Protective Effect of Field Horsetail Polyphenolic Extract on Erythrocytes and Their Membranes
Abstract
:1. Introduction
2. Results
2.1. Phenolic Content
2.2. Antiradical Activity
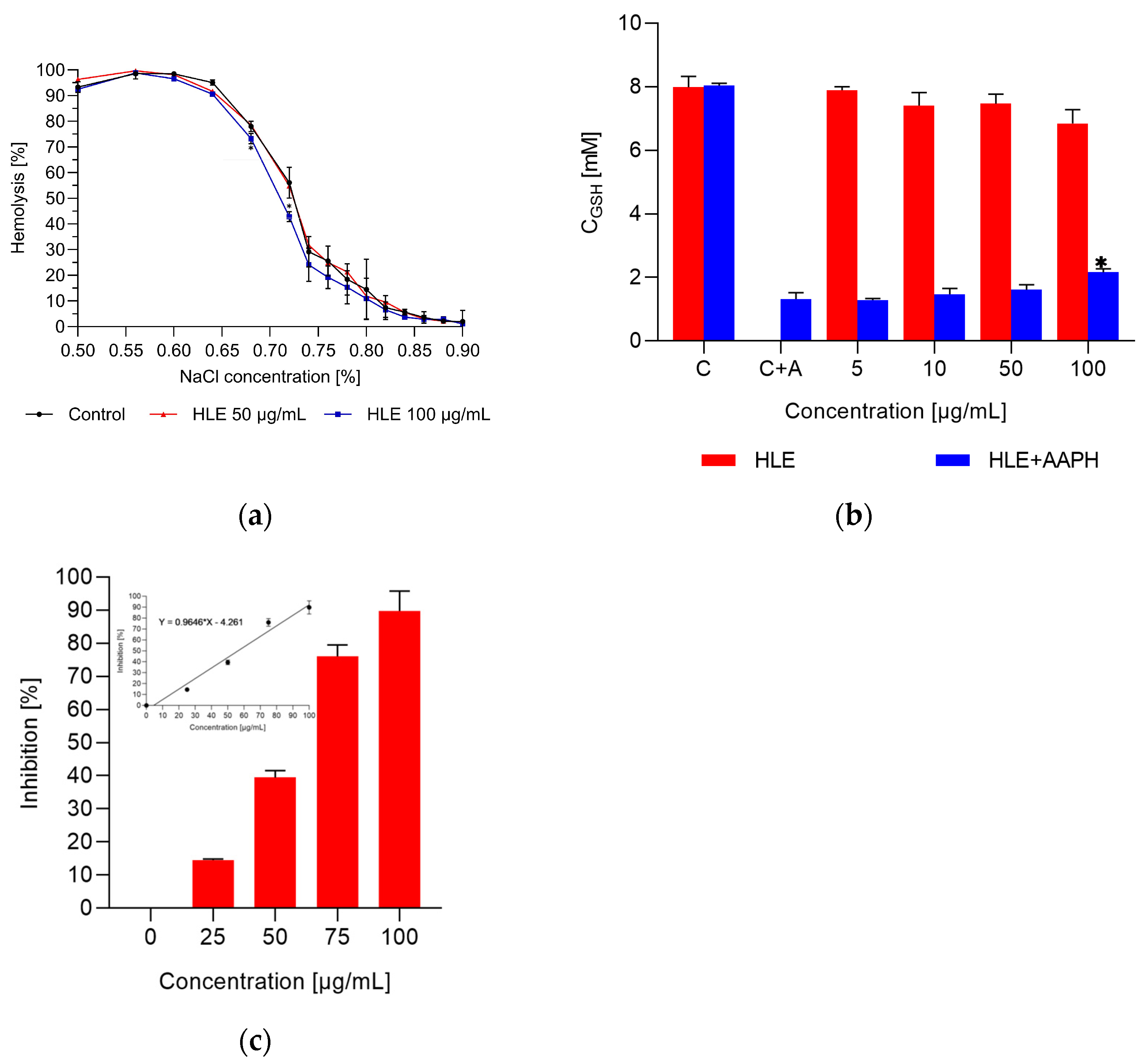
2.3. Interaction of Extract with Erythrocytes and HMEC-1 Cells
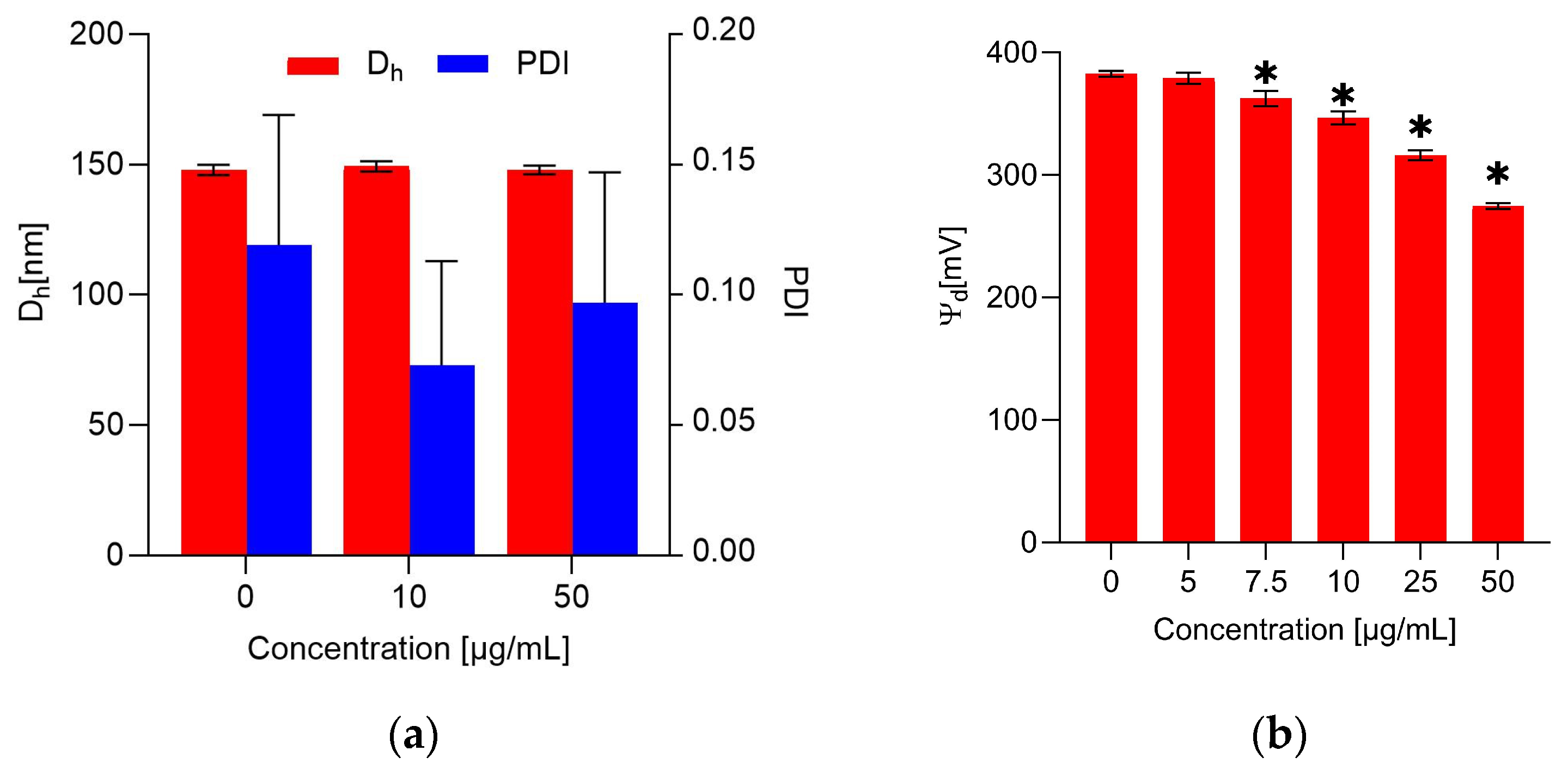
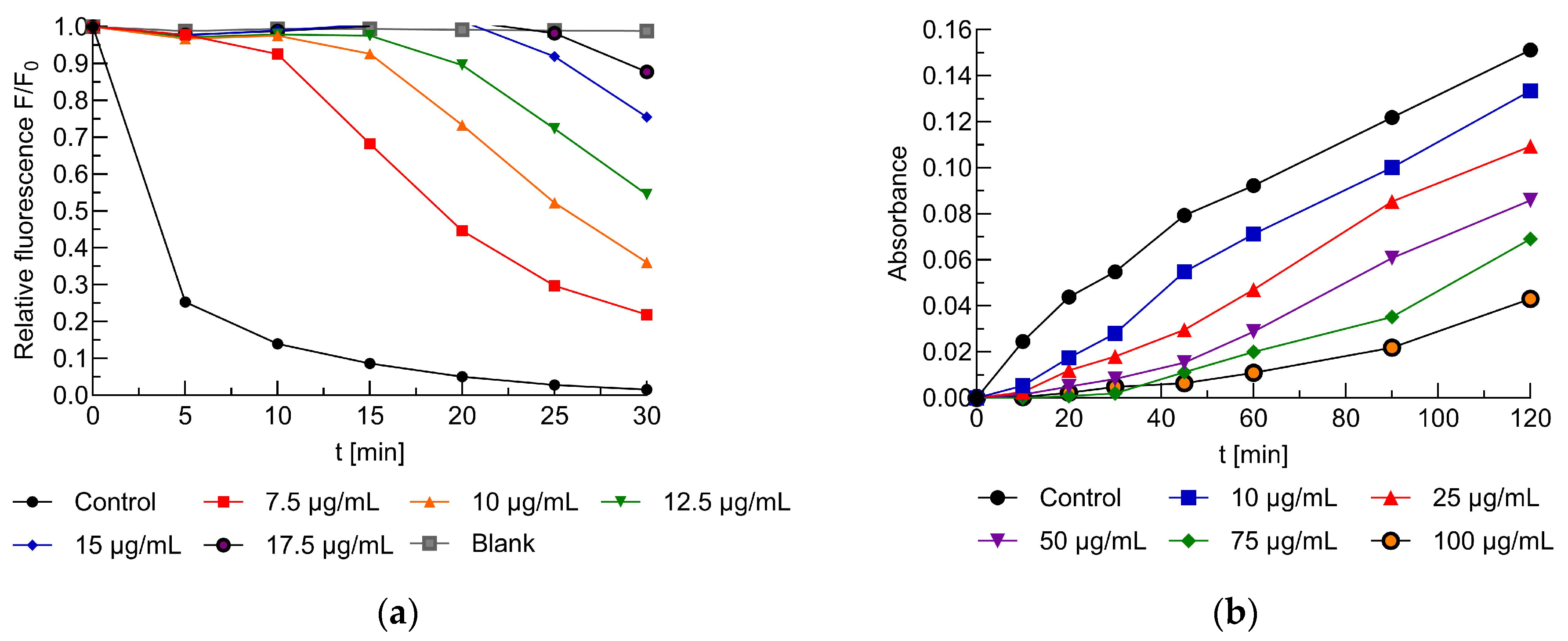
2.4. The Impact of the Extract on the Erythrocyte Membrane and Model Lipid Membrane
2.5. Antioxidant Activity of the Extracts
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Reagents
4.1.2. Plant Material and Extraction Procedure
4.1.3. Red Blood Cells, HMEC-1 Cells, and Model Membranes
4.2. Methods
4.2.1. Phenolic Content
UPLC-DAD, UPLC–ESI–MS
4.2.2. Antiradical Activity
4.2.3. Interaction of Extract with Erythrocytes and HMEC-1 Cells
Hemolysis and Osmotic Resistance of Erythrocytes
MTT Cytotoxicity Assay
Concentrations of Reduced Glutathione in Red Blood Cells Exposed to Free Radicals
Protection Against Free-Radical-Induced Hemolysis
Fluorometric Determination of Erythrocyte Transmembrane Potential
Microscopic Studies of Erythrocyte Shapes
4.2.4. The Impact of the Extract on the Erythrocyte Membrane and Model Lipid Membrane
Determination of the Hydrodynamic Diameter of Liposomes and the Polydispersity Index
Fluorometric Dipole Potential Measurement
Packing Order and Fluidity of the Membrane
4.2.5. Antioxidant Activity of the Extracts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omotayo, O.; Maduka, C.P.; Muonde, M.; Olorunsogo, T.O.; Ogugua, J.O. The rise of non-communicable diseases: A global health review of challenges and prevention strategies. Int. Med. Sci. Res. J. 2024, 4, 74–88. [Google Scholar] [CrossRef]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Cyboran-Mikołajczyk, S.; Solarska-Ściuk, K.; Mieszała, K.; Glatzel-Plucińska, N.; Matczak, K.; Kleszczyńska, H. The Impact of O-Glycosylation on Cyanidin Interaction with RBCs and HMEC-1 Cells—Structure–Activity Relationships. Int. J. Mol. Sci. 2019, 20, 1928. [Google Scholar] [CrossRef]
- Okamoto, K.; Maruyama, T.; Kaji, Y.; Harada, M.; Mawatari, S.; Fujino, T.; Uyesaka, N. Verapamil prevents impairment in filterability of human erythrocytes exposed to oxidative stress. Jpn. J. Physiol. 2004, 54, 39–46. [Google Scholar] [CrossRef]
- Skverchinskaya, E.A.; Tapinova, O.D.; Filatov, N.A.; Besedina, N.A.; Mindukshev, I.V.; Bukatin, A.S. Investigation of Erythrocyte Transport through Microchannels After the Induction of Oxidative Stress with Tert-Butyl Peroxide. Tech. Phys. 2020, 65, 1491–1496. [Google Scholar] [CrossRef]
- Bungau, S.; Popa, V.-C. Between Religion and Science: Some Aspects: Concerning Illness and Healing in Antiquity. Transylv. Rev. 2015, 24, 3–19. [Google Scholar]
- Chemjong, M.; Kumari Yadav, N.A.; Sarkate, A.; Yaqoob, M. Bioactive Compounds, Types, Stability and Health Benefits. Plant Arch. 2021, 21, 1863–1869. [Google Scholar] [CrossRef]
- Mondal, S.; Soumya, N.P.P.; Mini, S.; Sivan, S.K. Bioactive compounds in functional food and their role as therapeutics. Bioact. Compd. Health Dis. 2021, 4, 24. [Google Scholar] [CrossRef]
- Amato, A. Natural Compounds and Healthy Foods: Useful Tools against Onset and Progression of Chronic Diseases. Nutrients 2023, 15, 2898. [Google Scholar] [CrossRef]
- Kaźmierczak, T.; Cyboran-Mikołajczyk, S.; Trochanowska-Pauk, N.; Walski, T.; Nowicka, P.; Bonarska-Kujawa, D. Insights on the Mechanisms of the Protective Action of Naringenin, Naringin and Naringin Dihydrochalcone on Blood Cells in Terms of Their Potential Anti-Atherosclerotic Activity. Molecules 2025, 30, 547. [Google Scholar] [CrossRef] [PubMed]
- Tomou, E.-M.; Papakyriakopoulou, P.; Skaltsa, H.; Valsami, G.; Kadoglou, N.P.E. Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin. Molecules 2023, 28, 2387. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, D.; Różański, H. Equisetum arvense L. field horsetail (Equisetaceae Michx. ex DC.). Herbalism 2023, 9, 166–177. [Google Scholar] [CrossRef]
- Parameshwaran, K.; Almaghrabi, M.; Govindarajulu, M.; Clark, R.C.; Dhanasekaran, M. Bioactives and Pharmacology of Equisetum arvense L. In Bioactives and Pharmacology of Medicinal Plants; Apple Academic Press: Cambridge, MA, USA, 2022; ISBN 978-1-003-28170-2. [Google Scholar]
- Makia, R.; Sammarrae, K.W.A.; Halbosiy, M.M.A.; Mashhadani, M.H.A.; Makia, R.; Sammarrae, K.W.A.; Halbosiy, M.M.A.; Mashhadani, M.H.A. Pharmacology of the species Equisetum (Equisetum arvense). GSC Biol. Pharm. Sci. 2022, 18, 290–294. [Google Scholar] [CrossRef]
- Makia, R.; Al-Halbosiy, M.M.; Al-Mashhadani, M.H. Phytochemistry of the Genus Equisetum (Equisetum arvense). GSC Biol. Pharm. Sci. 2022, 18, 283–289. [Google Scholar] [CrossRef]
- Ng, C.X.; Affendi, M.M.; Chong, P.P.; Lee, S.H. The Potential of Plant-Derived Extracts and Compounds to Augment Anticancer Effects of Chemotherapeutic Drugs. Nutr. Cancer 2022, 74, 3058–3076. [Google Scholar] [CrossRef]
- Eslamiyan, F.; Mehrabiyan, S.; Majd, A. Evaluation of antimicrobial activity of aqueous extract, ethanol, methanol and ashes two species ramosissimum and telmateia of Equisetum arvense on several bacterial species and Yeast. Rep. Health Care 2015, 1, 120–123. [Google Scholar]
- Sravanthi Pammi, S.S.; Giri, A. Phytochemicals and Their Antimicrobial Activity: An Update on Their Mode of Action. Int. J. Clin. Exp. Med. Res. 2021, 5, 41–69. [Google Scholar] [CrossRef]
- Taylor, A.; Bonafos, R.; Chovelon, M.; Parvaud, C.E.; Furet, A.; Aveline, N.; Bertrand, C.; Marchand, P.A. Equisetum arvense (horsetail) Extract: The First Approved Basic Substance Allowed for EU Crop Protection. Int. J. Bio-Resour. Stress Manag. 2022, 13, 566–577. [Google Scholar]
- Roumili, I.; Cheniti, W.; Baghiani, A.; Charef, N.; Arrar, L. HPLC Analysis, Acute Toxicity and Assessment of Antioxidant and Antiinflammatory Capacity of Different Extracts of Equisetum arvense. EBSCOhost. Available online: https://openurl.ebsco.com/contentitem/doi:10.38150%2Fsajeb.12(3).p318-326?sid=ebsco:plink:crawler&id=ebsco:doi:10.38150%2Fsajeb.12(3).p318-326 (accessed on 27 November 2024).
- Bokelmann, J.M. 57 - Horsetail (Equisetum arvense): Above-Ground Parts. In Medicinal Herbs in Primary Care; Elsevier: Amsterdam, The Netherlands, 2022; pp. 457–461. ISBN 9780323846769. [Google Scholar] [CrossRef]
- Luanda, A.; Ripanda, A.; Makangara, J.J. Therapeutic potential of Equisetum arvense L. for management of medical conditions. Phytomedicine Plus 2023, 3, 100444. [Google Scholar] [CrossRef]
- Guilherme di Camillo Orfali, A.C.D.; Jo, F.B.M.P.; Priolli, A. Review of anticancer mechanisms of isoquercitin. World J. Clin. Oncol. 2016, 7, 189–199. [Google Scholar] [CrossRef]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Podolak, I. A Flavonoid on the Brain: Quercetin as a Potential Therapeutic Agent in Central Nervous System Disorders. Life 2022, 12, 591. [Google Scholar] [CrossRef]
- Michala, A.-S.; Pritsa, A. Quercetin: A Molecule of Great Biochemical and Clinical Value and Its Beneficial Effect on Diabetes and Cancer. Diseases 2022, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Wang, Y.; Zhang, S.; Wang, Y.; Zhang, J.; Cao, J.; Sun, L. Caffeoyl substitution changes the inhibition mode of tartaric acid against α-amylase: Analysis of the enzyme inhibition by four caffeic and tartaric acid derivates. LWT 2020, 133, 109942. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Simin, N.; Cvejic, J.; Jovin, E.; Orcic, D.; Bozin, B. Phenolic Compounds in Field Horsetail (Equisetum arvense L.) as Natural Antioxidants. Molecules 2008, 13, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Nosrati Gazafroudi, K.; Mailänder, L.K.; Daniels, R.; Kammerer, D.R.; Stintzing, F.C. From Stem to Spectrum: Phytochemical Characterization of Five Equisetum Species and Evaluation of Their Antioxidant Potential. Molecules 2024, 29, 2821. [Google Scholar] [CrossRef]
- Veit, M.; Geiger, H.; Czygan, F.-C.; Markham, K.R. Malonylated flavone 5-O-glucosides in the barren sprouts of Equisetum arvense. Phytochemistry 1990, 29, 2555–2560. [Google Scholar] [CrossRef]
- Glaser, R. Mechanisms of electromechanical coupling in membranes demonstrated by transmembrane potential-dependent shape transformations of human erythrocytes. Bioelectrochem. Bioenerg. 1993, 30, 103–109. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Pasławski, R.; Pasławska, U.; Nowak, K.; Płóciennik, M.; Męczarska, K.; Oszmiański, J.; Bonarska-Kujawa, D.; Kowalczyk, P.; Wawrzyńska, M. Comparison of Osmotic Resistance, Shape and Transmembrane Potential of Erythrocytes Collected from Healthy and Fed with High Fat-Carbohydrates Diet (HF-CD) Pigs—Protective Effect of Cistus incanus L. Extracts. Materials 2021, 14, 1050. [Google Scholar] [CrossRef]
- Watson, W.H.; Chen, Y.; Jones, D.P. Redox state of glutathione and thioredoxin in differentiation and apoptosis. BioFactors 2003, 17, 307–314. [Google Scholar] [CrossRef]
- Ishkaeva, R.A.; Zoughaib, M.; Laikov, A.V.; Angelova, P.R.; Abdullin, T.I. Probing Cell Redox State and Glutathione-Modulating Factors Using a Monochlorobimane-Based Microplate Assay. Antioxidants 2022, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.V.; van Hoek, A.; Visser, A.J.; Frank, J.; Apell, H.J.; Clarke, R.J. Time-resolved fluorescence investigations of the interaction of the voltage-sensitive probe RH421 with lipid membranes and proteins. Biochemistry 1995, 34, 11777–11784. [Google Scholar] [CrossRef]
- do Canto, A.M.T.M.; Robalo, J.R.; Santos, P.D.; Carvalho, A.J.P.; Ramalho, J.P.P.; Loura, L.M.S. Diphenylhexatriene membrane probes DPH and TMA-DPH: A comparative molecular dynamics simulation study. Biochim. Biophys. Acta BBA Biomembr. 2016, 1858, 2647–2661. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; De Stasio, G.; d’Ubaldo, A.; Gratton, E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 1990, 57, 1179–1186. [Google Scholar] [CrossRef]
- Leite, N.B.; Martins, D.B.; Alvares, D.S.; Cabrera, M.P.d.S. Quercetin induces lipid domain-dependent permeability. Chem. Phys. Lipids 2022, 242, 105160. [Google Scholar] [CrossRef]
- Han, J.; Amau, M.; Okamoto, Y.; Suga, K.; Umakoshi, H. Investigation of Quercetin interaction behaviors with lipid bilayers: Toward understanding its antioxidative effect within biomembrane. J. Biosci. Bioeng. 2021, 132, 49–55. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlęga, B.; Ignacy Gruszecki, W.; Misiak, L.; Paduch, R.; Piersiak, T.; Zarzyka, B.; Pawelec, J.; Gawron, A. Modification of membranes by quercetin, a naturally occurring flavonoid, via its incorporation in the polar head group. Biochim. Biophys. Acta BBA Biomembr. 2007, 1768, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska-Pawlęga, B.; Gruszecki, W.I.; Misiak, L.E.; Gawron, A. The study of the quercetin action on human erythrocyte membranes. Biochem. Pharmacol. 2003, 66, 605–612. [Google Scholar] [CrossRef]
- Strugała, P.; Tronina, T.; Huszcza, E.; Gabrielska, J. Bioactivity In Vitro of Quercetin Glycoside Obtained in Beauveria bassiana Culture and Its Interaction with Liposome Membranes. Mol. Basel Switz. 2017, 22, 1520. [Google Scholar] [CrossRef]
- Meleleo, D.; Avato, P.; Conforti, F.; Argentieri, M.P.; Messina, G.; Cibelli, G.; Mallamaci, R. Interaction of Quercetin, Cyanidin, and Their O-Glucosides with Planar Lipid Models: Implications for Their Biological Effects. Membranes 2023, 13, 600. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Pruchnik, H.; Oszmiański, J.; Sarapuk, J.; Kleszczyńska, H. Changes Caused by Fruit Extracts in the Lipid Phase of Biological and Model Membranes. Food Biophys. 2011, 6, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bonarska-Kujawa, D.; Cyboran-Mikołajczyk, S.; Kleszczyńska, H. Molecular mechanism of action of chlorogenic acid on erythrocyte and lipid membranes. Mol. Membr. Biol. 2015, 32, 46–54. [Google Scholar] [CrossRef]
- Alyasiri, T.; Hassan, A.A.; Adil, H.; Alsayed, R.; Makia, R.; Salman, H.; Kadhom, M.; Yousif, E. The antibacterial and antioxidant activities of combined Equisetum arvense extract with TiO2 nanoparticles in PMAA films. Results Chem. 2024, 11, 101829. [Google Scholar] [CrossRef]
- Dormousoglou, M.; Efthimiou, I.; Antonopoulou, M.; Fetzer, D.L.; Hamerski, F.; Corazza, M.L.; Papadaki, M.; Santzouk, S.; Dailianis, S.; Vlastos, D. Investigation of the genotoxic, antigenotoxic and antioxidant profile of different extracts from Equsetum arvense L. Antioxidants 2022, 11, 1393. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abo-EL-Sooud, K.; Aleya, L.; Bungǎu, S.G.; Najda, A.; Saluja, R. Alleviation of Drugs and Chemicals Toxicity: Biomedical Value of Antioxidants. Oxid. Med. Cell. Longev. 2018, 2018, 6276438. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Zakhary, N.I.; Aleya, L.; Bungǎu, S.G.; Bohara, R.A.; Siddiqi, N.J. Aging, Metabolic, and Degenerative Disorders: Biomedical Value of Antioxidants. Oxid. Med. Cell. Longev. 2018, 2018, 2098123. [Google Scholar] [CrossRef] [PubMed]
- Pallag, A.; Bungau, S.; Tit, D.M.; Jurca, T.; Sirbu, V.; Honiges, A.; Horhogea, C. Comparative Study of Polyphenols, Flavonoids and Chlorophylls in Equisetum arvense L. Populations. Rev. Chim. 2016, 67, 530–533. [Google Scholar]
- Gasiorowski, K.; Szyba, K.; Brokos, B.; Kołaczyńska, B.; Jankowiak-Włodarczyk, M.; Oszmiański, J. Antimutagenic Activity of Anthocyanins Isolated from Aronia Melanocarpa Fruits. Cancer Lett. 1997, 119, 37–46. [Google Scholar] [CrossRef]
- Solarska-Ściuk, K.; Adach, K.; Cyboran-Mikołajczyk, S.; Bonarska-Kujawa, D.; Rusak, A.; Cwynar-Zając, Ł.; Machałowski, T.; Jesionowski, T.; Grzywacz, K.; Fijałkowski, M. Are Biogenic and Pyrogenic Mesoporous SiO2 Nanoparticles Safe for Normal Cells? Mol. Basel Switz. 2021, 26, 1427. [Google Scholar] [CrossRef]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Maddy, A.H.; Dunn, M.J.; Kelly, P.G. The characterisation of membrane proteins by centrifugation and gel electrophoresis a comparison of proteins prepared by different methods. Biochim. Biophys. Acta BBA Biomembr. 1972, 288, 263–276. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Męczarska, K.; Cyboran-Mikołajczyk, S.; Włoch, A.; Bonarska-Kujawa, D.; Oszmiański, J.; Kleszczyńska, H. Polyphenol content and bioactivity of Saskatoon (Amelanchier alnifolia Nutt.) leaves and berries. Acta Pol. Pharm. 2017, 74, 660–669. [Google Scholar]
- Cyboran-Mikołajczyk, S.; Matczak, K.; Olchowik-Grabarek, E.; Sękowski, S.; Nowicka, P.; Krawczyk-Łebek, A.; Kostrzewa-Susłow, E. The influence of the chlorine atom on the biological activity of 2′-hydroxychalcone in relation to the lipid phase of biological membranes–Anticancer and antimicrobial activity. Chem. Biol. Interact. 2024, 398, 111082. [Google Scholar] [CrossRef]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.; Gratton, E. Laurdan and Prodan as Polarity-Sensitive Fluorescent Membrane Probes; Springer: Berlin/Heidelberg, Germany, 1998; Volume 8. [Google Scholar]
- Lakowicz, J.R. Fluorescence Polarization. In Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 1983; pp. 111–153. ISBN 978-1-4615-7658-7. [Google Scholar]

| Compounds | Content mg/g | Rt min | λmax nm | Molecular ion [M-H]− m/z | MS2 m/z |
|---|---|---|---|---|---|
| Caffeoyl tartrate isomer | 3.66 | 3.47 | 327 | 311 | 179 |
| Caffeoyl tartrate isomer | 50.34 | 3.57 | 327 | 311 | 179 |
| Caffeoylshikimic acid isomer Kaempherol diglycoside | 3.81 | 4.96 | 327 | 335 | 179 |
| Quercetin dihexoside | 4.91 | 5.03 | 342 | 609 | 285 |
| Quercetin-glucoside | 24.88 | 5.76 | 350 | 625 | 301 |
| Quercetin-3-O-6-acetylglucoside isomer | 22.89 | 7.32 | 351 | 463 | 301 |
| Quercetin-3-O-6-acetylglucoside isomer | 81.58 | 7.95 | 353 | 505 | 301 |
| Quercetin-3-O-6-acetylglucoside isomer | 3.83 | 8.32 | 353 | 505 | 301 |
| Kaempferol-3-O-6-acetylglucoside | 1.23 | 8.96 | 353 | 505 | 301 |
| Dicaffeoyl tartaric acid isomer | 1.31 | 9.32 | 340 | 489 | 285 |
| Dicaffeoyl tartaric acid isomer | 15.92 | 9.55 | 328 | 473 | 179 |
| Total | 219.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Męczarska, K.; Cyboran-Mikołajczyk, S.; Solarska-Ściuk, K.; Oszmiański, J.; Siejak, K.; Bonarska-Kujawa, D. Protective Effect of Field Horsetail Polyphenolic Extract on Erythrocytes and Their Membranes. Int. J. Mol. Sci. 2025, 26, 3213. https://doi.org/10.3390/ijms26073213
Męczarska K, Cyboran-Mikołajczyk S, Solarska-Ściuk K, Oszmiański J, Siejak K, Bonarska-Kujawa D. Protective Effect of Field Horsetail Polyphenolic Extract on Erythrocytes and Their Membranes. International Journal of Molecular Sciences. 2025; 26(7):3213. https://doi.org/10.3390/ijms26073213
Chicago/Turabian StyleMęczarska, Katarzyna, Sylwia Cyboran-Mikołajczyk, Katarzyna Solarska-Ściuk, Jan Oszmiański, Katarzyna Siejak, and Dorota Bonarska-Kujawa. 2025. "Protective Effect of Field Horsetail Polyphenolic Extract on Erythrocytes and Their Membranes" International Journal of Molecular Sciences 26, no. 7: 3213. https://doi.org/10.3390/ijms26073213
APA StyleMęczarska, K., Cyboran-Mikołajczyk, S., Solarska-Ściuk, K., Oszmiański, J., Siejak, K., & Bonarska-Kujawa, D. (2025). Protective Effect of Field Horsetail Polyphenolic Extract on Erythrocytes and Their Membranes. International Journal of Molecular Sciences, 26(7), 3213. https://doi.org/10.3390/ijms26073213









