Anti-Fibrotic Effect of SDF-1β Overexpression in Bleomycin-Injured Rat Lung
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Primary IPF Lung Fibroblasts
2.3. Plasmid
2.4. In Vitro Transfection
2.5. Wound-Healing Assay
2.6. Animals
2.7. Bleomycin Instillation
2.8. pSDF-1 In Vivo Electroporation-Mediated SDF-1 Gene Transfer
2.9. Assessment
2.10. Hydroxyproline Assay
2.11. Morphological Analyses at Light and Electron Microscopic Level
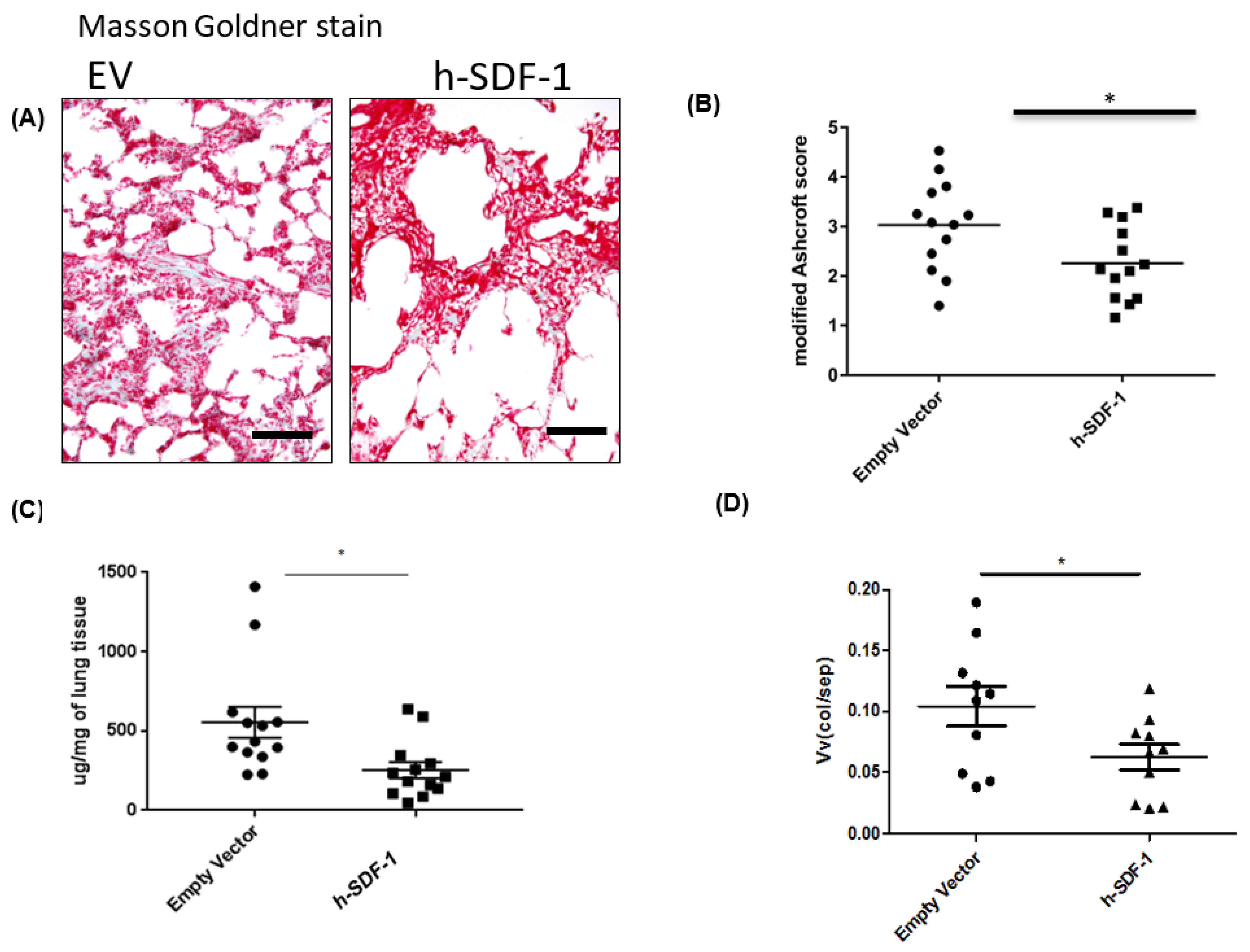
2.12. Masson–Goldner Staining
2.13. Western Blot
2.14. Flow Cytometry
2.15. ELISA
2.16. Statistics
3. Results
3.1. Efficient Electroporation-Mediated pSDF-1β Gene Transfer in Bleomycin-Injured Rat Lungs
3.2. pSDF-1 Gene Transfer-Reduced Total Collagen Content and Improved Histology in Bleomycin-Injured Rat Lung
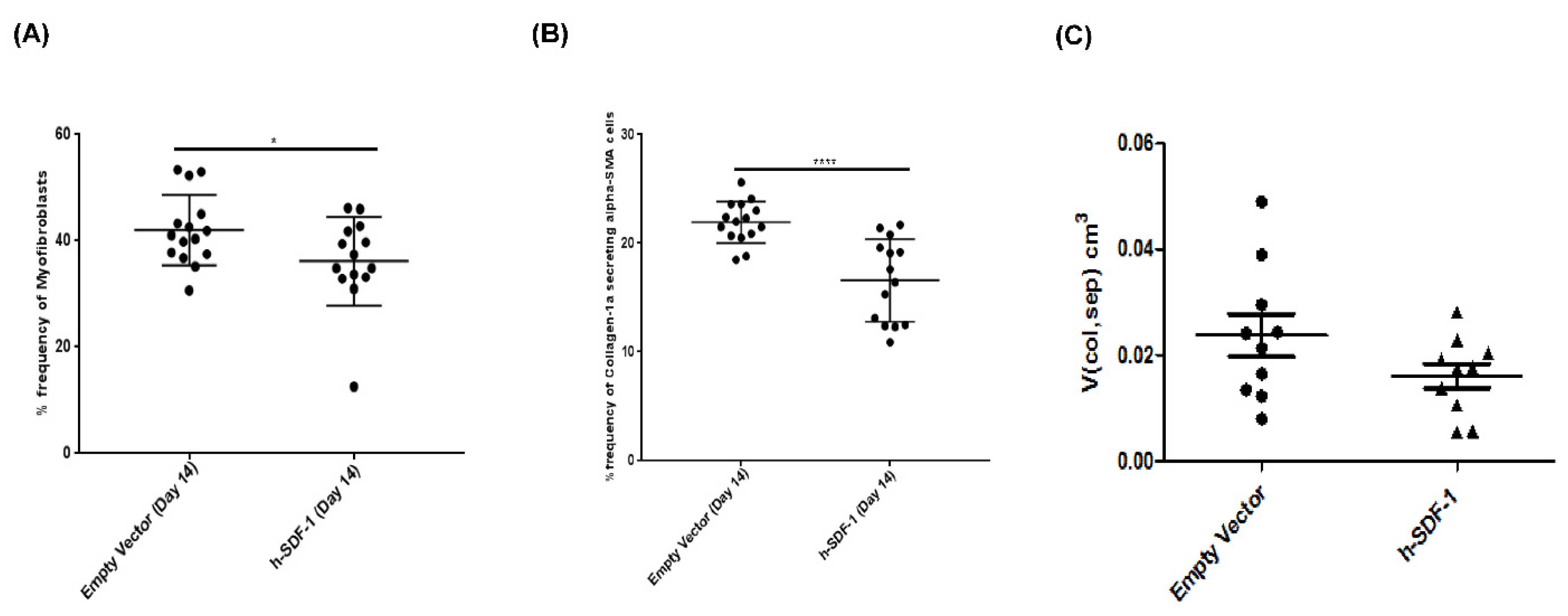
3.3. Composition of the Parenchymatous Lung Tissue after pSDF-1β Gene Transfer in Bleomycin-Injured Rat Lung
3.4. pSDF-1β Overexpression Reduces Collagen Producing myofibroblasts in the Bleomycin-Injured Rat Lung
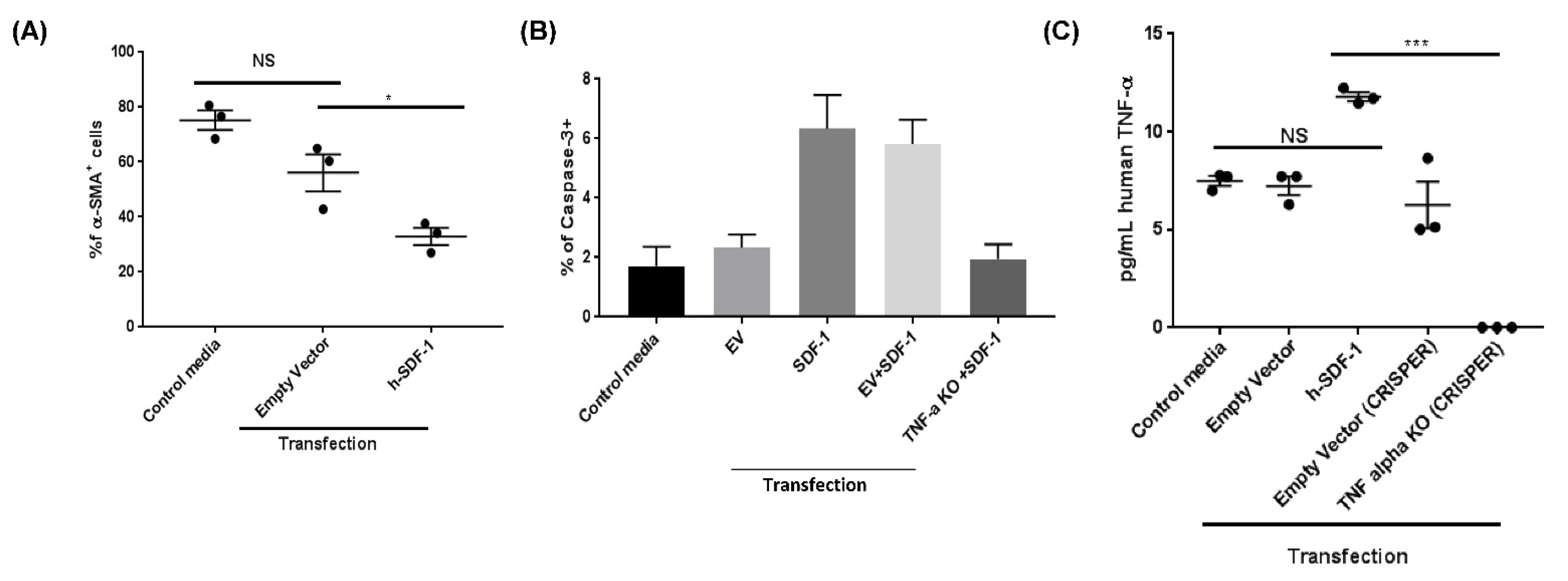
3.5. pSDF-1β Overexpression Induces Apoptosis in the Myofibroblasts in the Bleomycin-Injured rAT Lung
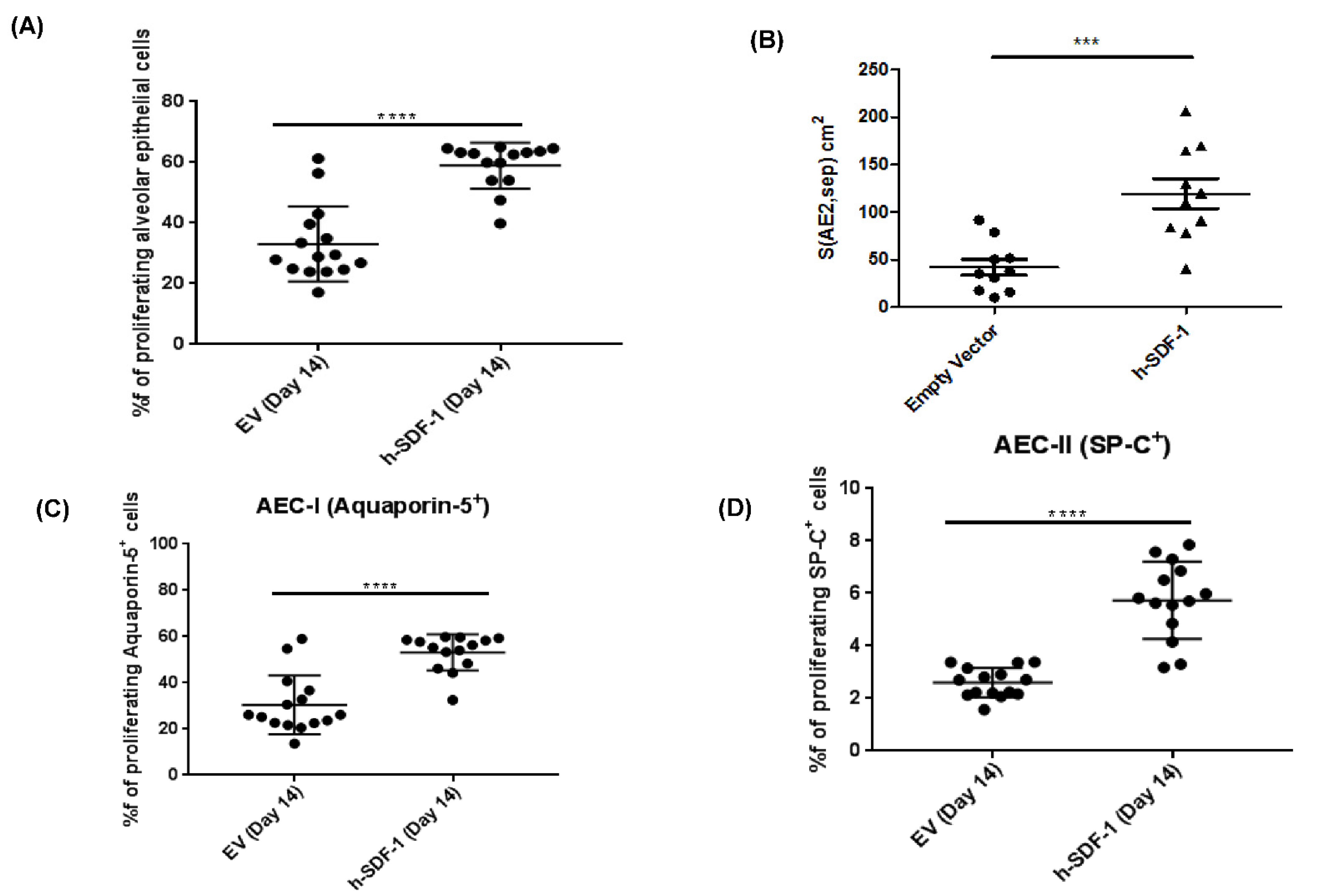
3.6. pSDF-1β Overexpression Increases Number of Alveolar Epithelial Cells, Increases the Epithelial Basal Lamina Covering Epithelial Cells and Stimulates Proliferation of Alveolar Epithelial Cells in the Bleomycin-Injured Rat Lung
3.7. pSDF-1β Induces TNF-α-Mediated Apoptosis in IPF Lung Fibroblasts In Vitro
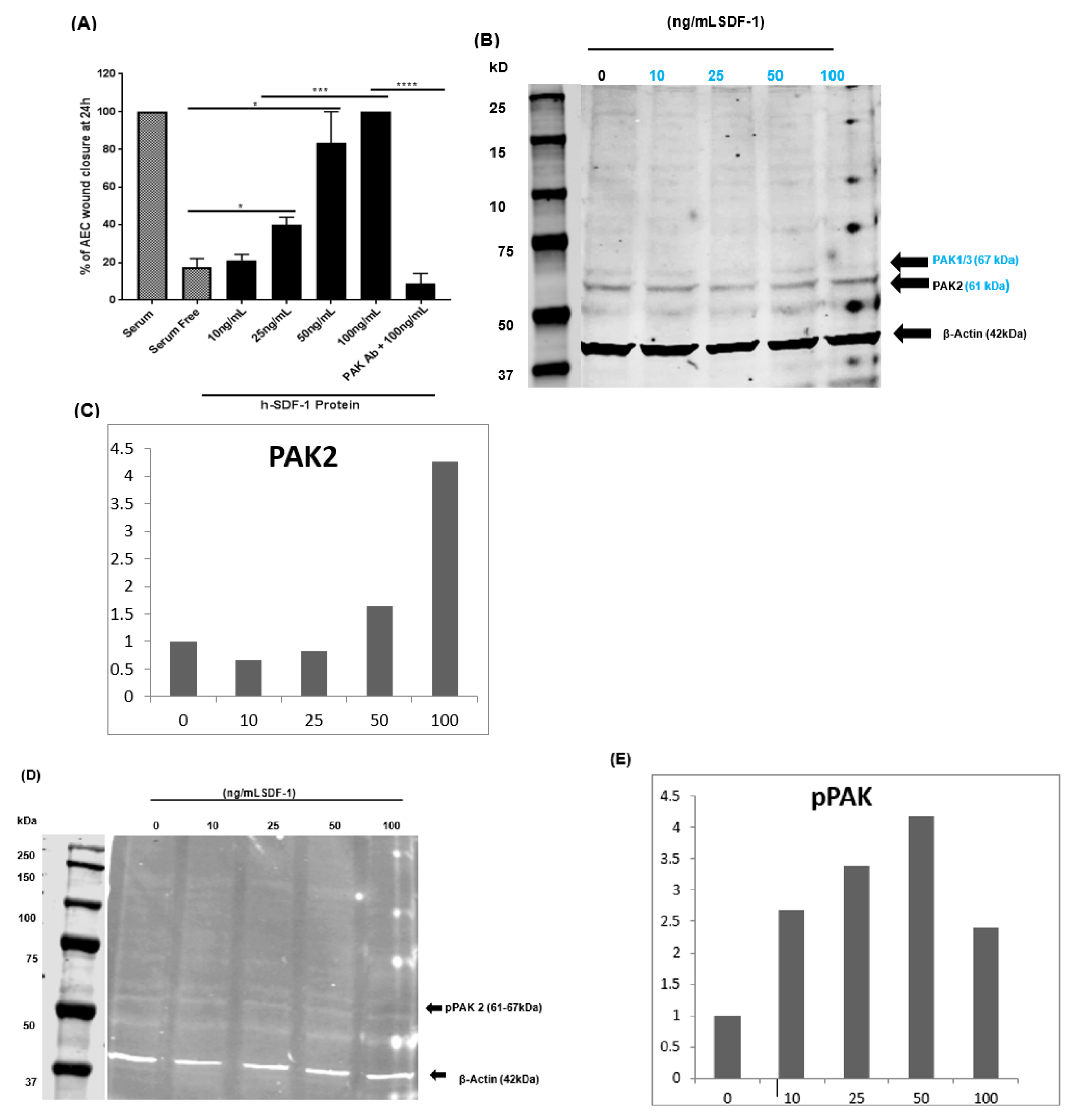
3.8. pSDF-1 Improves In Vitro Alveolar Epithelial Wound Repair via the PAK 2 Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Martinez, F.J.; Chisholm, A.; Collard, H.R.; Flaherty, K.R.; Myers, J.; Raghu, G.; Walsh, S.L.; White, E.S.; Richeldi, L. The diagnosis of idiopathic pulmonary fibrosis: Current and future approaches. Lancet Respir. Med. 2017, 5, 61–71. [Google Scholar] [CrossRef]
- Olson, A.L.; Gifford, A.H.; Inase, N.; Fernández Pérez, E.R.; Suda, T. The epidemiology of idiopathic pulmonary fibrosis and interstitial lung diseases at risk of a progressive-fibrosing phenotype. Eur. Respir. Rev. 2018, 27, 180077. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.; Langford, B.; Diamantopoulos, A.; Rohr, K.; Baldwin, M.; Inoue, Y. Estimating long-term survival in progressive fibrosing interstitial lung disease (PF-ILD) other than IPF using matched IPF data. Eur. Respir. J. 2021, 58, OA4238. [Google Scholar] [CrossRef]
- Langford, B.; Diamantopoulos, A.; Maher, T.M.; Inoue, Y.; Rohr, K.B.; Baldwin, M. Using Data on Survival with Idiopathic Pulmonary Fibrosis to Estimate Survival with Other Types of Progressive Fibrosis Interstitial Lung Disease: A Bayesian Framework. Adv. Ther. 2022, 39, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Wolters, P.J.; Collard, H.R.; Jones, K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 2014, 9, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef]
- Todd, N.W.; Luzina, I.G.; Atamas, S.P. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 2012, 5, 11. [Google Scholar] [CrossRef]
- Habiel, D.M.; Hogaboam, C.M. Heterogeneity of Fibroblasts and Myofibroblasts in Pulmonary Fibrosis. Curr. Pathobiol. Rep. 2017, 5, 101–110. [Google Scholar] [CrossRef]
- Barron, L.; Gharib, S.A.; Duffield, J.S. Lung Pericytes and Resident Fibroblasts: Busy Multitaskers. Am. J. Pathol. 2016, 186, 2519–2531. [Google Scholar] [CrossRef]
- El Agha, E.; Kramann, R.; Schneider, R.K.; Li, X.; Seeger, W.; Humphreys, B.D.; Bellusci, S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell 2017, 21, 166–177. [Google Scholar] [CrossRef]
- Phan, S.H. Genesis of the myofibroblast in lung injury and fibrosis. Proc. Am. Thorac. Soc. 2012, 9, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.-L.; Gabbiani, G. The Myofibroblast: One Function, Multiple Origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Zuba-Surma, E.; Kucia, M.; Reca, R.; Wojakowski, W.; Ratajczak, J. The pleiotropic effects of the SDF-1–CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia 2006, 20, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Kollet, O.; Lapidot, T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp. Hematol. 2006, 34, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mora, A.; Shim, H.; Stecenko, A.; Brigham, K.L.; Rojas, M. Role of the SDF-1/CXCR4 Axis in the Pathogenesis of Lung Injury and Fibrosis. Am. J. Respir. Cell Mol. Biol. 2007, 37, 291–299. [Google Scholar] [CrossRef]
- Xie, L.; Zhao, L. Role of CXCL12/CXCR4-Mediated Circulating Fibrocytes in Pulmonary Fibrosis. J. Biomed. Mater. Res. A 2017, 2, 134–139. [Google Scholar] [CrossRef]
- Chow, L.N.; Schreiner, P.; Ng, B.Y.Y.; Lo, B.; Hughes, M.R.; Scott, R.W.; Gusti, V.; Lecour, S.; Simonson, E.; Manisali, I.; et al. Impact of a CXCL12/CXCR4 Antagonist in Bleomycin (BLM) Induced Pulmonary Fibrosis and Carbon Tetrachloride (CCl4) Induced Hepatic Fibrosis in Mice. PLoS ONE 2016, 11, e0151765. [Google Scholar] [CrossRef]
- Griffiths, K.; Habiel, D.M.; Jaffar, J.; Binder, U.; Darby, W.G.; Hosking, C.G.; Skerra, A.; Westall, G.P.; Hogaboam, C.M.; Foley, M. Anti-fibrotic Effects of CXCR4-Targeting i-body AD-114 in Preclinical Models of Pulmonary Fibrosis. Sci. Rep. 2018, 8, 3212. [Google Scholar] [CrossRef]
- Makino, H.; Aono, Y.; Azuma, M.; Kishi, M.; Yokota, Y.; Kinoshita, K.; Takezaki, A.; Kishi, J.; Kawano, H.; Ogawa, H.; et al. Antifibrotic effects of CXCR4 antagonist in bleomycin-induced pulmonary fibrosis in mice. J. Med. Investig. 2013, 60, 127–137. [Google Scholar] [CrossRef]
- Weigold, F.; Günther, J.; Pfeiffenberger, M.; Cabral-Marques, O.; Siegert, E.; Dragun, D.; Philippe, A.; Regensburger, A.-K.; Recke, A.; Yu, X.; et al. Antibodies against chemokine receptors CXCR3 and CXCR4 predict progressive deterioration of lung function in patients with systemic sclerosis. Arthritis Res. Ther. 2018, 20, 52. [Google Scholar] [CrossRef] [Green Version]
- Thelen, M.; Thelen, S. CXCR7, CXCR4 and CXCL12: An eccentric trio? J. Neuroimmunol. 2008, 198, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Gazdhar, A.; Susuri, N.; Hostettler, K.; Gugger, M.; Knudsen, L.; Roth, M.; Ochs, M.; Geiser, T. HGF Expressing Stem Cells in Usual Interstitial Pneumonia Originate from the Bone Marrow and Are Antifibrotic. PLoS ONE 2013, 8, e65453. [Google Scholar] [CrossRef]
- Tamm, M.; Roth, M.; Malouf, M.; Chhajed, P.; Johnson, P.; Black, J.; Glanville, A. Primary Fibroblast cell cultures from transbronchial biopsies of lung transplant recipients. Transplantation 2001, 71, 337–339. [Google Scholar] [CrossRef]
- Gazdhar, A.; Temuri, A.; Knudsen, L.; Gugger, M.; Schmid, R.A.; Ochs, M.; Geiser, T. Targeted gene transfer of hepatocyte growth factor to alveolar type II epithelial cells reduces lung fibrosis in rats. Hum. Gene Ther. 2013, 24, 105–116. [Google Scholar] [CrossRef]
- Tschanz, S.; Schneider, J.P.; Knudsen, L. Design-based stereology: Planning, volumetry and sampling are crucial steps for a successful study. Ann. Anat.-Anat. Anz. 2014, 196, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hübner, R.-H.; Gitter, W.; Mokhtari, N.E.E.; Mathiak, M.; Both, M.; Bolte, H.; Freitag-Wolf, S.; Bewig, B. Standardized quantification of pulmonary fibrosis in histological samples. BioTechniques 2008, 44, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Tschanz, S.A.; Burri, P.H.; Weibel, E.R. A simple tool for stereological assessment of digital images: The STEPanizer. J. Microsc. 2011, 243, 47–59. [Google Scholar] [CrossRef]
- Lutz, D.; Gazdhar, A.; Lopez-Rodriguez, E.; Ruppert, C.; Mahavadi, P.; Günther, A.; Klepetko, W.; Bates, J.H.; Smith, B.; Geiser, T.; et al. Alveolar Derecruitment and Collapse Induration as Crucial Mechanisms in Lung Injury and Fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 52, 232–243. [Google Scholar] [CrossRef]
- Tamò, L.; Simillion, C.; Hibaoui, Y.; Feki, A.; Gugger, M.; Prasse, A.; Jäger, B.; Goldmann, T.; Geiser, T.; Gazdhar, A. Gene Network Analysis of Interstitial Macrophages After Treatment with Induced Pluripotent Stem Cells Secretome (iPSC-cm) in the Bleomycin Injured Rat Lung. Stem Cell Rev. Rep. 2018, 14, 412–424. [Google Scholar] [CrossRef]
- Hostettler, K.E.; Gazdhar, A.; Khan, P.; Savic, S.; Tamo, L.; Lardinois, D.; Roth, M.; Tamm, M.; Geiser, T. Multipotent mesenchymal stem cells in lung fibrosis. PLoS ONE 2017, 12, e0181946. [Google Scholar] [CrossRef] [Green Version]
- Janowski, M. Functional diversity of SDF-1 splicing variants. Cell Adh. Migr. 2009, 3, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, K.; Gupta, S.K. Cloning and Relative Expression Analysis of Rat Stromal Cell Derived Factor-1 (SDF-1): SDF-1 α mRNA Is Selectively Induced in Rat Model of Myocardial Infarction. Inflammation 2001, 25, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Tesz, G.J.; Guntur, K.V.P.; Czech, M.P. Tumor Necrosis Factor-α Induces Caspase-mediated Cleavage of Peroxisome Proliferator-activated Receptor γ in Adipocytes. J. Biol. Chem. 2009, 284, 17082–17091. [Google Scholar] [CrossRef] [PubMed]
- Jarrah, A.A.; Schwarskopf, M.; Wang, E.R.; LaRocca, T.; Dhume, A.; Zhang, S.; Hadri, L.; Hajjar, R.J.; Schecter, A.D.; Tarzami, S.T. SDF-1 induces TNF-mediated apoptosis in cardiac myocytes. Apoptosis 2018, 23, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Araki, K.; Vesin, C.; Garcia, I.; Kapanci, Y.; Whitsett, J.A.; Piguet, P.F.; Vassalli, P. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J. Clin. Investig. 1995, 96, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.K.; Eliseeva, S.; Rahimi, H.; Schwarz, E.M.; Georas, S.N. Restrictive lung disease in TNF-transgenic mice: Correlation of pulmonary function testing and micro-CT imaging. Exp. Lung Res. 2019, 45, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Redente, E.F.; Keith, R.C.; Janssen, W.; Henson, P.M.; Ortiz, L.A.; Downey, G.P.; Bratton, D.L.; Riches, D.W.H. Tumor Necrosis Factor-α Accelerates the Resolution of Established Pulmonary Fibrosis in Mice by Targeting Profibrotic Lung Macrophages. Am. J. Respir. Cell Mol. Biol. 2014, 50, 825–837. [Google Scholar] [CrossRef]
- Phan, S.H.; Kunkel, S.L. Lung Cytokine Production in Bleomycin-Induced Pulmonary Fibrosis. Exp. Lung Res. 1992, 18, 29–43. [Google Scholar] [CrossRef]
- Ilhan, A.; Nabokikh, A.; Maj, M.; Vidakovic, M.; Nielsen, J.H.; Prikoszovich, T.; Niederle, B.; Base, W.; Luger, A.; Wagner, L. CXCL12/SDF-1 over-expression in human insulinomas and its biological relevance. Mol. Cell. Endocrinol. 2009, 298, 1–10. [Google Scholar] [CrossRef]
- Colamussi, M.L.; Secchiero, P.; Zella, D.; Curreli, S.; Mirandola, P.; Capitani, S.; Zauli, G. Stromal derived factor-1α induces apoptosis in activated primary CD4+ T cells. AIDS 2000, 14, 748–750. [Google Scholar] [CrossRef]
- Han, Y.; He, T.; Huang, D.; Pardo, C.A.; Ransohoff, R.M. TNF-α mediates SDF-1α–induced NF-κB activation and cytotoxic effects in primary astrocytes. J. Clin. Investig. 2001, 108, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Pardo, A. Revealing the Pathogenic and Aging-related Mechanisms of the Enigmatic Idiopathic Pulmonary Fibrosis. An Integral Model. Am. J. Respir. Crit. Care Med. 2014, 189, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Johanesen, P.A.; Wendt, M.K.; Binion, D.G.; Dwinell, M.B. CXCL12 activation of CXCR4 regulates mucosal host defense through stimulation of epithelial cell migration and promotion of intestinal barrier integrity. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288, G316–G326. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chai, L.; Chen, L.; Chen, W.; Ge, L.; Li, X.; Li, H.; Li, S.; Cao, C. Stromal cell-derived factor 1 (SDF-1) accelerated skin wound healing by promoting the migration and proliferation of epidermal stem cells. Vitr. Cell. Dev. Biol.-Anim. 2015, 51, 578–585. [Google Scholar] [CrossRef]
- Gericke, A.; Wang, X.; Ackermann, M.; Neufurth, M.; Wiens, M.; Schröder, H.C.; Pfeiffer, N.; Müller, W.E.G. Utilization of metabolic energy in treatment of ocular surface disorders: Polyphosphate as an energy source for corneal epithelial cell proliferation. RSC Adv. 2019, 9, 22531–22539. [Google Scholar] [CrossRef]
- Kanter, J.A.; Sun, H.; Chiu, S.; DeCamp, M.M.; Sporn, P.H.; Sznajder, J.I.; Bharat, A. Decreased CXCL12 is associated with impaired alveolar epithelial cell migration and poor lung healing after lung resection. Surgery 2015, 158, 1073–1080, discussion 1080–1072. [Google Scholar] [CrossRef]
- Wu, Y.; Yoder, A. Chemokine Coreceptor Signaling in HIV-1 Infection and Pathogenesis. PLoS Pathog. 2009, 5, e1000520. [Google Scholar] [CrossRef]
- Gao, C.; Ma, T.; Pang, L.; Xie, R. Activation of P21-activated protein kinase 2 is an independent prognostic predictor for patients with gastric cancer. Diagn. Pathol. 2014, 9, 55. [Google Scholar] [CrossRef]
- Lovisa, S. Epithelial-to-Mesenchymal Transition in Fibrosis: Concepts and Targeting Strategies. Front. Pharmacol. 2021, 12, 737570. [Google Scholar] [CrossRef]
- Hewlett, J.C.; Kropski, J.A.; Blackwell, T.S. Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018, 71–72, 112–127. [Google Scholar] [CrossRef]
- Penn, M.S.; Mendelsohn, F.O.; Schaer, G.L.; Sherman, W.; Farr, M.; Pastore, J.; Rouy, D.; Clemens, R.; Aras, R.; Losordo, D.W. An Open-Label Dose Escalation Study to Evaluate the Safety of Administration of Nonviral Stromal Cell-Derived Factor-1 Plasmid to Treat Symptomatic Ischemic Heart Failure. Circ. Res. 2013, 112, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.; Bansal, R.; Prakash, J. Drug targeting to myofibroblasts: Implications for fibrosis and cancer. Adv. Drug Deliv. Rev. 2017, 121, 101–116. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fytianos, K.; Schliep, R.; Mykoniati, S.; Khan, P.; Hostettler, K.E.; Tamm, M.; Gazdhar, A.; Knudsen, L.; Geiser, T. Anti-Fibrotic Effect of SDF-1β Overexpression in Bleomycin-Injured Rat Lung. Pharmaceutics 2022, 14, 1803. https://doi.org/10.3390/pharmaceutics14091803
Fytianos K, Schliep R, Mykoniati S, Khan P, Hostettler KE, Tamm M, Gazdhar A, Knudsen L, Geiser T. Anti-Fibrotic Effect of SDF-1β Overexpression in Bleomycin-Injured Rat Lung. Pharmaceutics. 2022; 14(9):1803. https://doi.org/10.3390/pharmaceutics14091803
Chicago/Turabian StyleFytianos, Kleanthis, Ronja Schliep, Sofia Mykoniati, Petra Khan, Katrin E. Hostettler, Michael Tamm, Amiq Gazdhar, Lars Knudsen, and Thomas Geiser. 2022. "Anti-Fibrotic Effect of SDF-1β Overexpression in Bleomycin-Injured Rat Lung" Pharmaceutics 14, no. 9: 1803. https://doi.org/10.3390/pharmaceutics14091803
APA StyleFytianos, K., Schliep, R., Mykoniati, S., Khan, P., Hostettler, K. E., Tamm, M., Gazdhar, A., Knudsen, L., & Geiser, T. (2022). Anti-Fibrotic Effect of SDF-1β Overexpression in Bleomycin-Injured Rat Lung. Pharmaceutics, 14(9), 1803. https://doi.org/10.3390/pharmaceutics14091803





