Abstract
Uranium mononitride (UN) is a candidate fuel material for light water reactors with higher uranium (U) loading and thermal conductivity than uranium dioxide (UO2). However, the sintering of UN pellets is challenging as the UN powder particles oxidize rapidly at high temperatures unless the oxygen concentration is extremely low. Oxidation during sintering either reduces the relative density of the sintered UN pellet or disintegrates the sintered UN pellet to powder. To address this problem, the present work developed a rapid sintering method for producing highly densified UN surrogate pellets with minimal oxidation. Cerium nitride (CeN) is used as a surrogate for UN to reduce radiation hazards. With the custom-developed fast-heating system, the sintering process was completed within 150 s. The sintering atmosphere was flowing nitrogen (N2). The sintered CeN pellet density was 95% of the theoretical density (TD) or higher. The microstructure was uniform with a 10–25 µm grain size as demonstrated by scanning electron microscopy (SEM) and contained trivial levels of oxides as demonstrated by X-ray diffraction (XRD). The resultant pellets indicate that the rapid sintering method is a promising method to make UN fuel pellets with equivalent or higher density to pellets made by conventional sintering methods, while also being more efficient in time and costs.
1. Introduction
The 2011 tsunami caused by the Great East Japan Earthquake that damaged the Fukushima Daiichi nuclear power plant kickstarted the U.S. Department of Energy’s (DOE) Advanced Fuels Campaign to identify and develop a new fuel that can help to integrate a more accident-tolerant system in operating commercial light water reactors (LWRs) [1]. Several fuel candidates have been investigated over the last decade including uranium silicide (U3Si2), uranium carbide (UC), and uranium mononitride (UN). The candidate fuel of particular interest recently to cooperating DOE national labs and fuel vendors is UN. The advantages that UN has over traditional uranium dioxide (UO2) fuel are higher uranium (U) loading, which can result in longer fuel cycles, and higher thermal conductivity, which is beneficial in accident scenarios. However, UN suffers from oxidation in both air and water, which can cause problems during fabrication [1]. The oxidation of UN can be quite rapid and pyrophoric when the powder is fine or at high temperatures [2,3,4]. Therefore, the UN powder processing and sintering process must be performed in inert atmospheres with a minimal amount of oxidizing agent. This is particularly important for the mass fabrication of UN pellets. A highly densified UN pellet is desired [4] as it would be more oxidation-resistant as fewer pore defects would react with oxidizing agents.
A variety of sintering methods have been used to make actinide and lanthanide nitride fuel pellets, including tungsten refractory metal furnaces, tube furnaces, hot extrusion, hot pressing, hot isostatic pressing (HIP), and spark plasma sintering (SPS) [1,3,4,5,6,7,8]. Using a tungsten refractory metal furnace or tube furnace, UN pellets achieved 95% or higher of theoretical density (TD) with a sintering profile of 2500 °C for 2 to 6 h [3], and (U, Nd)N pellets achieved ~95%TD with a sintering profile of 1800 °C for 4 h [9]. However, most studies in the open literature reported that such sintering methods cannot achieve enough densification if the temperature was lower—at sintering temperatures of 1700 °C or lower, the density of UN or (U, Dy)N pellet was less than 85%TD [5,7,9]. What’s more, the process generally requires a long sintering time, which results in heavy oxidation due to the difficulties in retaining an inert atmosphere for many hours. Conversely, pressure-assisted methods, i.e., hot extrusion, hot pressing, HIP, and SPS, can make the pellets more densified at lower temperatures as the applied pressure facilitates the consolidation of the powders. Using SPS, UN pellets achieved densities of 95–99%TD at 1650 °C for three minutes with a pressure of 134 MPa for a total sintering time of around 33 min [1,4,6]. Using HIP, UN pellets achieved a density of 99.8%TD at 1650 °C with a pressure of 207 MPa [3]. The hot extrusion of UN pellets resulted in heavy cracking but showed promising densification at 2200 °C [3]. A study of a surrogate material, (Dy, Zr)N, found that hot pressing at 2000 °C and 26 MPa would lead to 99%TD [7]. However, there is room for improvement as the pressure-assisted sintering method lacks production efficiency, both in throughput and energy requirements, as a result of the technique’s operation. Moreover, with pressure-assisted methods, it is difficult to make complex structures and multiple samples at the same time due to the reliance on graphite or tungsten carbide dies.
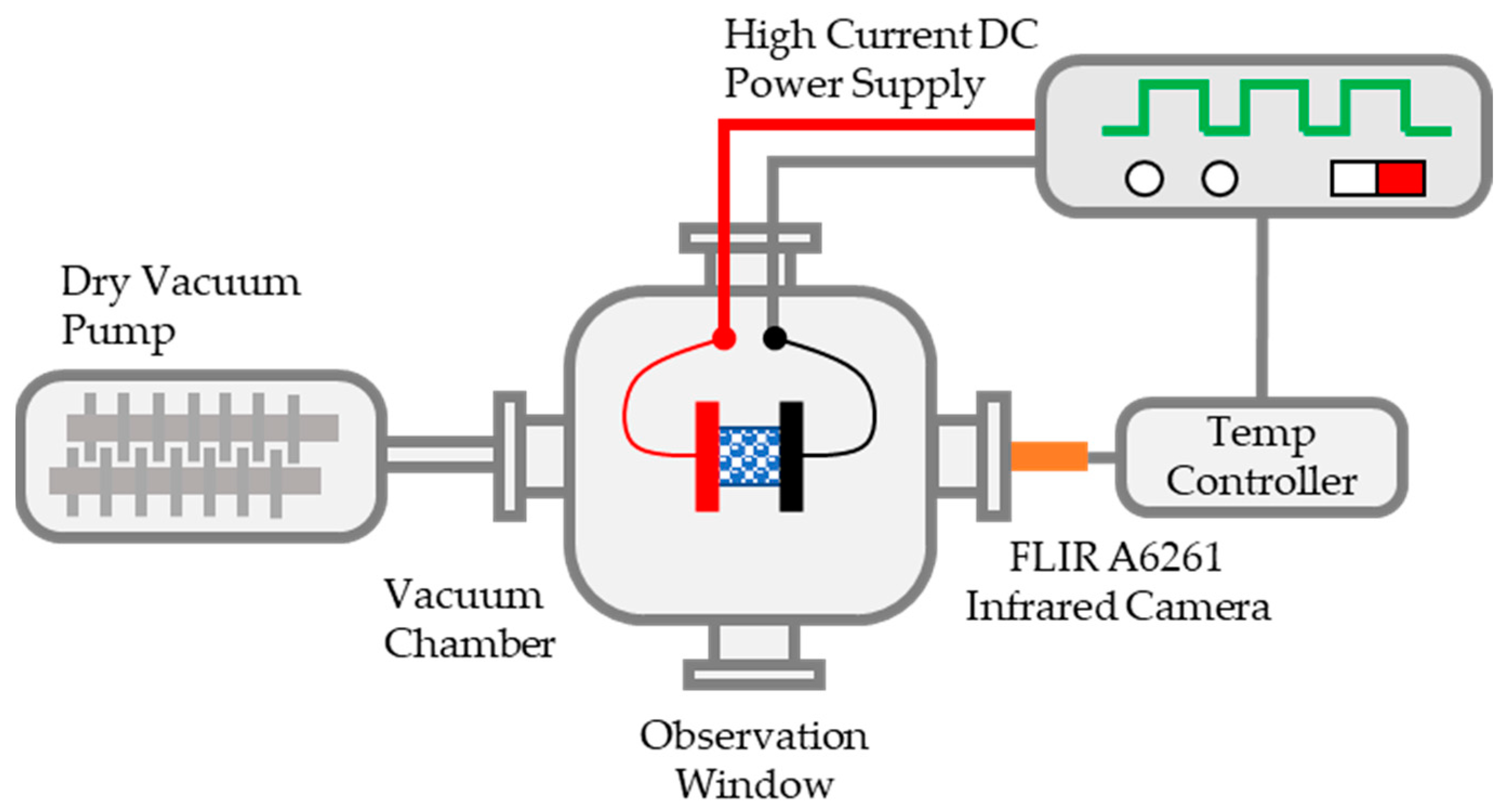
The present work aims to develop a rapid sintering method for UN pellets using a custom-designed fast-heating system. A schematic diagram of the assembly is shown in Figure 1. The system is composed of a heating stage, 10-kW DC power supply (programmable with 0–30 volts of direct current [VDC]), high-vacuum pump, infrared camera (700–2500 °C), and LabView-programmed temperature controller. The high-vacuum pump allows the working atmosphere to be vacuum or flowing argon/nitrogen/air/specialty gas. The heating mechanism is based on the well-known joule heating effect (i.e., the heating element is wired to the DC power supply and is powered/heated when the power turns on). The power supply works in either constant current or constant voltage mode, depending on the requirement. The heating element acts as a resistance, and the power follows Ohm’s law power formula. Due to the joule-heating effect, the resistor heats up rapidly. The sample to be heated can be the heating element itself, in which case the sample is wired to the DC power supply; or the sample can be heated up by the heat conduction of heating elements by being sandwiched between the heating elements. This fast-heating mechanism has been used in many other applications, such as pulsed electric current sintering [10], in situ heating transmission electron microscopy [11,12], flash sintering [13,14,15,16,17,18], and ultrafast high-temperature sintering [19].
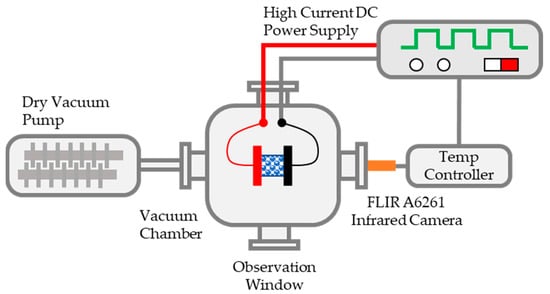
Figure 1.
Schematic diagram of the fast-heating system.
As UN is radioactive, a non-radioactive surrogate material is preferred for the convenience of operation. Cerium nitride (CeN) was used as the surrogate in this study due to its similar thermophysical properties (Melting point: UN is 2757 °C [20] and CeN is 2480 °C [21]. Coefficient of thermal expansion (CTE): UN is 7.481·10-6 K-1 [22] and CeN is 30·10-6 K-1 [23]. Thermal conductivity (k): UN is 25 W/m-K (at 1500 °C) [24] and CeN is unknown) and crystal structure. Cerium oxide (CeO2) has been commonly used as a surrogate for UO2 due to the similarity of chemical properties between Ce and U [25,26,27]. The oxidation properties between CeN and UN differ in that the former is far more oxygen-sensitive [28] than the latter. A density greater than 95%TD would indicate the pellet is densified enough to serve as a fuel pellet [1]. The threshold for oxide content in UN for performance is 1.17 at.% [29]. However, considering that CeN has a higher propensity for oxygen than UN, a realistic goal for oxide content is between 5–10 at.%. The present work showed that the rapid sintering method leads to minimal oxidation of CeN, being a promising indicator that the UN would be well-sintered by the method with even less oxidation occurring.
2. Materials and Methods
2.1. Materials
The CeN starting material was certified 99.5% purity−100 mesh (<149 µm) powder obtained from American Elements. To prevent oxidation, the as-received CeN powder was stored in the argon-filled glovebox where the oxygen and moisture level was less than 1 ppm as detected by the oxygen and moisture sensors integrated into the glovebox.
2.2. Green Body Fabrication
The as-received powder was milled in a planetary ball mill to break up large agglomerates. Alumina jars (100 mL) and balls were used as grinding media and isopropanol was used as the grinding fluid. The ball-to-powder mass ratio was 4:1 and the rotatory speed was 200 rpm for 8 h with 25 min cycles and 5 min cooldown times. To prevent the powder from oxidizing, the jar was vacuum-sealed before being transferred from the glovebox. After milling, the powder was dried at 100 °C in a muffle furnace within the argon-filled glovebox for 4 h. The powder was then pushed through a 500-mesh (25 μm) sieve and weighed. The balls were also weighed before and after milling to determine any possible contamination of the powder from the balls.
The milled powder was pressed into a pellet shape using a uniaxial press at the pressing pressure of 250 MPa with a 19 mm die inside the argon-filled glovebox. Each pellet was around 2 g. This amount of powder corresponded to around 2 mm thickness for the pellet. The green body was vacuum-sealed in a plastic bag and then transferred out of the glovebox to be sintered.
2.3. Rapid Sintering
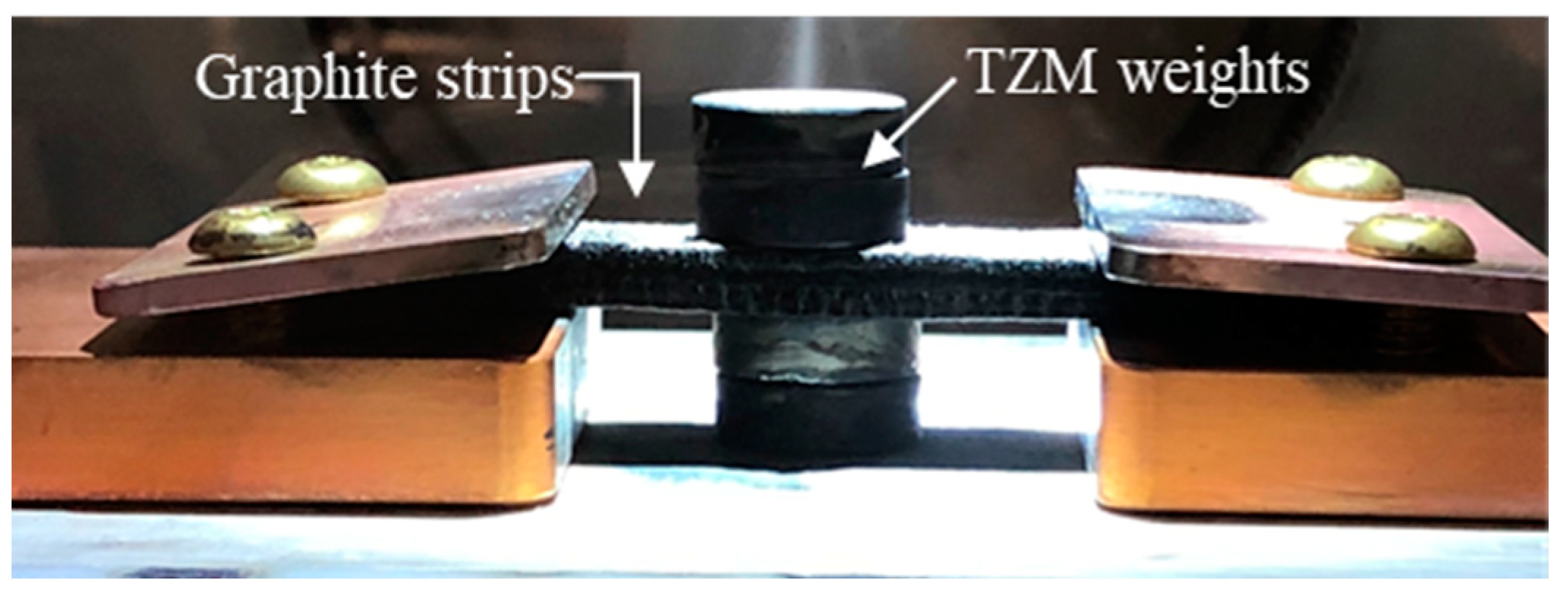
Each pellet was placed on the heating stage of the fast-heating system between a pair of graphite strips with both ends connected to the power supply, as shown in Figure 2. Weights made of tungsten-zirconium-molybdenum (TZM) alloy, which has an exceptionally high melting point, were placed on the top and bottom of the graphite strips to improve the contact and thermal conduction between the pellet and strips. Before heating, the vacuum chamber was vacuumed by the pump, and pure nitrogen gas of 5N (99.999%) purity was provided. The sintering atmosphere was flowing nitrogen. The green-body pellets were tested with various heating profiles to determine the optimal sintering parameters. The heating rate was applied at 35 and 160 °C/s, the dwell temperature was varied from 1800 to 2500 °C, and the dwell time was varied from 10 to 30 s. The temperature of the graphite foam strip was measured using the infrared camera. The temperature controller had minor overshoot with high starting ramp rates but was effective in keeping the dwell temperature within ±5 °C of the target dwell temperature for the duration of the experiments. After sintering, the pellet was immediately transferred to the glovebox for storage.
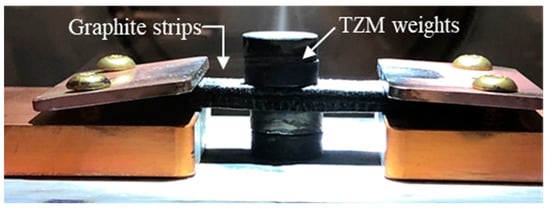
Figure 2.
Heating stage configuration. The sample is placed in between the graphite foam strips and pressed by the TZM weights to improve the contact and heat conduction.
2.4. Characterization
The density of the sintered pellets was measured using an Archimedes density measurement. The sample was immersed in isopropanol as the working fluid. The TD of CeN was taken as 7.89 g/cm3 [30]. The powder particles and sintered pellets were analyzed using a Hitachi S-4800 SEM. The SEM was operated with an accelerating voltage of 5.0 kV. The powder particles and sintered pellets were also analyzed using X-ray diffraction (XRD) with a Panalytical Empyrean diffractometer. To prevent oxidation, the powder was placed in an air-tight sample holder for the XRD measurement. However, in practice, the sintered pellets were exposed to air for the XRD measurement as the sample holder did not fit. The XRD scanned five times on each sample, with Cu Kα radiation, across a 2θ range of 30° to 90° with a step size of 0.013° and a hold time of 178.3 s. Rietveld refinement in Highscore Plus software was performed for phase fitting.
3. Results
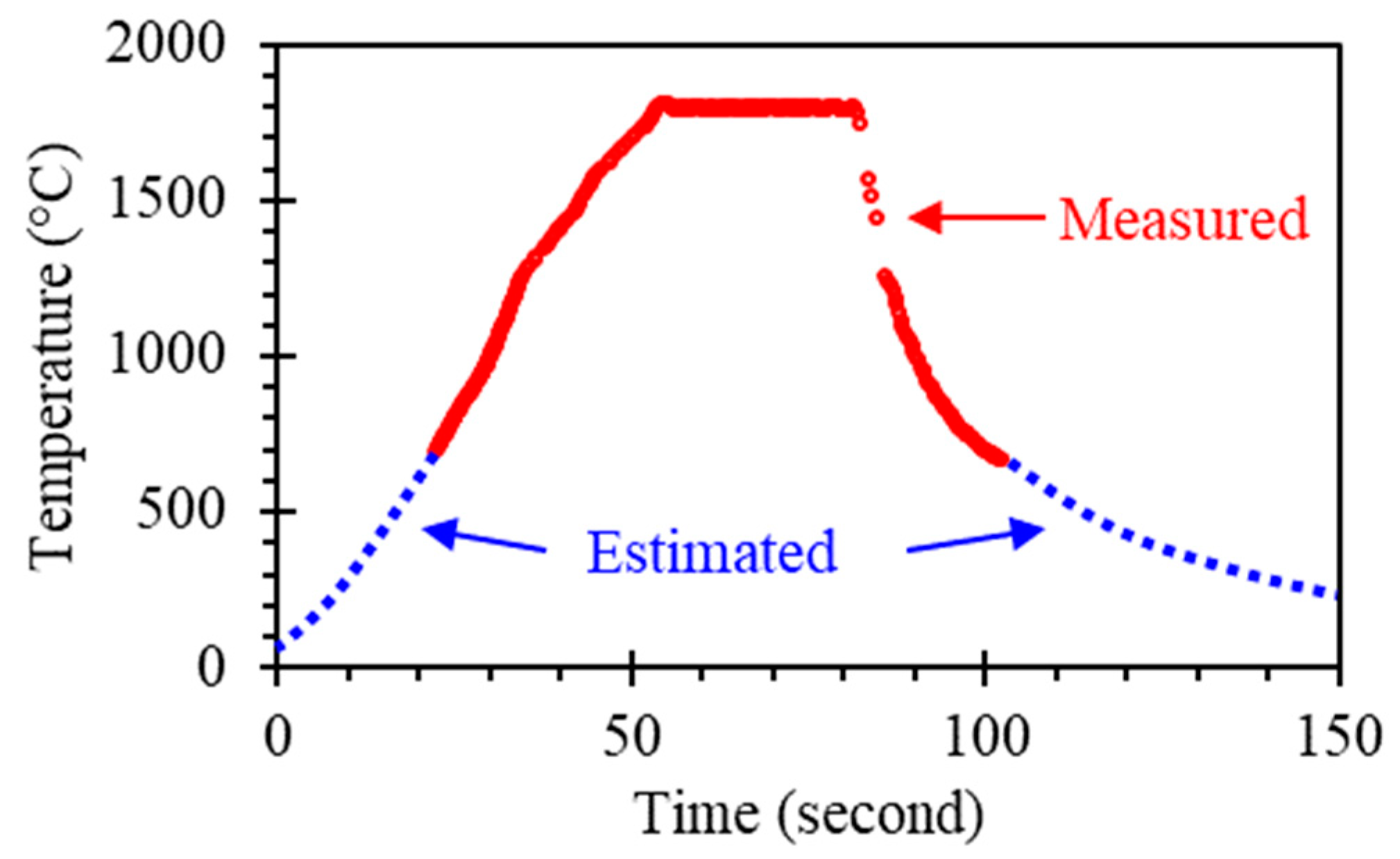
Multiple CeN pellets were sintered following the temperature profile as plotted in Figure 3. The maximum temperature, i.e., the temperature during the dwell at 55–85 s, was 1800 °C. The heating rate was limited to 35 °C/s as a larger heating rate, though capable, induced thermal shock cracking. It was found that the maximum temperature was limited to 1800 °C as higher temperatures resulted in melting, as shown in Table 1. A total of 11 sintering attempts were made at the dwell temperature of 1800 °C, but 3 failures occurred due to issues with heat conduction through the sample. This issue will be resolved by modifying the heating stage configuration in the future. Sample tests with shorter dwell times (10–20 s) or thicker samples (2–3 mm) had issues with sintering completeness. Note that Table 1 only includes the densities of the successfully sintered pellets.
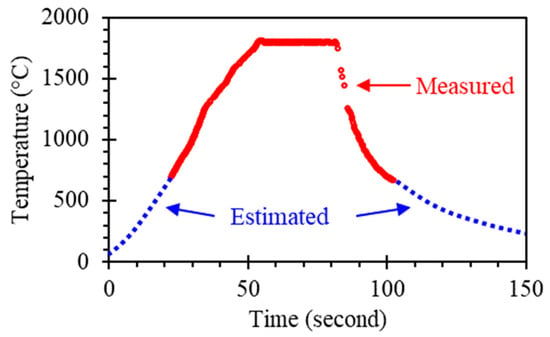
Figure 3.
Measured temperature profile for CeN sintering. The dwell time at 1800 °C is approximately 30 s. Temperature below 700 °C is estimated as they are outside the measurement range. See supplementary Data S1.

Table 1.
Samples tested at various maximum temperatures in this study.
3.1. Ball-Milled Powder
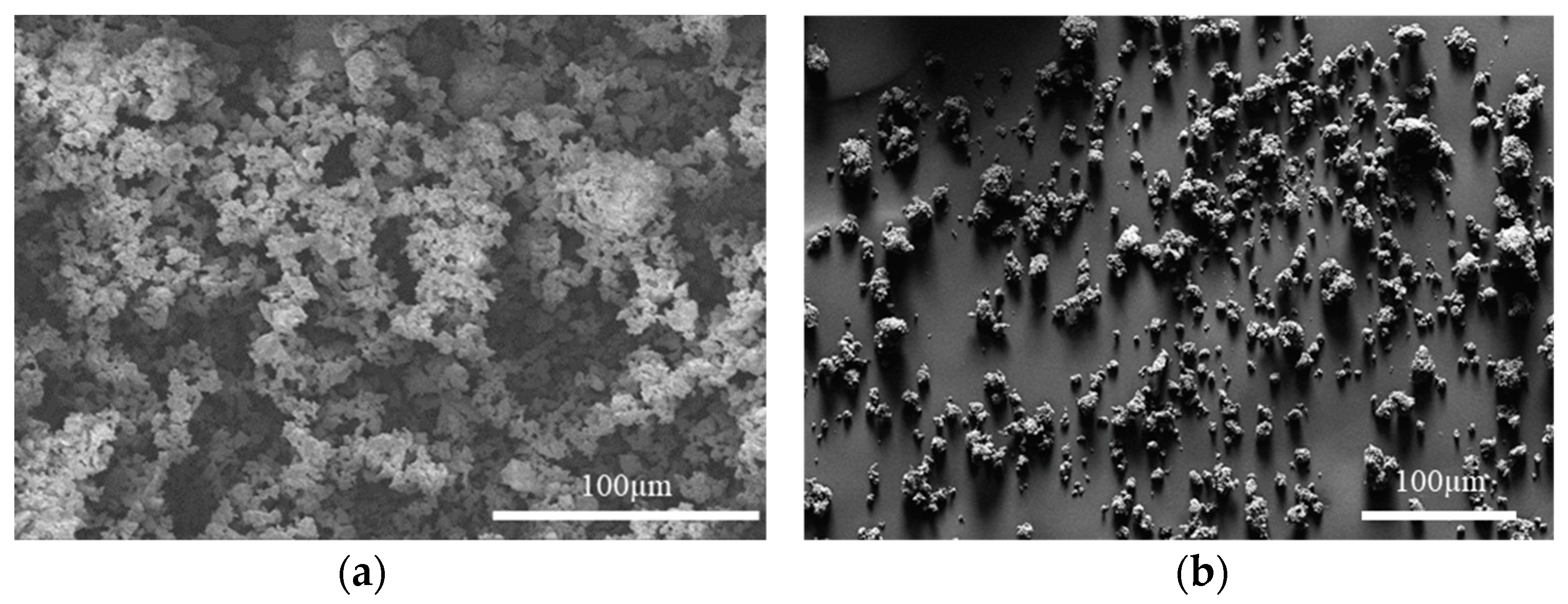
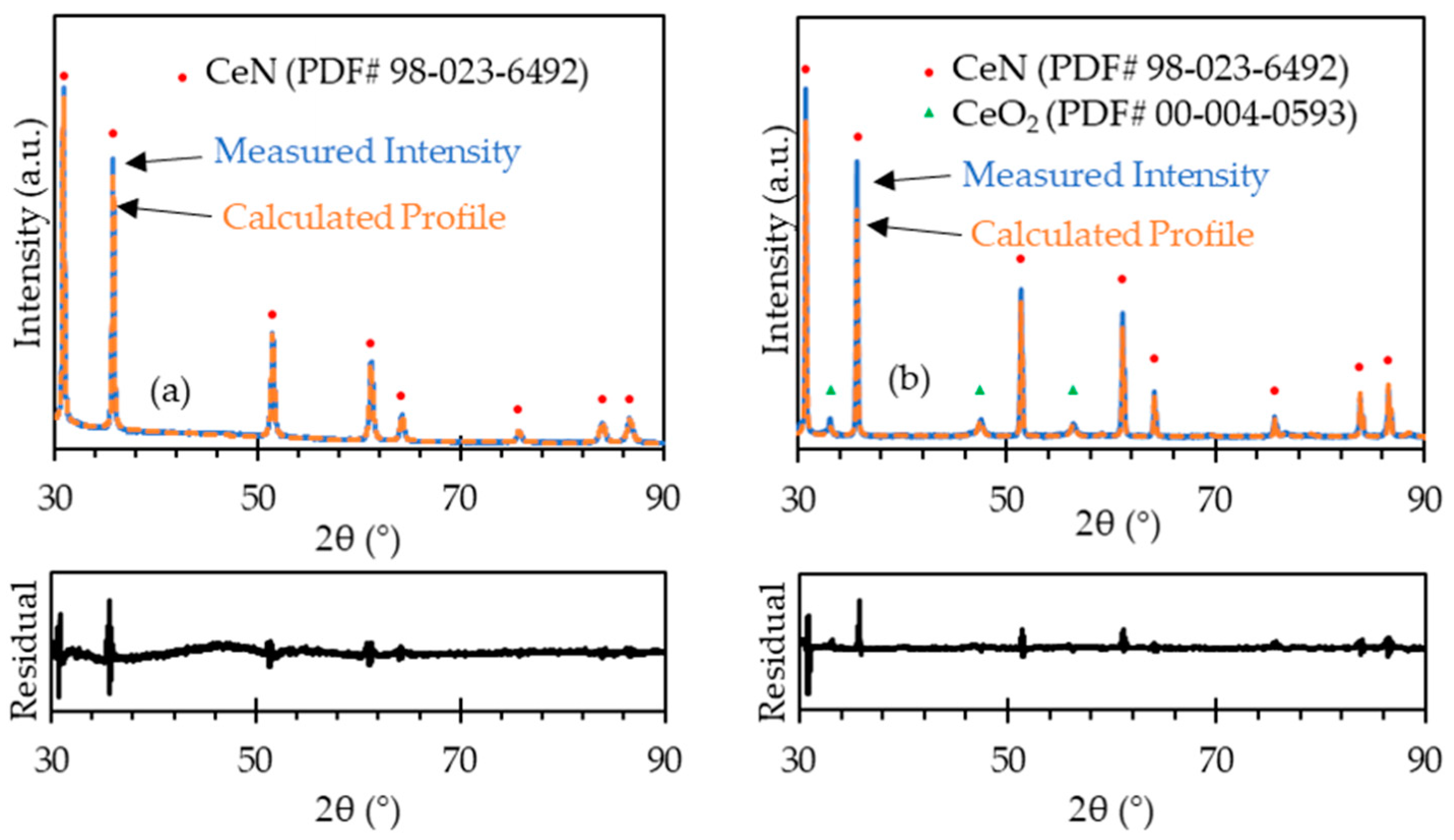
The as-received powder particles were agglomerated, with the agglomerates being hundreds of microns in diameter, as shown in Figure 4a. Such agglomerates would make the sintered samples poorly densified as well as result in abnormal grain growth. The milled powder particles were less agglomerated, as shown in Figure 4b. The milled powder particles were as small as 1 µm with small agglomerates of 10–30 µm. It should be noted that, due to the fine particle size, the milled powder became pyrophoric in air when it was loaded from the sample container to the SEM observation chamber. The fast oxidation may have changed the particle shape to some extent. In addition, as the milling was carried out in an argon-filled jar with an oxygen level of less than 1 ppm, the CeN phase was not affected by oxygen. As shown in Figure 5a, the XRD pattern was identified as 100% CeN phase, which demonstrates that the milling did not lead to oxidation.
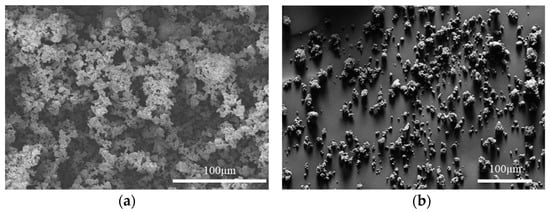
Figure 4.
SEM secondary electron images showing (a) as-received CeN powder particles in large agglomerates and (b) milled CeN powder particles in small agglomerates.
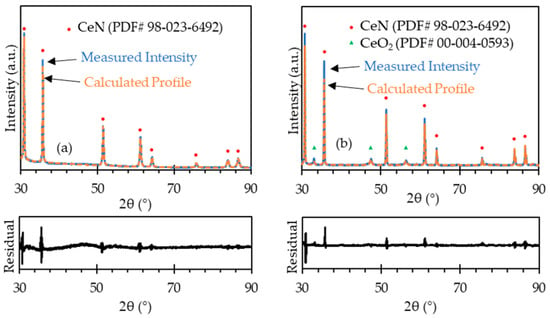
Figure 5.
XRD patterns with profile fitting residual of (a) milled CeN powder and (b) the as-sintered CeN pellet. See supplementary Data S2.
3.2. As-Sintered Pellets
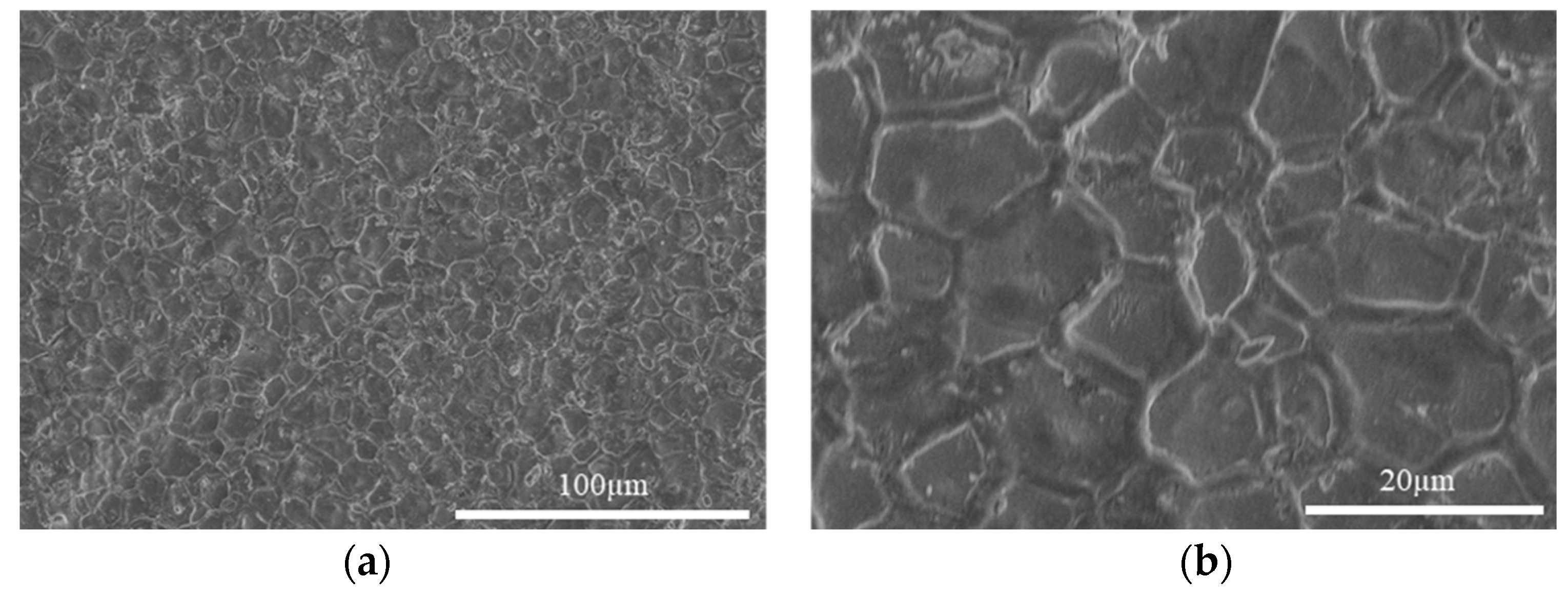
The measured density of the pellets sintered at 1800 °C, as listed in Table 1, was generally between 92–98% TD. The microstructure of the sintered pellets is shown in Figure 6. The grain size is approximately 10–25 µm. The grain boundaries appear wide, indicating the formation of oxide on the grain boundaries. The SEM images were taken after the pellets were exposed to air for some time (several hours), so the oxide formation proceeded from the grain boundaries on the polished surface during this time. The XRD pattern of the sintered pellet along with the calculated profile and difference plot, as shown in Figure 5b, contains peaks of both CeN and CeO2. The Rietveld refinement identified that the pattern is composed of 94 mol% CeN and 6 mol% CeO2 with a weighted profile R-factor (Rwp) value of 9.58, indicating the profile fits the pattern reasonably well. The parameters used in the Highscore Plus software for fitting include Scale Factor, Flat Background, More Background, Specimen Displacement, Lattice Parameters, and Caglioti W. It is likely that the sintering process results in slight oxidation on the surface, but the XRD measurement might oxidize the surface as the sample was exposed to air, which is different to that of the CeN powder measurement (see Section 2.4), which was protected in an argon atmosphere. Therefore, it is unclear if the oxides were mostly formed by sintering or during the XRD measurement. Nevertheless, the effect is minor as only 6 mol% CeO2 was identified.
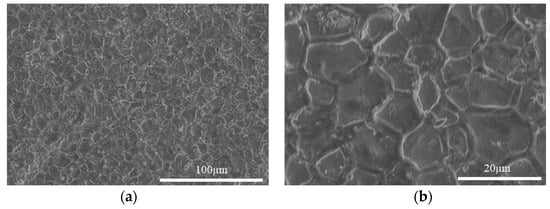
Figure 6.
SEM secondary electron images showing the microstructure of as-sintered CeN pellets at low (a) and high (b) magnifications.
4. Discussion
4.1. Rapid Sintering Mechanism
The sintering profile, as shown in Figure 3, finishes in 150 s with 30 s of dwell at 1800 °C. Compared with the traditional sintering methods (furnaces, hot extrusion, hot pressing, SPS, and HIP, as mentioned above), which take tens of minutes to hours, this method is rapid. It is well known that the driving force behind sintering is the reduction of the total interfacial or surface energy among powder particles, so whether a sample is sintered or not is determined by the energy applied to the sample. Heat input, therefore, is essential. The heat transfer principle of the fast-heating system in this study, i.e., solid-solid heat conduction, is a highly efficient method of heat transfer. Traditional furnaces rely on heat radiation and/or convection and, thus, the heat transfer efficiency is lower. For hot extrusion, hot pressing, SPS, and HIP, the heat transfer principle is solid-solid heat conduction with/without heat radiation, and the working temperature is limited depending on the specific models. This is because the die is not functional at higher temperatures. Thus, the methods take a longer time to supply the heat energy. The traditional methods also need additional time for heating and cooling cycles, which adds to the total time of sintering, whereas the fast-heating system has rapid heating and cooling.
Sintering using the fast-heating system has other advantages in addition to the rapid timeframe and solid-solid heat conduction. The efficiency of the system is high, with high throughput and low energy requirements. The entire process from loading the sample, sintering, and taking the sample out can take between 5–10 min depending on operator speed and dwell temperature, which affects the time needed for cooling. Multiple samples can be loaded into the device at the same time provided they are small enough to fit in the strip (2 cm × 5 cm). The strip size can be enlarged depending on the need and can make larger samples at a time. As mentioned above, the DC power supply is 10 kW. By contrast, a 1700 °C tube furnace is roughly 8000 kW, and the sintering takes ~10 h. Therefore, the fast-heating system requires less than 1% of the energy required by conventional furnace sintering. Because the system does not use a die, complex structures that can fit in the carbon foam strip can be sintered and the only consumable is the carbon foam which needs to be replaced after each experiment.
The fast-heating system not only lowers time and energy consumption but also eliminates carbon contamination. Carbon contamination resulting from the graphite strip was not observed in the samples. For a spark plasma sintering, the carbon contamination issue, caused by the interdiffusion between the graphite die and sample, has been a concern. The pressure-less mode of the fast-heating system minimizes the contact and time of contact between the graphite strip and sample, thus reducing the diffusion and reaction.
At the same time, there are some small drawbacks to the fast-heating system sintering method that may require system modification. The heat conduction penetration in the current setup is not high and thus thicker samples did not sinter as well with the same short dwell times as thin samples. The thickest sample successfully sintered was 3 mm—although thicker samples have not currently been attempted.
4.2. Maximum Sintering Temperature
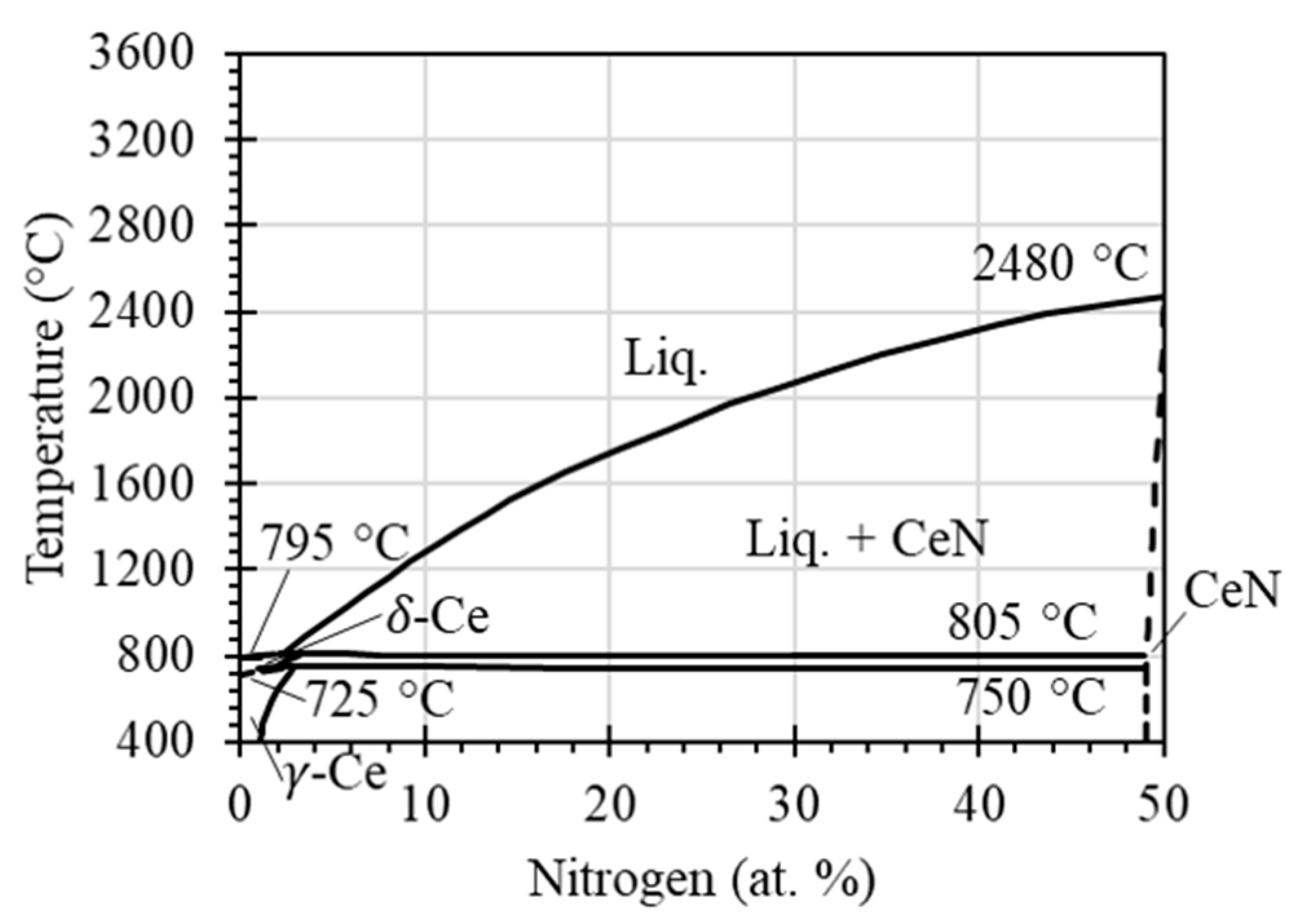
It was found that the pellet melted at temperatures above 1800 °C, whereas the melting point of CeN according to the open literature is 2480 °C. This is not because the temperature measurement was faulty, as the infrared camera was calibrated with an uncertainty of ±2 °C. Instead, this phenomenon is due to CeN not being a line compound. The CeN phase tolerates excess Ce atoms at lower temperatures, as shown in the phase diagram of Figure 7. With the increase in temperature, phase transformation of CeN to CeN+L (liquid) occurs, leading to melting, especially when the temperature approaches ~2000 °C according to the phase diagram. For the CeN powder used, the temperature of 1800 °C was determined as the boundary of solid to liquid in this study.
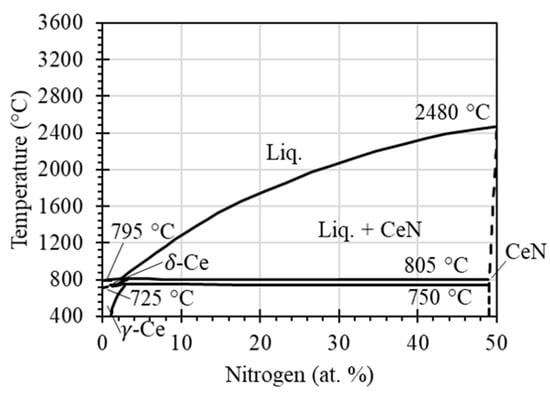
Figure 7.
Equilibrium phase diagram of Ce-N, adapted from the literature (Gschneidener and Verkade, 1975, [32]).
Conversely, the UN species would not encounter the same melting problem. First, the melting point of UN (2757 °C) is higher than that of CeN, so the maximum sintering temperature of UN would be 1800 °C or higher. The ideal sintering temperature for UN would need to be tested in the next phase of the study. Second, UN is a line compound, so it does not encounter phase transformation to liquid below its melting point. The CeN species might not be the ideal surrogate for UN in this case, but the similar thermophysical and chemical properties, particularly the oxidation behavior, make CeN a good choice for the investigation of the sintering process.
4.3. Oxidation Effect
Both UN and CeN are sensitive to air and oxygen, as mentioned above, and both have been observed to experience oxidation and disintegration either in pellet or powder form. Both nitrides will oxidize in air at room temperature within hours and especially quickly at high temperatures during the sintering process. In an inert atmosphere, temperature and time are critical for the oxidation reaction. Sintering must be done at high temperatures to facilitate the interdiffusion of particles and grains. Therefore, reducing the time of sintering is the only effective way to mitigate oxidation. The result of sintered pellets is promising, as shown in the XRD pattern (Figure 5) and microstructure (Figure 6). The sintered CeN pellets exhibit uniform microstructure with no visible pores and contain no more than 6 mol% (this value could be even lower as the sample surface could be oxidized during the XRD measurement) CeO2, which is an oxidation product of CeN. No other phases, such as Ce2O3, were detected by XRD because the final product of oxidation in CeN is CeO2 [28]. Additionally, the conditions in the system only allowed for CeO2 to form as the final product (for example, a reducing agent would be needed to form Ce2O3 from CeO2 [31]). Such a low level of oxides supports the hypothesis that a rapid sintering process leads to a well-sintered pellet with trivial levels of oxides. The surface of the sintered pellet would be disintegrated into powder if the surface encountered severe oxidation.
The XRD pattern also supports the contention that the CeN phase was well retained, and phase transformation or decomposition did not occur. This is consistent with the equilibrium phase diagram of Ce-N as shown in Figure 7. It should be noted that CeN2 and Ce3N4 have been founded in other studies: CeN2 was found at high pressures between 30 and 300 atm [32], and Ce3N4 was found in activated nitrogen conditions [33]. These phases are uncommon and neither the nitrogen pressure nor activated nitrogen conditions were present, so they were not found in this study.
5. Conclusions
The rapid sintering method used in this study to sinter CeN pellets proved effective at producing highly densified samples while minimizing oxidation during the process. The CeN pellets produced were densified and had favorable and uniform microstructure. The densities of the pellets exceeded 95%TD and the grains were consistently between 10 and 25 µm. The fast-heating system proved effective in producing these pellets within minutes. The optimal temperature profile for sintering CeN in the fast-heating system while retaining stoichiometry is 1800 °C and 30 s at temperature. It is reasonable to conclude, based on the study, that it would be possible to sinter UN in the fast-heating system in a similar way to the way CeN was sintered. Further work should be conducted to test the fabrication potential of UN in the fast-heating system, sintering multiple samples at a time, and sintering complex structures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ceramics5040072/s1, Supplementary Data S1, CeN Temperature Profile for FHS; Supplementary Data S2, CeN XRD Data.
Author Contributions
The author contributions to the study are as follows: Y.X., conceptualization, methodology, resources, writing, supervision, funding acquisition; L.J., software, validation, formal analysis, investigation, data curation, writing, visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported through the Idaho National Laboratory (INL) Laboratory Directed Research & Development (LDRD) Program under DOE Idaho Operations Office Contract DE-AC07-05ID14517 and Purdue University. L.J. appreciates the U.S. Nuclear Regulatory Commission (NRC) Fellowship award.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is available as supplementary files.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Watkins, J.K.; Gonzales, A.; Wagner, A.R.; Sooby, E.S.; Jaques, B.J. Challenges and Opportunities to Alloyed and Composite Fuel Architectures to Mitigate High Uranium Density Fuel Oxidation: Uranium Mononitride. J. Nucl. Mater. 2021, 553, 153048. [Google Scholar] [CrossRef]
- Dell, R.M.; Wheeler, V.J.; Mciver, E.J. Oxidation of Uranium Mononitride and Uranium Monocarbide. Trans. Faraday Soc. 1966, 62, 3591–3606. [Google Scholar] [CrossRef]
- Metroka, R.R. Fabrication of Uranium Mononitride Compacts; National Aeronautics and Space Administration: Cleveland, OH, USA, 1970. [Google Scholar]
- Johnson, K.; Ström, V.; Wallenius, J.; Lopes, D.A. Oxidation of Accident Tolerant Fuel Candidates. J. Nucl. Sci. Technol. 2017, 54, 280–286. [Google Scholar] [CrossRef]
- Choi, J.; Ebbinghaus, B.; Livermore, M.-L. Laboratory Directed Research and Development (LDRD) on Mono-Uranium Nitride Fuel Development for SSTAR and Space Applications; Lawrence Livermore National Lab (LLNL): Livermore, CA, USA, 2006. [Google Scholar]
- Johnson, K.D.; Wallenius, J.; Jolkkonen, M.; Claisse, A. Spark Plasma Sintering and Porosity Studies of Uranium Nitride. J. Nucl. Mater. 2016, 473, 13–17. [Google Scholar] [CrossRef]
- Butt, D.P.; Jaques, B. Synthesis and Optimization of the Sintering Kinetics of Actinide Nitrides; Boise State University: Boise, ID, USA, 2009. [Google Scholar]
- Jaques, B.J.; Watkins, J.; Croteau, J.R.; Alanko, G.A.; Tyburska-Pueschel, B.; Meyer, M.; Xu, P.; Lahoda, E.J.; Butt, D.P. Synthesis and Sintering of UN-UO2 Fuel Composites. J. Nucl. Mater. 2015, 466, 745. [Google Scholar] [CrossRef]
- Yamasaki, K.; Tamaki, Y.; Takano, M.; Akabori, M.; Minatq, K.; Arai, Y.; Takano, M.; Minato, K. Fabrication of Lanthanide Nitride Pellets and Simulated Burnup Fuels. In Proceedings of the Symposium on Nitride Fuel Cycle Technology, Kashiwa, Japan, 28 July 2004. [Google Scholar]
- Munir, Z.A.; Quach, D.V.; Ohyanagi, M. Electric Current Activation of Sintering: A Review of the Pulsed Electric Current Sintering Process. J. Am. Ceram. Soc. 2011, 94, 1–19. [Google Scholar] [CrossRef]
- Saka, H.; Kamino, T.; Arai, S.; Sasaki, K. In Situ Heating Transmission Electron Microscopy. MRS Bull. 2008, 33, 93–100. [Google Scholar] [CrossRef]
- Li Phuah, X.; Jian, J.; Wang, H.; Wang, X.; Zhang, X.; Wang, H. Ultra-High Heating Rate Effects on the Sintering of Ceramic Nanoparticles: An in situ TEM Study. Mater. Res. Lett. 2021, 9, 373–381. [Google Scholar] [CrossRef]
- Yu, M.; Grasso, S.; Mckinnon, R.; Saunders, T.; Reece, M.J. Review of Flash Sintering: Materials, Mechanisms and Modelling. Adv. Appl. Ceram. 2017, 116, 24–60. [Google Scholar] [CrossRef]
- Cologna, M.; Francis, J.S.C.; Raj, R. Field Assisted and Flash Sintering of Alumina and Its Relationship to Conductivity and MgO-Doping. J. Eur. Ceram. Soc. 2011, 31, 2827–2837. [Google Scholar] [CrossRef]
- Ji, W.; Parker, B.; Falco, S.; Zhang, J.Y.; Fu, Z.Y.; Todd, R.I. Ultra-Fast Firing: Effect of Heating Rate on Sintering of 3YSZ, with and without an Electric Field. J. Eur. Ceram. Soc. 2017, 37, 2547–2551. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, J.; Wang, W.; Fu, Z.; Todd, R. The Microstructural Origin of Rapid Densification in 3YSZ during Ultra-Fast Firing with or without an Electric Field. J. Eur. Ceram. Soc. 2020, 40, 5829–5836. [Google Scholar] [CrossRef]
- Grasso, S.; Sakka, Y.; Rendtorff, N.; Hu, C.; Maizza, G.; Borodianska, H.; Vasylkiv, O. Modeling of the Temperature Distribution of Flash Sintered Zirconia. J. Ceram. Soc. Jpn. 2011, 119, 144–146. [Google Scholar] [CrossRef]
- Eqbal, A.; Arya, K.S.; Chakrabarti, T. In-Depth Study of the Evolving Thermal Runaway and Thermal Gradient in the Dog Bone Sample during Flash Sintering Using Finite Element Analysis. Ceram. Int. 2020, 46, 10370–10378. [Google Scholar] [CrossRef]
- Wang, C.; Ping, W.; Bai, Q.; Cui, H.; Hensleigh, R.; Wang, R.; Brozena, A.H.; Xu, Z.; Dai, J.; Pei, Y.; et al. A General Method to Synthesize and Sinter Bulk Ceramics in Seconds. Science 2020, 368, 521–526. [Google Scholar] [CrossRef]
- Olson, W.M.; Mulford, R.N.R. The Decomposition Pressure and Melting Point of Uranium Mononitride. J. Phys. Chem. 1963, 67, 952–954. [Google Scholar] [CrossRef]
- O’Dell, K.D.; Hensley, E.B. The Decomposition Pressure, Congruent Melting Point and Electrical Resistivity of Cerium Nitride. J. Phys. Chem. Solids 1972, 33, 443–449. [Google Scholar] [CrossRef]
- Hayes, S.L.; Thomas, J.K.; Peddicord, K.L. Material Property Correlations for Uranium Mononitride I. Physical Properties. J. Nucl. Mater. 1990, 171, 262–270. [Google Scholar] [CrossRef]
- Schram, R.P.C.; Boshoven, J.G.; Cordfunke, E.H.P.; Konings, R.J.M.; van der Laan, R.R. Enthalpy Increment Measurements of Cerium Mononitride, CeN. J. Alloys Compd. 1997, 252, 20–23. [Google Scholar] [CrossRef]
- Suzuki, Y.; Arai, Y. Thermophysical and Thermodynamic Properties of Actinide Mononitrides and Their Solid Solutions. J. Alloys Compd. 1998, 271–273, 577–582. [Google Scholar] [CrossRef]
- Cavender, D.P.; Mireles, O.R.; Frendi, A. Design of a Uranium Dioxide Spheroidization System. In Proceedings of the Proceedings of Nuclear and Emerging Technologies for Space, Albuquerque, NM, USA, 25 February 2013. [Google Scholar]
- Porfirio, T.C.; Muccillo, E.N.S.; Muccillo, R. Electric Field-Assisted Synthesis/Sintering Cerium Oxide: 5 Wt.% Gadolinium Oxide. J. Eur. Ceram. Soc. 2021, 41, 7105–7110. [Google Scholar] [CrossRef]
- Roleček, J.; Foral, Š.; Katovský, K.; Salamon, D. A Feasibility Study of Using CeO2 as a Surrogate Material during the Investigation of UO2 Thermal Conductivity Enhancement. Adv. Appl. Ceram. 2017, 116, 123–131. [Google Scholar] [CrossRef]
- Wan, Y.; Yi, T.; Fu, Y.; Zheng, F.; Yang, M.; He, Z.; Cao, L. Structure and Oxidation Properties of CeN Thin Films Prepared by DC Reactive Magnetron Sputtering. Surf. Coat Technol. 2020, 381, 125168. [Google Scholar] [CrossRef]
- Rogozkin, B.D.; Stepennova, N.M.; Bergman, G.A.; Proshkin, A.A. Thermochemical Stability, Radiation Testing, Fabrication, And Reprocessing of Mononitride Fuel. At. Énergiya 2003, 95, 428–438. [Google Scholar] [CrossRef]
- Lide, D.R.; Baysinger, G.; Berger, L.I.; Goldberg, R.N.; Kehiaian, H.V.; Kuchitsu, K.; Roth, D.L.; Zwillinger, D. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Jorge, A.B.; Fraxedas, J.; Cantarero, A.; Williams, A.J.; Rodgers, J.; Attfield, J.P.; Fuertes, A. Nitrogen Doping of Ceria. Chem. Mater. 2008, 20, 1682–1684. [Google Scholar] [CrossRef]
- Gschneidner, K.A., Jr.; Verkade, M.E. Selected Cerium Phase Diagrams; White Plains: New York, NY, USA, 1975; p. 10604. [Google Scholar]
- Sharan, A.; Lany, S. Computational Discovery of Stable and Metastable Ternary Oxynitrides. J. Chem. Phys. 2021, 154, 234706. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).