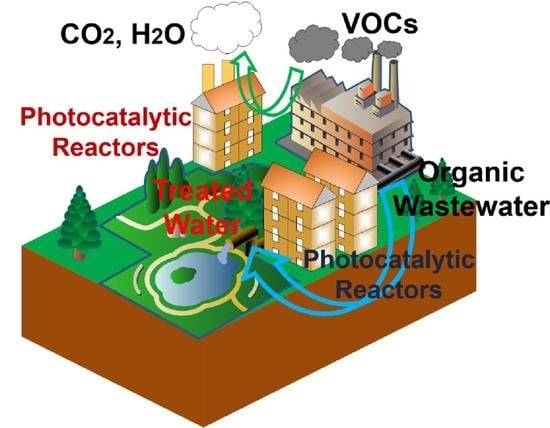
Photocatalytic Reactor as a Bridge to Link the Commercialization of Photocatalyst in Water and Air Purification
Abstract
:1. Introduction
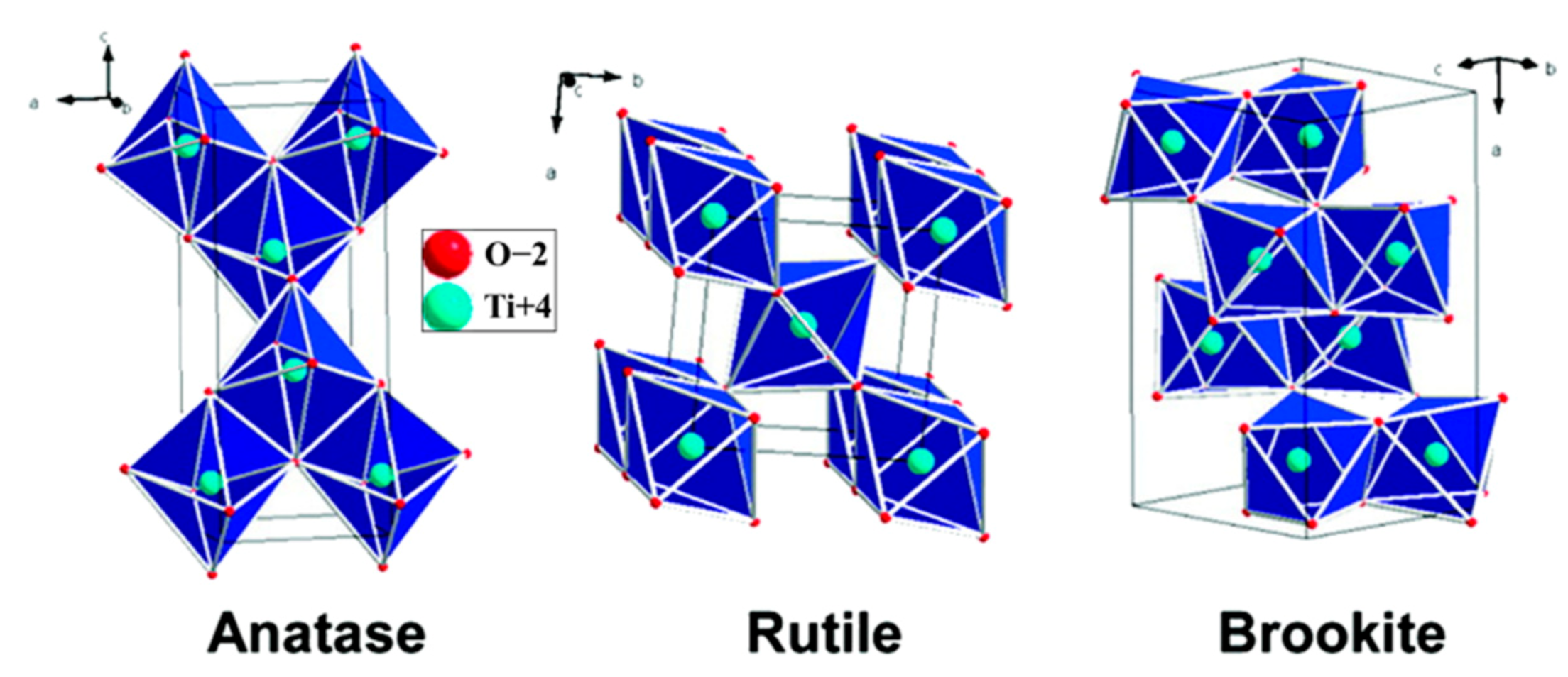
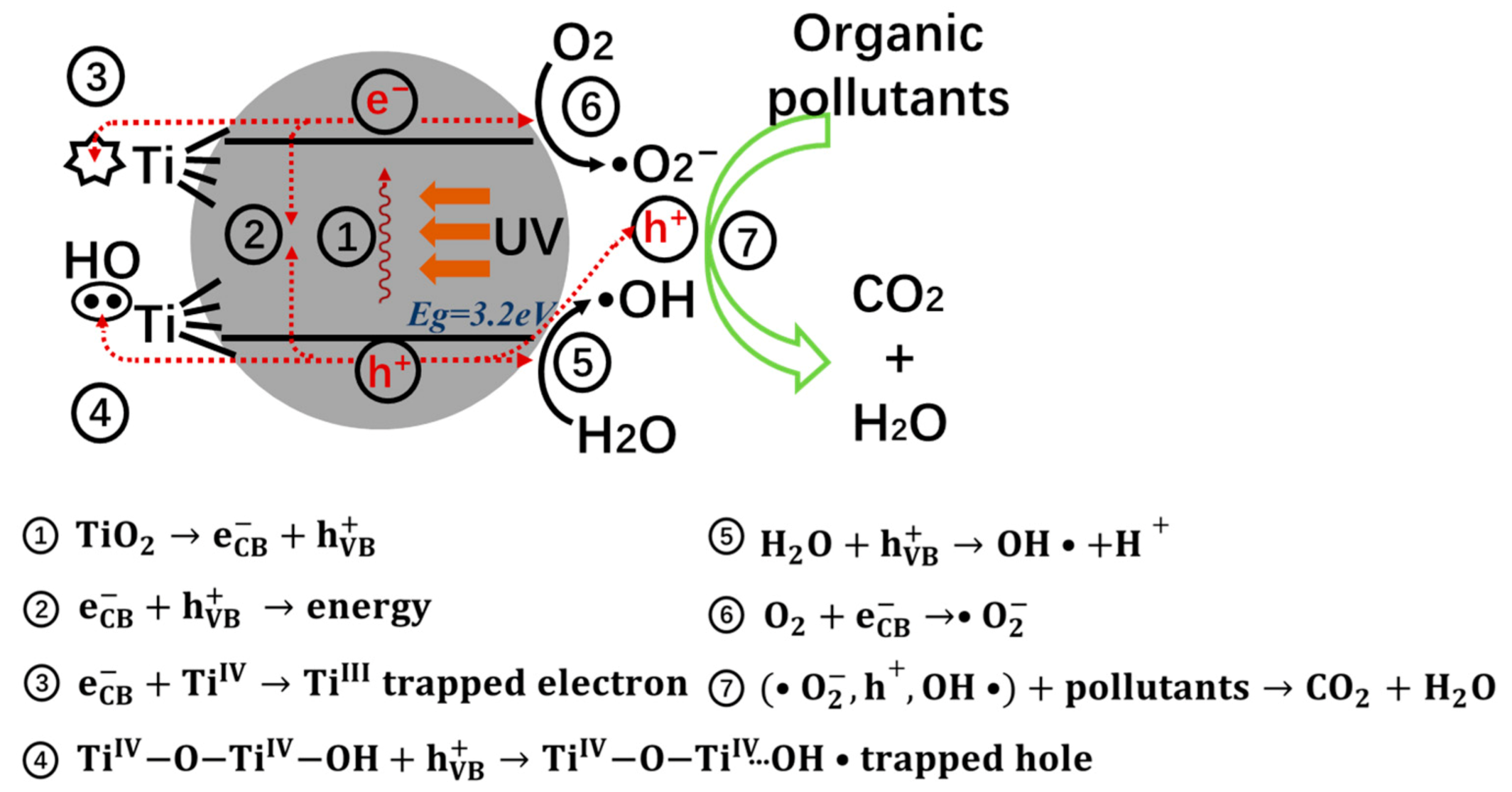
2. Photocatalysis Mechanism of TiO2
3. Types of TiO2-Based Photoreactors in Environmental Remediation
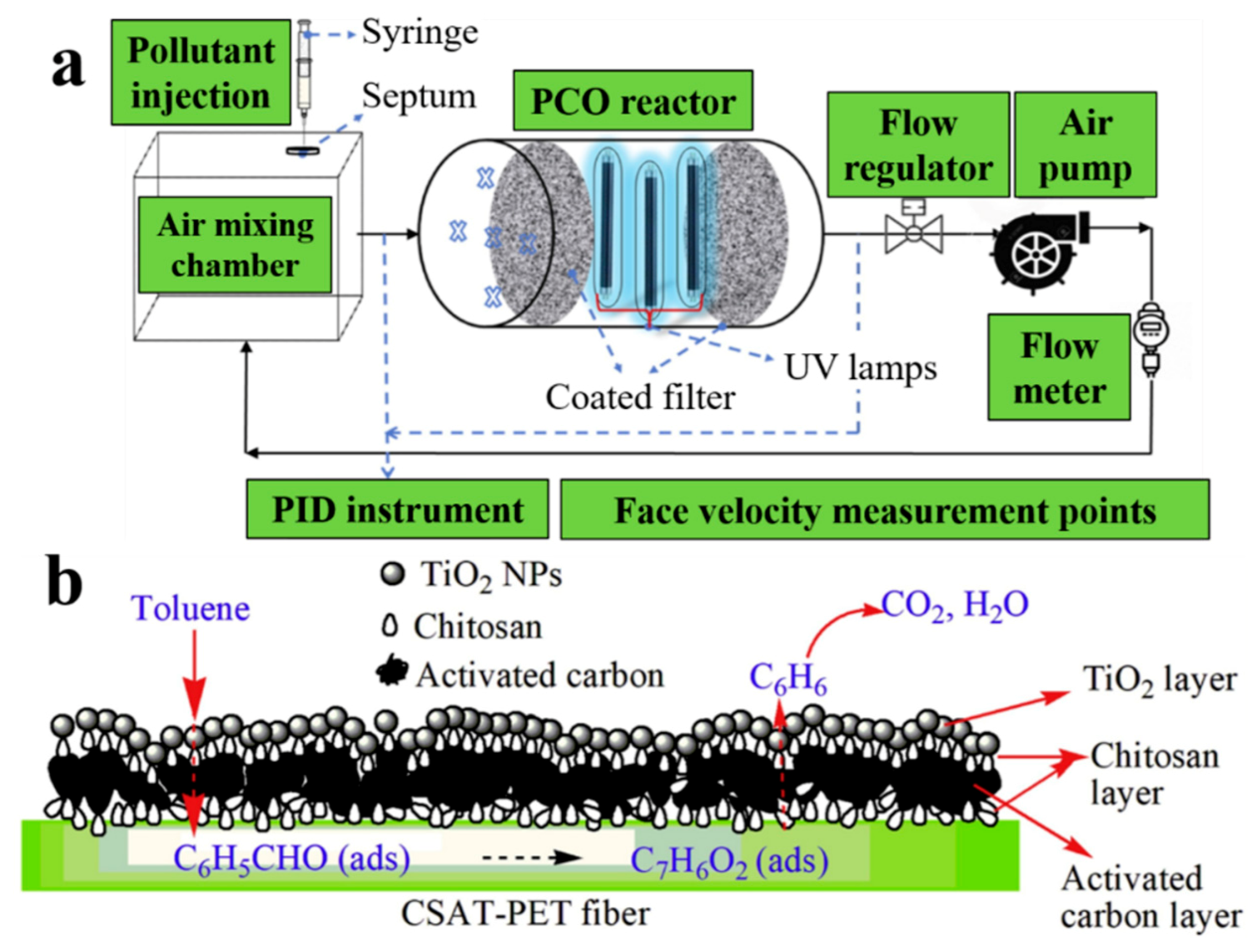
3.1. Application of TiO2-Based Photoreactors for Air Purification
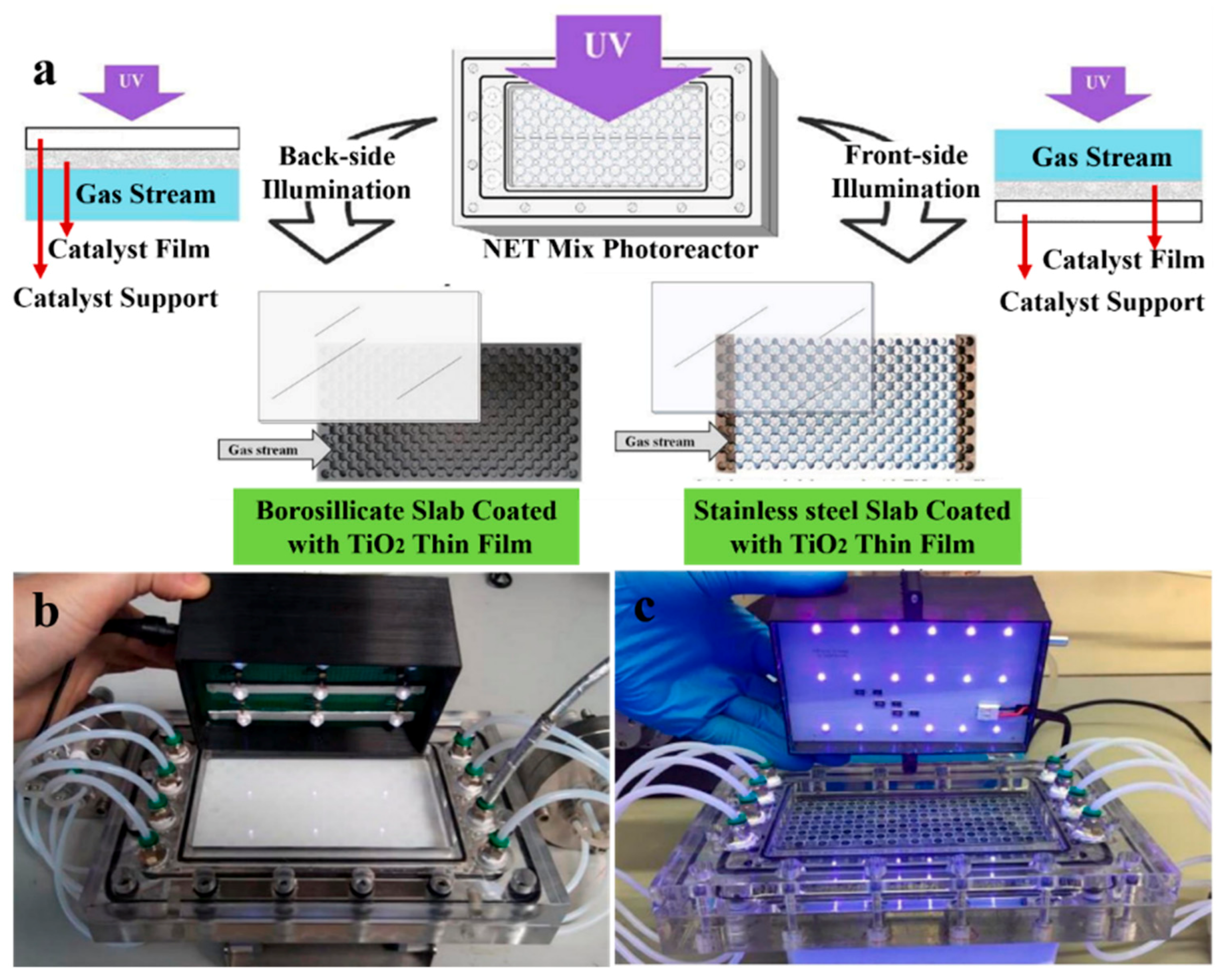
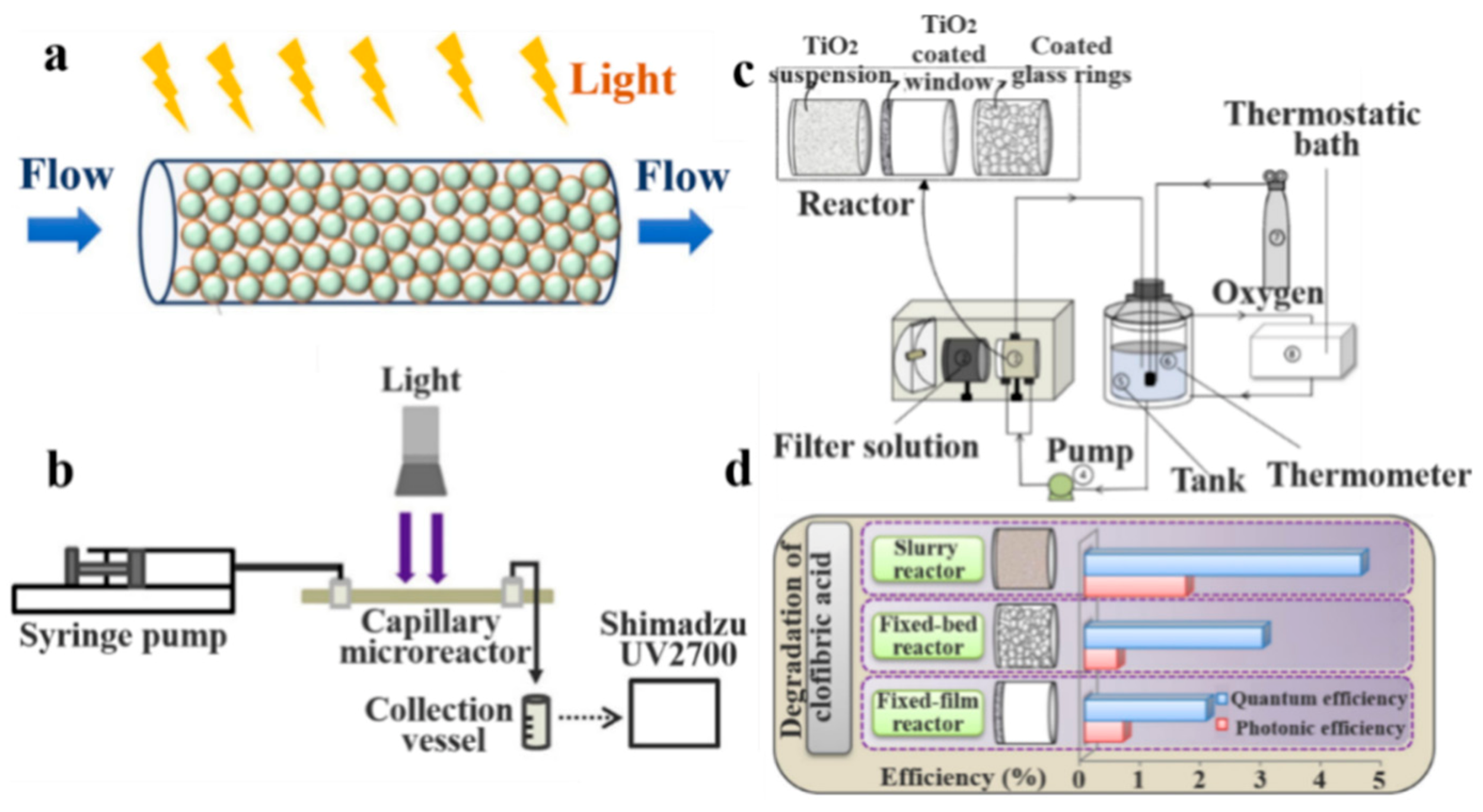
3.1.1. Packed Bed Reactor

3.1.2. Film Reactor
3.2. Application of TiO2-Based Photoreactors for Water Purification
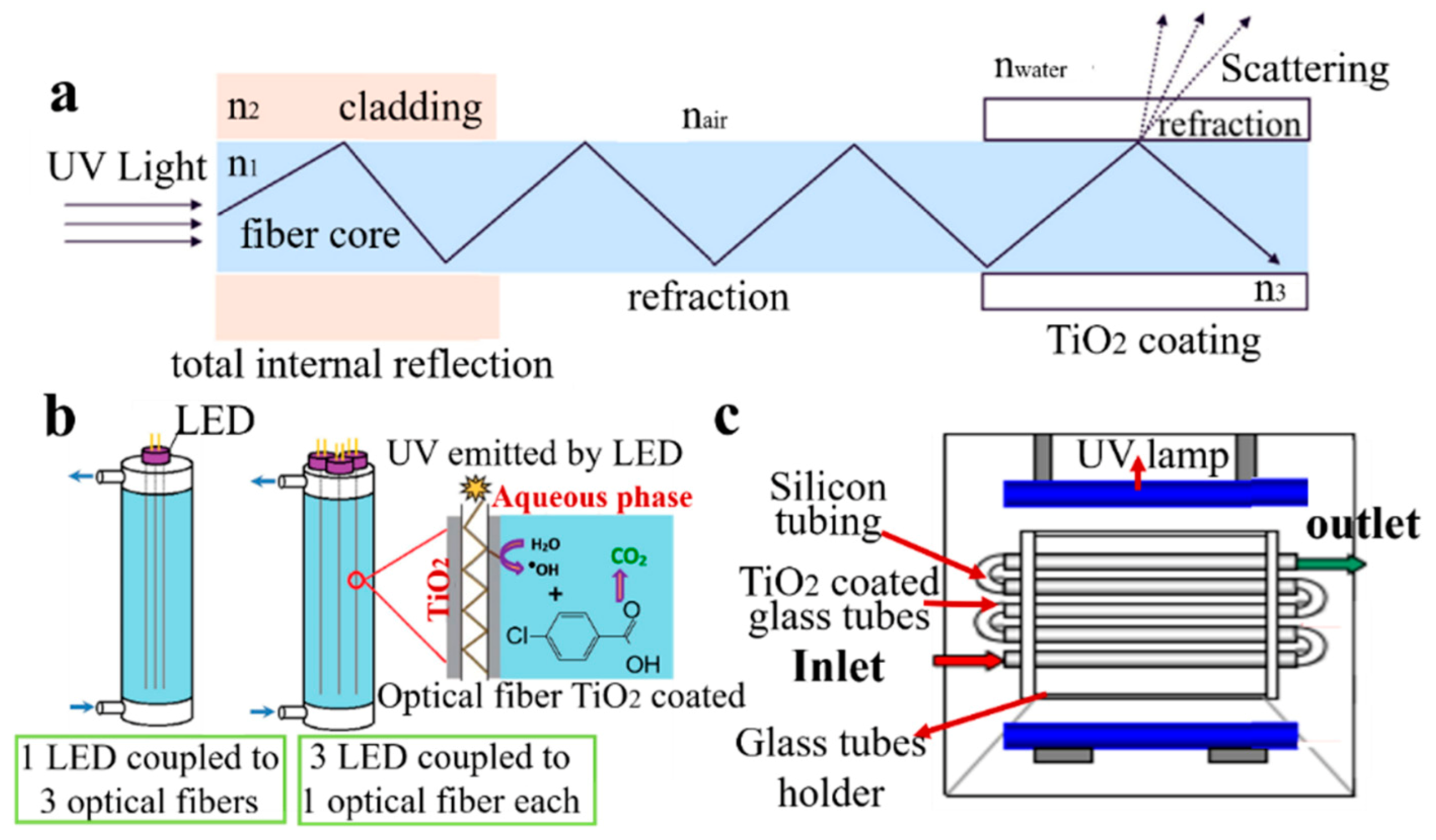
3.2.1. Membrane Reactor
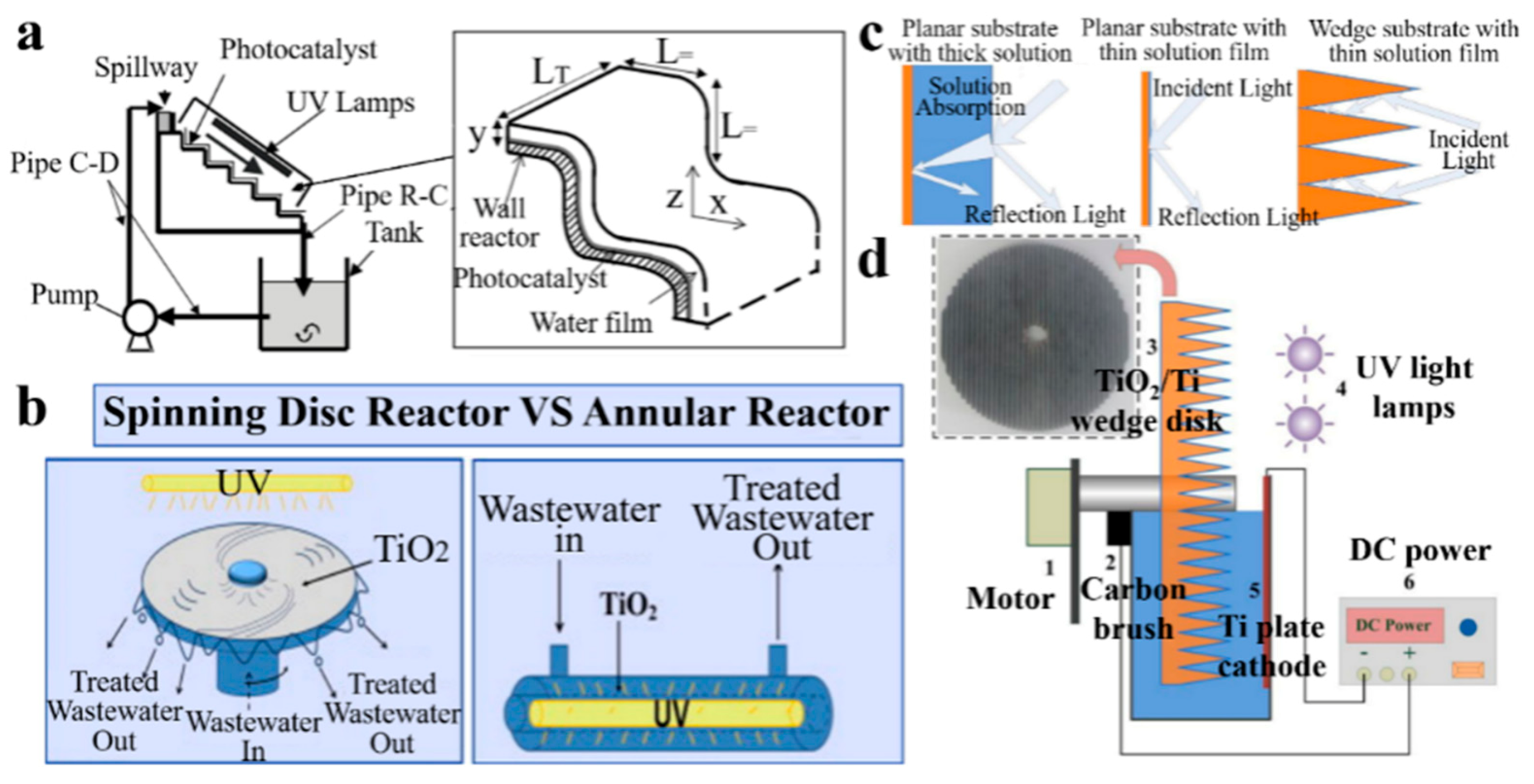
3.2.2. Film Reactor
3.2.3. Packed-Bed Reactor
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental implications of hydroxyl radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.I.; Green, P.; Balk, D.; Fekete, B.M.; Revenga, C.; Todd, M.; Montgomery, M. Urban growth, climate change, and freshwater availability. Proc. Natl. Acad. Sci. USA 2011, 108, 6312–6317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Simonich, S.L.; Hites, R.A. Organic pollutant accumulation in vegetation. Environ. Sci. Technol. 1995, 29, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhu, L.; Peh, C.K.; Ho, G.W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 2019, 12, 841–864. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Mauter, M.S.; Zucker, I.; Perreault, F.; Werber, J.R.; Kim, J.-H.; Elimelech, M. The role of nanotechnology in tackling global water challenges. Nat. Sustain. 2018, 1, 166–175. [Google Scholar] [CrossRef]
- Zhang, N.; Ishag, A.; Li, Y.; Wang, H.; Guo, H.; Mei, P.; Meng, Q.; Sun, Y. Recent investigations and progress in environmental remediation by using covalent organic framework-based adsorption method: A review. J. Clean. Prod. 2020, 277, 123360. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.A.; O’shea, K. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Nanayakkara, C.E.; Grassian, V.H. Titanium dioxide photocatalysis in atmospheric chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Rezania, S.; Kim, K.-H.; Sanaye, R.; Amani, A.M. Recent advances in photocatalytic removal of organic and inorganic pollutants in air. J. Clean. Prod. 2021, 278, 123895. [Google Scholar] [CrossRef]
- Gao, G.; Xi, Q.; Zhou, H.; Zhao, Y.; Wu, C.; Wang, L.; Guo, P.; Xu, J. Novel inorganic perovskite quantum dots for photocatalysis. Nanoscale 2017, 9, 12032–12038. [Google Scholar] [CrossRef]
- Sundar, K.P.; Kanmani, S. Progression of Photocatalytic reactors and it’s comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M. Recent advancements in engineering approach towards design of photo-reactors for selective photocatalytic CO2 reduction to renewable fuels. J. CO2 Util. 2019, 29, 205–239. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X. The technology horizon for photocatalytic water treatment: Sunrise or sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Weon, S.; Choi, J.; Park, T.; Choi, W. Freestanding doubly open-ended TiO2 nanotubes for efficient photocatalytic degradation of volatile organic compounds. Appl. Catal. B 2017, 205, 386–392. [Google Scholar] [CrossRef]
- Yue, D.; Zhang, T.; Kan, M.; Qian, X.; Zhao, Y. Highly photocatalytic active thiomolybdate [Mo3S13]2−clusters/BiOBr nanocomposite with enhanced sulfur tolerance. Appl. Catal. B 2016, 183, 1–7. [Google Scholar] [CrossRef]
- Yue, D.; Zhang, Z.; Tian, Z.; Zhang, T.; Kan, M.; Qian, X.; Zhao, Y. Highly photocatalytic active thiomolybdate [Mo3S13]2−clusters/Bi2WO6 nanocomposites. Catal. Today 2016, 274, 22–27. [Google Scholar] [CrossRef]
- Weon, S.; Choi, W. TiO2 nanotubes with open channels as deactivation-resistant photocatalyst for the degradation of volatile organic compounds. Environ. Sci. Technol. 2016, 50, 2556–2563. [Google Scholar] [CrossRef]
- Yue, D.; Qian, X.; Zhao, Y. Photocatalytic remediation of ionic pollutant. Sci. Bull. 2015, 60, 1791–1806. [Google Scholar] [CrossRef] [Green Version]
- da Costa Filho, B.M.; Araujo, A.L.; Padrão, S.P.; Boaventura, R.A.; Dias, M.M.; Lopes, J.C.B.; Vilar, V.J. Effect of catalyst coated surface, illumination mechanism and light source in heterogeneous TiO2 photocatalysis using a mili-photoreactor for n-decane oxidation at gas phase. Chem. Eng. J. 2019, 366, 560–568. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Cristóvão, R.O.; Mendes, A.; Boaventura, R.A.; Vilar, V.J. Photocatalytic membrane reactor performance towards oxytetracycline removal from synthetic and real matrices: Suspended vs immobilized TiO2-P25. Chem. Eng. J. 2019, 378, 122114. [Google Scholar] [CrossRef]
- Moradi, M.; Moussavi, G. Enhanced treatment of tannery wastewater using the electrocoagulation process combined with UVC/VUV photoreactor: Parametric and mechanistic evaluation. Chem. Eng. J. 2019, 358, 1038–1046. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Checa, M.; Figueredo, M.; Aguinaco, A.; Beltrán, F. Graphene oxide/titania photocatalytic ozonation of primidone in a visible LED photoreactor. J. Hazard. Mater. 2019, 369, 70–78. [Google Scholar] [CrossRef]
- Zhang, L.; Kanki, T.; Sano, N.; Toyoda, A. Development of TiO2 photocatalyst reaction for water purification. Sep. Purif. Technol. 2003, 31, 105–110. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X. Anatase/Bronze TiO2 heterojunction: Enhanced photocatalysis and prospect in photothermal catalysis. Chem. Res. Chin. Univ. 2020, 36, 992–999. [Google Scholar] [CrossRef]
- Abdullah, F.; Bakar, N.A.; Bakar, M.A. Current advancements on the fabrication, modification, and industrial application of zinc oxide as photocatalyst in the removal of organic and inorganic contaminants in aquatic systems. J. Hazrd. Mater. 2022, 424, 127416. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, Y.; Zhang, S. Editorial: Photocatalysis for Environmental Applications. Front. Chem. 2019, 7, 303. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, R.; Cui, W.; Dong, X.a.; Wang, H.; Kim, K.-H.; Chu, Y.; Sheng, J.; Sun, Y.; Dong, F. Synergistic photocatalytic decomposition of a volatile organic compound mixture: High efficiency, reaction mechanism, and long-term stability. ACS Catal. 2020, 10, 7230–7239. [Google Scholar] [CrossRef]
- Lin, B.; Li, H.; An, H.; Hao, W.; Wei, J.; Dai, Y.; Ma, C.; Yang, G. Preparation of 2D/2D g-C3N4 nanosheet@ ZnIn2S4 nanoleaf heterojunctions with well-designed high-speed charge transfer nanochannels towards high-efficiency photocatalytic hydrogen evolution. Appl. Catal. B 2018, 220, 542–552. [Google Scholar] [CrossRef]
- Hu, J.; Zhong, Z.; Zhang, F.; Xing, W.; Jin, W.; Xu, N. High-efficiency, synergistic ZnO-coated SiC photocatalytic filter with antibacterial properties. Ind. Eng. Chem. Res 2016, 55, 6661–6670. [Google Scholar] [CrossRef]
- Dhiman, P.; Naushad, M.; Batoo, K.M.; Kumar, A.; Sharma, G.; Ghfar, A.A.; Kumar, G.; Singh, M. Nano FexZn1−xO as a tuneable and efficient photocatalyst for solar powered degradation of bisphenol A from aqueous environment. J. Clean. Prod. 2017, 165, 1542–1556. [Google Scholar] [CrossRef]
- Ren, J.; Si, M.; Zhang, L.; Zhang, J.; Lu, C. Research progress on photocatalysis and its application in treatment of uranium-containing wastewater. Mod. Chem. Ind. 2020, 40, 62–66. [Google Scholar]
- Haibao, H.; Daiqi, Y.E. Synergetic Decomposition of VOCs using Plasma/Photocatalysis System. Environ.Sci.Technol 2007, 30, 95–98. [Google Scholar]
- Taiming, X.; Gang, C.; Bingye, N. Study on air purification by plasma combined with photocatalysis. Chin. J. Chem. Eng. 2007, 1, 105–107. [Google Scholar]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of produced water with photocatalysis: Recent advances, affecting factors and future research prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Maezawa, A.; Nakadoi, H.; Suzuki, K.; Furusawa, T.; Suzuki, Y.; Uchida, S. Treatment of dye wastewater by using photo-catalytic oxidation with sonication. Ultrason. Sonochem. 2007, 14, 615–620. [Google Scholar] [CrossRef]
- Marcì, G.; García-López, E.; Palmisano, L. Mechanistic aspects of oxalic acid oxidation by photocatalysis and ozonation. J. Appl. Electrochem. 2008, 38, 1029–1033. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Kajitvichyanukul, P. Enhancement of chromium removal efficiency on adsorption and photocatalytic reduction using a bio-catalyst, titania-impregnated chitosan/xylan hybrid film. J. Clean. Prod. 2016, 130, 126–136. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic reactors with suspended and immobilized TiO2: Comparative efficiency evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Palmisano, L.; Augugliaro, V.; Campostrini, R.; Schiavello, M. A Proposal for the Quantitative Assessment of Heterogeneous Photocatalytic Processes. J. Catal. 1993, 143, 149–154. [Google Scholar] [CrossRef]
- Han, Y.; Yue, D.; Kan, M.; Wu, Y.; Zeng, J.; Bian, Z.; Zhao, Y.; Qian, X. [Mo3S13]2−modified TiO2 coating on non-woven fabric for efficient photocatalytic mineralization of acetone. Appl. Catal. B 2019, 245, 190–196. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, Y.; Burda, C. TiO2 nanoparticles as functional building blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Bettini, L.G.; Diamanti, M.V.; Sansotera, M.; Pedeferri, M.P.; Navarrini, W.; Milani, P. Immobilized TiO2 nanoparticles produced by flame spray for photocatalytic water remediation. J. Nanopart. Res. 2016, 18, 238. [Google Scholar] [CrossRef] [Green Version]
- Corradi, A.B.; Bondioli, F.; Focher, B.; Ferrari, A.M.; Grippo, C.; Mariani, E.; Villa, C. Conventional and microwave-hydrothermal synthesis of TiO2 nanopowders. J. Am. Ceram. Soc. 2005, 88, 2639–2641. [Google Scholar] [CrossRef]
- Gotić, M.; Ivanda, M.; Sekulić, A.; Musić, S.; Popović, S.; Turković, A.; Furić, K. Microstructure of nanosized TiO2 obtained by sol-gel synthesis. Mater. Lett. 1996, 28, 225–229. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Junkar, I.; Kulkarni, M.; Drašler, B.; Rugelj, N.; Mazare, A.; Flašker, A.; Drobne, D.; Humpolíček, P.; Resnik, M.; Schmuki, P. Influence of various sterilization procedures on TiO2 nanotubes used for biomedical devices. Bioelectrochemistry 2016, 109, 79–86. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, M.; Choung, S.-J.; Ogino, K.; Miyata, S.; Kim, M.-S.; Park, J.-Y.; Kim, J.-B. The preparation of TiO2 nanometer photocatalyst film by a hydrothermal method and its sterilization performance for Giardia lamblia. Water Res. 2004, 38, 713–719. [Google Scholar] [CrossRef]
- Sichel, C.; Fernández-Ibáñez, P.; De Cara, M.; Tello, J. Lethal synergy of solar UV-radiation and H2O2 on wild Fusarium solani spores in distilled and natural well water. Water Res. 2009, 43, 1841–1850. [Google Scholar] [CrossRef]
- Yui, T.; Kan, A.; Saitoh, C.; Koike, K.; Ibusuki, T.; Ishitani, O. Photochemical reduction of CO2 using TiO2: Effects of organic adsorbates on TiO2 and deposition of Pd onto TiO2. ACS Appl. Mater. Interfaces 2011, 3, 2594–2600. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.-R.; Fu, X.; Xu, Y.-J. TiO2−graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2−graphene truly different from other TiO2−carbon composite materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, Z.; Wang, J.; Elzatahry, A.A.; Zhao, D. A perspective on mesoporous TiO2 materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Dai, W.; Tao, Y.; Zou, H.; Xiao, S.; Li, G.; Zhang, D.; Li, H. Gas-phase photoelectrocatalytic oxidation of NO via TiO2 nanorod array/FTO photoanodes. Environ. Sci. Technol. 2020, 54, 5902–5912. [Google Scholar] [CrossRef]
- Dambournet, D.; Belharouak, I.; Amine, K. Tailored preparation methods of TiO2 anatase, rutile, brookite: Mechanism of formation and electrochemical properties. Chem. Mater. 2010, 22, 1173–1179. [Google Scholar] [CrossRef]
- Deng, D.; Kim, M.G.; Lee, J.Y.; Cho, J. Green energy storage materials: Nanostructured TiO2 and Sn-based anodes for lithium-ion batteries. Energy Environ. Sci. 2009, 2, 818–837. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Krivec, M.; Zǎgar, K.; Suhadolnik, L.; Ceh, M.; Dražić, G. Highly efficient TiO2-based microreactor for photocatalytic applications. ACS Appl. Mater. Interfaces 2013, 5, 9088–9094. [Google Scholar] [CrossRef]
- Dhada, I.; Nagar, P.K.; Sharma, M. Challenges of TiO2-based photooxidation of volatile organic compounds: Designing, coating, and regenerating catalyst. Ind. Eng. Chem. Res. 2015, 54, 5381–5387. [Google Scholar] [CrossRef]
- Mirzaei, M.; Dabir, B.; Dadvar, M.; Jafarikojour, M. Photocatalysis of phenol using a spinning disc photoreactor immobilized with TiO2 nanoparticles: Hydrodynamic modeling and reactor optimization. Ind. Eng. Chem. Res. 2017, 56, 1739–1749. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Qian, X.; Ren, M.; Yue, D.; Zhu, Y.; Han, Y.; Bian, Z.; Zhao, Y. Mesoporous TiO2 films coated on carbon foam based on waste polyurethane for enhanced photocatalytic oxidation of VOCs. Appl. Catal. B 2017, 212, 1–6. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase–A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef] [Green Version]
- Weon, S.; He, F.; Choi, W. Status and challenges in photocatalytic nanotechnology for cleaning air polluted with volatile organic compounds: Visible light utilization and catalyst deactivation. Environ. Sci. Nano 2019, 6, 3185–3214. [Google Scholar] [CrossRef]
- Cui, W.; Wang, H.; Li, J.; Sun, Y.; Wang, H.; Zhang, Y.; Huang, H.; Dong, F. Promoting ring-opening efficiency for suppressing toxic intermediates during photocatalytic toluene degradation via surface oxygen vacancies. Sci. Bull. 2019, 64, 669–678. [Google Scholar]
- Teixeira, S.; Martins, P.; Lanceros-Méndez, S.; Kühn, K.; Cuniberti, G. Reusability of photocatalytic TiO2 and ZnO nanoparticles immobilized in poly (vinylidene difluoride)-co-trifluoroethylene. Appl. Surf. Sci. 2016, 384, 497–504. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kumar, P.; Szulejko, J.E.; Adelodun, A.A.; Junaid, M.F.; Uchimiya, M.; Chambers, S. Toward a better understanding of the impact of mass transit air pollutants on human health. Chemosphere 2017, 174, 268–279. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.-H.; Uchimiya, M.; Kumar, P.; Das, S.; Bhattacharya, S.S.; Szulejko, J. The micro-environmental impact of volatile organic compound emissions from large-scale assemblies of people in a confined space. Environ. Res. 2016, 151, 304–312. [Google Scholar] [CrossRef]
- Urkasame, K.; Yoshida, S.; Takanohashi, T.; Iwamura, S.; Ogino, I.; Mukai, S.R. Development of TiO2–SiO2 photocatalysts having a microhoneycomb structure by the ice templating method. ACS Omega 2018, 3, 14274–14279. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Silva, C.G.; Silva, A.M.; Vilar, V.J.; Boaventura, R.A.; Faria, J.L. Photocatalytic activity of TiO2-coated glass raschig rings on the degradation of phenolic derivatives under simulated solar light irradiation. Chem. Eng. J. 2013, 224, 32–38. [Google Scholar] [CrossRef]
- Salvadó-Estivill, I.; Hargreaves, D.M.; Li Puma, G. Evaluation of the intrinsic photocatalytic oxidation kinetics of indoor air pollutants. Environ. Sci. Technol. 2007, 41, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- López de León, L.R.; Deaton, K.E.; Deshusses, M.A. Miniaturized biotrickling filters and capillary microbioreactors for process intensification of VOC treatment with intended application to indoor air. Environ. Sci. Technol. 2018, 53, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Nzila, A.; Razzak, S.A.; Zhu, J. Bioaugmentation: An emerging strategy of industrial wastewater treatment for reuse and discharge. Int. J. Environ. Res. Public Health 2016, 13, 846. [Google Scholar] [CrossRef]
- Bueno-Alejo, C.J.; Hueso, J.L.; Mallada, R.; Julian, I.; Santamaria, J. High-radiance LED-driven fluidized bed photoreactor for the complete oxidation of n-hexane in air. Chem. Eng. J. 2019, 358, 1363–1370. [Google Scholar] [CrossRef] [Green Version]
- Maciuca, A.; Batiot-Dupeyrat, C.; Tatibouët, J.-M. Synergetic effect by coupling photocatalysis with plasma for low VOCs concentration removal from air. Appl. Catal. B 2012, 125, 432–438. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Merabet, S.; Wolbert, D. Modeling and simulation of VOCs removal by nonthermal plasma discharge with photocatalysis in a continuous reactor: Synergetic effect and mass transfer. Chem. Eng. J. 2014, 258, 119–127. [Google Scholar] [CrossRef]
- Vincent, G.; Marquaire, P.-M.; Zahraa, O. Photocatalytic degradation of gaseous 1-propanol using an annular reactor: Kinetic modelling and pathways. J. Hazard. Mater. 2009, 161, 1173–1181. [Google Scholar] [CrossRef]
- Vincent, G.; Marquaire, P.-M.; Zahraa, O. Abatement of volatile organic compounds using an annular photocatalytic reactor: Study of gaseous acetone. J. Photochem. Photobiol. A 2008, 197, 177–189. [Google Scholar] [CrossRef]
- Doucet, N.; Bocquillon, F.; Zahraa, O.; Bouchy, M. Kinetics of photocatalytic VOCs abatement in a standardized reactor. Chemosphere 2006, 65, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.-B.; Xi, Z.-G.; Chao, F.-H.; Zhang, W.; Zhang, H.-S.; Yang, D.-F. Decomposition of low-concentration gas-phase toluene using plasma-driven photocatalyst reactor. Atmos. Environ. 2007, 41, 6853–6859. [Google Scholar] [CrossRef]
- Nie, L.; Duan, B.; Lu, A.; Zhang, L. Pd/TiO2@ carbon microspheres derived from chitin for highly efficient photocatalytic degradation of volatile organic compounds. ACS Sustain. Chem. Eng. 2018, 7, 1658–1666. [Google Scholar] [CrossRef]
- Meng, F.; Song, M.; Wei, Y.; Wang, Y. The contribution of oxygen-containing functional groups to the gas-phase adsorption of volatile organic compounds with different polarities onto lignin-derived activated carbon fibers. Environ. Sci. Pollut. Res. 2019, 26, 7195–7204. [Google Scholar] [CrossRef]
- Kamravaei, S.; Shariaty, P.; Jahandar Lashaki, M.; Atkinson, J.D.; Hashisho, Z.; Phillips, J.H.; Anderson, J.E.; Nichols, M. Effect of beaded activated carbon fluidization on adsorption of volatile organic compounds. Ind. Eng. Chem. Res 2017, 56, 1297–1305. [Google Scholar] [CrossRef]
- Mohan, L.; Shiva Nagendra, S.M.; Maiya, M.P. Photocatalytic degradation of gaseous toluene using self-assembled air filter based on chitosan/activated carbon/TiO2. J. Environ. Chem. Eng. 2019, 7, 103455–103467. [Google Scholar] [CrossRef]
- Pillejera, M.K.V.; Chen, W.-H.; De Luna, M.D.G. Bamboo Torrefaction in a High Gravity (Higee) Environment Using a Rotating Packed Bed. ACS Sustain. Chem. Eng. 2017, 5, 7052–7062. [Google Scholar] [CrossRef]
- Ibhadon, A.; Arabatzis, I.; Falaras, P.; Tsoukleris, D. The design and photoreaction kinetic modeling of a gas-phase titania foam packed bed reactor. Chem. Eng. J. 2007, 133, 317–323. [Google Scholar] [CrossRef]
- Zazueta, A.L.L.; Destaillats, H.; Puma, G.L. Radiation field modeling and optimization of a compact and modular multi-plate photocatalytic reactor (MPPR) for air/water purification by Monte Carlo method. Chem. Eng.J. 2013, 217, 475–485. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Y.; Xu, Q.; Mo, J. A mass transfer based method for measuring the reaction coefficients of a photocatalyst. Atmos. Environ. 2007, 41, 1221–1229. [Google Scholar] [CrossRef]
- Shiraishi, F.; Maruoka, D.; Tanoue, Y. A better UV light and TiO2-PET sheet arrangement for enhancing photocatalytic decomposition of volatile organic compounds. Sep. Purif. Technol. 2017, 175, 185–193. [Google Scholar] [CrossRef]
- da Costa Filho, B.M.; Araujo, A.L.; Silva, G.V.; Boaventura, R.A.; Dias, M.M.; Lopes, J.C.; Vilar, V.J. Intensification of heterogeneous TiO2 photocatalysis using an innovative micro-meso-structured-photoreactor for n-decane oxidation at gas phase. Chem. Eng. J. 2017, 310, 331–341. [Google Scholar] [CrossRef]
- Blommaerts, N.; Asapu, R.; Claes, N.; Bals, S.; Lenaerts, S.; Verbruggen, S.W. Gas phase photocatalytic spiral reactor for fast and efficient pollutant degradation. Chem. Eng. J. 2017, 316, 850–856. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, R.; Zhao, R. A model for analyzing the performance of photocatalytic air cleaner in removing volatile organic compounds. Atmos. Environ. 2003, 37, 3395–3399. [Google Scholar] [CrossRef]
- Sun, H.; Wang, S.; Ang, H.M.; Tadé, M.O.; Li, Q. Halogen element modified titanium dioxide for visible light photocatalysis. Chem. Eng. J. 2010, 162, 437–447. [Google Scholar] [CrossRef]
- Kamat, P.V. Photochemistry on nonreactive and reactive (semiconductor) surfaces. Chem. Rev. 1993, 93, 267–300. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Huang, Z.F.; Song, J.; Pan, L.; Zhang, X.; Wang, L.; Zou, J.J. Tungsten oxides for photocatalysis, electrochemistry, and phototherapy. Adv. Mater. 2015, 27, 5309–5327. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Monteiro, R.A.R.; Miranda, S.M.; Rodrigues-Silva, C.; Faria, J.L.; Silva, A.M.T.; Boaventura, R.A.R.; Vilar, V.J.P. Gas phase oxidation of n -decane and PCE by photocatalysis using an annular photoreactor packed with a monolithic catalytic bed coated with P25 and PC500. Appl. Catal. B Environ. 2015, 165, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Lugo-Vega, C.S.; Serrano-Rosales, B.; de Lasa, H. Immobilized particle coating for optimum photon and TiO2 utilization in scaled air treatment photo reactors. Appl. Catal. B Environ. 2016, 198, 211–223. [Google Scholar] [CrossRef]
- Srikanth, B.; Goutham, R.; Narayan, R.B.; Ramprasath, A.; Gopinath, K.; Sankaranarayanan, A. Recent advancements in supporting materials for immobilised photocatalytic applications in waste water treatment. J. Environ. Manag. 2017, 200, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Odling, G.; Robertson, N. Bridging the gap between laboratory and application in photocatalytic water purification. Catal. Sci. Technol. 2019, 9, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Maeng, S.K.; Cho, K.; Jeong, B.; Lee, J.; Lee, Y.; Lee, C.; Choi, K.J.; Hong, S.W. Substrate-immobilized electrospun TiO2 nanofibers for photocatalytic degradation of pharmaceuticals: The effects of pH and dissolved organic matter characteristics. Water Res. 2015, 86, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Motegh, M.; van Ommen, J.R.; Appel, P.W.; Kreutzer, M.T. Scale-Up Study of a Multiphase Photocatalytic Reactor Degradation of Cyanide in Water over TiO2. Environ. Sci. Technol. 2014, 48, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ibáñez, P.; Sichel, C.; Polo-López, M.; de Cara-García, M.; Tello, J. Photocatalytic disinfection of natural well water contaminated by Fusarium solani using TiO2 slurry in solar CPC photo-reactors. Catal. Today 2009, 144, 62–68. [Google Scholar] [CrossRef]
- Burdyny, T.; Riordon, J.; Dinh, C.-T.; Sargent, E.H.; Sinton, D. Self-assembled nanoparticle-stabilized photocatalytic reactors. Nanoscale 2016, 8, 2107–2115. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, G.; Huang, T.; Chen, J.; Wang, Q.; Meng, Q. Photocatalytic membrane reactor for degradation of acid red B wastewater. Chem. Eng. J. 2010, 156, 571–577. [Google Scholar] [CrossRef]
- Li, Q.; Jia, R.; Shao, J.; He, Y. Photocatalytic degradation of amoxicillin via TiO2 nanoparticle coupling with a novel submerged porous ceramic membrane reactor. J. Clean. Prod. 2019, 209, 755–761. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Wang, W.Y.; Irawan, A.; Ku, Y. Photocatalytic degradation of Acid Red 4 using a titanium dioxide membrane supported on a porous ceramic tube. Water. Res. 2008, 42, 4725–4732. [Google Scholar] [CrossRef] [PubMed]
- Li Puma, G.; Yue, P.L. A novel fountain photocatalytic reactor for water treatment and purification: Modeling and design. Ind. Eng. Chem. Res. 2001, 40, 5162–5169. [Google Scholar] [CrossRef]
- O’Neal Tugaoen, H.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Compact light-emitting diode optical fiber immobilized TiO2 reactor for photocatalytic water treatment. Sci. Total Environ. 2018, 613–614, 1331–1338. [Google Scholar] [CrossRef]
- Hurtado, L.; Solís-Casados, D.; Escobar-Alarcón, L.; Romero, R.; Natividad, R. Multiphase photo-capillary reactors coated with TiO2 films: Preparation, characterization and photocatalytic performance. Chem. Eng. J. 2016, 304, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Ling, C.M.; Mohamed, A.R.; Bhatia, S. Performance of photocatalytic reactors using immobilized TiO2 film for the degradation of phenol and methylene blue dye present in water stream. Chemosphere 2004, 57, 547–554. [Google Scholar] [CrossRef]
- Natarajan, K.; Natarajan, T.S.; Bajaj, H.C.; Tayade, R.J. Photocatalytic reactor based on UV-LED/TiO2 coated quartz tube for degradation of dyes. Chem. Eng. J. 2011, 178, 40–49. [Google Scholar] [CrossRef]
- Stephan, B.; Ludovic, L.; Dominique, W. Modelling of a falling thin film deposited photocatalytic step reactor for water purification: Pesticide treatment. Chem. Eng. J. 2011, 169, 216–225. [Google Scholar] [CrossRef]
- Chan, A.H.; Chan, C.K.; Barford, J.P.; Porter, J.F. Solar photocatalytic thin film cascade reactor for treatment of benzoic acid containing wastewater. Water Res. 2003, 37, 1125–1135. [Google Scholar] [CrossRef]
- Boiarkina, I.; Norris, S.; Patterson, D.A. Investigation into the effect of flow structure on the photocatalytic degradation of methylene blue and dehydroabietic acid in a spinning disc reactor. Chem. Eng. J. 2013, 222, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Boiarkina, I.; Norris, S.; Patterson, D.A. The case for the photocatalytic spinning disc reactor as a process intensification technology: Comparison to an annular reactor for the degradation of methylene blue. Chem. Eng. J. 2013, 225, 752–765. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zhang, H.; He, Y.; Tang, T.; Ying, D.; Wang, Y.; Sun, T.; Jia, J. Novel wedge structured rotating disk photocatalytic reactor for post-treatment of actual textile wastewater. Chem. Eng. J. 2015, 268, 10–20. [Google Scholar] [CrossRef]
- Nogueira, R.F.; Jardim, W.F. TiO2-fixed-bed reactor for water decontamination using solar light. Sol. Energy 1996, 56, 471–477. [Google Scholar] [CrossRef]
- Mehrvar, M.; Anderson, W.A.; Moo-Young, M. Preliminary analysis of a tellerette packed-bed photocatalytic reactor. Adv. Environ. Res. 2002, 6, 411–418. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Hu, W.; Li, D.; Zha, F.; Lei, Z. Facile fabrication of underwater superoleophobic TiO2 coated mesh for highly efficient oil/water separation. Colloids Surf. A 2016, 489, 441–446. [Google Scholar] [CrossRef]
- Kovacic, M.; Salaeh, S.; Kusic, H.; Suligoj, A.; Kete, M.; Fanetti, M.; Stangar, U.L.; Dionysiou, D.D.; Bozic, A.L. Solar-driven photocatalytic treatment of diclofenac using immobilized TiO2-based zeolite composites. Environ. Sci. Pollut. Res. 2016, 23, 17982–17994. [Google Scholar] [CrossRef]
- Imoberdorf, G.E.; Vella, G.; Sclafani, A.; Rizzuti, L.; Alfano, O.M.; Cassano, A.E. Radiation model of a TiO2-coated, quartz wool, packed-bed photocatalytic reactor. AlChE J. 2010, 56, 1030–1044. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Sun, J.; Tang, Z. Capillary microphotoreactor packed with TiO2-coated glass beads: An efficient tool for photocatalytic reaction. Chem. Eng. Process. 2020, 147, 107746–107754. [Google Scholar] [CrossRef]
- Sraw, A.; Kaur, T.; Pandey, Y.; Sobti, A.; Wanchoo, R.K.; Toor, A.P. Fixed bed recirculation type photocatalytic reactor with TiO2 immobilized clay beads for the degradation of pesticide polluted water. J. Environ. Chem. Eng. 2018, 6, 7035–7043. [Google Scholar] [CrossRef]
- Sarkar, S.; Chakraborty, S.; Bhattacharjee, C. Photocatalytic degradation of pharmaceutical wastes by alginate supported TiO2 nanoparticles in packed bed photo reactor (PBPR). Ecotoxicol. Environ. Saf. 2015, 121, 263–270. [Google Scholar] [CrossRef]
- Cerrato, G.; Bianchi, C.; Galli, F.; Pirola, C.; Morandi, S.; Capucci, V. Micro-TiO2 coated glass surfaces safely abate drugs in surface water. J. Hazard. Mater. 2019, 363, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-J.; Tsai, M.-T.; Huang, Y.-H. Mineralization and defluoridation of 2, 2, 3, 3-tetrafluoro-1-propanol (TFP) by UV oxidation in a novel three-phase fluidized bed reactor (3P-FBR). Water Res. 2013, 47, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, C.; Skillen, N.; Adams, M.; Robertson, P.K. Photocatalytic reactors for environmental remediation: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1002–1017. [Google Scholar] [CrossRef]
- Marugan, J.; Van Grieken, R.; Pablos, C.; Satuf, M.L.; Cassano, A.E.; Alfano, O.M. Photocatalytic inactivation of Escherichia coli aqueous suspensions in a fixed-bed reactor. Catal. Today 2015, 252, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Cloteaux, A.; Gérardin, F.; Thomas, D.; Midoux, N.; André, J.-C. Fixed bed photocatalytic reactor for formaldehyde degradation: Experimental and modeling study. Chem. Eng. J. 2014, 249, 121–129. [Google Scholar] [CrossRef]
- Nam, W.; Woo, K.; Han, G. Photooxidation of anionic surfactant (sodium lauryl sulfate) in a three-phase fluidized bed reactor using TiO2/SiO2 photocatalyst. J. Ind. Eng. Chem. 2009, 15, 348–353. [Google Scholar] [CrossRef]
- Surenjan, A.; Pradeep, T.; Philip, L. Application and performance evaluation of a cost-effective vis-LED based fluidized bed reactor for the treatment of emerging contaminants. Chemosphere 2019, 228, 629–639. [Google Scholar] [CrossRef]




| Photoreactor | Radiation Parameters | Photocatalysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Volume (L) | Flow Rate (L/min) | Light Source | Intensity (W m−2) | Period (min) | Materials and Dosage (g) | Pollutant | Concentration (mg/L) | Efficiency (%) | Ref. |
| Packed bed reactor | 0.1 | - | UV-Vis | 10 | 300 | FSP a-made TiO2 0.00040 | RhB | 4.8 | 70 | [53] |
| Fluidized bed reactor | 0.0025 | 0.1 | UV LED | 0.22 | 30 | P25 0.00075 | n-hexane | 200 | 80 | [85] |
| DBD b reactor | 0.23 | 5 | UV lamp | 41.1 | - | TiO2 0.0057 | Isovaleraldehyde | 50 | 85 | [86] |
| Film reactor | 5.57 | 2000 | UV | 15 | 480 | TiO2-PET - | toluene | 1.0 | 99 | [101] |
| Annular reactor | 0.22 | 0.22 | Sunlight | 18.9 | 4320 | TiO2-P25 0.075 | n-decane | 1.86 | 83 | [102] |
| glass spiral reactor | 0.033 | 1.6 | UVA lamp | 42 | 16.7 | TiO2 0.0066 | acetaldehyde | 200 | 100 | [103] |
| Film reactor | 0.22 | 0.075 | UV | 38.4 | - | PC500 0.0523 | n-decane | 71 | 100 | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ma, Y.; Li, K.; Chen, S.; Yue, D. Photocatalytic Reactor as a Bridge to Link the Commercialization of Photocatalyst in Water and Air Purification. Catalysts 2022, 12, 724. https://doi.org/10.3390/catal12070724
Li Y, Ma Y, Li K, Chen S, Yue D. Photocatalytic Reactor as a Bridge to Link the Commercialization of Photocatalyst in Water and Air Purification. Catalysts. 2022; 12(7):724. https://doi.org/10.3390/catal12070724
Chicago/Turabian StyleLi, Yunzhang, Youjia Ma, Kan Li, Suhong Chen, and Dongting Yue. 2022. "Photocatalytic Reactor as a Bridge to Link the Commercialization of Photocatalyst in Water and Air Purification" Catalysts 12, no. 7: 724. https://doi.org/10.3390/catal12070724
APA StyleLi, Y., Ma, Y., Li, K., Chen, S., & Yue, D. (2022). Photocatalytic Reactor as a Bridge to Link the Commercialization of Photocatalyst in Water and Air Purification. Catalysts, 12(7), 724. https://doi.org/10.3390/catal12070724