The Role of Deep Hypothermia in Cardiac Surgery
Abstract
:1. Introduction
2. History
3. Indications for the Use of DH in Cardiac Surgery
- -
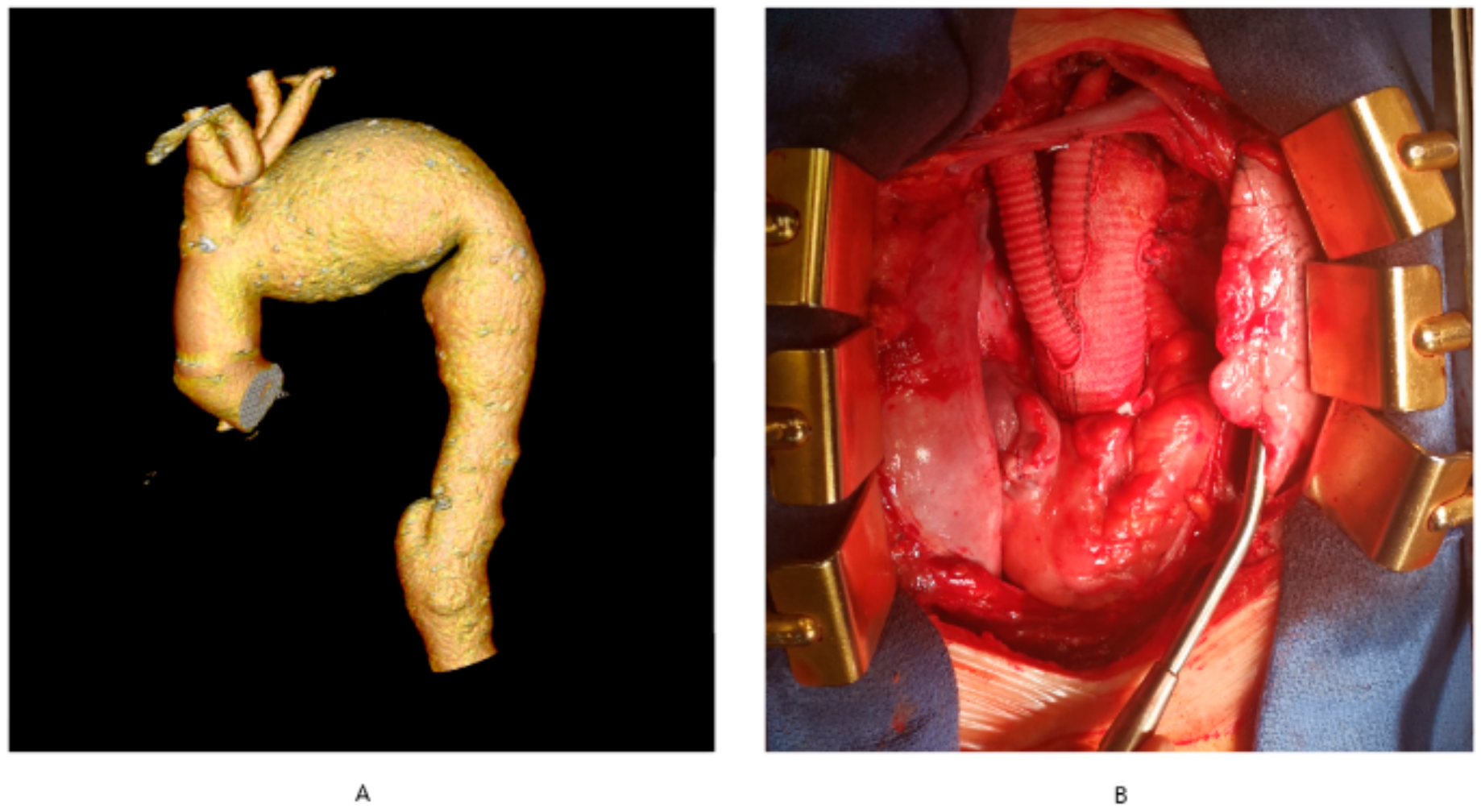
- complex aortic arch surgery (Figure 1A,B)
- -
- chronic type-A aortic dissection
- -
- pulmonary embolism surgery
- -
- complex thoraco-abdominal aneurysm surgery
- -
- surgery with co-existing massive calcifications of the ascending aorta precluding cross-clamping (porcelain aorta).
- -
- surgery for acute type-A aortic dissection
- -
- mild hypothermia is used for corrections of simple defects, where circulatory arrest is not required, and cardiac arrest does not exceed 20 min,
- -
- moderate hypothermia without circulatory arrest is used in older infants with a bodyweight above 10 kg and a complex heart defect, in whom the cardiac arrest duration needed for correction exceeds 20 min,
- -
- DH is used to correct congenital heart defects in infants below 10 kg in bodyweight, and selected older infants.
4. Physiological Effects of Hypothermia
- -
- 28 °C—50%
- -
- 18 °C—19%
- -
- 8 °C—11%
- -
- 15 °C—30 min
- -
- 10 °C—40 min
5. The Rules of Cooling and Rewarming
6. Neuroprotection during DH
- -
- -
- cerebrospinal fluid drainage;
- -
- pharmacotherapy.
- -
- Anesthetics agents.
- -
- Steroids.
- -
- Other drugs.
7. Monitoring during DH
7.1. Physical
- -
- temperature at the arterial outlet of the oxygenator (a surrogate for cerebral perfusate temperature) and two anatomical sites (nasopharyngeal cavity, tympanic membrane, bladder, esophageal or rectal);
- -
- arterial blood pressure at three different sites (right radial artery, left radial artery, femoral artery);
- -
- assessing the function of the central nervous system:
- ◦
- electric: electroencephalogram (EEG) or somatosensory evoked potentials (SSEP);
- ◦
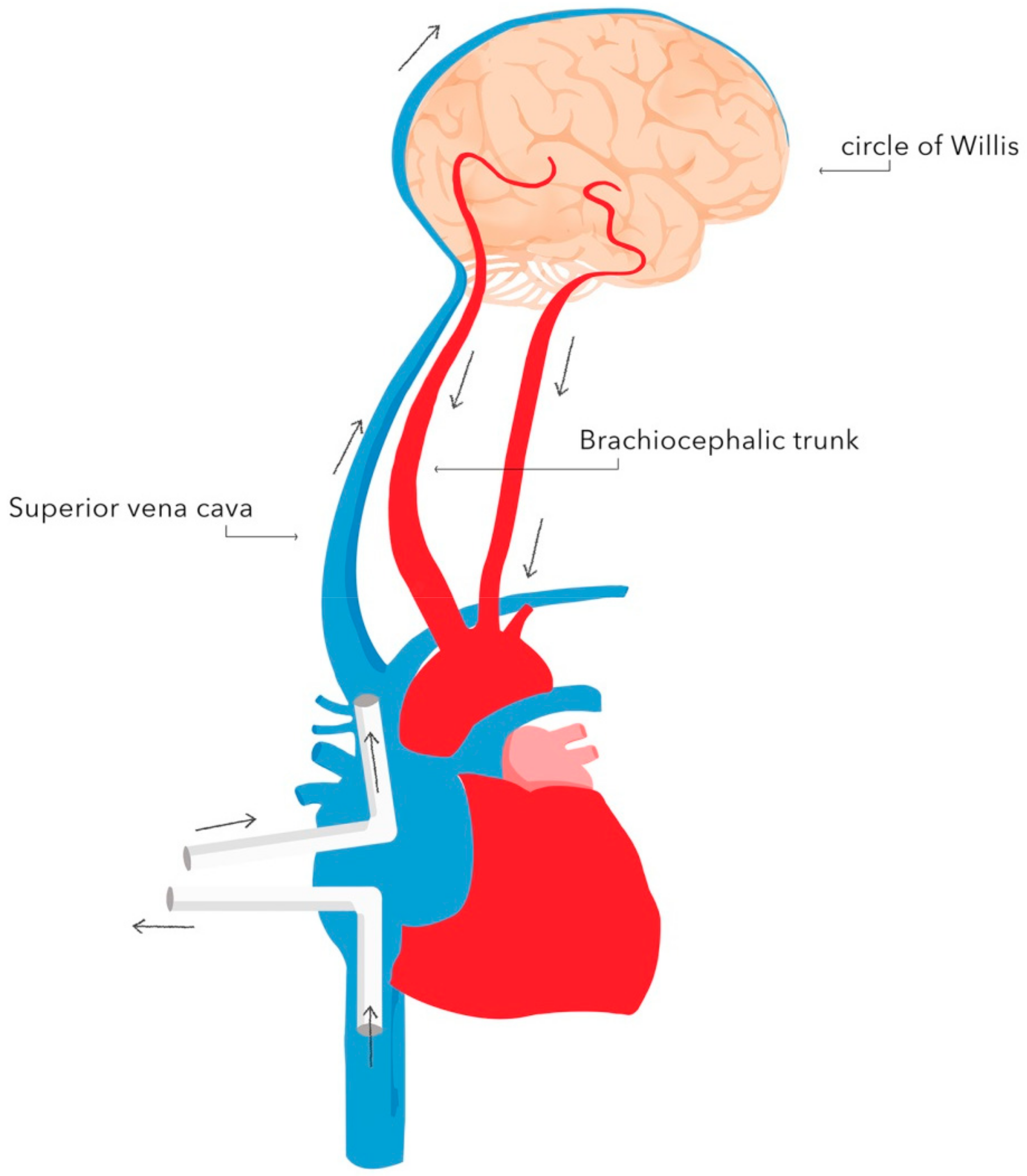
- oxygen delivery: jugular venous bulb saturation (JVBS), near-infrared spectroscopy (NIRS), transcranial doppler sonography.
7.2. Biochemical
- -
- Blood glucose concentration—DH induces hyperglycemia, which is amplified by administering steroids during circulatory arrest. There are two mechanisms of the adverse impact of hyperglycemia: increased intracellular acidosis which enhances apoptosis, and triggering the release of amino acids that adversely affect the ischemic nervous tissue [61]. Therefore, a restrictive approach to blood glucose concentrations below 180 mg/dL is recommended during DH [62].
- -
- Hematocrit—although not routinely practiced, hemodilution to a hematocrit of 20% is assumed to improve flow in the microcirculation, while values of 30% may be beneficial [63]. During rewarming, under normothermic conditions, hemodilution can significantly impair the oxygen flow to the tissues and may be associated with hemostasis impairments. [64,65].
- -
- Arterial blood gas analysis—in hypothermia, the solubility of carbon dioxide increases, resulting in a decrease in the partial pressure of CO2 and thus alkalosis. Two different approaches to achieving an acid–base balance during hypothermia have been developed. The pH-stat perfusion strategy involves measuring pH and pCO2 corrected to the patient’s current (low) temperature and maintaining a pH of 7.4 and pCO2 of 40 mmHg during the surgery. The alpha-stat strategy is to keep the pH at 7.4 and pCO2 at 40 mmHg by measuring these values from a blood sample heated to 37 °C. In order to preserve cerebral self-regulation and intracellular electrochemical neutrality in patients in induced mild/moderate hypothermia, the use of an alpha-stat strategy appears to be acceptable [66,67]. However, for induced circulatory arrest in deep hypothermia, the pH-stat strategy should be preferred to maximize brain protection, enhance cerebral blood flow, cerebral oxygenation, and improve brain cooling [68,69,70].
8. Advantages and Drawbacks of DH
8.1. Advantages
- -
- enables complex surgical interventions involving the aortic arch during circulatory arrest;
- -
- indispensable for pulmonary embolism surgery;
- -
- in infant congenital defect surgery, it improves the technical aspect, as the CPB cannulas limiting the operating field can be temporarily removed during circulatory arrest;
- -
- protects the tissues (particularly nervous tissue) from ischemic damage by reducing the cellular metabolism.
8.2. Drawbacks
- -
- as DH does not stop the cellular metabolism completely, the time of its application is limited;
- -
- DH requires advanced monitoring of physical and biochemical parameters;
- -
- Risk of excessive bleeding due to coagulopathy;
- -
- to limit the rate of neurological adverse events, the use of neuroprotective measures such as pharmacotherapy and selective organ perfusion techniques is necessary.
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, T.D.; Bannon, P.G.; Bavaria, J.; Coselli, J.S.; Elefteriades, J.A.; Griepp, R.B.; Hughes, G.C.; LeMaire, S.A.; Kazui, T.; Kouchoukos, N.T.; et al. Consensus on Hypothermia in Aortic Arch Surgery. Ann. Cardiothorac. Surg. 2013, 2, 163–168. [Google Scholar] [CrossRef]
- McCullough, J.N.; Zhang, N.; Reich, D.L.; Juvonen, T.S.; Klein, J.J.; Spielvogel, D.; Ergin, M.A.; Griepp, R.B. Cerebral Metabolic Suppression during Hypothermic Circulatory Arrest in Humans. Ann. Thorac. Surg. 1999, 67, 1895–1899; discussion 1919–1921. [Google Scholar] [CrossRef]
- Adams, F. The Genuine Works of Hippocrates; William Wood: New York, NY, USA, 1929. [Google Scholar]
- Bigelow, W.G.; Lindsay, W.K.; Greenwood, W.F. Hypothermia: Its Possible Role in Cardiac Surgery: An Investigation of Factors Governing Survival in Dogs at Low Body Temperatures. Ann. Surg. 1950, 132, 849–866. [Google Scholar] [CrossRef]
- Lewis, F.J.; Taufic, M.; Varco, R.L.; Niazi, S. The Surgical Anatomy of Atrial Septal Defects: Experiences with Repair under Direct Vision. Ann. Surg. 1955, 142, 401–415. [Google Scholar] [CrossRef]
- Lewis, F.J.; Taufic, M. Closure of Atrial Septal Defects with the Aid of Hypothermia; Experimental Accomplishments and the Report of One Successful Case. Surgery 1953, 33, 52–59. [Google Scholar]
- Swan, H.; Zeavin, I.; Blount, S.G.; Virtue, R.W. Surgery by Direct Vision in the Open Heart during Hypothermia. J. Am. Med. Assoc. 1953, 153, 1081–1085. [Google Scholar] [CrossRef]
- Swan, H.; Virtue, R.W.; Blount, S.G.; Kircher, L.T. Hypothermia in Surgery; Analysis of 100 Clinical Cases. Ann. Surg. 1955, 142, 382–400. [Google Scholar] [CrossRef] [PubMed]
- Cooley, D.A.; Mahaffey, D.E.; De Bakey, M.E. Total Excision of the Aortic Arch for Aneurysm. Surg. Gynecol. Obstet. 1955, 101, 667–672. [Google Scholar] [PubMed]
- Sealy, W.C.; Brown, I.W.; Young, W.G. A Report on the Use of Both Extracorporeal Circulation and Hypothermia for Open Heart Surgery. Ann. Surg. 1958, 147, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Piwnica, A.; Lenfant, C.; Sprovieri, L.; Laurent, D.; Blondeau, P.; Dubost, C. Deep Hypothermia with Total Circulatory Arrest. Trans. Am. Soc. Artif. Intern. Organs 1960, 6, 227–239. [Google Scholar] [PubMed]
- Horiuchi, T.; Koyamada, K.; Matano, I.; Mohri, H.; Komatsu, T.; Honda, T.; Abe, T.; Ishitoya, T.; Sagawa, Y.; Matsuzawa, K.; et al. Radical Operation for Ventricular Septal Defect in Infancy. J. Thorac. Cardiovasc. Surg. 1963, 46, 180–190. [Google Scholar] [CrossRef]
- Barnard, C.N.; Schrire, V. The Surgical Treatment of Acquired Aneurysm of the Throracic Aorta. Thorax 1963, 18, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Barratt-Boyes, B.G.; Simpson, M.; Neutze, J.M. Intracardiac Surgery in Neonates and Infants Using Deep Hypothermia with Surface Cooling and Limited Cardiopulmonary Bypass. Circulation 1971, 43, I25–I30. [Google Scholar] [CrossRef]
- Griepp, R.B.; Stinson, E.B.; Hollingsworth, J.F.; Buehler, D. Prosthetic Replacement of the Aortic Arch. J. Thorac. Cardiovasc. Surg. 1975, 70, 1051–1063. [Google Scholar] [CrossRef]
- Jain, V.; Langham, M.C.; Wehrli, F.W. MRI Estimation of Global Brain Oxygen Consumption Rate. J. Cereb. Blood Flow Metab. 2010, 30, 1598–1607. [Google Scholar] [CrossRef]
- Norwood, W.I.; Norwood, C.R. Influence of Hypothermia on Intracellular PH during Anoxia. Am. J. Physiol. 1982, 243, C62–C65. [Google Scholar] [CrossRef] [PubMed]
- Jonas, R.A.; Bellinger, D.C.; Rappaport, L.A.; Wernovsky, G.; Hickey, P.R.; Farrell, D.M.; Newburger, J.W. Relation of PH Strategy and Developmental Outcome after Hypothermic Circulatory Arrest. J. Thorac. Cardiovasc. Surg. 1993, 106, 362–368. [Google Scholar] [CrossRef]
- Mezrow, C.K.; Midulla, P.S.; Sadeghi, A.M.; Gandsas, A.; Wang, W.; Dapunt, O.E.; Zappulla, R.; Griepp, R.B. Evaluation of Cerebral Metabolism and Quantitative Electroencephalography after Hypothermic Circulatory Arrest and Low-Flow Cardiopulmonary Bypass at Different Temperatures. J. Thorac. Cardiovasc. Surg. 1994, 107, 1006–1019. [Google Scholar] [CrossRef]
- Michenfelder, J.D.; Milde, J.H. The Effect of Profound Levels of Hypothermia (below 14 °C) on Canine Cerebral Metabolism. J. Cereb. Blood Flow Metab. 1992, 12, 877–880. [Google Scholar] [CrossRef]
- Englum, B.R.; Andersen, N.D.; Husain, A.M.; Mathew, J.P.; Hughes, G.C. Degree of Hypothermia in Aortic Arch Surgery—Optimal Temperature for Cerebral and Spinal Protection: Deep Hypothermia Remains the Gold Standard in the Absence of Randomized Data. Ann. Cardiothorac. Surg. 2013, 2, 184–193. [Google Scholar] [CrossRef]
- Kayatta, M.O.; Chen, E.P. Optimal Temperature Management in Aortic Arch Operations. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 639–650. [Google Scholar] [CrossRef]
- Gambert, S.; Bès-Houtmann, S.; Vandroux, D.; Tissier, C.; Vergely-Vandriesse, C.; Rochette, L.; Athias, P. Deep Hypothermia during Ischemia Improves Functional Recovery and Reduces Free-Radical Generation in Isolated Reperfused Rat Heart. J. Heart Lung Transplant. 2004, 23, 487–491. [Google Scholar] [CrossRef]
- Alva, N.; Palomeque, J.; Carbonell, T. Oxidative Stress and Antioxidant Activity in Hypothermia and Rewarming: Can RONS Modulate the Beneficial Effects of Therapeutic Hypothermia? Oxid. Med. Cell. Longev. 2013, 2013, 957054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Ibarra, F.P.; Varon, J.; López-Meza, E.G. Therapeutic Hypothermia: Critical Review of the Molecular Mechanisms of Action. Front. Neurol. 2011, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelman, R.; Baker, R.A.; Likosky, D.S.; Grigore, A.; Dickinson, T.A.; Shore-Lesserson, L.; Hammon, J.W. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: Clinical Practice Guidelines for Cardiopulmonary Bypass—Temperature Management during Cardiopulmonary Bypass. J. ExtraCorpor. Technol. 2015, 47, 145–154. [Google Scholar]
- Harrington, D.K.; Fragomeni, F.; Bonser, R.S. Cerebral Perfusion. Ann. Thorac. Surg. 2007, 83, S799–S804; discussion S824–S831. [Google Scholar] [CrossRef]
- Ergin, M.A.; Griepp, E.B.; Lansman, S.L.; Galla, J.D.; Levy, M.; Griepp, R.B. Hypothermic Circulatory Arrest and Other Methods of Cerebral Protection during Operations on the Thoracic Aorta. J. Card. Surg. 1994, 9, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, B.A.; Elefteriades, J.A. Deep Hypothermic Circulatory Arrest. Ann. Cardiothorac. Surg. 2013, 2, 303–315. [Google Scholar] [CrossRef]
- Newburger, J.W.; Jonas, R.A.; Wernovsky, G.; Wypij, D.; Hickey, P.R.; Kuban, K.C.; Farrell, D.M.; Holmes, G.L.; Helmers, S.L.; Constantinou, J.; et al. A Comparison of the Perioperative Neurologic Effects of Hypothermic Circulatory Arrest versus Low-Flow Cardiopulmonary Bypass in Infant Heart Surgery. N. Engl. J. Med. 1993, 329, 1057–1064. [Google Scholar] [CrossRef]
- Higami, T.; Kozawa, S.; Asada, T.; Obo, H.; Gan, K.; Iwahashi, K.; Nohara, H. Retrograde Cerebral Perfusion versus Selective Cerebral Perfusion as Evaluated by Cerebral Oxygen Saturation during Aortic Arch Reconstruction. Ann. Thorac. Surg. 1999, 67, 1091–1096. [Google Scholar] [CrossRef]
- Kazui, T.; Kimura, N.; Yamada, O.; Komatsu, S. Surgical Outcome of Aortic Arch Aneurysms Using Selective Cerebral Perfusion. Ann. Thorac. Surg. 1994, 57, 904–911. [Google Scholar] [CrossRef]
- Bachet, J. What Is the Best Method for Brain Protection in Surgery of the Aortic Arch? Selective Antegrade Cerebral Perfusion. Cardiol. Clin. 2010, 28, 389–401. [Google Scholar] [CrossRef]
- Halkos, M.E.; Kerendi, F.; Myung, R.; Kilgo, P.; Puskas, J.D.; Chen, E.P. Selective Antegrade Cerebral Perfusion via Right Axillary Artery Cannulation Reduces Morbidity and Mortality after Proximal Aortic Surgery. J. Thorac. Cardiovasc. Surg. 2009, 138, 1081–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagl, C.; Ergin, M.A.; Galla, J.D.; Lansman, S.L.; McCullough, J.N.; Spielvogel, D.; Sfeir, P.; Bodian, C.A.; Griepp, R.B. Neurologic Outcome after Ascending Aorta–Aortic Arch Operations: Effect of Brain Protection Technique in High-Risk Patients. J. Thorac. Cardiovasc. Surg. 2001, 121, 1107–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Immer, F.F.; Moser, B.; Krähenbühl, E.S.; Englberger, L.; Stalder, M.; Eckstein, F.S.; Carrel, T. Arterial Access through the Right Subclavian Artery in Surgery of the Aortic Arch Improves Neurologic Outcome and Mid-Term Quality of Life. Ann. Thorac. Surg. 2008, 85, 1614–1618; discussion 1618. [Google Scholar] [CrossRef] [PubMed]
- Budde, J.M.; Serna, D.L.; Osborne, S.C.; Steele, M.A.; Chen, E.P. Axillary Cannulation for Proximal Aortic Surgery Is as Safe in the Emergent Setting as in Elective Cases. Ann. Thorac. Surg. 2006, 82, 2154–2159; discussion 2159–2160. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y. A Reappraisal of Retrograde Cerebral Perfusion. Ann. Cardiothorac. Surg. 2013, 2, 316–325. [Google Scholar] [CrossRef]
- Wong, C.H.; Bonser, R.S. Retrograde Cerebral Perfusion: Clinical and Experimental Aspects. Perfusion 1999, 14, 247–256. [Google Scholar] [CrossRef]
- Bavaria, J.E.; Woo, Y.J.; Hall, R.A.; Carpenter, J.P.; Gardner, T.J. Retrograde Cerebral and Distal Aortic Perfusion during Ascending and Thoracoabdominal Aortic Operations. Ann. Thorac. Surg. 1995, 60, 345–352; discussion 352–353. [Google Scholar] [CrossRef]
- Kitamura, M.; Hashimoto, A.; Aomi, S.; Imamaki, M.; Koyanagi, H. Medium-Term Results after Surgery for Aortic Arch Aneurysm with Hypothermic Cerebral Perfusion. Eur. J. Cardio-Thorac. Surg. 1995, 9, 697–700. [Google Scholar] [CrossRef]
- Safi, H.J.; Brien, H.W.; Winter, J.N.; Thomas, A.C.; Maulsby, R.L.; Doerr, H.K.; Svensson, L.G. Brain Protection via Cerebral Retrograde Perfusion during Aortic Arch Aneurysm Repair. Ann. Thorac. Surg. 1993, 56, 270–276. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ueno, A.; Wada, T.; Kimoto, S. A New and Simple Method of Preventing Spinal Cord Damage Following Temporary Occlusion of the Thoracic Aorta by Draining the Cerebrospinal Fluid. J. Cardiovasc. Surg. 1960, 1, 188–197. [Google Scholar]
- Wortmann, M.; Böckler, D.; Geisbüsch, P. Perioperative Cerebrospinal Fluid Drainage for the Prevention of Spinal Ischemia after Endovascular Aortic Repair. Gefässchirurgie 2017, 22, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slupe, A.M.; Kirsch, J.R. Effects of Anesthesia on Cerebral Blood Flow, Metabolism, and Neuroprotection. J. Cereb. Blood Flow Metab. 2018, 38, 2192–2208. [Google Scholar] [CrossRef] [PubMed]
- Kurth, C.D.; Priestley, M.; Watzman, H.M.; McCann, J.; Golden, J. Desflurane Confers Neurologic Protection for Deep Hypothermic Circulatory Arrest in Newborn Pigs. Anesthesiology 2001, 95, 959–964. [Google Scholar] [CrossRef]
- Hoff, J.T.; Smith, A.L.; Hankinson, H.L.; Nielsen, S.L. Barbiturate Protection from Cerebral Infarction in Primates. Stroke 1975, 6, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Michenfelder, J.D. A Valid Demonstration of Barbiturate-Induced Brain Protection in Man—At Last. Anesthesiology 1986, 64, 140–142. [Google Scholar] [CrossRef]
- Al-Hashimi, S.; Zaman, M.; Waterworth, P.; Bilal, H. Does the Use of Thiopental Provide Added Cerebral Protection during Deep Hypothermic Circulatory Arrest? Interact. Cardiovasc. Thorac. Surg. 2013, 17, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, A.; Naik, S.; Klein, N.; Levinsky, R.J.; Strobel, S.; Elliott, M. Interleukin-8 Release and Neutrophil Degranulation after Pediatric Cardiopulmonary Bypass. J. Thorac. Cardiovasc. Surg. 1993, 105, 234–241. [Google Scholar] [CrossRef]
- Bronicki, R.A.; Backer, C.L.; Baden, H.P.; Mavroudis, C.; Crawford, S.E.; Green, T.P. Dexamethasone Reduces the Inflammatory Response to Cardiopulmonary Bypass in Children. Ann. Thorac. Surg. 2000, 69, 1490–1495. [Google Scholar] [CrossRef]
- Shum-Tim, D.; Tchervenkov, C.I.; Jamal, A.M.; Nimeh, T.; Luo, C.Y.; Chedrawy, E.; Laliberte, E.; Philip, A.; Rose, C.P.; Lavoie, J. Systemic Steroid Pretreatment Improves Cerebral Protection after Circulatory Arrest. Ann. Thorac. Surg. 2001, 72, 1465–1471; discussion pp. 1471–1472. [Google Scholar] [CrossRef]
- Shinzawa, M.; Yoshitani, K.; Minatoya, K.; Irie, T.; Ogino, H.; Ohnishi, Y. Changes of Motor Evoked Potentials during Descending Thoracic and Thoracoabdominal Aortic Surgery with Deep Hypothermic Circulatory Arrest. J. Anesth. 2012, 26, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Guérit, J.M.; Verhelst, R.; Rubay, J.; el Khoury, G.; Noirhomme, P.; Baele, P.; Dion, R. The Use of Somatosensory Evoked Potentials to Determine the Optimal Degree of Hypothermia during Circulatory Arrest. J. Card. Surg. 1994, 9, 596–603. [Google Scholar] [CrossRef]
- Shaaban Ali, M.; Harmer, M.; Latto, I.P. Jugular Bulb Oximetry during Cardiac Surgery: Jugular Bulb Oximetry. Anaesthesia 2001, 56, 24–37. [Google Scholar] [CrossRef]
- Denault, A.; Deschamps, A.; Murkin, J.M. A Proposed Algorithm for the Intraoperative Use of Cerebral Near-Infrared Spectroscopy. Semin. Cardiothorac. Vasc. Anesth. 2007, 11, 274–281. [Google Scholar] [CrossRef]
- Gottlieb, E.A.; Fraser, C.D.; Andropoulos, D.B.; Diaz, L.K. Bilateral Monitoring of Cerebral Oxygen Saturation Results in Recognition of Aortic Cannula Malposition during Pediatric Congenital Heart Surgery. Pediatr. Anesth. 2006, 16, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Sheinberg, R.; Yee, M.-S.; Ono, M.; Zheng, Y.; Hogue, C.W. Cerebral Near-Infrared Spectroscopy Monitoring and Neurologic Outcomes in Adult Cardiac Surgery Patients: A Systematic Review. Anesth. Analg. 2013, 116, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, G.A.; Cook, D.J.; Fulgham, J.R.; Oliver, W.C.; Proper, J.A. The Relationship between Cerebral Blood Flow and Transcranial Doppler Blood Flow Velocity during Hypothermic Cardiopulmonary Bypass in Adults. Anesth. Analg. 1996, 82, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.A.; Rubens, F.D.; Wozny, D.; Nathan, H.J. Cerebral Emboli Detected by Transcranial Doppler During Cardiopulmonary Bypass Are Not Correlated with Postoperative Cognitive Deficits. Stroke 2010, 41, 2229–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Yan, J.; Shi, H. Hyperglycemia as a Risk Factor of Ischemic Stroke. J. Drug Metab. Toxicol. 2013, 4, 4. [Google Scholar] [CrossRef]
- Lam, A.M.; Winn, H.R.; Cullen, B.F.; Sundling, N. Hyperglycemia and Neurological Outcome in Patients with Head Injury. J. Neurosurg. 1991, 75, 545–551. [Google Scholar] [CrossRef]
- Duebener, L.F.; Sakamoto, T.; Hatsuoka, S.; Stamm, C.; Zurakowski, D.; Vollmar, B.; Menger, M.D.; Schäfers, H.J.; Jonas, R.A. Effects of Hematocrit on Cerebral Microcirculation and Tissue Oxygenation during Deep Hypothermic Bypass. Circulation 2001, 104, I260–I264. [Google Scholar] [CrossRef] [Green Version]
- Ranucci, M.; Carboni, G.; Cotza, M.; Bianchi, P.; Di Dedda, U.; Aloisio, T. Surgical and Clinical Outcome Research (SCORE) Group Hemodilution on Cardiopulmonary Bypass as a Determinant of Early Postoperative Hyperlactatemia. PLoS ONE 2015, 10, e0126939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranucci, M.; Baryshnikova, E.; Ciotti, E.; Ranucci, M.; Silvetti, S. Hemodilution on Cardiopulmonary Bypass: Thromboelastography Patterns and Coagulation-Related Outcomes. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1588–1594. [Google Scholar] [CrossRef]
- Murkin, J.M.; Farrar, J.K.; Tweed, W.A.; McKenzie, F.N.; Guiraudon, G. Cerebral Autoregulation and Flow/Metabolism Coupling during Cardiopulmonary Bypass: The Influence of PaCO2. Anesth. Analg. 1987, 66, 825–832. [Google Scholar] [CrossRef]
- Duebener, L.F.; Hagino, I.; Sakamoto, T.; Mime, L.B.; Stamm, C.; Zurakowski, D.; Schäfers, H.-J.; Jonas, R.A. Effects of PH Management during Deep Hypothermic Bypass on Cerebral Microcirculation: Alpha-Stat versus PH-Stat. Circulation 2002, 106, I103–I108. [Google Scholar] [PubMed]
- Aoki, M.; Nomura, F.; Stromski, M.E.; Tsuji, M.K.; Fackler, J.C.; Hickey, P.R.; Holtzman, D.H.; Jonas, R.A. Effects of PH on Brain Energetics after Hypothermic Circulatory Arrest. Ann. Thorac. Surg. 1993, 55, 1093–1103. [Google Scholar] [CrossRef]
- Hiramatsu, T.; Miura, T.; Forbess, J.M.; Du Plessis, A.; Aoki, M.; Nomura, F.; Holtzman, D.; Jonas, R.A. PH Strategies and Cerebral Energetics before and after Circulatory Arrest. J. Thorac. Cardiovasc. Surg. 1995, 109, 948–957; discussion 957–958. [Google Scholar] [CrossRef] [Green Version]
- Kurth, C.D.; O’Rourke, M.M.; O’Hara, I.B. Comparison of PH-Stat and Alpha-Stat Cardiopulmonary Bypass on Cerebral Oxygenation and Blood Flow in Relation to Hypothermic Circulatory Arrest in Piglets. Anesthesiology 1998, 89, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.Y.; Pan, W.; Lemaire, S.A.; Pisklak, P.; Lee, V.-V.; Bracey, A.W.; Elayda, M.A.; Preventza, O.; Price, M.D.; Collard, C.D.; et al. Moderate Hypothermia during Aortic Arch Surgery Is Associated with Reduced Risk of Early Mortality. J. Thorac. Cardiovasc. Surg. 2013, 146, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englberger, L.; Suri, R.M.; Greason, K.L.; Burkhart, H.M.; Sundt, T.M.; Daly, R.C.; Schaff, H.V. Deep Hypothermic Circulatory Arrest Is Not a Risk Factor for Acute Kidney Injury in Thoracic Aortic Surgery. J. Thorac. Cardiovasc. Surg. 2011, 141, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Harky, A.; Jahangeer, S.; Adams, B.; Bashir, M. Varying Evidence on Deep Hypothermic Circulatory Arrest in Thoracic Aortic Aneurysm Surgery. Tex. Heart Inst. J. 2018, 45, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouchoukos, N.T.; Scharff, J.R.; Castner, C.F. Repair of Primary or Complicated Aortic Coarctation in the Adult with Cardiopulmonary Bypass and Hypothermic Circulatory Arrest. J. Thorac. Cardiovasc. Surg. 2015, 149, S83–S85. [Google Scholar] [CrossRef] [Green Version]
- Urbanski, P.P.; Raad, M.; Wagner, M.; Heinz, N.; Reents, W.; Diegeler, A. Cardiac Surgery in Patients with a Porcelain Aorta in the Era of Transcatheter Valve Implantation. Eur. J. Cardio-Thorac. Surg. 2013, 44, 48–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, B.; Liu, J.; Wu, Y.; Wang, G.; Feng, Z.; Liu, M.; Long, C.; Song, Y. Perfusion Techniques for Pulmonary Thromboendarterectomy under Deep Hypothermia Circulatory Arrest: A Case Series. J. ExtraCorpor. Technol. 2006, 38, 302–306. [Google Scholar]
- Choi, C.; Maus, T. Pulmonary Thromboendarterectomy Requiring Cardiopulmonary Bypass and Deep Hypothermic Circulatory Arrest in a Patient with Congenital Afibrinogenemia. J. Cardiothorac. Vasc. Anesth. 2021, 35, 593–596. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, J.; Liu, X.; Sun, G.; Zheng, X.; Han, Y.; Zhai, Z.; Li, A.; Lin, F.; Liu, P. Impact of Pulmonary Thromboendarterectomy on Tricuspid Regurgitation in Patients with Chronic Thromboembolic Pulmonary Hypertension: A Single-Center Prospective Cohort Experience. J. Thorac. Dis. 2020, 12, 758–764. [Google Scholar] [CrossRef]
- Hayashi, Y.; Ito, T.; Maekawa, A.; Sawaki, S.; Hoshino, S.; Tokoro, M.; Yanagisawa, J. Reoperative cardiac surgery after previous coronary artery bypass grafting. Kyobu Geka 2014, 67, 433–437; discussion pp. 438–441. [Google Scholar]
- Kızıltan, H.T.; İdem, A.; Salihi, S.; Demir, A.S.; Korkmaz, A.A.; Güden, M. Mitral Valve Surgery Using Video-Assisted Right Minithoracotomy and Deep Hypothermic Perfusion in Patients with Previous Cardiac Operations. J. Cardiothorac. Surg. 2015, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Comentale, G.; Giordano, R.; Palma, G. Comparison of the Different Cardioplegic Strategies in Cardiac Valves Surgery: Who Wins the “Arm-Wrestling”? J. Thorac. Dis. 2018, 10, 714–717. [Google Scholar] [CrossRef] [Green Version]

| Stages | Body Core Temperature | |
|---|---|---|
| Standard Values | Cardiac Surgery | |
| Mild (°C) | 35–32 | 34–28.1 |
| Moderate (°C) | 31.9–28 | 28–20.1 |
| Deep (°C) | 27.9–20.1 | 20–14.1 |
| Profound (°C) | ≤20 | ≤14 |
| Name | Year | Development |
|---|---|---|
| Hippocrates | 4th century BC | Hypothermia used to support the treatment of tetanus |
| Larrey | 1812 | Local hypothermia used to alleviate the pain during amputations of extremities |
| Bigelow | 1950 | Safe circulatory arrest in dogs for 15 min at a temperature of 20 °C |
| Lewis | 1952 | First successful surgery in human patient with the use of hypothermia |
| Swan | 1955 | 100 open heart surgeries with the use of hypothermia |
| Cooley | 1955 | Use of deep hypothermia for cerebral protection during aortic arch surgery |
| Sealy, Brown, Young | 1958 | Clinical use of concomitant surface cooling and cardiopulmonary bypass |
| Dubost | 1960 | Deep hypothermia with circulatory arrest |
| Mohri | 1963 | Technique of surface cooling to 17–20 °C |
| Barnard, Schrir | 1963 | Successful use of deep hypothermia and cardiopulmonary bypass during ascending aorta and aortic arch surgery |
| Barratt-Boyes | 1972 | Technique of surface cooling enabling open heart correction with circulatory arrest and subsequent re-warming in extracorporeal circulation |
| Symptoms | Mild | Moderate | Deep |
|---|---|---|---|
| Neuro-muscular | ataxia dysarthria shivering | stiffness of muscles and joints | muscle contraction |
| Neurological | confusion amnesia apathy limited awareness | limited consciousness | dilated pupils coma loss of self-regulation |
| Circulatory | tachycardia vascular constriction blood pressure increase | bradycardia widening of QRS complexes elevation/depression of ST segment T-wave inversion AV block QT segment prolongation | serious bradycardia asystole ventricular fibrillation |
| Respiratory | tachypnae HbO2 curve shifts to the left | bradypnae bronchial constriction | lactic acidosis HbO2 curve shifts to the right |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gocoł, R.; Hudziak, D.; Bis, J.; Mendrala, K.; Morkisz, Ł.; Podsiadło, P.; Kosiński, S.; Piątek, J.; Darocha, T. The Role of Deep Hypothermia in Cardiac Surgery. Int. J. Environ. Res. Public Health 2021, 18, 7061. https://doi.org/10.3390/ijerph18137061
Gocoł R, Hudziak D, Bis J, Mendrala K, Morkisz Ł, Podsiadło P, Kosiński S, Piątek J, Darocha T. The Role of Deep Hypothermia in Cardiac Surgery. International Journal of Environmental Research and Public Health. 2021; 18(13):7061. https://doi.org/10.3390/ijerph18137061
Chicago/Turabian StyleGocoł, Radosław, Damian Hudziak, Jarosław Bis, Konrad Mendrala, Łukasz Morkisz, Paweł Podsiadło, Sylweriusz Kosiński, Jacek Piątek, and Tomasz Darocha. 2021. "The Role of Deep Hypothermia in Cardiac Surgery" International Journal of Environmental Research and Public Health 18, no. 13: 7061. https://doi.org/10.3390/ijerph18137061
APA StyleGocoł, R., Hudziak, D., Bis, J., Mendrala, K., Morkisz, Ł., Podsiadło, P., Kosiński, S., Piątek, J., & Darocha, T. (2021). The Role of Deep Hypothermia in Cardiac Surgery. International Journal of Environmental Research and Public Health, 18(13), 7061. https://doi.org/10.3390/ijerph18137061







