García-Menaya, J.M.; Cordobés-Durán, C.; García-Martín, E.; Agundez, J. Pharmacogenetic Factors Affecting Asthma Treatment Response. Potential Implications for Drug Therapy. Front. Pharmacol. 2019, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Oesch, F.; Fabian, E.; Landsiedel, R. Xenobiotica-metabolizing enzymes in the lung of experimental animals, man and in human lung models. Arch. Toxicol. 2019, 93, 3419–3489. [Google Scholar] [CrossRef]
- Mishra, B.; Singh, J. Novel drug delivery systems and significance in respiratory diseases. Target. Chronic Inflamm. Lung Dis. Using Adv. Drug Deliv. Syst. 2020, 57–95. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M.; et al. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release 2018, 269, 374–392. [Google Scholar] [CrossRef]
- Borghardt, J.M.; Kloft, C.; Sharma, A. Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes. Can. Respir. J. 2018, 2018, 2732017. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Wang, X.; Zhang, S.; Gao, P.; Shi, Z. The Role of Pulmonary Surfactants in the Treatment of Acute Respiratory Distress Syndrome in COVID-19. Front. Pharmacol. 2021, 12, 698905. [Google Scholar] [CrossRef]
- van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef]
- Liang, Z.; Ni, R.; Zhou, J.; Mao, S. Recent advances in controlled pulmonary drug delivery. Drug Discov. Today 2015, 20, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wang, Z.; Wei, X.; Shi, J.; Li, C. Antibodies against polyethylene glycol in human blood: A literature review. J. Pharmacol. Toxicol. Methods 2020, 102, 106678. [Google Scholar] [CrossRef] [PubMed]
- Lubich, C.; Allacher, P.; de la Rosa, M.; Bauer, A.; Prenninger, T.; Horling, F.M.; Siekmann, J.; Oldenburg, J.; Scheiflinger, F.; Reipert, B.M. The Mystery of Antibodies Against Polyethylene Glycol (PEG)—What do we Know? Pharm. Res. 2016, 33, 2239–2249. [Google Scholar] [CrossRef]
- Moghimi, S.M. Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines. Mol. Ther. 2021, 29, 898–900. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef]
- Kiyono, H.; Azegami, T. The mucosal immune system: From dentistry to vaccine development. Proc. Jpn. Acad. Ser. B 2015, 91, 423–439. [Google Scholar] [CrossRef]
- Ardain, A.; Marakalala, M.; Leslie, A. Tissue-resident innate immunity in the lung. Immunology 2019, 159, 245–256. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Marsland, B.J. Lung Homeostasis: Influence of Age, Microbes, and the Immune System. Immunity 2017, 46, 549–561. [Google Scholar] [CrossRef]
- Shamji, M.H.; Valenta, R.; Jardetzky, T.; Verhasselt, V.; Durham, S.R.; Würtzen, P.A.; van Neerven, R.J. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy 2021, 76, 3627–3641. [Google Scholar] [CrossRef]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S41–S52. [Google Scholar] [CrossRef]
- Woof, J.M.; Kerr, M.A. The function of immunoglobulin A in immunity. J. Pathol. 2006, 208, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Karra, N.; Swindle, E.; Morgan, H. Drug delivery for traditional and emerging airway models. Organs-on-a-Chip 2019, 1, 100002. [Google Scholar] [CrossRef]
- Huckaby, J.T.; Lai, S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018, 124, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Darquenne, C. Deposition Mechanisms. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.; Gaber, D.M.; Elzoghby, A.O.; Helmy, M.W.; Abdallah, O.Y. Promoted Antitumor Activity of Myricetin against Lung Carcinoma Via Nanoencapsulated Phospholipid Complex in Respirable Microparticles. Pharm. Res. 2020, 37, 82. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zhang, W. Applications of Chitosan in Pulmonary Drug Delivery. Role Nov. Drug Deliv. Veh. Nanobiomed. 2020. [Google Scholar] [CrossRef]
- Rasul, R.M.; Muniandy, M.T.; Zakaria, Z.; Shah, K.; Chee, C.F.; Dabbagh, A.; Rahman, N.A.; Wong, T.W. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr. Polym. 2020, 250, 116800. [Google Scholar] [CrossRef]
- Luo, T.; Loira-Pastoriza, C.; Patil, H.P.; Ucakar, B.; Muccioli, G.G.; Bosquillon, C.; Vanbever, R. PEGylation of paclitaxel largely improves its safety and anti-tumor efficacy following pulmonary delivery in a mouse model of lung carcinoma. J. Control. Release 2016, 239, 62–71. [Google Scholar] [CrossRef]
- De Leo, V.; Ruscigno, S.; Trapani, A.; Di Gioia, S.; Milano, F.; Mandracchia, D.; Comparelli, R.; Castellani, S.; Agostiano, A.; Trapani, G.; et al. Preparation of drug-loaded small unilamellar liposomes and evaluation of their potential for the treatment of chronic respiratory diseases. Int. J. Pharm. 2018, 545, 378–388. [Google Scholar] [CrossRef]
- Leal, J.; Peng, X.; Liu, X.; Arasappan, D.; Wylie, D.C.; Schwartz, S.H.; Fullmer, J.J.; McWilliams, B.C.; Smyth, H.D.; Ghosh, D. Peptides as surface coatings of nanoparticles that penetrate human cystic fibrosis sputum and uniformly distribute in vivo following pulmonary delivery. J. Control. Release 2020, 322, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.; Forier, K.; Braeckmans, K.; Schneider, M. Mucus-penetrating solid lipid nanoparticles for the treatment of cystic fibrosis: Proof of concept, challenges and pitfalls. Eur. J. Pharm. Biopharm. 2018, 124, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Steiner, V.; Öhlinger, K.; Corzo, C.; Salar-Behzadi, S.; Fröhlich, E. Cytotoxicity screening of emulsifiers for pulmonary application of lipid nanoparticles. Eur. J. Pharm. Sci. 2019, 136, 104968. [Google Scholar] [CrossRef] [PubMed]
- Lavorini, F.; Buttini, F.; Usmani, O.S. 100 Years of Drug Delivery to the Lungs; Barrett, J., Page, C., Michel, M., Eds.; Springer: Cham, Switzerland, 2019; Volume 260, pp. 143–159. [Google Scholar]
- Surasarang, S.H.; Florova, G.; Komissarov, A.; Shetty, S.; Idell, S.; Williams, R.O. Formulation for a novel inhaled peptide therapeutic for idiopathic pulmonary fibrosis. Drug Dev. Ind. Pharm. 2017, 44, 184–198. [Google Scholar] [CrossRef]
- Doty, A.; Schroeder, J.; Vang, K.; Sommerville, M.; Taylor, M.; Flynn, B.; Lechuga-Ballesteros, D.; Mack, P. Drug Delivery from an Innovative LAMA/LABA Co-suspension Delivery Technology Fixed-Dose Combination MDI: Evidence of Consistency, Robustness, and Reliability. AAPS PharmSciTech 2017, 19, 837–844. [Google Scholar] [CrossRef]
- Gibbons, A.; McElvaney, N.G.; Cryan, S.-A. A Dry Powder Formulation of Liposome-Encapsulated Recombinant Secretory Leukocyte Protease Inhibitor (rSLPI) for Inhalation: Preparation and Characterisation. AAPS PharmSciTech 2010, 11, 1411–1421. [Google Scholar] [CrossRef]
- Wang, J.-L.; Hanafy, M.S.; Xu, H.; Leal, J.; Zhai, Y.; Ghosh, D.; Iii, R.O.W.; Smyth, H.D.C.; Cui, Z. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int. J. Pharm. 2021, 596, 120215. [Google Scholar] [CrossRef]
- Khatib, I.; Khanal, D.; Ruan, J.; Cipolla, D.; Dayton, F.; Blanchard, J.D.; Chan, H.-K. Ciprofloxacin nanocrystals liposomal powders for controlled drug release via inhalation. Int. J. Pharm. 2019, 566, 641–651. [Google Scholar] [CrossRef]
- Ourique, A.; Chaves, P.D.S.; Souto, G.D.; Pohlmann, A.; Guterres, S.; Beck, R.C.R. Redispersible liposomal-N-acetylcysteine powder for pulmonary administration: Development, in vitro characterization and antioxidant activity. Eur. J. Pharm. Sci. 2014, 65, 174–182. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Cheng, P.; Yang, P.; Shi, P.; Kong, L.; Liu, H. Preparation of Curcumin Solid Lipid Nanoparticles Loaded with Flower-Shaped Lactose for Lung Inhalation and Preliminary Evaluation of Cytotoxicity In Vitro. Evidence-Based Complement. Altern. Med. 2021, 2021, 4828169. [Google Scholar] [CrossRef]
- Chao, Y.-S.; Grobelna, A. Curosurf (poractant alfa) for the Treatment of Infants At Risk For or Experiencing Respiratory Distress Syndrome: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018. [Google Scholar]
- Ramanathan, R.; Rasmussen, M.R.; Gerstmann, D.R.; Finer, N.; Sekar, K. The North American Study Group A Randomized, Multicenter Masked Comparison Trial of Poractant Alfa (Curosurf) versus Beractant (Survanta) in the Treatment of Respiratory Distress Syndrome in Preterm Infants. Am. J. Perinatol. 2004, 21, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Bancalari, E. On “A Randomized, Multicenter Masked Comparison Trial of Poractant Alfa (Curosurf) versus Beractant (Survanta) in the Treatment of Respiratory Distress Syndrome in Preterm Infants” (Am J Perinatol 2004;21:109-120). Am. J. Perinatol. 2004, 21, 307–309. [Google Scholar] [CrossRef] [PubMed]
- VanDevanter, D.; Geller, D. Tobramycin administered by the TOBI® Podhaler® for persons with cystic fibrosis: A review. Med. Devices Évid. Res. 2011, 4, 179–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geller, D.E.; Weers, J.; Heuerding, S. Development of an Inhaled Dry-Powder Formulation of Tobramycin Using PulmoSphere™ Technology. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Khan, O.; Chaudary, N. The Use of Amikacin Liposome Inhalation Suspension (Arikayce) in the Treatment of Refractory Nontuberculous Mycobacterial Lung Disease in Adults. Drug Des. Dev. Ther. 2020, 14, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Bilton, D.; Chalmers, J.D.; Davis, A.M.; Froehlich, J.; Gonda, I.; Thompson, B.; Wanner, A.; O’Donnell, A.E. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): Two phase 3, randomised controlled trials. Lancet Respir. Med. 2019, 7, 213–226. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Cipolla, D.; Thompson, B.; Davis, A.M.; O’Donnell, A.; Tino, G.; Gonda, I.; Haworth, C.; Froehlich, J. Changes in respiratory symptoms during 48-week treatment with ARD-3150 (inhaled liposomal ciprofloxacin) in bronchiectasis: Results from the ORBIT-3 and -4 studies. Eur. Respir. J. 2020, 56, 2000110. [Google Scholar] [CrossRef]
- De Soyza, A.; Aksamit, T.; Bandel, T.-J.; Criollo, M.; Elborn, J.S.; Operschall, E.; Polverino, E.; Roth, K.; Winthrop, K.L.; Wilson, R. RESPIRE 1: A phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2018, 51, 1702052. [Google Scholar] [CrossRef]
- Aksamit, T.; De Soyza, A.; Bandel, T.-J.; Criollo, M.; Elborn, J.S.; Operschall, E.; Polverino, E.; Roth, K.; Winthrop, K.L.; Wilson, R. RESPIRE 2: A phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2018, 51, 1702053. [Google Scholar] [CrossRef]
- Aksamit, T.; Bandel, T.-J.; Criollo, M.; De Soyza, A.; Elborn, J.S.; Operschall, E.; Polverino, E.; Roth, K.; Winthrop, K.L.; Wilson, R. The RESPIRE trials: Two phase III, randomized, multicentre, placebo-controlled trials of Ciprofloxacin Dry Powder for Inhalation (Ciprofloxacin DPI) in non-cystic fibrosis bronchiectasis. Contemp. Clin. Trials 2017, 58, 78–85. [Google Scholar] [CrossRef]

| Advantages | Challenges | |
|---|---|---|
| 1 | Diverse drugs delivered | Airway clearance mechanisms |
| 2 | Improved drug stability | Airway structural barrier |
| 3 | Enhanced local drug retention | Premature particle deposition |
| 4 | Reduced systemic toxicity | Limited formulation excipients |
| 5 | Reduced immunogenicity | Inhaler selection and compatibility |
| Drug | Commercial Name | LNP Type | Disease | Status |
|---|---|---|---|---|
| SP-B and SP-C | Curosurf® | Liposome | Respiratory distress syndrome in premature infants | Approved 1999 [159,160]. |
| Tobramycin | TOBI® Podhaler® | LNP | Chronic pulmonary Pseudomonas aeruginosa (Pa) infection in CF patients. | Approved 2013 [162,163]. |
| Amikacin | Arikayce® | Liposome | Mycobacterium avium complex lung disease | Approved 2018 [164]. |
| Ciprofloxacin | Apulmiq (Linhaliq/ Pulmaquin) | Liposome | Chronic lung infections with pseudomonas aeruginosa with non-cystic fibrosis bronchiectasis | Two phase III clinical trials completed in 2016. Discontinued [165,166]. |
| Ciprofloxacin | Ciprofloxacin DPI/ BAYQ3939 | Lipid micro-particle | Non-cystic fibrosis bronchiectasis | Two phase III clinical trials completed in 2016. Not yet approved [167,168,169]. |
| CFTR mRNA | MRT5005 | LNP | CF | Phase I/II clinical trial [34,98]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leong, E.W.X.; Ge, R. Lipid Nanoparticles as Delivery Vehicles for Inhaled Therapeutics. Biomedicines 2022, 10, 2179. https://doi.org/10.3390/biomedicines10092179
Leong EWX, Ge R. Lipid Nanoparticles as Delivery Vehicles for Inhaled Therapeutics. Biomedicines. 2022; 10(9):2179. https://doi.org/10.3390/biomedicines10092179
Chicago/Turabian StyleLeong, Ellenmae W. X., and Ruowen Ge. 2022. "Lipid Nanoparticles as Delivery Vehicles for Inhaled Therapeutics" Biomedicines 10, no. 9: 2179. https://doi.org/10.3390/biomedicines10092179
APA StyleLeong, E. W. X., & Ge, R. (2022). Lipid Nanoparticles as Delivery Vehicles for Inhaled Therapeutics. Biomedicines, 10(9), 2179. https://doi.org/10.3390/biomedicines10092179









