Facing Foodborne Pathogen Biofilms with Green Antimicrobial Agents: One Health Approach
Abstract
:1. Introduction
1.1. The Importance of Food Safety and the Challenge of Bacterial Biofilms
1.2. Green Antimicrobial Agents as a Potential Solution
2. Bacterial Biofilms
2.1. Definition and Characteristics of Bacterial Biofilms
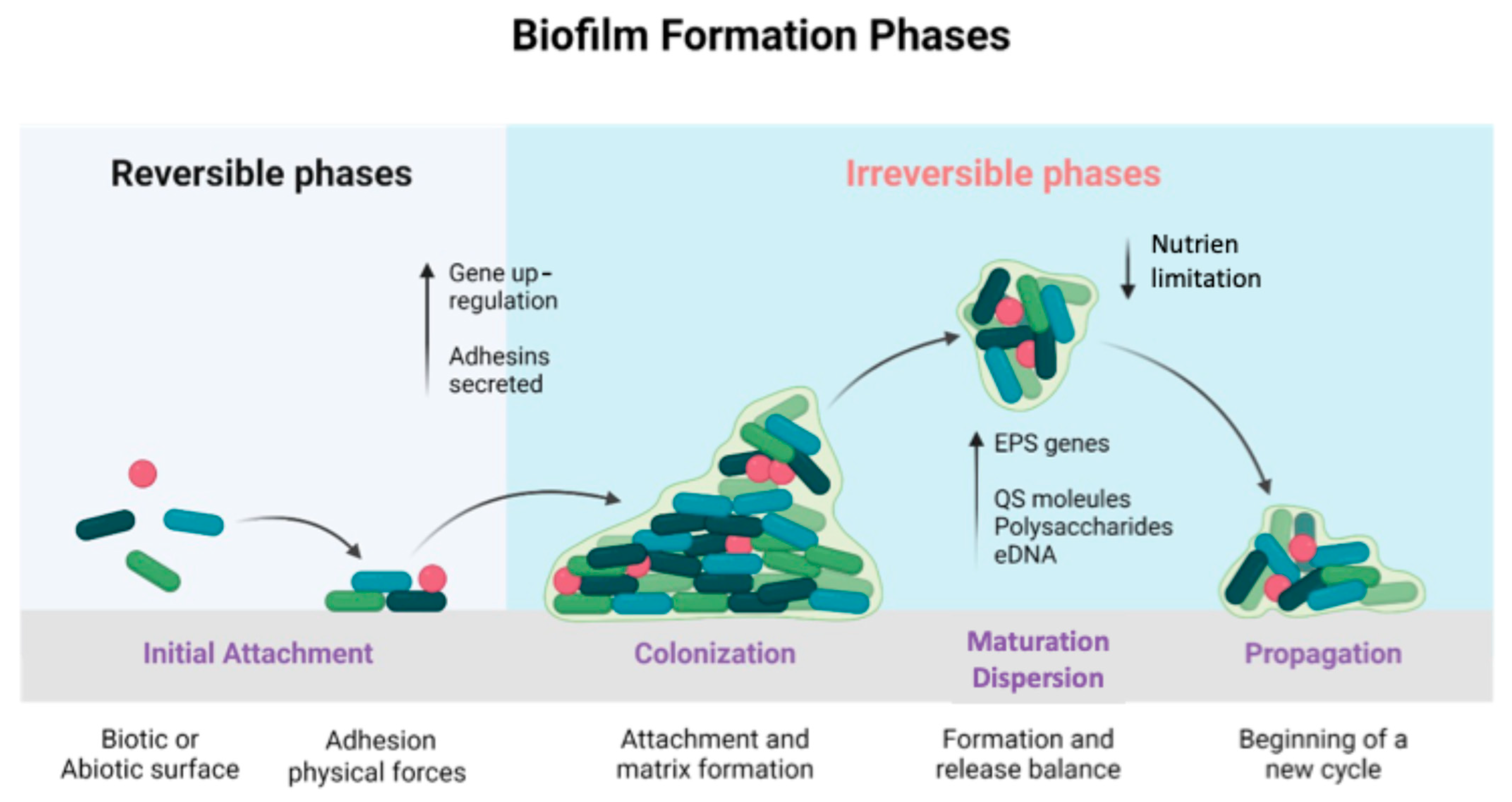
2.2. Formation, Structure, and Functions of Biofilms in Food Environments
2.3. Challenges Posed by Bacterial Biofilms in Food Safety
2.4. Antibiotic Resistance in Bacterial Biofilms
2.5. Mechanisms of Antibiotic Resistance in Biofilms
Key Mechanisms of Antibiotic Resistance in Biofilms
3. Novel Strategies and Mechanisms of Action: Green Antimicrobial Agents
3.1. Definition and Types of Green Antimicrobial Agents
3.2. Bacteriophages as Antimicrobial Agents
3.2.1. Mechanisms of Bacteriophages in Biofilm Control
3.2.2. Phage-Derived Enzymes as Antibiofilm Agents and Role in Biofilm Degradation
3.3. Plant Extracts: Essential Oils as Potential Antimicrobial Agents
3.4. Combining Green Antimicrobial Agents for Biofilm Control
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO; WHO. Food Control System Assessment Tool: Introduction and Glossary; Food Safety and Quality Series No. 7/1; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; ISBN 9789251316306. [Google Scholar]
- Nyarugwe, S.P.; Linnemann, A.R.; Ren, Y.; Bakker, E.J.; Kussaga, J.B.; Watson, D.; Fogliano, V.; Luning, P.A. An Intercontinental Analysis of Food Safety Culture in View of Food Safety Governance and National Values. Food Control 2020, 111, 107075. [Google Scholar] [CrossRef]
- Toushik, S.H.; Mizan, M.F.R.; Hossain, M.I.; Ha, S. Do Fighting with Old Foes: The Pledge of Microbe-Derived Biological Agents to Defeat Mono- and Mixed-Bacterial Biofilms Concerning Food Industries. Trends Food Sci. Technol. 2020, 99, 413–425. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Tao, Q.; Wu, Q.; Zhang, Z.; Liu, J.; Tian, C.; Huang, Z.; Malakar, P.K.; Pan, Y.; Zhao, Y. Meta-Analysis for the Global Prevalence of Foodborne Pathogens Exhibiting Antibiotic Resistance and Biofilm Formation. Front. Microbiol. 2022, 13, 906490. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef]
- Quested, T.E.; Cook, P.E.; Gorris, L.G.M.; Cole, M.B. Trends in Technology, Trade and Consumption Likely to Impact on Microbial Food Safety. Int. J. Food Microbiol. 2010, 139, S29–S42. [Google Scholar] [CrossRef]
- Spink, J.; Ortega, D.L.; Chen, C.; Wu, F. Food Fraud Prevention Shifts the Food Risk Focus to Vulnerability. Trends Food Sci. Technol. 2017, 62, 215–220. [Google Scholar] [CrossRef]
- Winkelstroter, L.K. Microbial Biofilms: The Challenge of Food Industry. Biochem. Mol. Biol. J. 2015, 1, 93227. [Google Scholar] [CrossRef]
- Bai, X.; Nakatsu, C.H.; Bhunia, A.K. Bacterial Biofilms and Their Implications in Pathogenesis and Food Safety. Foods 2021, 10, 2117. [Google Scholar] [CrossRef]
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef]
- Jacques, M.; Malouin, F. One Health-One Biofilm. Vet. Res. 2022, 53, 51. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M. Bacteriophages and the One Health Approach to Combat Multidrug Resistance: Is This the Way? Antibiotics 2020, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Adeeb, S.; Ali, M. Green Anti-Biofilm Agents: A Mini-Review. Indian J. Appl. Res. 2020, 10, 36106. [Google Scholar] [CrossRef]
- Lahiri, D.; Mukherjee, I.; Ghosh, S.; Biswas, S.; Nag, M.; Ray, R.R. Green-Synthesized Silver Nanoparticle as Effective Antibiofilm Agent. Am. J. Appl. Bio-Technol. Res. 2020, 1, 1–15. [Google Scholar] [CrossRef]
- Das, P.; Karankar, V.S. New Avenues of Controlling Microbial Infections through Anti-Microbial and Anti-Biofilm Potentials of Green Mono-and Multi-Metallic Nanoparticles: A Review. J. Microbiol. Methods 2019, 167, 105766. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Ullah, S.; Ahmad, I.; Qureshi, A.K.; Balkhair, K.S.; Abdur Rehman, M. Green Biocides, a Promising Technology: Current and Future Applications to Industry and Industrial Processes. J. Sci. Food Agric. 2014, 94, 388–403. [Google Scholar] [CrossRef]
- Rahmani, R.; Zarrini, G.; Sheikhzadeh, F.; Aghamohammadzadeh, N. Effective Phages as Green Antimicrobial Agents against Antibiotic-Resistant Hospital Escherichia coli. Jundishapur J. Microbiol. 2015, 8, e17744. [Google Scholar] [CrossRef]
- Monk, A.B.; Rees, C.D.; Barrow, P.; Hagens, S.; Harper, D.R. Bacteriophage Applications: Where Are We Now? Lett. Appl. Microbiol. 2010, 51, 260–270. [Google Scholar] [CrossRef]
- Kakasis, A.; Panitsa, G. Bacteriophage Therapy as an Alternative Treatment for Human Infections. A Comprehensive Review. Int. J. Antimicrob. Agents 2019, 53, 16–21. [Google Scholar] [CrossRef]
- Golkar, Z.; Bagasra, O.; Gene Pace, D. Bacteriophage Therapy: A Potential Solution for the Antibiotic Resistance Crisis. J. Infect. Dev. Ctries. 2014, 8, 129–136. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Garvey, M. Bacteriophages and Food Production: Biocontrol and Bio-Preservation Options for Food Safety. Antibiotics 2022, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.G.; El-Dougdoug, N.K. Controlling Foodborne Pathogens with Natural Antimicrobials by Biological Control and Antivirulence Strategies. Heliyon 2020, 6, e0520. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Song, X.; Chen, M.; Tian, S.; Lu, Z.; Sun, J.; Li, X.; Lu, Y.; Yuk, H.G. Combating Biofilms of Foodborne Pathogens with Bacteriocins by Lactic Acid Bacteria in the Food Industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1657–1676. [Google Scholar] [CrossRef]
- Bai, X.; Chen, X.; Zhang, D.; Liu, X.; Li, J. Targeted Phytogenic Compounds against Vibrio Parahaemolyticus Biofilms. Crit. Rev. Food Sci. Nutr. 2024, 65, 1761–1772. [Google Scholar] [CrossRef]
- Liu, C.M.; Aziz, M.; Park, D.E.; Wu, Z.; Stegger, M.; Li, M.; Wang, Y.; Schmidlin, K.; Johnson, T.J.; Koch, B.J.; et al. Using Source-Associated Mobile Genetic Elements to Identify Zoonotic Extraintestinal E. coli Infections. One Health 2023, 16, 100518. [Google Scholar] [CrossRef] [PubMed]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the Structure and Contribution towards Bacterial Resistance in Antibiotics. Med. Microecol. 2023, 16, 100518. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, S.Y. Review: Comparison of the Effectiveness of Decontaminating Strategies for Fresh Fruits and Vegetables and Related Limitations. Crit. Rev. Food Sci. Nutr. 2018, 58, 3189–3208. [Google Scholar] [CrossRef]
- Marić, S.; Vraneš, J. Characteristics and Significance of Microbial Biofilm Formation. Period. Biol. 2007, 109, 115–121. [Google Scholar]
- Stewart, P.S. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015, 3, 10-1128. [Google Scholar] [CrossRef]
- Svircev, A.; Roach, D.; Castle, A. Framing the Future with Bacteriophages in Agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Phillips, C.A. Bacterial Biofilms in Food Processing Environments: A Review of Recent Developments in Chemical and Biological Control. Int. J. Food Sci. Technol. 2016, 51, 1731–1743. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Rayner, J.; Veeh, R.; Flood, J. Prevalence of Microbial Biofilms on Selected Fresh Produce and Household Surfaces. Int. J. Food Microbiol. 2004, 95, 29–39. [Google Scholar] [CrossRef]
- Blaschek, H.P.; Wang, H.H.; Agle, M.E. Biofilms in the Food Environment; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Goel, N.; Fatima, S.W.; Kumar, S.; Sinha, R.; Khare, S.K. Antimicrobial Resistance in Biofilms: Exploring Marine Actinobacteria as a Potential Source of Antibiotics and Biofilm Inhibitors. Biotechnol. Rep. 2021, 30, e00613. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Tilahun, A.; Haddis, S.; Teshale, A.; Hadush, T. Review on Biofilm and Microbial Adhesion. Int. J. Microbiol. Res. 2016, 7, 63–73. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Parsek, M.R. New Insight into the Early Stages of Biofilm Formation. Proc. Natl. Acad. Sci. USA 2018, 115, 4317–4319. [Google Scholar] [CrossRef]
- Speziale, P.; Geoghegan, J.A. Biofilm Formation by Staphylococci and Streptococci: Structural, Functional, and Regulatory Aspects and Implications for Pathogenesis. Front. Cell Infect. Microbiol. 2015, 5, 31. [Google Scholar] [CrossRef]
- Nasser, A.; Dallal, M.M.S.; Jahanbakhshi, S.; Azimi, T.; Nikouei, L. Staphylococcus Aureus: Biofilm Formation and Strategies Against It. Curr. Pharm. Biotechnol. 2021, 23, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public. Health 2021, 18, 7100. [Google Scholar] [CrossRef]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. How Bacteria Stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef]
- Visnapuu, A.; Der Gucht, M.; Wagemans, J.; Lavigne, R. Deconstructing the Phage–Bacterial Biofilm Interaction as a Basis to Establish New Antibiofilm Strategies. Viruses 2022, 14, 1057. [Google Scholar] [CrossRef]
- Stinson, K.J. Peering into the Matrix: A Look at Biofilms and Their Inherent Antibiotic Resistance. SURG J. 2013, 6, 86–95. [Google Scholar] [CrossRef]
- Mah, T.F. Biofilm-Specific Antibiotic Resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Anderson, G.G.; O’Toole, G.A. Innate and Induced Resistance Mechanisms of Bacterial Biofilms. In Bacterial Biofilms; Springer: Berlin/Heidelberg, Germany, 2008; pp. 85–105. [Google Scholar]
- Dincer, S.; Uslu, F.M.; Delik, A. Antibiotic Resistance In Bacterial Biofilms; Humana Press: New York, NY, USA, 2020. [Google Scholar]
- Zeineldin, M.; Esmael, A.; Al-Hindi, R.R.; Alharbi, M.G.; Ashenafi Bekele, D.; Teklemariam, A.D. Beyond the Risk of Biofilms: An Up-and-Coming Battleground of Bacterial Life and Potential Antibiofilm Agents. Life 2023, 13, 503. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular Mechanisms of Biofilm-Based Antibiotic Resistance and Tolerance in Pathogenic Bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Rodis, N.; Tsapadikou, V.K.; Potsios, C.; Xaplanteri, P. A Review on Resistance Mechanisms in Bacterial Biofilm Formations. Recent Dev. Med. Med. Res. 2021, 3, 7–18. [Google Scholar]
- Stewart, P.S. Mechanisms of Antibiotic Resistance in Bacterial Biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Mazza, M.G. The Physics of Biofilms—An Introduction. J. Phys. D Appl. Phys. 2016, 49, 203001. [Google Scholar] [CrossRef]
- Flores-Encarnacion, M.; Nava-Nolazco, R.M.; Aguilar-Gutierrez, G.R.; Carreno-Lopez, R.; Carreno-Lóopez, R. The Effect of Thymus Vulgaris on Growth and Biofilm Formation of Uropathogenic Escherichia coli. Afr. J. Microbiol. Res. 2018, 12, 237–242. [Google Scholar] [CrossRef]
- Gan, T.; Gong, X. Characterization of the Physical Properties of Biofilms. Shengwu Gongcheng Xuebao/Chin. J. Biotechnol. 2017, 33, 1390–1399. [Google Scholar] [CrossRef]
- Kesel, S.; Grumbein, S.; Gümperlein, I.; Tallawi, M.; Marel, A.K.; Lieleg, O.; Opitz, M. Direct Comparison of Physical Properties of Bacillus Subtilis NCIB 3610 and B-1 Biofilms. Appl. Environ. Microbiol. 2016, 82, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Wentland, E.J.; Stewart, P.S.; Huang, C.T.; McFeters, G.A. Spatial Variations in Growth Rate within Klebsiella Pneumoniae Colonies and Biofilm. Biotechnol. Prog. 1996, 12, 316–321. [Google Scholar] [CrossRef]
- Roy, V.; Adams, B.L.; Bentley, W.E. Developing next Generation Antimicrobials by Intercepting AI-2 Mediated Quorum Sensing. Enzyme Microb. Technol. 2011, 49, 113–123. [Google Scholar] [CrossRef] [PubMed]
- France, M.T.; Ridenhour, B.J.; Forney, L.J. Effects of Spatial Structure and Reduced Growth Rates on Evolution in Bacterial Populations. In Grand. Challenges in Biology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Schembri, M.A.; Kjærgaard, K.; Klemm, P. Global Gene Expression in Escherichia coli Biofilms. Mol. Microbiol. 2003, 48, 253–267. [Google Scholar] [CrossRef]
- Resch, A.; Rosenstein, R.; Nerz, C.; Götz, F. Differential Gene Expression Profiling of Staphylococcus Aureus Cultivated under Biofilm and Planktonic Conditions. Appl. Environ. Microbiol. 2005, 71, 2663–2676. [Google Scholar] [CrossRef]
- Shemesh, M.; Tam, A.; Steinberg, D. Differential Gene Expression Profiling of Streptococus Mutans Cultured under Biofilm and Planktonic Conditions. Microbiology 2007, 153, 1307–1317. [Google Scholar] [CrossRef]
- Prigent-Combaret, C.; Vidal, O.; Dorel, C.; Lejeune, P. Abiotic Surface Sensing and Biofilm-Dependent Regulation of Gene Expression in Escherichia coli. J. Bacteriol. 1999, 181, 5993–6002. [Google Scholar] [CrossRef]
- Conlon, B.P.; Rowe, S.E.; Lewis, K. Persister Cells in Biofilm Associated Infections; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–9. [Google Scholar]
- Wuyts, J.; Van Dijck, P.; Holtappels, M. Fungal Persister Cells: The Basis for Recalcitrant Infections? PLoS Pathog. 2018, 14, e1007301. [Google Scholar] [CrossRef]
- Abrantes, J.A.; da Rocha Nogueira, J.M. Biofilme e Células Persisters: Da Persistência à Resistência Microbiana. Rev. Bras. Análises Clínicas 2022, 54, 2448–3877. [Google Scholar] [CrossRef]
- Roberts, M.E.; Stewart, P.S. Modelling Protection from Antimicrobial Agents in Biofilms through the Formation of Persister Cells. Microbiology 2005, 151, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Chen, Z.; Allan, E.; van der Mei, H.C.; Busscher, H.J. Emergent Heterogeneous Microenvironments in Biofilms: Substratum Surface Heterogeneity and Bacterial Adhesion Force-Sensing. FEMS Microbiol. Rev. 2018, 42, 259–272. [Google Scholar] [CrossRef]
- Martin, M.; Dragoš, A.; Otto, S.B.; Schäfer, D.; Brix, S.; Maróti, G.; Kovács, Á.T. Cheaters Shape the Evolution of Phenotypic Heterogeneity in Bacillus Subtilis Biofilms. ISME J. 2020, 14, 2302–2312. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Flint, S.; Li, Y.J.; Ou, K.; Yuan, L.; He, G.Q. Phenotypic and Genetic Heterogeneity within Biofilms with Particular Emphasis on Persistence and Antimicrobial Tolerance. Future Microbiol. 2017, 12, 1087–1107. [Google Scholar] [CrossRef]
- Obando, M.C.; Serra, D.O. Dissecting Cell Heterogeneities in Bacterial Biofilms and Their Implications for Antibiotic Tolerance. Curr. Opin. Microbiol. 2024, 78, 102450. [Google Scholar] [CrossRef]
- Vadakkan, K.; Choudhury, A.A.; Gunasekaran, R.; Hemapriya, J.; Vijayanand, S. Quorum Sensing Intervened Bacterial Signaling: Pursuit of Its Cognizance and Repression. J. Genet. Eng. Biotechnol. 2018, 16, 239–252. [Google Scholar] [CrossRef]
- Pan, J.; Ren, D. Quorum Sensing Inhibitors: A Patent Overview. Expert. Opin. Ther. Pat. 2009, 19, 1581–1601. [Google Scholar] [CrossRef]
- Maha Swetha, B.R.; Saravanan, M.; Piruthivraj, P. Emerging Trends in the Inhibition of Bacterial Molecular Communication: An Overview. Microb. Pathog. 2024, 186, 106495. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory Mechanisms and Promising Applications of Quorum Sensing-Inhibiting Agents in Control of Bacterial Biofilm Formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Das, A.; Mallik, S. Inhibition of Quorum Sensing in Pseudomonas Aeruginosa: A Review. Indian. J. Pharm. Sci. 2019, 81, 797–806. [Google Scholar] [CrossRef]
- Zhao, Y.; An, J.; Su, H.; Li, B.; Liang, D.; Huang, C. Antimicrobial Food Packaging Integrating Polysaccharide-Based Substrates with Green Antimicrobial Agents: A Sustainable Path. Food Res. Int. 2022, 155, 111096. [Google Scholar] [CrossRef]
- Sharma, D.; Shandilya, P.; Saini, N.K.; Singh, P.; Thakur, V.K.; Saini, R.V.; Mittal, D.; Chandan, G.; Saini, V.; Saini, A.K. Insights into the Synthesis and Mechanism of Green Synthesized Antimicrobial Nanoparticles, Answer to the Multidrug Resistance. Mater. Today Chem. 2021, 19, 100391. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-álvarez, J.; Vega-Fernández, L.; de Oca-Vásquez, G.M.; Vega-Baudrit, J.R. Green Synthesis of Gold and Silver Nanoparticles from Plant Extracts and Their Possible Applications as Antimicrobial Agents in the Agricultural Area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Talapko, J.; Škrlec, I. The Principles, Mechanisms, and Benefits of Unconventional Agents in the Treatment of Biofilm Infection. Pharmaceuticals 2020, 13, 299. [Google Scholar] [CrossRef]
- Wani, A.; Mange, H.; Vasudevan, A. Essential Oils: A Novel Approach for Anti-Microbial Therapy. Nat. Prod. J. 2022, 12, 1. [Google Scholar] [CrossRef]
- Chao, S.C.; Young, D.G.; Oberg, C.J. Screening for Inhibitory Activity of Essential Oils on Selected Bacteria, Fungi and Viruses. J. Essent. Oil Res. 2000, 12, 639–649. [Google Scholar] [CrossRef]
- Leyva-l, N.; Guti, E.P.; Vazquez-olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Gajic, I.; Kekic, D.; Jankovic, M.; Tomic, N.; Skoric, M.; Petrovic, M.; Mitic Culafic, D.; Opavski, N.; Ristivojevic, P.; Krstic Ristivojevic, M.; et al. Nature’s Arsenal: Uncovering Antibacterial Agents Against Antimicrobial Resistance. Antibiotics 2025, 14, 253. [Google Scholar] [CrossRef]
- Panda, S.K.; Buroni, S.; Swain, S.S.; Bonacorsi, A.; da Fonseca Amorim, E.A.; Kulshrestha, M.; da Silva, L.C.N.; Tiwari, V. Recent Advances to Combat ESKAPE Pathogens with Special Reference to Essential Oils. Front. Microbiol. 2022, 13, 1029098. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; De Biasi, M.G.; Mancusi, A. An Overview of the Potentialities of Antimicrobial Peptides Derived from Natural Sources. Antibiotics 2022, 11, 1483. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Priyadarshi, R.; Zhang, W.; Khezerlou, A.; Rhim, J.-W. Innovative Application of Laccase Enzyme in Food Packaging. Trends Food Sci. Technol. 2024, 151, 104623. [Google Scholar] [CrossRef]
- Lopes, B.S.; Hanafiah, A.; Nachimuthu, R.; Muthupandian, S.; Md Nesran, Z.N.; Patil, S. The Role of Antimicrobial Peptides as Antimicrobial and Antibiofilm Agents in Tackling the Silent Pandemic of Antimicrobial Resistance. Molecules 2022, 27, 2995. [Google Scholar] [CrossRef]
- Fernandes, S.; Gomes, I.B.; Simões, M.; Simões, L.C. Novel Chemical-Based Approaches for Biofilm Cleaning and Disinfection. Curr. Opin. Food Sci. 2024, 55, 101124. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Wang, Z.; Wei, J.; Hu, T.; Si, J.; Tao, G.; Zhang, L.; Xie, L.; Abdalla, A.E.; et al. A Combination Therapy of Phages and Antibiotics: Two Is Better than One. Int. J. Biol. Sci. 2021, 17, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Kim, Y.T.; Ryu, S.; Lee, J.H. Biocontrol and Rapid Detection of Food-Borne Pathogens Using Bacteriophages and Endolysins. Front. Microbiol. 2016, 7, 474. [Google Scholar] [CrossRef]
- Tan, L.-H.; Chan, K.-G.; Lee, L.-H. Application of Bacteriophage in Biocontrol of Major Foodborne Bacterial Pathogens. J. Mol. Biol. Mol. Imaging 2014, 1, 4658187. [Google Scholar]
- Aprea, G.; Zocchi, L.; Di Fabio, M.; De Santis, S.; Prencipe, V.A.; Migliorati, G. The Applications of Bacteriophages and Their Lysins as Biocontrol Agents against the Foodborne Pathogens Listeria Monocytogenes and Campylobacter: An Updated Look. Vet. Ital. 2018, 54, 293–303. [Google Scholar]
- Bhardwaj, N.; Bhardwaj, S.K.; Deep, A.; Dahiya, S.; Kapoor, S. Lytic Bacteriophages as Biocontrol Agents of Foodborne Pathogens. Asian J. Anim. Vet. Adv 2015, 10, 708–723. [Google Scholar] [CrossRef]
- Oluwarinde, B.O.; Ajose, D.J.; Abolarinwa, T.O.; Montso, P.K.; Du Preez, I.; Njom, H.A.; Ateba, C.N. Safety Properties of Escherichia coli O157:H7 Specific Bacteriophages: Recent Advances for Food Safety. Foods 2023, 12, 3989. [Google Scholar] [CrossRef]
- Romero-Calle, D.; Benevides, R.G.; Góes-Neto, A.; Billington, C. Bacteriophages as Alternatives to Antibiotics in Clinical Care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Brives, C.; Pourraz, J. Phage Therapy as a Potential Solution in the Fight against AMR: Obstacles and Possible Futures. Palgrave Commun. 2020, 6, 100. [Google Scholar] [CrossRef]
- Tian, S.; van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Recent Advances and Future Challenges in the Use of Nanoparticles for the Dispersal of Infectious Biofilms. J. Mater. Sci. Technol. 2021, 84, 208–218. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial Exo-Polysaccharides in Biofilms: Role in Antimicrobial Resistance and Treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Parasion, S.; Kwiatek, M.; Gryko, R.; Mizak, L.; Malm, A. Bacteriophages as an Alternative Strategy for Fighting Biofilm Development. Pol. J. Microbiol. 2014, 63, 137–145. [Google Scholar] [CrossRef]
- Danis-Wlodarczyk, K.M.; Wozniak, D.J.; Abedon, S.T. Treating Bacterial Infections with Bacteriophage-Based Enzybiotics: In Vitro, in Vivo and Clinical Application. Antibiotics 2014, 63, 137–145. [Google Scholar] [CrossRef]
- Pires, D.P.; Oliveira, H.; Melo, L.D.R.; Sillankorva, S.; Azeredo, J. Bacteriophage-Encoded Depolymerases: Their Diversity and Biotechnological Applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef]
- Kaplan, J.B. Therapeutic Potential of Biofilm-Dispersing Enzymes. Int. J. Artif. Organs 2009, 32, 545–554. [Google Scholar] [CrossRef]
- Pei, R.; Lamas-Samanamud, G.R. Inhibition of Biofilm Formation by T7 Bacteriophages Producing Quorum-Quenching Enzymes. Appl. Environ. Microbiol. 2014, 80, 5340–5348. [Google Scholar] [CrossRef]
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial Biofilm Destruction: A Focused Review on the Recent Use of Phage-Based Strategies with Other Antibiofilm Agents. Nanotechnol. Sci. Appl. 2021, 14, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, J.; García, P.; Drulis-Kawa, Z. Targeting Biofilms Using Phages and Their Enzymes. Curr. Opin. Biotechnol. 2021, 68, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Melo, L.D.R.; Vilas Boas, D.; Sillankorva, S.; Azeredo, J. Phage Therapy as an Alternative or Complementary Strategy to Prevent and Control Biofilm-Related Infections. Curr. Opin. Microbiol. 2017, 39, 48–56. [Google Scholar] [CrossRef]
- Mittal, R.P.; Rana, A.; Jaitak, V. Essential Oils: An Impending Substitute of Synthetic Antimicrobial Agents to Overcome Antimicrobial Resistance. Curr. Drug Targets 2019, 20, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Chen, B.; McClements, D.J. Improving the Efficacy of Essential Oils as Antimicrobials in Foods: Mechanisms of Action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Q.; Zhang, D.; Yan, W. Mechanisms and Control Strategies of Antibiotic Resistance in Pathological Biofilms. J. Microbiol. Biotechnol. 2020, 31, 1–7. [Google Scholar] [CrossRef]
- Said, M.B.; Trabelsi, D.; Achouri, F.; Saad, M.B.; Bousselmi, L.; Ghrabi, A. Application of Bacteriophage and Essential Oil to Monitor Bacterial Biofilm Formation. In Application of Bacteriophage and Essential Oil to Monitor Bacterial Biofilm Formation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 273–274. [Google Scholar]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid.-Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth. or Real. Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St. Rose, T.; Puchalski, K.; Langland, J. Antimicrobial Activity of the Volatile Substances from Essential Oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef]
- Yang, S.K.; Tan, N.P.; Chong, C.W.; Abushelaibi, A.; Lim, S.H.E.; Lai, K.S. The Missing Piece: Recent Approaches Investigating the Antimicrobial Mode of Action of Essential Oils. Evol. Bioinform. 2021, 17, 1176934320938391. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Shen, Y.; Loessner, M.J. Beyond Antibacterials—Exploring Bacteriophages as Antivirulence Agents. Curr. Opin. Biotechnol. 2021, 68, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Budzyńska, A.; Wiȩckowska-Szakiel, M.; Sadowska, B.; Kalemba, D.; Rózalska, B. Antibiofilm Activity of Selected Plant Essential Oils and Their Major Components. Pol. J. Microbiol. 2011, 60, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Millezi, A.F.; Piccoli, R.H.; Oliveira, J.M.; Pereira, M.O. Anti-Biofim and Antibacterial Effect of Essential Oils and Their Major Compounds. J. Essent. Oil-Bear. Plants 2016, 19, 624–631. [Google Scholar] [CrossRef]
- Cerri, A.C.; Esmerino, L.A. Atividade Antimicrobiana e Efeito Antibiofilme de Óleos Essenciais: Um Estudo Comparativo. Braz. J. Dev. 2022, 8, 73850–73863. [Google Scholar] [CrossRef]
- Reichling, J. Anti-Biofilm and Virulence Factor-Reducing Activities of Essential Oils and Oil Components as a Possible Option for Bacterial Infection Control. Planta Med. 2020, 86, 520–537. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Synergistic Interactions of Plant Essential Oils with Antimicrobial Agents: A New Antimicrobial Therapy. Crit. Rev. Food Sci. Nutr. 2020, 62, 1740–1751. [Google Scholar] [CrossRef]
- Solarte, A.L.; Astorga, R.J.; Aguiar, F.; Galán-Relaño, Á.; Maldonado, A.; Huerta, B. Combination of Antimicrobials and Essential Oils as an Alternative for the Control of Salmonella Enterica Multiresistant Strains Related to Foodborne Disease. Foodborne Pathog. Dis. 2017, 14, 558–563. [Google Scholar] [CrossRef]
- Zhang, H.; Tikekar, R.V.; Ding, Q.; Gilbert, A.R.; Wimsatt, S.T. Inactivation of Foodborne Pathogens by the Synergistic Combinations of Food Processing Technologies and Food-Grade Compounds. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2110–2138. [Google Scholar] [CrossRef]
- Lai, E.P.C.; Iqbal, Z.; Avis, T.J. Combating Antimicrobial Resistance in Foodborne Microorganisms. J. Food Prot. 2016, 79, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Q.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus-Derived Components for Inhibiting Biofilm Formation in the Food Industry. World J. Microbiol. Biotechnol. 2024, 40, 117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Jiang, Y.H.; Li, H.W.; Li, X.Z.; Zhang, Q.L. Purification and Characterization of Lactobacillus Plantarum-Derived Bacteriocin with Activity against Staphylococcus Argenteus Planktonic Cells and Biofilm. J. Food Sci. 2022, 87, 2718–2731. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Choi, M.W.; Byun, K.H.; Kim, B.H.; Song, M.S.; Kang, I.; Ha, S. Do Characterization of Salmonella Ser. Enteritidis-Specific Bacteriophages and Biocontrol Strategy to Reduce, S. Enteritidis on Egg Products Using Bacteriophages and Essential Oil Compounds. Food Control 2024, 160, 110304. [Google Scholar] [CrossRef]
- Viazis, S.; Akhtar, M.; Feirtag, J.; Diez-Gonzalez, F. Reduction of Escherichia coli O157:H7 Viability on Leafy Green Vegetables by Treatment with a Bacteriophage Mixture and Trans-Cinnamaldehyde. Food Microbiol. 2011, 28, 149–157. [Google Scholar] [CrossRef]
- Ban, G.H.; Kang, D.H. Effect of Sanitizer Combined with Steam Heating on the Inactivation of Foodborne Pathogens in a Biofilm on Stainless Steel. Food Microbiol. 2016, 55, 47–54. [Google Scholar] [CrossRef]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination Strategies to Enhance the Efficacy of Antimicrobial Peptides against Bacterial Biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef]
- Oliveira, A.; Ribeiro, H.G.; Silva, A.C.; Silva, M.D.; Sousa, J.C.; Rodrigues, C.F.; Melo, L.D.R.; Henriques, A.F.; Sillankorva, S. Synergistic Antimicrobial Interaction between Honey and Phage against Escherichia coli Biofilms. Front. Microbiol. 2017, 8, 2407. [Google Scholar] [CrossRef]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef]
- Alves, D.; Cerqueira, M.A.; Pastrana, L.M.; Sillankorva, S. Entrapment of a Phage Cocktail and Cinnamaldehyde on Sodium Alginate Emulsion-Based Films to Fight Food Contamination by Escherichia coli and Salmonella Enteritidis. Food Res. Int. 2020, 128, 108791. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Wang, J.; Ahn, J. Synergistic Antimicrobial Activity of Essential Oils in Combination wit+h Phage Endolysin against Salmonella Typhimurium in Cooked Ground Beef. Food Control 2024, 157, 110187. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Bhaskar, R.; Han, S.S. Bacteriophages: Natural Antimicrobial Bioadditives for Food Preservation in Active Packaging. Int. J. Biol. Macromol. 2024, 276, 133945. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vivas, J.; Elexpuru-Zabaleta, M.; Samano, M.L.; Barrera, A.P.; Forbes-Hernández, T.Y.; Giampieri, F.; Battino, M. Phages and Enzybiotics in Food Biopreservation. Molecules 2021, 26, 5138. [Google Scholar] [CrossRef] [PubMed]
- Neeraj, M.; Ritu, K.S. Brajesh Varshney Biopolymers as Antimicrobial Food Packaging. In Green Biopolymers for Packaging Applications; Aman, U., Shakeel, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–13. [Google Scholar]
- Basak, E.O.; Nurten, T.; Hatice, S.A. Polysaccharides from Fruit and Vegetable Wastes and Their Food Applications: A Review. Int. J. Biol. Macromol. 2024, 276, 134007. [Google Scholar]
- Sarangi, P.K.; Srivastava, R.K.; Vivekanand, V.; Goksen, G.; Sahoo, U.K.; Thakur, T.K.; Debeaufort, F.; Uysal-Unalan, I.; Pugazhendhi, A. Recovery of Green Phenolic Compounds from Lignin-Based Source: Role of Ferulic Acid Esterase towards Waste Valorization and Bioeconomic Perspectives. Env. Environ. Res. 2024, 256, 119218. [Google Scholar] [CrossRef]
| Type of Agent | Main Mechanism | Typical Application | Example–Reference |
|---|---|---|---|
| Essential Oils | Membrane disruption, QS inhibition | Food preservation, surfaces | Carvacrol, eugenol [87] |
| Plant Extracts | Polyphenols, antioxidants, antimicrobial action | In vitro biofilms, packaging | Green tea, grape, rosemary [88] |
| Antimicrobial Peptides (AMPs) | Membrane interaction → pore formation or lysis | Combined with enzymes or nanoparticles | Nisin, defensins, bacteriocins [89] |
| Bacteriophages | Specific bacterial lysis; endolysin release | Food surfaces, infections | Listeria in deli meats [6] |
| Enzymes (e.g., endolysins, dispersins) | EPS matrix degradation | Combined with phages or EO | Dispersin B + phage [90] |
| Green Nanoparticles | Targeted delivery + catalytic activity | Encapsulated formulations | AgNPs with EO [91] |
| Microbial Biosurfactants | Surface tension reduction; antiadhesion | Biofilms, formulations | Rhamnolipids [92] |
| Combination | Target Bacteria | Application Matrix | Synergistic Effect |
|---|---|---|---|
| Phage + Honey [136] | E. coli | In vitro biofilm | Enhanced killing vs. phage alone |
| Phage + Endolysin [137] | L. monocytogenes | Food-contact surface | Biofilm disruption > 70% |
| Phages + Cinnamaldehyde [138] | E. coli, Salmonella | Alginate-based films | Significant CFU reduction |
| EO + Phage endolysin [139] | Salmonella Typhimurium | Cooked ground beef | Increased antimicrobial activity |
| Phages + Enzymes + EO [140] | Multiple pathogens | Active packaging | EPS matrix degradation + phage delivery |
| Phages+ Enzybiotics [141] | Listeria, Salmonella | Food biofilms | Specificity + matrix penetration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao Godinez, A.K.; Villicaña, C.; Basilio Heredia, J.; Valdez-Torres, J.B.; Muy-Rangel, M.; León-Félix, J. Facing Foodborne Pathogen Biofilms with Green Antimicrobial Agents: One Health Approach. Molecules 2025, 30, 1682. https://doi.org/10.3390/molecules30081682
Kao Godinez AK, Villicaña C, Basilio Heredia J, Valdez-Torres JB, Muy-Rangel M, León-Félix J. Facing Foodborne Pathogen Biofilms with Green Antimicrobial Agents: One Health Approach. Molecules. 2025; 30(8):1682. https://doi.org/10.3390/molecules30081682
Chicago/Turabian StyleKao Godinez, Ana Karina, Claudia Villicaña, José Basilio Heredia, José Benigno Valdez-Torres, Maria Muy-Rangel, and Josefina León-Félix. 2025. "Facing Foodborne Pathogen Biofilms with Green Antimicrobial Agents: One Health Approach" Molecules 30, no. 8: 1682. https://doi.org/10.3390/molecules30081682
APA StyleKao Godinez, A. K., Villicaña, C., Basilio Heredia, J., Valdez-Torres, J. B., Muy-Rangel, M., & León-Félix, J. (2025). Facing Foodborne Pathogen Biofilms with Green Antimicrobial Agents: One Health Approach. Molecules, 30(8), 1682. https://doi.org/10.3390/molecules30081682








