Health Risk Assessments and Microbial Community Analyses of Groundwater from a Heavy Metal-Contaminated Site in Hezhou City, Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Analytical Methods
2.4. Health Risk Assessment
2.4.1. Determination of Exposure
2.4.2. Characterization of Health Risks
3. Results and Discussions
3.1. Distribution Characteristics of Heavy Metals in Groundwater
3.2. Correlation Analysis
3.3. Human Health Risk Assessment
3.4. Microbial Community Analysis in Groundwater
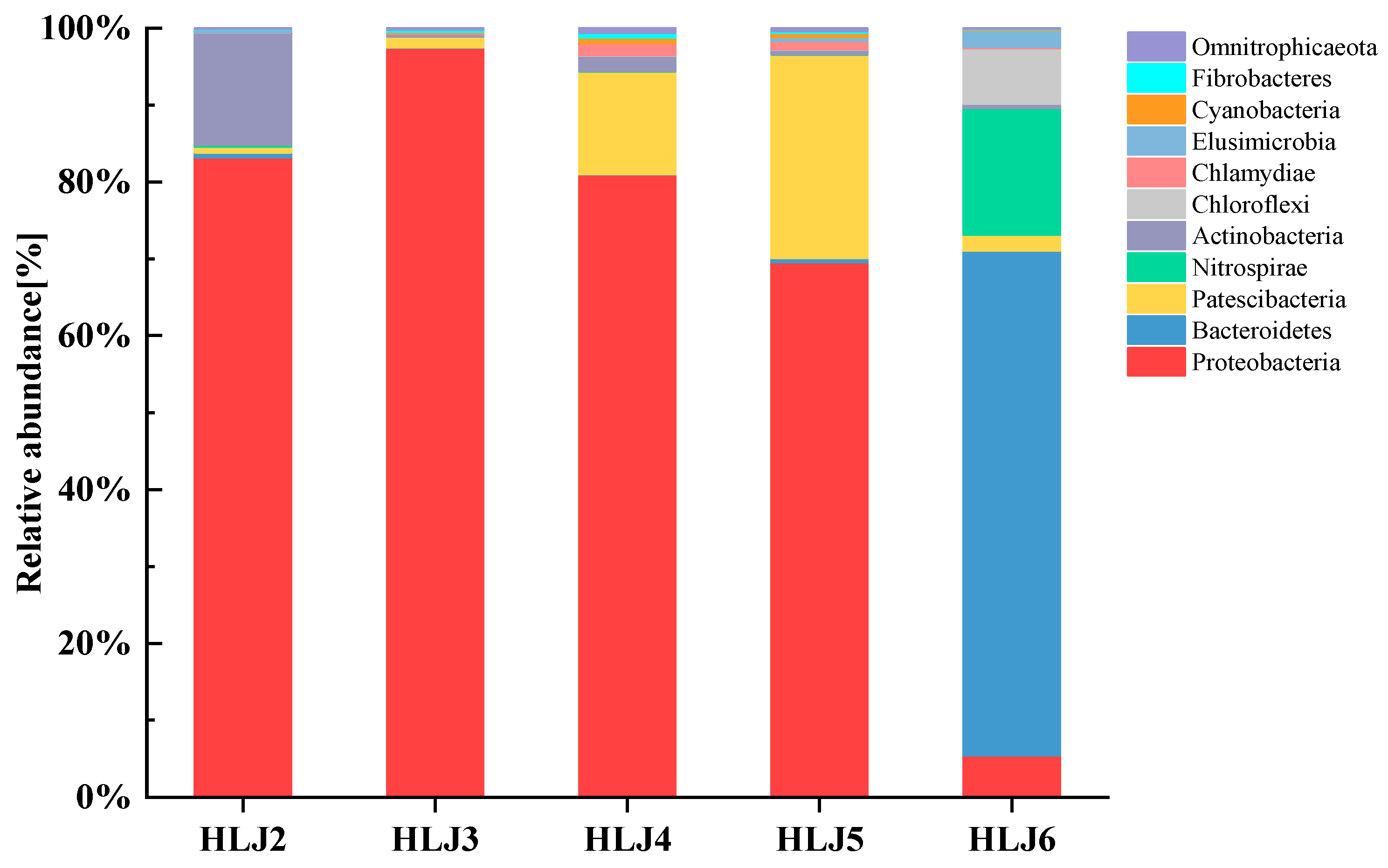
3.4.1. Microbial Community Composition Based on Phylum and Genus Levels
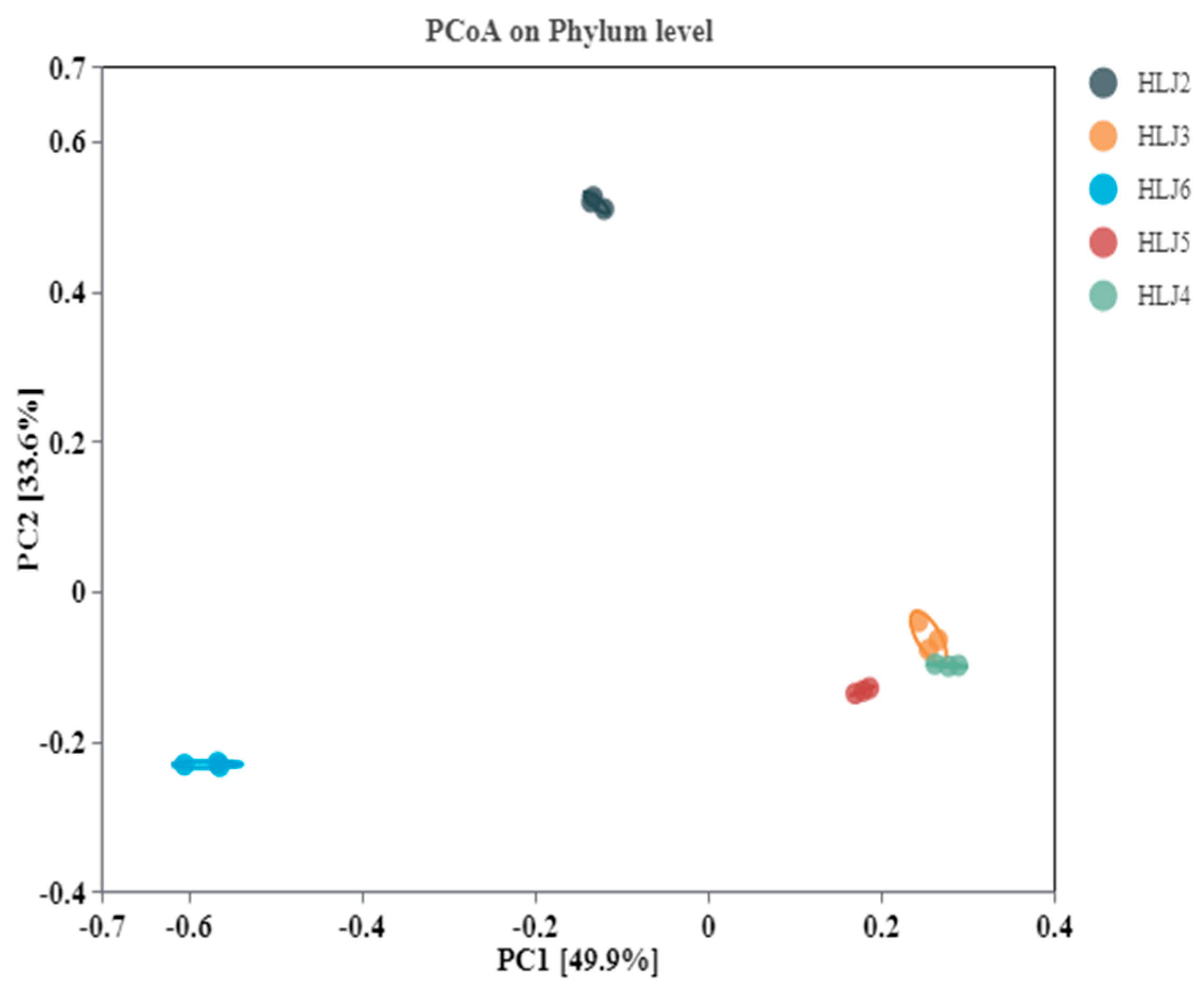
3.4.2. Microbial Community Diversity
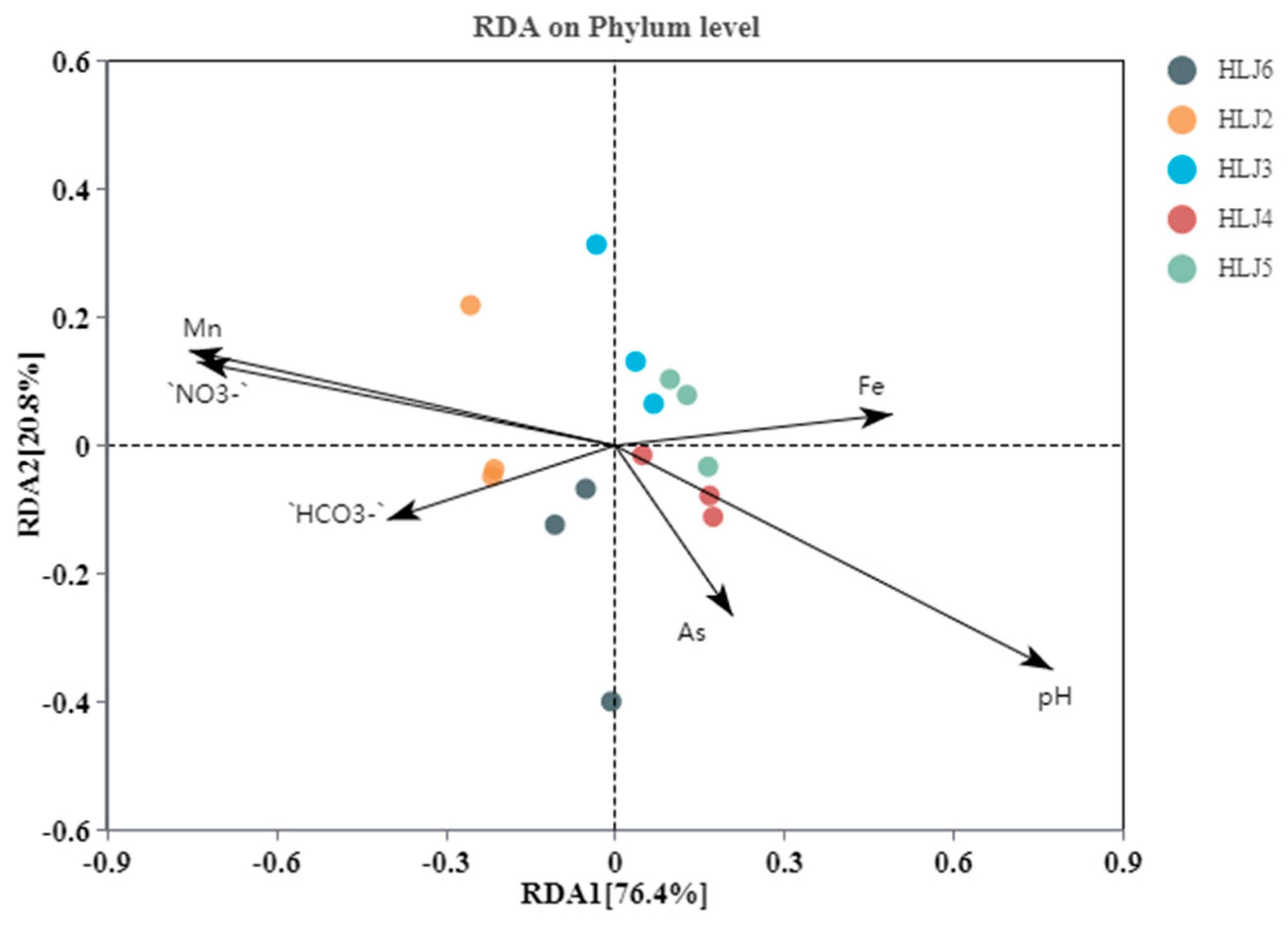
3.4.3. Correlation between Bacterial Community and Environmental Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinsri, P.; Shrestha, S.; KC, S.; Mohanasundaram, S.; Virdis, S.G.P.; Nguyen, T.P.L.; Chaowiwat, W. Assessing the future climate change, land use change, and abstraction impacts on groundwater resources in the Tak Special Economic Zone, Thailand. Environ. Res. 2022, 211, 113026. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Q.; Yang, Y.; Xie, C.; Ma, H. Hydrogeochemical controls on arsenic contamination potential and health threat in an intensive agricultural area, northern China. Environ. Pollut. 2020, 256, 113455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Tan, X.; Zhao, W.; Feng, L.; He, S.; Wei, L.; Yang, L.; Wang, K.; Zhao, Q. Efficiency assessment of ZVI-based media as fillers in permeable reactive barrier for multiple heavy metal-contaminated groundwater remediation. J. Hazard. Mater. 2022, 424, 127605. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Liu, S.; Dai, C.; Tao, A.; Tan, B.; Ma, G.; Chalov, R.S.; Chalov, S.R. Heavy Metal Distribution and Groundwater Quality Assessment for a Coastal Area on a Chinese Island. Pol. J. Environ. Stud. 2017, 26, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, C.; Ma, R. Review: Safe and sustainable groundwater supply in China. Hydrogeol. J. 2018, 26, 1301–1324. [Google Scholar] [CrossRef]
- Shrestha, S.; Kafle, R.; Pandey, V.P. Evaluation of index-overlay methods for groundwater vulnerability and risk assessment in Kathmandu Valley, Nepal. Sci. Total Environ. 2017, 575, 779–790. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, H.; Zhou, Y.; Chen, J.; Wang, H.; Ren, W.; Qu, W. Cumulative health risk assessment of multiple chemicals in groundwater based on deterministic and Monte Carlo models in a large semiarid basin. J. Clean. Prod. 2022, 352, 131567. [Google Scholar] [CrossRef]
- Miao, F.; Zhang, Y.; Li, Y.; Liang, X.; Lin, Q.; Zhou, Y. Establishing a weighted methodology for human health risk assessment of cadmium based on its equilibrium speciation in groundwater. J. Clean. Prod. 2021, 322, 129053. [Google Scholar] [CrossRef]
- Zhang, L.M.; Yang, Q.C.; Wang, H.; Gu, Q.B.; Zhang, Y.L. Genetic interpretation and health risk assessment of arsenic in Hetao Plain of inner Mongolia, China. Environ. Res. 2022, 208, 112680. [Google Scholar] [CrossRef]
- Shikha, D.; Singh, P.K. In situ phytoremediation of heavy metal-contaminated soil and groundwater: A green inventive approach. Environ. Sci. Pollut. Res. 2021, 28, 4104–4124. [Google Scholar] [CrossRef]
- Gómez-Gener, L.; Siebers, A.R.; Arce, M.I.; Arnon, S.; Bernal, S.; Bolpagni, R.; Datry, T.; Gionchetta, G.; Grossart, H.; Mendoza-Lera, C.; et al. Towards an improved understanding of biogeochemical processes across surface-groundwater interactions in intermittent rivers and ephemeral streams. Earth Sci. Rev. 2021, 220, 103724. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B.; Darbha, G.K. The groundwater arsenic contamination in the Bengal Basin—A review in brief. Chemosphere 2022, 299, 134369. [Google Scholar] [CrossRef]
- He, W.; Lin, X.; Shi, Z.; Yu, J.; Ke, S.; Lu, X.; Deng, Z.; Wu, Y.; Wang, L.; He, Q.; et al. Nutrient removal performance and microbial community analysis of amended bioretention column for rainwater runoff treatment. J. Clean. Prod. 2022, 374, 133974. [Google Scholar] [CrossRef]
- Meng, D.; Li, J.; Liu, T.; Liu, Y.; Yan, M.; Hu, J.; Li, X.; Liu, X.; Liang, Y.; Liu, H.; et al. Effects of redox potential on soil cadmium solubility: Insight into microbial community. J. Environ. Sci. 2019, 75, 224–232. [Google Scholar] [CrossRef]
- Wang, C.; Jia, Y.; Wang, Q.; Yan, F.; Wu, M.; Li, X.; Fang, W.; Xu, F.; Liu, H.; Qiu, Z. Responsive change of crop-specific soil bacterial community to cadmium in farmlands surrounding mine area of Southeast China. Environ. Res. 2022, 214, 113748. [Google Scholar] [CrossRef]
- Akash, S.; Sivaprakash, B.; Raja, V.C.V.; Rajamohan, N.; Muthusamy, G. Remediation techniques for uranium removal from polluted environment—Review on methods, mechanism and toxicology. Environ. Pollut. 2022, 302, 119068. [Google Scholar] [CrossRef]
- Hur, M.; Park, S.-J. Identification of microbial profiles in heavy-metal-contaminated soil from full-length 16S rRNA reads sequenced by a PacBio system. Microorganisms 2019, 7, E357. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.W.; Wang, J.L.; Lv, Y.; Liu, X.Y.; Zhong, J.; Cui, X.L.; Zhang, M.J.; Ma, D.Z.; Yan, X.; Zhu, X.Z. Effects of Heavy Metals/Metalloids and Soil Properties on Microbial Communities in Farmland in the Vicinity of a Metals Smelter. Front. Microbiol. 2021, 12, 707786. [Google Scholar] [CrossRef]
- Kadim, M.K.; Risjani, Y. Biomarker for monitoring heavy metal pollution in aquatic environment: An overview toward molecular perspectives. Emerging Contam. 2022, 8, 195–205. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Ren, L.; Zhou, Y.; Gao, J.; Luo, L.; Yang, Y.; Peng, Q.; Huang, H.; Chen, A. Diagnosis of soil contamination using microbiological indices: A review on heavy metal pollution. J. Environ. Manag. 2019, 242, 121–130. [Google Scholar] [CrossRef]
- Liang, D.; Song, J.; Xia, J.; Chang, J.; Kong, F.; Sun, H.; Qiong, W.; Cheng, D.; Zhang, Y. Effects of heavy metals and hyporheic exchange on microbial community structure and functions in hyporheic zone. J. Environ. Manag. 2022, 303, 114201. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Vaidya, B.P.; Goodey, N.M.; Krumins, J.A. Soil microbial response to metal contamination in a vegetated and urban brownfield. J. Environ. Manag. 2019, 244, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Xie, T.; Wang, X.; Bai, J.; Tang, L.; Zhao, H.; Wei, W.; Wang, M.; Zhao, Y. Metagenomic analysis of microbial community and function involved in cd-contaminated soil. BMC Microbiol. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhu, Y.; Dong, H.; Li, J.; Zhang, W.; Shao, Y.; Shao, Y. Effects of heavy metals and antibiotics on antibiotic resistance genes and microbial communities in soil. Process Saf. Environ. Prot. 2023, 169, 418–427. [Google Scholar] [CrossRef]
- Ying, X.; Mengxue, L.; Xinyue, D.; Yiming, M.; Yongle, Z.; Jiaxi, T. Health Risk and Vulnerability Assessment of Fluorine Chemical Park and Surrounding Groundwater. J. Environ. Sci. Beijing China 2020, 40, 2300–2310. (In Chinese). Available online: https://kns.cnki.net/kcms/detail/11.1843.x.20200515.2310.002.html (accessed on 29 November 2022).
- Giri, S.; Singh, A.K. Risk assessment, statistical source identification and seasonal fluctuation of dissolved metals in the Subarnarekha River, India. J. Hazard. Mater. 2014, 265, 305–314. [Google Scholar] [CrossRef]
- Yu, H.; Lin, M.; Peng, W.; He, C. Seasonal changes of heavy metals and health risk assessment based on Monte Carlo simulation in alternate water sources of the Xinbian River in Suzhou City, Huaibei Plain, China. Ecotoxicol. Environ. Safe. 2022, 236, 113445. [Google Scholar] [CrossRef]
- Ma, J.; Yan, Y.; Chen, X.; Niu, Z.; Yu, R.; Hu, G. Incorporating bioaccessibility and source apportionment into human health risk assessment of heavy metals in urban dust of Xiamen, China. Ecotoxicol. Environ. Safe. 2021, 228, 112985. [Google Scholar] [CrossRef]
- Han, J.; Lee, S.; Mammadov, Z.; Kim, M.; Mammadov, G.; Ro, H. Source apportionment and human health risk assessment of trace metals and metalloids in surface soils of the Mugan Plain, the Republic of Azerbaijan. Environ. Pollut. 2021, 290, 118058. [Google Scholar] [CrossRef]
- Huanhuan, S.; Yujie, P.; Min, Z.; Changsheng, H.; Qingqin, H.; Pengcheng, P.; Hongxia, P. Source analysis and health risk assessment of heavy metals in groundwater in Leizhou Peninsula. J. Environ. Sci. Beijing China 2021, 42, 4246–4256. (In Chinese). Available online: https://kns.cnki.net/kcms/detail/11.1895.X.20210323.1343.038.html (accessed on 29 November 2022).
- Wang, Z.; Su, Q.; Wang, S.; Gao, Z.; Liu, J. Spatial distribution and health risk assessment of dissolved heavy metals in groundwater of eastern China coastal zone. Environ. Pollut. 2021, 290, 118016. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K.; Mahato, M.K. Monte Carlo simulation-based probabilistic health risk assessment of metals in groundwater via ingestion pathway in the mining areas of Singhbhum copper belt, India. Int. J. Environ. Health Res 2020, 30, 447–460. [Google Scholar] [CrossRef]
- Zhenyan, W.; Shu, W.; Tengfei, F.; Zongjun, G.; Xingyong, X.; Qiao, S.; Wenquan, L.; Guangquan, C. Characteristics and health risk assessment of heavy metals in groundwater in Qinhuangdao coastal zone. Environ. Chem. Beijing China 2021, 40, 1157–1166. (In Chinese). Available online: https://kns.cnki.net/kcms/detail/11.1844.X.20210421.1831.022.html (accessed on 29 November 2022).
- Hoang, H.; Chiang, C.; Lin, C.; Wu, C.; Lee, C.; Cheruiyot, N.K.; Tran, H.; Bui, X. Human health risk simulation and assessment of heavy metal contamination in a river affected by industrial activities. Environ. Pollut. 2021, 285, 117414. [Google Scholar] [CrossRef]
- Jinmei, Z.; Zhongcheng, J.; Guangli, X.; Xiaoqun, Q.; Qibo, H.; Liankai, Z. Distribution of metal elements in groundwater around iron mines and health risk assessment. China Environ. Sci. Chin. Ed. 2019, 39, 1934–1944. [Google Scholar] [CrossRef]
- Jinmei, Z.; Zhongcheng, J.; Guangli, X.; Xiaoqun, Q.; Qibo, H.; Liankai, Z. Groundwater quality analysis and health risk assessment in Xiangshui area of Chongzuo. J. Environ. Sci. Beijing China 2019, 40, 2675–2685. (In Chinese). Available online: https://kns.cnki.net/kcms/detail/11.1895.X.20190120.1729.022.html (accessed on 29 November 2022).
- Jun, J.; Husheng, X.; Qiao, L.; Hongfei, T.; Youwei, J.; Aihemati, M.H. Geochemical characteristics of groundwater in the Kuitun River Basin and its impact on arsenic transport. Environ. Chem. Beijing China 2021, 40, 1775–1786. (In Chinese). Available online: https://kns.cnki.net/kcms/detail/11.1844.X.20210608.1108.014.html (accessed on 29 November 2022).
- Qiao, W.; Cao, W.; Gao, Z.; Pan, D.; Ren, Y.; Li, Z.; Zhang, Z. Contrasting behaviors of groundwater arsenic and fluoride in the lower reaches of the Yellow River basin, China: Geochemical and modeling evidences. Sci. Total Environ. 2022, 851, 158134. [Google Scholar] [CrossRef]
- Ewusi, A.; Sunkari, E.D.; Seidu, J.; Coffie-Anum, E. Hydrogeochemical characteristics, sources and human health risk assessment of heavy metal dispersion in the mine pit water–surface water–groundwater system in the largest manganese mine in Ghana. Environ. Technol. Innov. 2022, 26, 102312. [Google Scholar] [CrossRef]
- Xiaodong, W.; Wei, T.; Xueyan, Z. Distribution characteristics and health risk assessment of metal elements in groundwater in Ningxia. J. Environ. Sci. Beijing China 2022, 43, 329–338. (In Chinese). Available online: https://kns.cnki.net/kcms/detail/11.1895.X.20210708.1724.033.html (accessed on 29 November 2022).
- Haohui, Z.; Junxiang, S.; Yonghai, J.; Yongfeng, J.; Xiujin, L. Health risk assessment and microbial community analysis of petroleum hydrocarbons in groundwater of a polluted site. Res. Environ. Sci. 2022, 35, 1063–1071. (In Chinese). Available online: https://kns.cnki.net/kcms/detail/11.1827.x.20210918.0613.001.html (accessed on 29 November 2022).
- Hemmat-Jou, M.H.; Safari-Sinegani, A.A.; Che, R.; Mirzaie-Asl, A.; Tahmourespour, A.; Tahmasbian, I. Toxic trace element resistance genes and systems identified using the shotgun metagenomics approach in an Iranian mine soil. Environ. Sci. Pollut. Res. 2021, 28, 4845–4856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Q.; Shi, H. Distribution and population structure characteristics of microorganisms in urban sewage system. Appl. Microbiol. Biotechnol. 2015, 99, 7723–7734. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zou, D.; Zheng, X.; Liu, F.; Li, L.; Xiao, Z. Effects of antibiotics on anaerobic digestion of sewage sludge: Performance of anaerobic digestion and structure of the microbial community. Sci. Total Environ. 2022, 845, 157384. [Google Scholar] [CrossRef]
- HongE, Y.; Wan, Z.; Kim, Y.; Yu, J. Submerged zone and vegetation drive distribution of heavy metal fractions and microbial community structure: Insights into stormwater biofiltration system. Sci. Total Environ. 2022, 853, 158367. [Google Scholar] [CrossRef]
- Johnson, H.; Cho, H.; Choudhary, M. Bacterial heavy metal resistance genes and bioremediation potenital. Comput. Mol. Biosci. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Huang, H.; Mou, L.; Ru, J.; Zhao, J.; Xiao, S. Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci. Total Environ. 2018, 637–638, 1400–1412. [Google Scholar] [CrossRef]
- Zheng, T.; Deng, Y.; Wang, Y.; Jiang, H.; Xie, X.; Gan, Y. Microbial sulfate reduction facilitates seasonal variation of arsenic concentration in groundwater of Jianghan Plain, Central China. Sci. Total Environ. 2020, 735, 139327. [Google Scholar] [CrossRef]
- Pi, K.; Wang, Y.; Xie, X.; Ma, T.; Su, C.; Liu, Y. Role of sulfur redox cycling on arsenic mobilization in aquifers of Datong Basin, northern China. Appl. Geochem. 2017, 77, 31–43. [Google Scholar] [CrossRef]
- Kumar, N.; Couture, R.; Millot, R.; Battaglia-Brunet, F.; Rose, J. Microbial Sulfate Reduction Enhances Arsenic Mobility Downstream of Zerovalent-Iron-Based Permeable Reactive Barrier. Environ. Sci. Technol. 2016, 50, 7610–7617. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, J.; Yan, W.; Zhang, T.C.; Chen, C. Response of microbial community to different land-use types, nutrients and heavy metals in urban river sediment. J. Environ. Manag. 2022, 321, 115855. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, X.; Huang, Z.; Li, H.; Huang, J.; Corti, G.; Wu, Z.; Qin, X.; Zhang, Y.; Ye, X.; et al. A field study on the composition, structure, and function of endophytic bacterial community of Robinia pseudoacacia at a composite heavy metals tailing. Sci. Total Environ. 2022, 850, 157874. [Google Scholar] [CrossRef]
- Li, Y.; Gong, X.; Xiong, J.; Sun, Y.; Shu, Y.; Niu, D.; Lin, Y.; Wu, L.; Zhang, R. Different dissolved organic matters regulate the bioavailability of heavy metals and rhizosphere microbial activity in a plant-wetland soil system. J. Environ. Chem. Eng. 2021, 9, 106823. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, J.; Lu, J.; Sun, Y. Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef]
- Kasemodel, M.C.; Sakamoto, I.K.; Varesche, M.B.A.; Rodrigues, V.G.S. Potentially toxic metal contamination and microbial community analysis in an abandoned Pb and Zn mining waste deposit. Sci. Total Environ. 2019, 675, 367–379. [Google Scholar] [CrossRef]
- Weng, T.; Liu, C.; Kao, Y.; Hsiao, S.S. Isotopic evidence of nitrogen sources and nitrogen transformation in arsenic-contaminated groundwater. Sci. Total Environ. 2017, 578, 167–185. [Google Scholar] [CrossRef]


| Heavy Metals | Monitoring Well Number | Standard * | ||||
|---|---|---|---|---|---|---|
| HLJ2 | HLJ3 | HLJ4 | HLJ5 | HLJ6 | ||
| Mn | 22,130 ± 138.41 | 370 ± 1.34 | 250 ± 0.30 | 330 ± 1.78 | 250 ± 1.93 | 100 |
| Cr | 0.38 ± 0.15 | 0.28 ± 0.16 | 0.09 ± 0.05 | 0.19 ± 0.05 | 0.25 ± 0.10 | 5 |
| As | 16.13 ± 1.14 | 19,407.34 ± 124.03 | 31,733.05 ± 458.42 | 106,242.67 ± 1482.80 | 111,640.67 ± 1286.29 | 10 |
| Cd | 408.33 ± 11.76 | 19.85 ± 1.26 | 14.31 ± 0.44 | 23.35 ± 0.54 | 18.58 ± 0.82 | 5 |
| Pb | 8.95 ± 0.24 | 0.26 ± 0.04 | 0.53 ± 0.22 | 2.31 ± 0.10 | 1 ± 0.67 | 10 |
| Cu | 1054.92 ± 39.75 | 0.28 ± 0.22 | 2.6 ± 1.05 | 8.43 ± 1.57 | 4.16 ± 0.52 | 1000 |
| Ni | 223.53 ± 7.86 | 4.46 ± 0.11 | 3.82 ± 0.27 | 1.8 ± 0.01 | 1.66 ± 0.15 | 20 |
| Heavy Metals | Cr | As | Cd | Ni | Cu | Pb | Mn |
|---|---|---|---|---|---|---|---|
| Cr | 1.000 | −0.182 | 0.621 * | 0.282 | 0.318 | 0.325 | 0.564 * |
| As | 1.000 | −0.414 | −0.950 ** | −0.111 | −0.157 | −0.671 ** | |
| Cd | 1.000 | 0.443 | 0.746** | 0.746 ** | 0.854 ** | ||
| Ni | 1.000 | 0.132 | 0.182 | 0.682 ** | |||
| Cu | 1.000 | 0.968 ** | 0.407 | ||||
| Pb | 1.000 | 0.439 | |||||
| Mn | 1.000 |
| Cr | As | Cd | Ni | Cu | Pb | Mn | |
|---|---|---|---|---|---|---|---|
| pH | −0.286 | 0.097 | −0.699 * | −0.122 | −0.954 ** | −0.894 ** | −0.432 |
| F- | 0.418 | −0.589 * | 0.596 * | 0.554 * | −0.021 | 0.043 | 0.864 ** |
| Cl- | 0.493 | −0.568 * | 0.607 * | 0.586 * | −0.029 | 0.036 | 0.854 ** |
| NO3- | 0.054 | −0.871 ** | 0.239 | 0.861 ** | 0.207 | 0.250 | 0.371 |
| SO42- | 0.257 | 0.182 | 0.093 | −0.182 | −0.411 | −0.375 | 0.307 |
| K+ | 0.461 | −0.521 * | 0.375 | 0.479 | −0.143 | −0.082 | 0.739 ** |
| Na+ | 0.229 | −0.757 ** | 0.021 | 0.768 ** | −0.375 | −0.300 | 0.479 |
| Route of Exposure | Heavy Metals | Monitoring Well Number | ||||
|---|---|---|---|---|---|---|
| HLJ2 | HLJ3 | HLJ4 | HLJ5 | HLJ6 | ||
| Drinking water ingestion | Cr | 2.67 × 10−6 | 1.96 × 10−6 | 6.54 × 10−7 | 1.34 × 10−6 | 1.80 × 10−6 |
| As | 3.42 × 10−4 | 4.12 × 10−1 | 6.74 × 10−1 | 2.26 | 2.37 | |
| Cd | 3.53 × 10−2 | 1.71 × 10−3 | 1.24 × 10−3 | 2.02 × 10−3 | 1.60 × 10−3 | |
| TCR | 3.56 × 10−2 | 4.14 × 10−1 | 6.75 × 10−1 | 2.26 | 2.37 | |
| Dermal contact | Cr | 1.29 × 10−6 | 9.47 × 10−7 | 3.17 × 10−7 | 6.48 × 10−7 | 8.70 × 10−7 |
| As | 9.09 × 10−6 | 1.09 × 10−2 | 1.79 × 10−2 | 5.99 × 10−2 | 6.29 × 10−2 | |
| Cd | 2.66 × 10−5 | 1.29 × 10−6 | 9.31 × 10−7 | 1.52 × 10−6 | 1.21 × 10−6 | |
| TCR | 3.70 × 10−5 | 1.09 × 10−2 | 1.79 × 10−2 | 5.99 × 10−2 | 6.29 × 10−2 | |
| Route of Exposure | Heavy Metals | Monitoring Well Number | ||||
|---|---|---|---|---|---|---|
| HLJ2 | HLJ 3 | HLJ 4 | HLJ 5 | HLJ 6 | ||
| Drinking water ingestion | Ni | 5.13 × 10−1 | 1.02 × 10−2 | 8.76 × 10−3 | 4.13 × 10−3 | 3.82 × 10−3 |
| Cu | 1.21 | 3.22 × 10−4 | 2.98 × 10−3 | 9.68 × 10−3 | 4.77 × 10−3 | |
| Pb | 2.94 × 10−1 | 8.66 × 10−3 | 1.73 × 10−2 | 7.56 × 10−2 | 3.29 × 10−2 | |
| Mn | 2.21 × 10 | 3.70 × 10−1 | 2.48 × 10−1 | 3.31 × 10−1 | 2.53 × 10−1 | |
| HI | 2.41 × 10 | 3.89 × 10−1 | 2.77 × 10−1 | 4.20 × 10−1 | 2.94 × 10−1 | |
| Dermal contact | Ni | 1.10 × 10−3 | 2.20 × 10−5 | 1.88 × 10−5 | 8.86 × 10−6 | 8.18 × 10−6 |
| Cu | 2.34 × 10−2 | 6.22 × 10−6 | 5.76 × 10−5 | 1.87 × 10−4 | 9.21 × 10−5 | |
| Pb | 6.80 × 10−6 | 2.01 × 10−7 | 4.01 × 10−7 | 1.75 × 10−6 | 7.63 × 10−7 | |
| Mn | 3.27 × 10−1 | 5.48 × 10−3 | 3.67 × 10−3 | 4.90 × 10−3 | 3.75 × 10−3 | |
| HI | 3.52 × 10−1 | 5.51 × 10−3 | 3.75 × 10−3 | 5.10 × 10−3 | 3.85 × 10−3 | |
| Monitoring Well | Chao1 | Shannon | Simpson |
|---|---|---|---|
| HLJ2 | 2746 | 0.0955 | 3.88 |
| HLJ3 | 4936 | 0.0761 | 4.73 |
| HLJ4 | 4987 | 0.0364 | 5.39 |
| HLJ5 | 5179 | 0.042 | 5.18 |
| HLJ6 | 3276 | 0.1047 | 4.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Zhang, K.; Wang, Y.; Zhang, B.; Mao, K.; Zhang, H. Health Risk Assessments and Microbial Community Analyses of Groundwater from a Heavy Metal-Contaminated Site in Hezhou City, Southwest China. Int. J. Environ. Res. Public Health 2023, 20, 604. https://doi.org/10.3390/ijerph20010604
Xu M, Zhang K, Wang Y, Zhang B, Mao K, Zhang H. Health Risk Assessments and Microbial Community Analyses of Groundwater from a Heavy Metal-Contaminated Site in Hezhou City, Southwest China. International Journal of Environmental Research and Public Health. 2023; 20(1):604. https://doi.org/10.3390/ijerph20010604
Chicago/Turabian StyleXu, Mingjie, Kuankuan Zhang, Yiduo Wang, Bin Zhang, Kang Mao, and Hua Zhang. 2023. "Health Risk Assessments and Microbial Community Analyses of Groundwater from a Heavy Metal-Contaminated Site in Hezhou City, Southwest China" International Journal of Environmental Research and Public Health 20, no. 1: 604. https://doi.org/10.3390/ijerph20010604





