The Complexity of Interferon Signaling in Host Defense against Protozoan Parasite Infection
Abstract
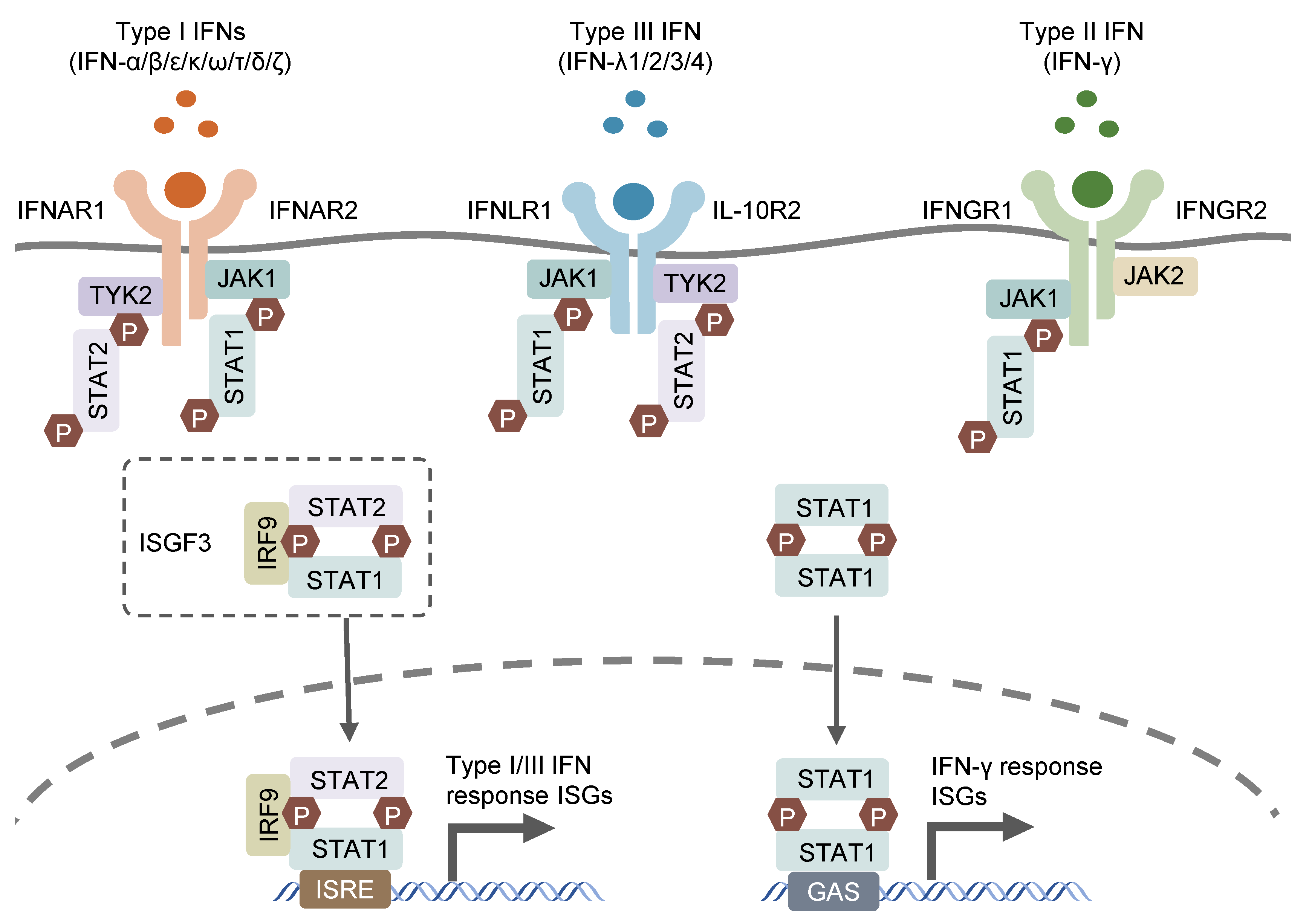
:1. Introduction
2. IFN-γ and Intracellular Protozoan Parasites
2.1. IFN-γ Production in Protozoan Parasite Infection
| Protective Effects | |||
| Parasite Species | Treatments and Findings | Effects | Ref. |
| P. falciparum | Recombinant IFN-γ in human hepatocyte cell culture | Hepatic schizont development ↓ | [45] |
| IFN-γ production in infected children | Occurrence of high-density and clinical episode of infection ↓ | [37] | |
| High IFN-γ level in infected patients | Occurrence of cerebral malaria ↓ | [46] | |
| P. berghei | Recombinant IFN-γ in rats, mice and human hepatocyte cell culture | Hepatic schizont development ↓ | [45] |
| Monoclonal IFN-γ neutralizing antibody in mice | Parasitemia ↑ | [47] | |
| Early IFN-γ production in infected mice | Occurrence of cerebral malaria ↓ | [48] | |
| P. vivax | Recombinant IFN-γ in chimpanzees | Parasitemia ↓ | [45] |
| P. Chabaudi | Recombinant IFN-γ in mice. | Parasitemia ↓ Intraerythrocytic parasites ↓ | [45] |
| IFN-γ−/− mice | Parasitemia ↑ | [49] | |
| P. cynomolgi | Recombinant IFN-γ in rhesus monkey | Hepatic schizont development ↓ | [45] |
| P. yoelii | Recombinant IFN-γ in mice | Parasitemia ↓ | [50] |
| IFN-γR−/− mice | Infection burden ↑ | [51] | |
| IFN-γ−/− mice | Parasitemia ↑ | [49] | |
| T. b. brucei | IFN-γ−/− mice | Parasitemia ↑ Survival time ↓ | [33] |
| T. b. rhodesiense | IFN-γ−/− mice | Parasitemia ↑ Survival time ↓ | [52] |
| L. major | IFN-γR−/− mice | Larger and progressing lesions | [53] |
| L. amazonesis | IFN-γ−/− mice | Devastating lesions in late infection stages | [54] |
| T. gondii | IFN-γ−/− mice | Survival ↓ Infection burden ↑ | [55] |
| C. parvum | Recombinant IFN-γ in intestinal enterocytes cell culture | Infection burden ↓ | [56] |
| IFN-γ−/− mice | Survival ↓ Occurrence of chronic infection ↑ | [57] | |
| Pathogenic Effects | |||
| Parasite Species | Treatments and Findings | Effects | Ref. |
| P. berghei | Late IFN-γ production in infected mice | Occurrence of cerebral malaria ↑ | [48] |
| Large amount of IFN-γ produced in infected mice | Susceptibility to cerebral malaria ↑ | [58,59] | |
| IFN-γR−/− mice | No cerebral malaria development | [60,61] | |
| IFN-γ−/− mice | No cerebral malaria development | [62,63] | |
| Monoclonal IFN-γ neutralizing antibody in mice | Occurrence of cerebral malaria ↓ Survival time ↑ | [64] | |
| P. yoelii, P. chabaudi, P. berghei | Overproduction of IFN-γ | Development of Tfh and GC B cell response ↓ Humoral immunity ↓ Autoimmune anemia ↑ | [65,66] [67,68] [69] |
| T. congolense | Overproducing IFN-γ in mice | Survival time ↓ | [70] |
| Reducing production of IFN-γ in mice | Survival time ↑ | [71] | |
| Monoclonal IFN-γ neutralizing antibody in mice | Susceptibility ↓ | [72] | |
2.2. IFN-γ in Host Defense against Protozoan Parasites
2.3. IFN-γ in the Pathogenesis of Protozoan Infection
3. Type I IFNs and Intracellular Protozoan Parasites
3.1. Type I IFN Production in Protozoan Parasite Infection
3.2. Type I IFN in Host Defense against Protozoan Parasites
3.3. Type I IFNs in the Pathogenesis of Protozoan Infection
4. Type III IFNs and Intracellular Protozoan Parasites
5. Crosstalk of IFNs
6. Conclusions and Speculations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isaacs, A.; Lindenmann, J. Virus Interference. I. The Interferon. Proc. R. Soc. Lond. B Biol. Sci. 1957, 147, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.; Kindsvogel, W.; Xu, W.; Henderson, K.; Schlutsmeyer, S.; Whitmore, T.E.; Kuestner, R.; Garrigues, U.; Birks, C.; Roraback, J.; et al. IL-28, IL-29 and Their Class II Cytokine Receptor IL-28R. Nat. Immunol. 2003, 4, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J.A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-Λs Mediate Antiviral Protection through a Distinct Class II Cytokine Receptor Complex. Nat. Immunol. 2003, 4, 69–77. [Google Scholar] [CrossRef]
- Blouin, C.M.; Lamaze, C. Interferon Gamma Receptor: The Beginning of the Journey. Front. Immunol. 2013, 4, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, Interferon-like Cytokines, and Their Receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I Interferons in Infectious Disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G. Interleukin-18: Biological Properties and Role in Disease Pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like Receptor Recognizes Bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Bosisio, D.; Polentarutti, N.; Sironi, M.; Bernasconi, S.; Miyake, K.; Webb, G.R.; Martin, M.U.; Mantovani, A.; Muzio, M. Stimulation of Toll-like Receptor 4 Expression in Human Mononuclear Phagocytes by Interferon-γ: A Molecular Basis for Priming and Synergism with Bacterial Lipopolysaccharide. Blood 2002, 99, 3427–3431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignali, D.A.A.; Kuchroo, V.K. IL-12 Family Cytokines: Immunological Playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Prokunina-Olsson, L.; Muchmore, B.; Tang, W.; Pfeiffer, R.M.; Park, H.; Dickensheets, H.; Hergott, D.; Porter-Gill, P.; Mumy, A.; Kohaar, I.; et al. A Variant Upstream of IFNL3 (IL28B) Creating a Novel Interferon Gene IFNL4 Is Associated with Impaired Clearance of Hepatitis C Virus. Nat. Genet. 2013, 45, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Mesev, E.V.; LeDesma, R.A.; Ploss, A. Decoding Type I and III Interferon Signalling during Viral Infection. Nat. Microbiol. 2019, 4, 914–924. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. IFNγ: Signalling, Epigenetics and Roles in Immunity, Metabolism, Disease and Cancer Immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Schnepf, D.; Staeheli, P. Interferon-λ Orchestrates Innate and Adaptive Mucosal Immune Responses. Nat. Rev. Immunol. 2019, 19, 614–625. [Google Scholar] [CrossRef] [PubMed]
- de Weerd, N.A.; Nguyen, T. The Interferons and Their Receptors—Distribution and Regulation. Immunol. Cell Biol. 2012, 90, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Reuter, A.; Soubies, S.; Härtle, S.; Schusser, B.; Kaspers, B.; Staeheli, P.; Rubbenstroth, D. Antiviral Activity of Lambda Interferon in Chickens. J. Virol. 2014, 88, 2835–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommereyns, C.; Paul, S.; Staeheli, P.; Michiels, T. IFN-Lambda (IFN-λ) Is Expressed in a Tissue-Dependent Fashion and Primarily Acts on Epithelial Cells In Vivo. PLoS Pathog. 2008, 4, e1000017. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, M.; Shoukry, N.H. Type III Interferons in Hepatitis C Virus Infection. Front. Immunol. 2016, 7, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesler, D.A.; Reiss, C.S. The Role of IFN-γ in Immune Responses to Viral Infections of the Central Nervous System. Cytokine Growth Factor Rev. 2002, 13, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Brown, H.M.; Hwang, S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune Netw. 2018, 18, e33. [Google Scholar] [CrossRef] [PubMed]
- Boxx, G.M.; Cheng, G. The Roles of Type I Interferon in Bacterial Infection. Cell Host Microbe 2016, 19, 760–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramharter, M.; Willheim, M.; Winkler, H.; Wahl, K.; Lagler, H.; Graninger, W.; Winkler, S. Cytokine Profile of Plasmodium Falciparum-Specific T Cells in Non-Immune Malaria Patients. Parasite Immunol. 2003, 25, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Riley, E.M. Activation of Human NK Cells by Plasmodium-Infected Red Blood Cells. Methods Mol. Biol. 2013, 923, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Barakat, F.M.; McDonald, V.; Di Santo, J.P.; Korbel, D.S. Roles for NK Cells and an NK Cell-Independent Source of Intestinal Gamma Interferon for Innate Immunity to Cryptosporidium Parvum Infection. Infect. Immun. 2009, 77, 5044–5049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onyilagha, C.; Kuriakose, S.; Ikeogu, N.; Kung, S.K.P.; Uzonna, J.E. NK Cells Are Critical for Optimal Immunity to Experimental Trypanosoma Congolense Infection. J. Immunol. 2019, 203, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Artavanis-Tsakonas, K.; Riley, E.M. Innate Immune Response to Malaria: Rapid Induction of IFN-γ from Human NK Cells by Live Plasmodium Falciparum-Infected Erythrocytes. J. Immunol. 2002, 169, 2956–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, I.; Salaiza, N.; Aguirre, M.; Delgado, J.; Carrillo-Carrasco, N.; Kobeh, L.G.; Ruiz, A.; Cervantes, R.; Torres, A.P.; Cabrera, N.; et al. Leishmania Lipophosphoglycan (LPG) Activates NK Cells through Toll-like Receptor-2. Mol. Biochem. Parasitol. 2003, 130, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Filipe-Santos, O.; Pescher, P.; Breart, B.; Lippuner, C.; Aebischer, T.; Glaichenhaus, N.; Späth, G.F.; Bousso, P. A Dynamic Map of Antigen Recognition by CD4 T Cells at the Site of Leishmania Major Infection. Cell Host Microbe 2009, 6, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Walther, M.; Jeffries, D.; Finney, O.C.; Njie, M.; Ebonyi, A.; Deininger, S.; Lawrence, E.; Ngwa-Amambua, A.; Jayasooriya, S.; Cheeseman, I.H.; et al. Distinct Roles for FOXP3+ and FOXP3− CD4+ T Cells in Regulating Cellular Immunity to Uncomplicated and Severe Plasmodium Falciparum Malaria. PLoS Pathog. 2009, 5, e1000364. [Google Scholar] [CrossRef] [PubMed]
- Ong’echa, J.M.O.; Lal, A.A.; Terlouw, D.J.; Ter Kuile, F.O.; Kariuki, S.K.; Udhayakumar, V.; Orago, A.S.S.; Hightower, A.W.; Nahlen, B.L.; Shi, Y.P. Association of Interferon-Gamma Responses to Pre-Erythrocytic Stage Vaccine Candidate Antigens of Plasmodium Falciparum in Young Kenyan Children with Improved Hemoglobin Levels: XV. Asembo Bay Cohort Project. Am. J. Trop. Med. Hyg. 2003, 68, 590–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Kang, H.; Kikuchi, T.; Suzuki, Y. Gamma Interferon Production, but Not Perforin-Mediated Cytolytic Activity, of T Cells Is Required for Prevention of Toxoplasmic Encephalitis in BALB/c Mice Genetically Resistant to the Disease. Infect. Immun. 2004, 72, 4432–4438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namangala, B.; Noël, W.; De Baetselier, P.; Brys, L.; Beschin, A. Relative Contribution of Interferon-γ and Interleukin-10 to Resistance to Murine African Trypanosomosis. J. Infect. Dis. 2001, 183, 1794–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.L.; Sack, B.K.; Baldwin, M.; Vaughan, A.M.; Kappe, S.H.I. Interferon-Mediated Innate Immune Responses against Malaria Parasite Liver Stages. Cell Rep. 2014, 7, 436–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soulard, V.; Roland, J.; Sellier, C.; Gruner, A.C.; Leite-de-Moraes, M.; Franetich, J.-F.; Rénia, L.; Cazenave, P.-A.; Pied, S. Primary Infection of C57BL/6 Mice with Plasmodium Yoelii Induces a Heterogeneous Response of NKT Cells. Infect. Immun. 2007, 75, 2511–2522. [Google Scholar] [CrossRef] [Green Version]
- Hviid, L.; Kurtzhals, J.A.L.; Adabayeri, V.; Loizon, S.; Kemp, K.; Goka, B.Q.; Lim, A.; Mercereau-Puijalon, O.; Akanmori, B.D.; Behr, C. Perturbation and Proinflammatory Type Activation of Vδ1+ Γδ T Cells in African Children with Plasmodium Falciparum Malaria. Infect. Immun. 2001, 69, 3190–3196. [Google Scholar] [CrossRef] [Green Version]
- D’Ombrain, M.C.; Robinson, L.J.; Stanisic, D.I.; Taraika, J.; Bernard, N.; Michon, P.; Mueller, I.; Schofield, L. Association of Early Interferon-γ Production with Immunity to Clinical Malaria: A Longitudinal Study among Papua New Guinean Children. Clin. Infect. Dis. 2008, 47, 1380–1387. [Google Scholar] [CrossRef] [Green Version]
- Pamplona, A.; Silva-Santos, B. Γδ T Cells in Malaria: A Double-edged Sword. FEBS J. 2021, 288, 1118–1129. [Google Scholar] [CrossRef]
- Suzuki, Y.; Claflin, J.; Wang, X.; Lengi, A.; Kikuchi, T. Microglia and Macrophages as Innate Producers of Interferon-Gamma in the Brain Following Infection with Toxoplasma Gondii. Int. J. Parasitol. 2005, 35, 83–90. [Google Scholar] [CrossRef]
- Wang, X.; Suzuki, Y. Microglia Produce IFN-γ Independently from T Cells During Acute Toxoplasmosis in the Brain. J. Interferon Cytokine Res. 2007, 27, 599–605. [Google Scholar] [CrossRef]
- Klose, C.S.N.; Flach, M.; Möhle, L.; Rogell, L.; Hoyler, T.; Ebert, K.; Fabiunke, C.; Pfeifer, D.; Sexl, V.; Fonseca-Pereira, D.; et al. Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages. Cell 2014, 157, 340–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, D.; Speranza, F.; Giola, M.; Matteelli, A.; Tambini, R.; Biondi, G. Role of Th1 and Th2 Cytokines in Immune Response to Uncomplicated Plasmodium Falciparum Malaria. Clin. Vaccine Immunol. 2002, 9, 348–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loevenich, K.; Ueffing, K.; Abel, S.; Hose, M.; Matuschewski, K.; Westendorf, A.M.; Buer, J.; Hansen, W. DC-Derived IL-10 Modulates Pro-Inflammatory Cytokine Production and Promotes Induction of CD4+IL-10+ Regulatory T Cells during Plasmodium Yoelii Infection. Front. Immunol. 2017, 8, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, D.L.; Denton, S.L.; Fettel, K.D.; Sondgeroth, K.S.; Munoz Gutierrez, J.; Bangoura, B.; Dunay, I.R.; Gigley, J.P. Innate Lymphoid Cells in Protection, Pathology, and Adaptive Immunity During Apicomplexan Infection. Front. Immunol. 2019, 10, 196. [Google Scholar] [CrossRef] [Green Version]
- Doolan, D.L.; Hoffman, S.L. The Complexity of Protective Immunity against Liver-Stage Malaria. J. Immunol. 2000, 165, 1453–1462. [Google Scholar] [CrossRef] [Green Version]
- Prakash, D.; Fesel, C.; Jain, R.; Cazenave, P.-A.; Mishra, G.C.; Pied, S. Clusters of Cytokines Determine Malaria Severity in Plasmodium Falciparum–Infected Patients from Endemic Areas of Central India. J. Infect. Dis. 2006, 194, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Schofield, L.; Villaquiran, J.; Ferreira, A.; Schellekens, H.; Nussenzweig, R.; Nussenzweig, V. γ Interferon, CD8+ T Cells and Antibodies Required for Immunity to Malaria Sporozoites. Nature 1987, 330, 664–666. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Hansen, A.M.; Hee, L.; Ball, H.J.; Potter, S.M.; Walker, J.C.; Hunt, N.H. Early Cytokine Production Is Associated with Protection from Murine Cerebral Malaria. Infect. Immun. 2005, 73, 5645–5653. [Google Scholar] [CrossRef] [Green Version]
- van der Heyde, H.C.; Pepper, B.; Batchelder, J.; Cigel, F.; Weidanz, W.P. The Time Course of Selected Malarial Infections in Cytokine-Deficient Mice. Exp. Parasitol. 1997, 85, 206–213. [Google Scholar] [CrossRef]
- Shear, H.L.; Srinivasan, R.; Nolan, T.; Ng, C. Role of IFN-Gamma in Lethal and Nonlethal Malaria in Susceptible and Resistant Murine Hosts. J. Immunol. 1989, 143, 2038–2044. [Google Scholar] [CrossRef]
- Tsuji, M.; Miyahira, Y.; Nussenzweig, R.S.; Aguet, M.; Reichel, M.; Zavala, F. Development of Antimalaria Immunity in Mice Lacking IFN-Gamma Receptor. J. Immunol. 1995, 154, 5338–5344. [Google Scholar] [CrossRef] [PubMed]
- Hertz, C.J.; Filutowicz, H.; Mansfield, J.M. Resistance to the African Trypanosomes Is IFN-γ Dependent. J. Immunol. 1998, 161, 6775–6783. [Google Scholar] [CrossRef] [PubMed]
- Swihart, K.; Fruth, U.; Messmer, N.; Hug, K.; Behin, R.; Huang, S.; Del Giudice, G.; Aguet, M.; Louis, J.A. Mice from a Genetically Resistant Background Lacking the Interferon γ Receptor Are Susceptible to Infection with Leishmania Major but Mount a Polarized T Helper Cell 1-Type CD4+ T Cell Response. J. Exp. Med. 1995, 181, 961–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, R.O.; Rossi-Bergmann, B. Interferon-Gamma Is Required for the Late but Not Early Control of Leishmania Amazonensis Infection in C57Bl/6 Mice. Mem. Inst. Oswaldo Cruz 2007, 102, 79–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharton-Kersten, T.M.; Wynn, T.A.; Denkers, E.Y.; Bala, S.; Grunvald, E.; Hieny, S.; Gazzinelli, R.T.; Sher, A. In the Absence of Endogenous IFN-Gamma, Mice Develop Unimpaired IL-12 Responses to Toxoplasma Gondii While Failing to Control Acute Infection. J. Immunol. 1996, 157, 4045–4054. [Google Scholar] [CrossRef] [PubMed]
- Pollok, R.C.G.; Farthing, M.J.G.; Bajaj-Elliott, M.; Sanderson, I.R.; McDonald, V. Interferon Gamma Induces Enterocyte Resistance against Infection by the Intracellular Pathogen Cryptosporidium Parvum. Gastroenterology 2001, 120, 99–107. [Google Scholar] [CrossRef]
- Lacroix, S.; Mancassola, R.; Naciri, M.; Laurent, F. Cryptosporidium Parvum-Specific Mucosal Immune Response in C57BL/6 Neonatal and Gamma Interferon-Deficient Mice: Role of Tumor Necrosis Factor Alpha in Protection. Infect. Immun. 2001, 69, 1635–1642. [Google Scholar] [CrossRef] [Green Version]
- de Kossodo, S.; Grau, G.E. Profiles of Cytokine Production in Relation with Susceptibility to Cerebral Malaria. J. Immunol. 1993, 151, 4811–4820. [Google Scholar] [CrossRef]
- Kossodo, S.; Monso, C.; Juillard, P.; Velu, T.; Goldman, M.; Grau, G.E. Interleukin-10 Modulates Susceptibility in Experimental Cerebral Malaria. Immunology 1997, 91, 536–540. [Google Scholar] [CrossRef]
- Amani, V.; Vigário, A.M.; Belnoue, E.; Marussig, M.; Fonseca, L.; Mazier, D.; Rénia, L. Involvement of IFN-Gamma Receptor-Medicated Signaling in Pathology and Anti-Malarial Immunity Induced by Plasmodium Berghei Infection. Eur. J. Immunol. 2000, 30, 1646–1655. [Google Scholar] [CrossRef]
- Belnoue, E.; Potter, S.M.; Rosa, D.S.; Mauduit, M.; Grüner, A.C.; Kayibanda, M.; Mitchell, A.J.; Hunt, N.H.; Rénia, L. Control of Pathogenic CD8+ T Cell Migration to the Brain by IFN-γ during Experimental Cerebral Malaria. Parasite Immunol. 2008, 30, 544–553. [Google Scholar] [CrossRef]
- Yañez, D.M.; Manning, D.D.; Cooley, A.J.; Weidanz, W.P.; Heyde, H.C. van der Participation of Lymphocyte Subpopulations in the Pathogenesis of Experimental Murine Cerebral Malaria. J. Immunol. 1996, 157, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Mendez, A.; Greig, R.; Shaw, T.N.; de Souza, J.B.; Gwyer Findlay, E.; Stumhofer, J.S.; Hafalla, J.C.R.; Blount, D.G.; Hunter, C.A.; Riley, E.M.; et al. IFN-γ-Producing CD4+ T Cells Promote Experimental Cerebral Malaria by Modulating CD8+ T Cell Accumulation within the Brain. J. Immunol. 2012, 189, 968–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grau, G.E.; Heremans, H.; Piguet, P.F.; Pointaire, P.; Lambert, P.H.; Billiau, A.; Vassalli, P. Monoclonal Antibody against Interferon Gamma Can Prevent Experimental Cerebral Malaria and Its Associated Overproduction of Tumor Necrosis Factor. Proc. Natl. Acad. Sci. USA 1989, 86, 5572–5574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zander, R.A.; Obeng-Adjei, N.; Guthmiller, J.J.; Kulu, D.I.; Li, J.; Ongoiba, A.; Traore, B.; Crompton, P.D.; Butler, N.S. PD-1 Co-Inhibitory and OX40 Co-Stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell Host Microbe 2015, 17, 628–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryg-Cornejo, V.; Ioannidis, L.J.; Ly, A.; Chiu, C.Y.; Tellier, J.; Hill, D.L.; Preston, S.P.; Pellegrini, M.; Yu, D.; Nutt, S.L.; et al. Severe Malaria Infections Impair Germinal Center Responses by Inhibiting T Follicular Helper Cell Differentiation. Cell Rep. 2016, 14, 68–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeng-Adjei, N.; Portugal, S.; Tran, T.M.; Yazew, T.B.; Skinner, J.; Li, S.; Jain, A.; Felgner, P.L.; Doumbo, O.K.; Kayentao, K.; et al. Circulating Th1-Cell-Type Tfh Cells That Exhibit Impaired B Cell Help Are Preferentially Activated during Acute Malaria in Children. Cell Rep. 2015, 13, 425–439. [Google Scholar] [CrossRef] [Green Version]
- Guthmiller, J.J.; Graham, A.C.; Zander, R.A.; Pope, R.L.; Butler, N.S. Cutting Edge: IL-10 Is Essential for the Generation of Germinal Center B Cell Responses and Anti-Plasmodium Humoral Immunity. J. Immunol. 2017, 198, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Correa, J.; Guthmiller, J.J.; Vijay, R.; Fernandez-Arias, C.; Pardo-Ruge, M.A.; Gonzalez, S.; Butler, N.S.; Rodriguez, A. Plasmodium DNA-Mediated TLR9 Activation of T-Bet+ B Cells Contributes to Autoimmune Anaemia during Malaria. Nat. Commun. 2017, 8, 1282. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Pan, W.; Tabel, H. Experimental African Trypanosomiasis: IFN-γ Mediates Early Mortality. Eur. J. Immunol. 2003, 33, 108–118. [Google Scholar] [CrossRef]
- Barkhuizen, M.; Magez, S.; Ryffel, B.; Brombacher, F. Interleukin-12p70 Deficiency Increases Survival and Diminishes Pathology in Trypanosoma Congolense Infection. J. Infect. Dis. 2008, 198, 1284–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzonna, J.E.; Kaushik, R.S.; Gordon, J.R.; Tabel, H. Experimental Murine Trypanosoma Congolense Infections. I. Administration of Anti-IFN-γ Antibodies Alters Trypanosome-Susceptible Mice to a Resistant-Like Phenotype. J. Immunol. 1998, 161, 5507–5515. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Bhardwaj, J.; Goyal, M.; Prakash, K.; Adnan, M.; Alreshidi, M.M.; Patel, M.; Soni, A.; Redman, W. Immune Responses in Liver and Spleen against Plasmodium Yoelii Pre-Erythrocytic Stages in Swiss Mice Model. J. Adv. Res. 2020, 24, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A.; Hunt, N.H.; Butcher, G.A.; Cowden, W.B. Inhibition of Murine Malaria (Plasmodium Chabaudi) in Vivo by Recombinant Interferon-Gamma or Tumor Necrosis Factor, and Its Enhancement by Butylated Hydroxyanisole. J. Immunol. 1987, 139, 3493–3496. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.B.; Williamson, K.H.; Otani, T.; Playfair, J.H. Early Gamma Interferon Responses in Lethal and Nonlethal Murine Blood-Stage Malaria. Infect. Immun. 1997, 65, 1593–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kester, K.E.; Cummings, J.F.; Ofori-Anyinam, O.; Ockenhouse, C.F.; Krzych, U.; Moris, P.; Schwenk, R.; Nielsen, R.A.; Debebe, Z.; Pinelis, E.; et al. Randomized, Double-Blind, Phase 2a Trial of Falciparum Malaria Vaccines RTS,S/AS01B and RTS,S/AS02A in Malaria-Naive Adults: Safety, Efficacy, and Immunologic Associates of Protection. J. Infect. Dis. 2009, 200, 337–346. [Google Scholar] [CrossRef]
- Chuang, I.; Sedegah, M.; Cicatelli, S.; Spring, M.; Polhemus, M.; Tamminga, C.; Patterson, N.; Guerrero, M.; Bennett, J.W.; McGrath, S.; et al. DNA Prime/Adenovirus Boost Malaria Vaccine Encoding P. Falciparum CSP and AMA1 Induces Sterile Protection Associated with Cell-Mediated Immunity. PLoS ONE 2013, 8, e55571. [Google Scholar] [CrossRef]
- Seder, R.A.; Chang, L.-J.; Enama, M.E.; Zephir, K.L.; Sarwar, U.N.; Gordon, I.J.; Holman, L.A.; James, E.R.; Billingsley, P.F.; Gunasekera, A.; et al. Protection Against Malaria by Intravenous Immunization with a Nonreplicating Sporozoite Vaccine. Science 2013, 341, 1359–1365. [Google Scholar] [CrossRef] [Green Version]
- Stanisic, D.I.; Fink, J.; Mayer, J.; Coghill, S.; Gore, L.; Liu, X.Q.; El-Deeb, I.; Rodriguez, I.B.; Powell, J.; Willemsen, N.M.; et al. Vaccination with Chemically Attenuated Plasmodium Falciparum Asexual Blood-Stage Parasites Induces Parasite-Specific Cellular Immune Responses in Malaria-Naïve Volunteers: A Pilot Study. BMC Med. 2018, 16, 184. [Google Scholar] [CrossRef] [Green Version]
- Chawla, B.; Mahajan, B.; Oakley, M.; Majam, V.F.; Belmonte, A.; Sedegah, M.; Shimp, R.L.; Kaslow, D.C.; Kumar, S. Antibody-Dependent, Gamma Interferon-Independent Sterilizing Immunity Induced by a Subunit Malaria Vaccine. Infect. Immun. 2019, 87, e00236-19. [Google Scholar] [CrossRef]
- Carneiro, M.B.H.; Lopes, M.E.D.M.; Vaz, L.G.; Sousa, L.M.A.; dos Santos, L.M.; de Souza, C.C.; Campos, A.C.D.A.; Gomes, D.A.; Gonçalves, R.; Tafuri, W.L.; et al. IFN-γ-Dependent Recruitment of CD4+ T Cells and Macrophages Contributes to Pathogenesis During Leishmania Amazonensis Infection. J. Interferon Cytokine Res. 2015, 35, 935–947. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Liu, G.; Shi, M. Interferon Gamma in African Trypanosome Infections: Friends or Foes? Front. Immunol. 2017, 8, 1105. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.D. Interferon-Inducible Effector Mechanisms in Cell-Autonomous Immunity. Nat. Rev. Immunol. 2012, 12, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, C.; Köhler, G.; Müller, U.; Mossmann, H.; Schaub, G.A.; Brombacher, F. Defective Nitric Oxide Effector Functions Lead to Extreme Susceptibility of Trypanosoma Cruzi-Infected Mice Deficient in Gamma Interferon Receptor or Inducible Nitric Oxide Synthase. Infect. Immun. 1998, 66, 1208–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellouk, S.; Hoffman, S.L.; Liu, Z.Z.; de la Vega, P.; Billiar, T.R.; Nussler, A.K. Nitric Oxide-Mediated Antiplasmodial Activity in Human and Murine Hepatocytes Induced by Gamma Interferon and the Parasite Itself: Enhancement by Exogenous Tetrahydrobiopterin. Infect. Immun. 1994, 62, 4043–4046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, S.J.; Meltzer, M.S.; Hibbs, J.B.; Nacy, C.A. Activated Macrophages Destroy Intracellular Leishmania Major Amastigotes by an L-Arginine-Dependent Killing Mechanism. J. Immunol. 1990, 144, 278–283. [Google Scholar] [CrossRef]
- Scharton-Kersten, T.M.; Yap, G.; Magram, J.; Sher, A. Inducible Nitric Oxide Is Essential for Host Control of Persistent but Not Acute Infection with the Intracellular Pathogen Toxoplasma Gondii. J. Exp. Med. 1997, 185, 1261–1274. [Google Scholar] [CrossRef] [Green Version]
- Leitch, G.J.; He, Q. Reactive Nitrogen and Oxygen Species Ameliorate Experimental Cryptosporidiosis in the Neonatal BALB/c Mouse Model. Infect. Immun. 1999, 67, 5885–5891. [Google Scholar] [CrossRef] [Green Version]
- Kolios, G.; Rooney, N.; Murphy, C.T.; Robertson, D.A.; Westwick, J. Expression of Inducible Nitric Oxide Synthase Activity in Human Colon Epithelial Cells: Modulation by T Lymphocyte Derived Cytokines. Gut 1998, 43, 56–63. [Google Scholar] [CrossRef]
- Hayward, A.R.; Chmura, K.; Cosyns, M. Interferon-Gamma Is Required for Innate Immunity to Cryptosporidium Parvum in Mice. J. Infect. Dis. 2000, 182, 1001–1004. [Google Scholar] [CrossRef]
- Hunn, J.P.; Koenen-Waisman, S.; Papic, N.; Schroeder, N.; Pawlowski, N.; Lange, R.; Kaiser, F.; Zerrahn, J.; Martens, S.; Howard, J.C. Regulatory Interactions between IRG Resistance GTPases in the Cellular Response to Toxoplasma Gondii. EMBO J. 2008, 27, 2495–2509. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ferguson, D.J.P.; Wilson, D.C.; Howard, J.C.; Sibley, L.D.; Yap, G.S. Virulent Toxoplasma Gondii Evade Immunity-Related GTPase (IRG)-Mediated Parasite Vacuole Disruption Within Primed Macrophages. J. Immunol. 2009, 182, 3775–3781. [Google Scholar] [CrossRef] [Green Version]
- Halonen, S.K.; Taylor, G.A.; Weiss, L.M. Gamma Interferon-Induced Inhibition of Toxoplasma Gondii in Astrocytes Is Mediated by IGTP. Infect. Immun. 2001, 69, 5573–5576. [Google Scholar] [CrossRef] [Green Version]
- Martens, S.; Parvanova, I.; Zerrahn, J.; Griffiths, G.; Schell, G.; Reichmann, G.; Howard, J.C. Disruption of Toxoplasma Gondii Parasitophorous Vacuoles by the Mouse P47-Resistance GTPases. PLoS Pathog. 2005, 1, e24. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.M.; Shaw, M.H.; Ayala, C.; Coppens, I.; Taylor, G.A.; Ferguson, D.J.P.; Yap, G.S. Vacuolar and Plasma Membrane Stripping and Autophagic Elimination of Toxoplasma Gondii in Primed Effector Macrophages. J. Exp. Med. 2006, 203, 2063–2071. [Google Scholar] [CrossRef]
- Zhao, Y.; Wilson, D.; Matthews, S.; Yap, G.S. Rapid Elimination of Toxoplasma Gondii by Gamma Interferon-Primed Mouse Macrophages Is Independent of CD40 Signaling. Infect. Immun. 2007, 75, 4799–4803. [Google Scholar] [CrossRef] [Green Version]
- Liesenfeld, O.; Parvanova, I.; Zerrahn, J.; Han, S.-J.; Heinrich, F.; Muñoz, M.; Kaiser, F.; Aebischer, T.; Buch, T.; Waisman, A.; et al. The IFN-γ-Inducible GTPase, Irga6, Protects Mice against Toxoplasma Gondii but Not against Plasmodium Berghei and Some Other Intracellular Pathogens. PLoS ONE 2011, 6, e20568. [Google Scholar] [CrossRef]
- Santiago, H.C.; Feng, C.G.; Bafica, A.; Roffe, E.; Arantes, R.M.; Cheever, A.; Taylor, G.; Vierira, L.Q.; Aliberti, J.; Gazzinelli, R.T.; et al. Mice Deficient in LRG-47 Display Enhanced Susceptibility to Trypanosoma Cruzi Infection Associated with Defective Hemopoiesis and Intracellular Control of Parasite Growth. J. Immunol. 2005, 175, 8165–8172. [Google Scholar] [CrossRef] [Green Version]
- Khaminets, A.; Hunn, J.P.; Könen-Waisman, S.; Zhao, Y.O.; Preukschat, D.; Coers, J.; Boyle, J.P.; Ong, Y.-C.; Boothroyd, J.C.; Reichmann, G.; et al. Coordinated Loading of IRG Resistance GTPases on to the Toxoplasma Gondii Parasitophorous Vacuole. Cell. Microbiol. 2010, 12, 939–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.-H.; Shenoy, A.R.; Kumar, P.; Bradfield, C.J.; MacMicking, J.D. IFN-Inducible GTPases in Host Defense. Cell Host Microbe 2012, 12, 432–444. [Google Scholar] [CrossRef]
- Yamamoto, M.; Okuyama, M.; Ma, J.S.; Kimura, T.; Kamiyama, N.; Saiga, H.; Ohshima, J.; Sasai, M.; Kayama, H.; Okamoto, T.; et al. A Cluster of Interferon-γ-Inducible P65 GTPases Plays a Critical Role in Host Defense against Toxoplasma Gondii. Immunity 2012, 37, 302–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degrandi, D.; Kravets, E.; Konermann, C.; Beuter-Gunia, C.; Klümpers, V.; Lahme, S.; Rasch, E.; Mausberg, A.K.; Beer-Hammer, S.; Pfeffer, K. Murine Guanylate Binding Protein 2 (MGBP2) Controls Toxoplasma Gondii Replication. Proc. Natl. Acad. Sci. USA 2013, 110, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selleck, E.M.; Fentress, S.J.; Beatty, W.L.; Degrandi, D.; Pfeffer, K.; Virgin, H.W.; MacMicking, J.D.; Sibley, L.D. Guanylate-Binding Protein 1 (Gbp1) Contributes to Cell-Autonomous Immunity against Toxoplasma Gondii. PLoS Pathog. 2013, 9, e1003320. [Google Scholar] [CrossRef]
- Winter, S.V.; Niedelman, W.; Jensen, K.D.; Rosowski, E.E.; Julien, L.; Spooner, E.; Caradonna, K.; Burleigh, B.A.; Saeij, J.P.J.; Ploegh, H.L.; et al. Determinants of GBP Recruitment to Toxoplasma Gondii Vacuoles and the Parasitic Factors That Control It. PLoS ONE 2011, 6, e24434. [Google Scholar] [CrossRef] [Green Version]
- Selleck, E.M.; Orchard, R.C.; Lassen, K.G.; Beatty, W.L.; Xavier, R.J.; Levine, B.; Virgin, H.W.; Sibley, L.D. A Noncanonical Autophagy Pathway Restricts Toxoplasma Gondii Growth in a Strain-Specific Manner in IFN-γ-Activated Human Cells. mBio 2015, 6, e01157-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Sasai, M.; Ma, J.S.; Sakaguchi, N.; Ohshima, J.; Bando, H.; Saitoh, T.; Akira, S.; Yamamoto, M. P62 Plays a Specific Role in Interferon-γ-Induced Presentation of a Toxoplasma Vacuolar Antigen. Cell Rep. 2015, 13, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haldar, A.K.; Foltz, C.; Finethy, R.; Piro, A.S.; Feeley, E.M.; Pilla-Moffett, D.M.; Komatsu, M.; Frickel, E.-M.; Coers, J. Ubiquitin Systems Mark Pathogen-Containing Vacuoles as Targets for Host Defense by Guanylate Binding Proteins. Proc. Natl. Acad. Sci. USA 2015, 112, E5628–E5637. [Google Scholar] [CrossRef] [Green Version]
- Foltz, C.; Napolitano, A.; Khan, R.; Clough, B.; Hirst, E.M.; Frickel, E.-M. TRIM21 Is Critical for Survival of Toxoplasma Gondii Infection and Localises to GBP-Positive Parasite Vacuoles. Sci. Rep. 2017, 7, 5209. [Google Scholar] [CrossRef]
- Choi, J.; Park, S.; Biering, S.B.; Selleck, E.; Liu, C.Y.; Zhang, X.; Fujita, N.; Saitoh, T.; Akira, S.; Yoshimori, T.; et al. The Parasitophorous Vacuole Membrane of Toxoplasma Gondii Is Targeted for Disruption by Ubiquitin-like Conjugation Systems of Autophagy. Immunity 2014, 40, 924–935. [Google Scholar] [CrossRef] [Green Version]
- Sasai, M.; Sakaguchi, N.; Ma, J.S.; Nakamura, S.; Kawabata, T.; Bando, H.; Lee, Y.; Saitoh, T.; Akira, S.; Iwasaki, A.; et al. Essential Role for GABARAP Autophagy Proteins in Interferon-Inducible GTPase-Mediated Host Defense. Nat. Immunol. 2017, 18, 899–910. [Google Scholar] [CrossRef]
- Jabado, N.; Jankowski, A.; Dougaparsad, S.; Picard, V.; Grinstein, S.; Gros, P. Natural Resistance to Intracellular Infections. J. Exp. Med. 2000, 192, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Weinberg, E.D. Iron: Mammalian Defense Systems, Mechanisms of Disease, and Chelation Therapy Approaches. Blood Rev. 1995, 9, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Tremblay, M.L.; Govoni, G.; Gauthier, S.; Sebastiani, G.; Malo, D.; Skamene, E.; Olivier, M.; Jothy, S.; Gros, P. The Ity/Lsh/Bcg Locus: Natural Resistance to Infection with Intracellular Parasites Is Abrogated by Disruption of the Nramp1 Gene. J. Exp. Med. 1995, 182, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The Kynurenine Pathway: A Finger in Every Pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, E.R. Interferon Gamma Blocks the Growth of Toxoplasma Gondii in Human Fibroblasts by Inducing the Host Cells to Degrade Tryptophan. Proc. Natl. Acad. Sci. USA 1984, 81, 908–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Däubener, W.; Spors, B.; Hucke, C.; Adam, R.; Stins, M.; Kim, K.S.; Schroten, H. Restriction of Toxoplasma Gondii Growth in Human Brain Microvascular Endothelial Cells by Activation of Indoleamine 2,3-Dioxygenase. Infect. Immun. 2001, 69, 6527–6531. [Google Scholar] [CrossRef] [Green Version]
- Nagineni, C.N.; Pardhasaradhi, K.; Martins, M.C.; Detrick, B.; Hooks, J.J. Mechanisms of Interferon-Induced Inhibition of Toxoplasma Gondii Replication in Human Retinal Pigment Epithelial Cells. Infect. Immun. 1996, 64, 4188–4196. [Google Scholar] [CrossRef] [Green Version]
- Murray, H.W.; Szuro-Sudol, A.; Wellner, D.; Oca, M.J.; Granger, A.M.; Libby, D.M.; Rothermel, C.D.; Rubin, B.Y. Role of Tryptophan Degradation in Respiratory Burst-Independent Antimicrobial Activity of Gamma Interferon-Stimulated Human Macrophages. Infect. Immun. 1989, 57, 845–849. [Google Scholar] [CrossRef] [Green Version]
- Däubener, W.; Remscheid, C.; Nockemann, S.; Pilz, K.; Seghrouchni, S.; Mackenzie, C.; Hadding, U. Anti-Parasitic Effector Mechanisms in Human Brain Tumor Cells: Role of Interferon-Gamma and Tumor Necrosis Factor-Alpha. Eur. J. Immunol. 1996, 26, 487–492. [Google Scholar] [CrossRef]
- Gupta, S.L.; Carlin, J.M.; Pyati, P.; Dai, W.; Pfefferkorn, E.R.; Murphy, M.J. Antiparasitic and Antiproliferative Effects of Indoleamine 2,3-Dioxygenase Enzyme Expression in Human Fibroblasts. Infect. Immun. 1994, 62, 2277–2284. [Google Scholar] [CrossRef]
- Heseler, K.; Spekker, K.; Schmidt, S.K.; MacKenzie, C.R.; Däubener, W. Antimicrobial and Immunoregulatory Effects Mediated by Human Lung Cells: Role of IFN-Gamma-Induced Tryptophan Degradation. FEMS Immunol. Med. Microbiol. 2008, 52, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Divanovic, S.; Sawtell, N.M.; Trompette, A.; Warning, J.I.; Dias, A.; Cooper, A.M.; Yap, G.S.; Arditi, M.; Shimada, K.; DuHadaway, J.B.; et al. Opposing Biological Functions of Tryptophan Catabolizing Enzymes During Intracellular Infection. J. Infect. Dis. 2012, 205, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knubel, C.P.; Martínez, F.F.; Fretes, R.E.; Lujan, C.D.; Theumer, M.G.; Cervi, L.; Motrán, C.C. Indoleamine 2,3-dioxigenase (IDO) Is Critical for Host Resistance against Trypanosoma Cruzi. FASEB J. 2010, 24, 2689–2701. [Google Scholar] [CrossRef]
- Doolan, D.L.; Sedegah, M.; Hedstrom, R.C.; Hobart, P.; Yupin, C. Hoffman Circumventing Genetic Restriction of Protection against Malaria with Multigene DNA Immunization: CD8+ Cell-, Interferon Gamma-, and Nitric Oxide-Dependent Immunity. J. Exp. Med. 1996, 183, 1739–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Sa, Q.; Gehman, M.; Ochiai, E. Interferon-Gamma- and Perforin-Mediated Immune Responses for Resistance against Toxoplasma Gondii in the Brain. Expert Rev. Mol. Med. 2011, 13, e31. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Stevenson, M.M. Central Role of Endogenous Gamma Interferon in Protective Immunity against Blood-Stage Plasmodium Chabaudi AS Infection. Infect. Immun. 2000, 68, 4399–4406. [Google Scholar] [CrossRef] [Green Version]
- Wassmer, S.C.; Combes, V.; Candal, F.J.; Juhan-Vague, I.; Grau, G.E. Platelets Potentiate Brain Endothelial Alterations Induced by Plasmodium Falciparum. Infect. Immun. 2006, 74, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassmer, S.C.; Lépolard, C.; Traoré, B.; Pouvelle, B.; Gysin, J.; Grau, G.E. Platelets Reorient Plasmodium Falciparum-Infected Erythrocyte Cytoadhesion to Activated Endothelial Cells. J. Infect. Dis. 2004, 189, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Uzonna, J.E.; Kaushik, R.S.; Gordon, J.R.; Tabel, H. Cytokines and Antibody Responses during Trypanosoma Congolense Infections in Two Inbred Mouse Strains That Differ in Resistance. Parasite Immunol. 1999, 21, 57–71. [Google Scholar] [CrossRef]
- Tabel, H.; Kaushik, R.S.; Uzonna, J.E. Susceptibility and Resistance to Trypanosoma Congolense Infections. Microbes Infect. 2000, 2, 1619–1629. [Google Scholar] [CrossRef]
- Tabel, H.; Wei, G.; Shi, M. T Cells and Immunopathogenesis of Experimental African Trypanosomiasis. Immunol. Rev. 2008, 225, 128–139. [Google Scholar] [CrossRef]
- Stijlemans, B.; Beschin, A.; Magez, S.; Van Ginderachter, J.A.; De Baetselier, P. Iron Homeostasis and Trypanosoma Brucei Associated Immunopathogenicity Development: A Battle/Quest for Iron. Biomed Res. Int. 2015, 2015, 819389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teijaro, J.R.; Ng, C.; Lee, A.M.; Sullivan, B.M.; Sheehan, K.C.F.; Welch, M.; Schreiber, R.D.; de la Torre, J.C.; Oldstone, M.B.A. Persistent LCMV Infection Is Controlled by Blockade of Type I Interferon Signaling. Science 2013, 340, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, E.B.; Yamada, D.H.; Elsaesser, H.; Herskovitz, J.; Deng, J.; Cheng, G.; Aronow, B.J.; Karp, C.L.; Brooks, D.G. Blockade of Chronic Type I Interferon Signaling to Control Persistent LCMV Infection. Science 2013, 340, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Arimori, Y.; Nakamura, R.; Yamada, H.; Shibata, K.; Maeda, N.; Kase, T.; Yoshikai, Y. Type I Interferon Limits Influenza Virus-Induced Acute Lung Injury by Regulation of Excessive Inflammation in Mice. Antivir. Res. 2013, 99, 230–237. [Google Scholar] [CrossRef]
- Teles, R.M.B.; Graeber, T.G.; Krutzik, S.R.; Montoya, D.; Schenk, M.; Lee, D.J.; Komisopoulou, E.; Kelly-Scumpia, K.; Chun, R.; Iyer, S.S.; et al. Type I Interferon Suppresses Type II Interferon-Triggered Human Anti-Mycobacterial Responses. Science 2013, 339, 1448–1453. [Google Scholar] [CrossRef] [Green Version]
- Antonelli, L.R.V.; Rothfuchs, A.G.; Gonçalves, R.; Roffê, E.; Cheever, A.W.; Bafica, A.; Salazar, A.M.; Feng, C.G.; Sher, A. Intranasal Poly-IC Treatment Exacerbates Tuberculosis in Mice through the Pulmonary Recruitment of a Pathogen-Permissive Monocyte/Macrophage Population. J. Clin. Investig. 2010, 120, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.A.; Calderon, B.; Unanue, E.R. Type I Interferon Sensitizes Lymphocytes to Apoptosis and Reduces Resistance to Listeria Infection. J. Exp. Med. 2004, 200, 535–540. [Google Scholar] [CrossRef]
- Auerbuch, V.; Brockstedt, D.G.; Meyer-Morse, N.; O’Riordan, M.; Portnoy, D.A. Mice Lacking the Type I Interferon Receptor Are Resistant to Listeria Monocytogenes. J. Exp. Med. 2004, 200, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Liehl, P.; Zuzarte-Luís, V.; Chan, J.; Zillinger, T.; Baptista, F.; Carapau, D.; Konert, M.; Hanson, K.K.; Carret, C.; Lassnig, C.; et al. Host-Cell Sensors for Plasmodium Activate Innate Immunity against Liver-Stage Infection. Nat. Med. 2014, 20, 47–53. [Google Scholar] [CrossRef]
- Montes de Oca, M.; Kumar, R.; Rivera, F.D.L.; Amante, F.H.; Sheel, M.; Faleiro, R.J.; Bunn, P.T.; Best, S.E.; Beattie, L.; Ng, S.S.; et al. Type I Interferons Regulate Immune Responses in Humans with Blood-Stage Plasmodium Falciparum Infection. Cell Rep. 2016, 17, 399–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.C.; Nelson, C.S.; Wilson, E.B.; Hou, B.; DeFranco, A.L.; DeRisi, J.L. Splenic Red Pulp Macrophages Produce Type I Interferons as Early Sentinels of Malaria Infection but Are Dispensable for Control. PLoS ONE 2012, 7, e48126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, M.B.; Nguyen, Q.P.; Cordeiro, C.; Hassan, M.A.; Yang, N.; McKell, R.; Rosowski, E.E.; Julien, L.; Butty, V.; Dardé, M.-L.; et al. Transcriptional Analysis of Murine Macrophages Infected with Different Toxoplasma Strains Identifies Novel Regulation of Host Signaling Pathways. PLOS Pathog. 2013, 9, e1003779. [Google Scholar] [CrossRef]
- Beiting, D.P.; Peixoto, L.; Akopyants, N.S.; Beverley, S.M.; Wherry, E.J.; Christian, D.A.; Hunter, C.A.; Brodsky, I.E.; Roos, D.S. Differential Induction of TLR3-Dependent Innate Immune Signaling by Closely Related Parasite Species. PLoS ONE 2014, 9, e88398. [Google Scholar] [CrossRef] [Green Version]
- Koblansky, A.A.; Jankovic, D.; Oh, H.; Hieny, S.; Sungnak, W.; Mathur, R.; Hayden, M.S.; Akira, S.; Sher, A.; Ghosh, S. Recognition of Profilin by Toll-like Receptor 12 Is Critical for Host Resistance to Toxoplasma Gondii. Immunity 2013, 38, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Han, S.-J.; Melichar, H.J.; Coombes, J.L.; Chan, S.W.; Koshy, A.A.; Boothroyd, J.C.; Barton, G.M.; Robey, E.A. Internalization and TLR-Dependent Type I Interferon Production by Monocytes in Response to Toxoplasma Gondii. Immunol. Cell Biol. 2014, 92, 872–881. [Google Scholar] [CrossRef] [Green Version]
- Diefenbach, A.; Schindler, H.; Donhauser, N.; Lorenz, E.; Laskay, T.; MacMicking, J.; Röllinghoff, M.; Gresser, I.; Bogdan, C. Type 1 Interferon (IFNα/β) and Type 2 Nitric Oxide Synthase Regulate the Innate Immune Response to a Protozoan Parasite. Immunity 1998, 8, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Ives, A.; Ronet, C.; Prevel, F.; Ruzzante, G.; Fuertes-Marraco, S.; Schutz, F.; Zangger, H.; Revaz-Breton, M.; Lye, L.-F.; Hickerson, S.M.; et al. Leishmania RNA Virus Controls the Severity of Mucocutaneous Leishmaniasis. Science 2011, 331, 775–778. [Google Scholar] [CrossRef] [Green Version]
- Dias, B.T.; Goundry, A.; Vivarini, A.C.; Costa, T.F.R.; Mottram, J.C.; Lopes, U.G.; Lima, A.P.C.A. Toll-Like Receptor- and Protein Kinase R-Induced Type I Interferon Sustains Infection of Leishmania Donovani in Macrophages. Front. Immunol. 2022, 13, 801182. [Google Scholar] [CrossRef]
- Sonnenfeld, G.; Kierszenbaum, F. Increased Serum Levels of an Interferon-like Activity during the Acute Period of Experimental Infection with Different Strains of Trypanosoma Cruzi. Am. J. Trop. Med. Hyg. 1981, 30, 1189–1191. [Google Scholar] [CrossRef]
- Kierszenbaum, F.; Sonnenfeld, G. Characterization of the Antiviral Activity Produced during Trypanosoma Cruzi Infection and Protective Effects of Exogenous Interferon against Experimental Chagas’ Disease. J. Parasitol. 1982, 68, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Une, C.; Andersson, J.; Eloranta, M.-L.; Sunnemark, D.; Harris, R.A.; Örn, A. Enhancement of Natural Killer (NK) Cell Cytotoxicity and Induction of NK Cell-Derived Interferon-Gamma (IFN-γ) Display Different Kinetics during Experimental Infection with Trypanosoma Cruzi. Clin. Exp. Immunol. 2000, 121, 499–505. [Google Scholar] [CrossRef] [PubMed]
- de Avalos, S.V.; Blader, I.J.; Fisher, M.; Boothroyd, J.C.; Burleigh, B.A. Immediate/Early Response to Trypanosoma Cruzi Infection Involves Minimal Modulation of Host Cell Transcription. J. Biol. Chem. 2002, 277, 639–644. [Google Scholar] [CrossRef] [Green Version]
- Chessler, A.-D.C.; Unnikrishnan, M.; Bei, A.K.; Daily, J.P.; Burleigh, B.A. Trypanosoma Cruzi Triggers an Early Type I IFN Response In Vivo at the Site of Intradermal Infection. J. Immunol. 2009, 182, 2288–2296. [Google Scholar] [CrossRef] [Green Version]
- Koga, R.; Hamano, S.; Kuwata, H.; Atarashi, K.; Ogawa, M.; Hisaeda, H.; Yamamoto, M.; Akira, S.; Himeno, K.; Matsumoto, M.; et al. TLR-Dependent Induction of IFN-β Mediates Host Defense against Trypanosoma Cruzi. J. Immunol. 2006, 177, 7059–7066. [Google Scholar] [CrossRef] [Green Version]
- Barakat, F.M.; McDonald, V.; Foster, G.R.; Tovey, M.G.; Korbel, D.S. Cryptosporidium parvum Infection Rapidly Induces a Protective Innate Immune Response Involving Type I Interferon. J. Infect. Dis. 2009, 200, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Heo, I.; Dutta, D.; Schaefer, D.A.; Iakobachvili, N.; Artegiani, B.; Sachs, N.; Boonekamp, K.E.; Bowden, G.; Hendrickx, A.P.A.; Willems, R.J.L.; et al. Modelling Cryptosporidium Infection in Human Small Intestinal and Lung Organoids. Nat. Microbiol. 2018, 3, 814–823. [Google Scholar] [CrossRef]
- Gibson, A.R.; Sateriale, A.; Dumaine, J.E.; Engiles, J.B.; Pardy, R.D.; Gullicksrud, J.A.; O’Dea, K.M.; Doench, J.G.; Beiting, D.P.; Hunter, C.A.; et al. A Genetic Screen Identifies a Protective Type III Interferon Response to Cryptosporidium That Requires TLR3 Dependent Recognition. PLoS Pathog. 2022, 18, e1010003. [Google Scholar] [CrossRef]
- Costa, V.M.A.; Torres, K.C.L.; Mendonça, R.Z.; Gresser, I.; Gollob, K.J.; Abrahamsohn, I.A. Type I IFNs Stimulate Nitric Oxide Production and Resistance to Trypanosoma Cruzi Infection. J. Immunol. 2006, 177, 3193–3200. [Google Scholar] [CrossRef] [Green Version]
- Lopez, R.; Demick, K.P.; Mansfield, J.M.; Paulnock, D.M. Type I IFNs Play a Role in Early Resistance, but Subsequent Susceptibility, to the African Trypanosomes. J. Immunol. 2008, 181, 4908–4917. [Google Scholar] [CrossRef]
- Colina, R.; Costa-Mattioli, M.; Dowling, R.J.O.; Jaramillo, M.; Tai, L.-H.; Breitbach, C.J.; Martineau, Y.; Larsson, O.; Rong, L.; Svitkin, Y.V.; et al. Translational Control of the Innate Immune Response through IRF-7. Nature 2008, 452, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.; Gomez, M.A.; Larsson, O.; Shio, M.T.; Topisirovic, I.; Contreras, I.; Luxenburg, R.; Rosenfeld, A.; Colina, R.; McMaster, R.W.; et al. Leishmania Repression of Host Translation through MTOR Cleavage Is Required for Parasite Survival and Infection. Cell Host Microbe 2011, 9, 331–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Carvalho, L.P.; Bhattacharya, S.; Carbone, C.J.; Kumar, K.G.S.; Leu, N.A.; Yau, P.M.; Donald, R.G.K.; Weiss, M.J.; Baker, D.P.; et al. Mammalian Casein Kinase 1α and Its Leishmanial Ortholog Regulate Stability of IFNAR1 and Type I Interferon Signaling. Mol. Cell. Biol. 2009, 29, 6401–6412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosowski, E.E.; Nguyen, Q.P.; Camejo, A.; Spooner, E.; Saeij, J.P.J. Toxoplasma Gondii Inhibits Gamma Interferon (IFN-γ)- and IFN-β-Induced Host Cell STAT1 Transcriptional Activity by Increasing the Association of STAT1 with DNA. Infect. Immun. 2014, 82, 706–719. [Google Scholar] [CrossRef] [Green Version]
- Rocha, B.C.; Marques, P.E.; Leoratti, F.M.d.S.; Junqueira, C.; Pereira, D.B.; Antonelli, L.R.d.V.; Menezes, G.B.; Golenbock, D.T.; Gazzinelli, R.T. Type I Interferon Transcriptional Signature in Neutrophils and High Frequency of Low-Density Granulocytes Are Associated with Tissue Damage in Malaria. Cell Rep. 2015, 13, 2829–2841. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; DeOliveira, R.B.; Kalantari, P.; Parroche, P.; Goutagny, N.; Jiang, Z.; Chan, J.; Bartholomeu, D.C.; Lauw, F.; Hall, J.P.; et al. Innate Immune Recognition of an AT-Rich Stem-Loop DNA Motif in the Plasmodium Falciparum Genome. Immunity 2011, 35, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Haque, A.; Best, S.E.; Montes de Oca, M.; James, K.R.; Ammerdorffer, A.; Edwards, C.L.; de Labastida Rivera, F.; Amante, F.H.; Bunn, P.T.; Sheel, M.; et al. Type I IFN Signaling in CD8– DCs Impairs Th1-Dependent Malaria Immunity. J. Clin. Investig. 2014, 124, 2483–2496. [Google Scholar] [CrossRef] [Green Version]
- Khouri, R.; Bafica, A.; Silva, M.D.P.P.; Noronha, A.; Kolb, J.-P.; Wietzerbin, J.; Barral, A.; Barral-Netto, M.; Van Weyenbergh, J. IFN-Beta Impairs Superoxide-Dependent Parasite Killing in Human Macrophages: Evidence for a Deleterious Role of SOD1 in Cutaneous Leishmaniasis. J. Immunol. 2009, 182, 2525–2531. [Google Scholar] [CrossRef] [Green Version]
- Xin, L.; Vargas-Inchaustegui, D.A.; Raimer, S.S.; Kelly, B.C.; Hu, J.; Zhu, L.; Sun, J.; Soong, L. Type I IFN Receptor Regulates Neutrophil Functions and Innate Immunity to Leishmania Parasites. J. Immunol. 2010, 184, 7047–7056. [Google Scholar] [CrossRef] [Green Version]
- Une, C.; Andersson, J.; Örn, A. Role of IFN-α/β and IL-12 in the Activation of Natural Killer Cells and Interferon-γ Production during Experimental Infection with Trypanosoma Cruzi. Clin. Exp. Immunol. 2003, 134, 195–201. [Google Scholar] [CrossRef]
- Bankoti, R.; Gupta, K.; Levchenko, A.; Stäger, S. Marginal Zone B Cells Regulate Antigen-Specific T Cell Responses during Infection. J. Immunol. 2012, 188, 3961–3971. [Google Scholar] [CrossRef] [Green Version]
- Silva-Barrios, S.; Smans, M.; Duerr, C.U.; Qureshi, S.T.; Fritz, J.H.; Descoteaux, A.; Stäger, S. Innate Immune B Cell Activation by Leishmania Donovani Exacerbates Disease and Mediates Hypergammaglobulinemia. Cell Rep. 2016, 15, 2427–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stifter, S.A.; Feng, C.G. Interfering with Immunity: Detrimental Role of Type I IFNs during Infection. J. Immunol. 2015, 194, 2455–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.M.; Jones, M.B.; Ongoiba, A.; Bijker, E.M.; Schats, R.; Venepally, P.; Skinner, J.; Doumbo, S.; Quinten, E.; Visser, L.G.; et al. Transcriptomic Evidence for Modulation of Host Inflammatory Responses during Febrile Plasmodium Falciparum Malaria. Sci. Rep. 2016, 6, 31291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, W.O.; Pepper, M.; Liles, W.C. B Cell Intrinsic Expression of IFNλ Receptor Suppresses the Acute Humoral Immune Response to Experimental Blood-Stage Malaria. Virulence 2020, 11, 594–606. [Google Scholar] [CrossRef]
- Ferguson, S.H.; Foster, D.M.; Sherry, B.; Magness, S.T.; Nielsen, D.M.; Gookin, J.L. Interferon-Λ3 Promotes Epithelial Defense and Barrier Function Against Cryptosporidium Parvum Infection. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef]
- Manry, J.; Laval, G.; Patin, E.; Fornarino, S.; Itan, Y.; Fumagalli, M.; Sironi, M.; Tichit, M.; Bouchier, C.; Casanova, J.-L.; et al. Evolutionary Genetic Dissection of Human Interferons. J. Exp. Med. 2011, 208, 2747–2759. [Google Scholar] [CrossRef] [Green Version]
- Manry, J.; Laval, G.; Patin, E.; Fornarino, S.; Tichit, M.; Bouchier, C.; Barreiro, L.B.; Quintana-Murci, L. Evolutionary Genetics Evidence of an Essential, Nonredundant Role of the IFN-γ Pathway in Protective Immunity. Hum. Mutat. 2011, 32, 633–642. [Google Scholar] [CrossRef]



| Protective Effects | |||
| Parasite Species | Treatments and Findings | Effects | Ref. |
| P. yoelii | IFNαβR−/− mice | Liver infection burden ↑ Parasitemia ↑ | [34,140] |
| L. major | IFN-αβ neutralizing antibody in mice | Early parasite spreading ↑ | [125] |
| C. parvum | Recombinant IFN-αβ in murine enterocyte cell culture | Parasite development ↓ | [156] |
| IFN-αβ neutralizing antibody in mice | Oocyst reproduction ↑ Gut epithelium infection burden ↑ | [156] | |
| T. cruzi | IFNαβR−/− mice | Acute phase parasitemia ↑ | [159] |
| T. b. rhodesiense | IFNAR1−/− mice | Early parasitemia clearance ↓ | [160] |
| Pathogenic Effects | |||
| Parasite Species | Treatments and Findings | Effects | Ref. |
| P. chabaudi | IFNαβR−/− mice | Liver damage ↓ | [165] |
| P. berghei | IFNAR1−/− mice | Survival ↑ | [166] |
| CD11c-Ifnar1−/− mice | Neurological symptoms ↓ Survival ↑ | [167] | |
| L. amazonensis, L. brazilliensis | Recombinant IFN-β in human macrophage cell culture | Infection burden ↑ | [168] |
| IFNAR−/− mice | Infection burden ↓ | [169] | |
| C. parvum | IFNAR1−/− mice | Infection burden ↓ | [158] |
| T. b. rhodesiense | IFN-αβ hypersensitive mice | Late phase parasite burden ↑ | [160] |
| T. cruzi | IFNαβR−/− mice | Survival ↑ | [170] |
| Protective Effects | |||
| Parasite Species | Treatments and Findings | Effects | Ref. |
| C. parvum | IFNLR1−/− mice | Infection burden ↑ | [158] |
| IFN-λ neutralizing antibody in mice | Infection burden ↑ Oocyst reproduction ↑ | [176] | |
| Recombinant IFN-λ in human intestinal epithelial cell culture | parasite development ↓ | [176] | |
| Pathogenic Effects | |||
| Parasite Species | Treatments and Findings | Effects | Ref. |
| P. yoelli | IFNLR1−/− mice | Infection burden ↓ | [175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Graham, M.L.; Chen, X.-M. The Complexity of Interferon Signaling in Host Defense against Protozoan Parasite Infection. Pathogens 2023, 12, 319. https://doi.org/10.3390/pathogens12020319
Deng S, Graham ML, Chen X-M. The Complexity of Interferon Signaling in Host Defense against Protozoan Parasite Infection. Pathogens. 2023; 12(2):319. https://doi.org/10.3390/pathogens12020319
Chicago/Turabian StyleDeng, Silu, Marion L. Graham, and Xian-Ming Chen. 2023. "The Complexity of Interferon Signaling in Host Defense against Protozoan Parasite Infection" Pathogens 12, no. 2: 319. https://doi.org/10.3390/pathogens12020319
APA StyleDeng, S., Graham, M. L., & Chen, X.-M. (2023). The Complexity of Interferon Signaling in Host Defense against Protozoan Parasite Infection. Pathogens, 12(2), 319. https://doi.org/10.3390/pathogens12020319






