The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering
Abstract
:1. Introduction
2. Flame-Retardant Mechanism
2.1. Burning Mechanism
2.2. Flame-Retardant Mechanism
2.3. Standard Tests for Flame Retardancy
2.3.1. Limiting Oxygen Index (LOI)
2.3.2. Tests for Flammability of Plastic Materials
2.3.3. Cone Calorimetry
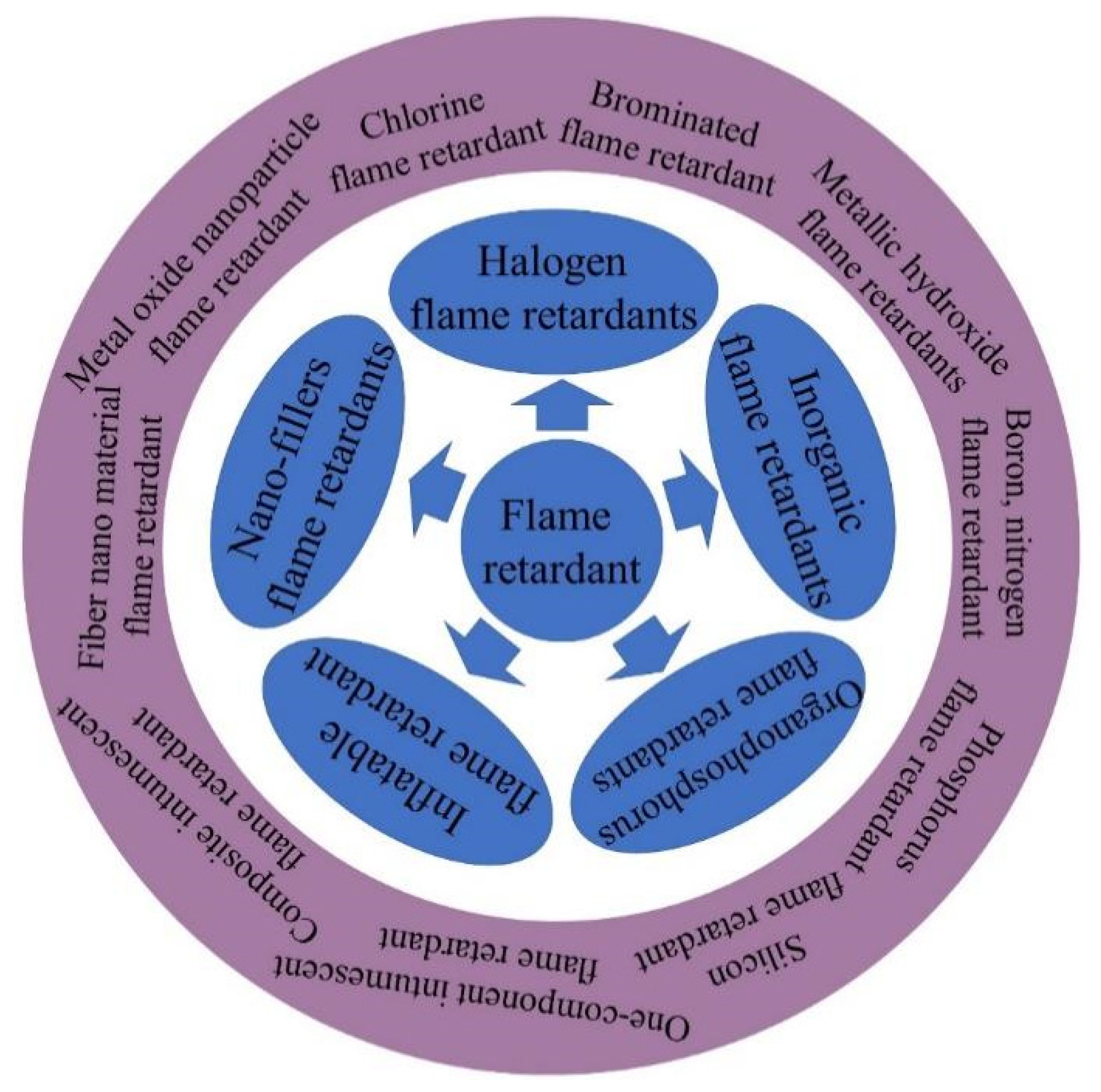
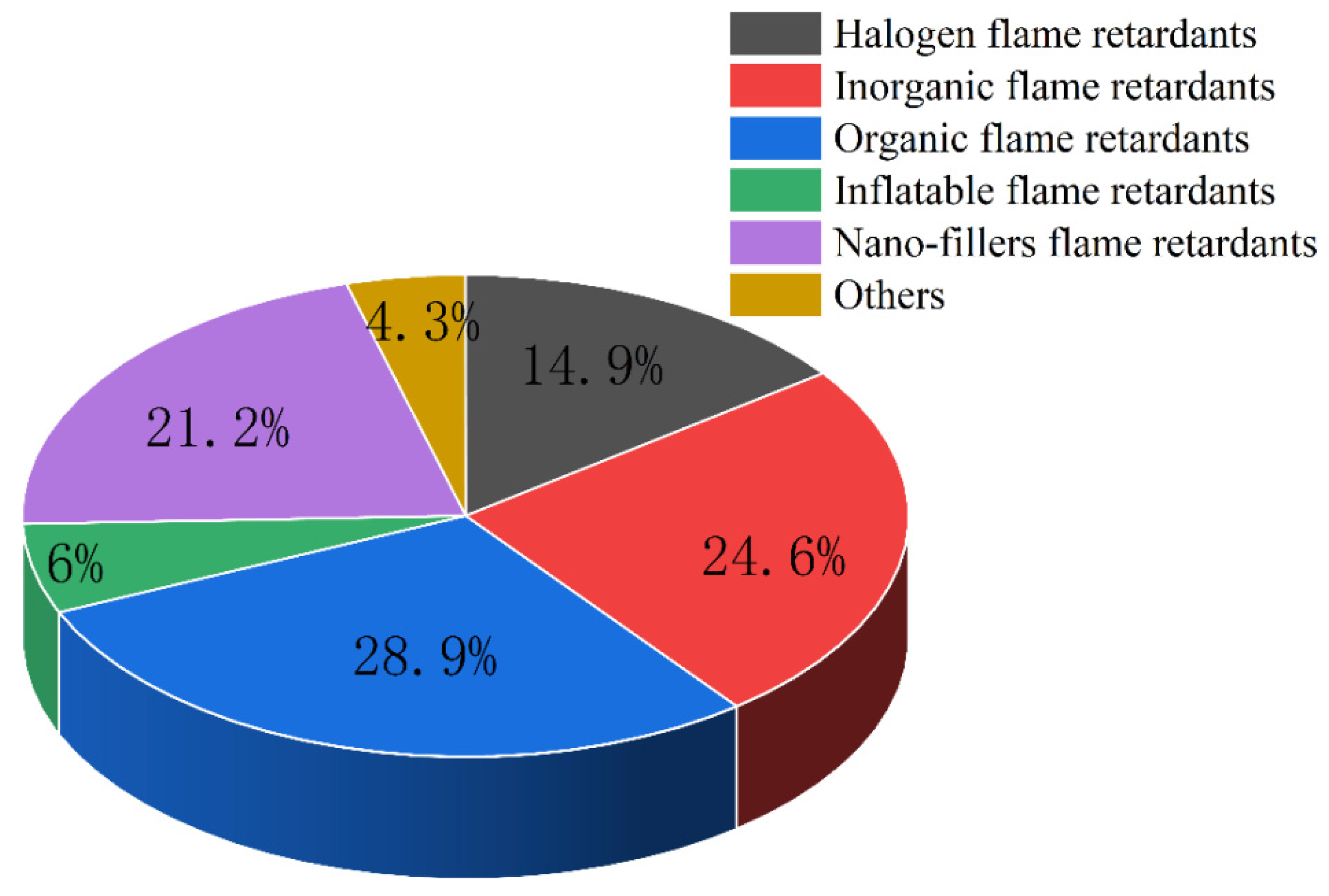
3. Classification of Flame-Retardant Polymer Materials
3.1. Halogen-Containing Flame Retardant
3.2. Inorganic Flame Retardants
3.2.1. Metallic Hydroxide Flame Retardants
3.2.2. Inorganic Phosphorus Flame Retardants
3.2.3. Boron-Containing Flame Retardants
3.2.4. Nitrogen-Containing Flame Retardants
3.3. Organic Flame Retardants
3.3.1. Organophosphorus Flame Retardants
3.3.2. Silicone Flame Retardants
3.4. Intumescent Flame Retardant
3.5. Nano Fillers-Containing Flame Retardants
4. Preparation of Flame-Retardant Polymer Materials
4.1. Halogen-Containing Flame-Retardant Composites
4.2. Inorganic-Containing Flame-Retardant Composites
4.3. Organic-Containing Flame-Retardant Composites
4.4. Expansion Flame-Retardant Composites
4.5. Nano Fillers-Containing Flame-Retardant Composites
5. Polymer-Based Flame-Retardant Composites and Application in Construction Engineering
5.1. Thermoplastic Flame-Retardant Composites
5.2. Thermosetting Flame-Retardant Composites
5.3. Application of Flame-Retardant Polymer Materials in Construction Engineering
6. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Li, J.; Zhu, Y.; Guo, Z.; Su, S. Increasing the Efficiency of Intumescent Flame Retardant Polypropylene Catalyzed by Polyoxometalate Based Ionic Liquid. J. Mater. Chem. A 2013, 1, 15242. [Google Scholar] [CrossRef]
- Dogan, M.; Dogan, S.D.; Savas, L.A.; Ozcelik, G.; Tayfun, U. Flame Retardant Effect of Boron Compounds in Polymeric Materials. Compos. Part B Eng. 2021, 222, 109088. [Google Scholar] [CrossRef]
- Dong, X.; Qin, R.; Nie, S.; Yang, J.; Zhang, C.; Wu, W. Fire Hazard Suppression of Intumescent Flame Retardant Polypropylene Based on a Novel Ni-Containing Char-Forming Agent. Polym. Advan. Technol. 2019, 30, 563–572. [Google Scholar] [CrossRef]
- Zhang, S.; Horrocks, A.R. A Review of Flame Retardant Polypropylene Fibres. Prog. Polym. Sci. 2003, 28, 1517–1538. [Google Scholar] [CrossRef]
- Gao, D.; Wen, X.; Guan, Y.; Czerwonko, W.; Li, Y.; Gao, Y.; Mijowska, E.; Tang, T. Flame Retardant Effect and Mechanism of Nanosized NiO as Synergist in PLA/APP/CSi-MCA Composites. Compos. Commun. 2020, 17, 170–176. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, L.; Fu, Z.; Shan, A.; Lavernia, E.J. Enhanced Thermal Stability and Ductility in a Nanostructured Ni-Based Alloy. Scr. Mater. 2017, 141, 1–5. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, X.; Liu, G. Synthesis of an Integrated Intumescent Flame Retardant and Its Flame Retardancy Properties for Polypropylene. Polym. Degrad. Stab. 2017, 138, 106–114. [Google Scholar] [CrossRef]
- Sun, J.; Kormakov, S.; Liu, Y.; Huang, Y.; Wu, D.; Yang, Z. Recent Progress in Metal-Based Nanoparticles Mediated Photodynamic Therapy. Molecules 2018, 23, 1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, X.; Liu, Y.; Xu, C.; Zhang, H.; Wu, D.; Tan, T.; Qin, X.; Sun, J.; Zhang, L. Preparation of Flexible and Elastic Thermal Conductive Nanocomposites Via Ultrasonic-Assisted Forced Infiltration. Compos. Sci. Technol. 2021, 202, 108582. [Google Scholar] [CrossRef]
- Sun, J.; Zhuang, J.; Liu, Y.; Xu, H.; Horne, J.; Wujcik, E.K.; Liu, H.; Ryu, J.E.; Wu, D.; Guo, Z. Development and Application of Hot Embossing in Polymer Processing: A Review. ES Mater. Manuf. 2019, 6, 3–17. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, J.; Lee, L.J.; Castro, J.M. Overview of Ultrasonic Assisted Manufacturing Multifunctional Carbon Nanotube Nanopaper Based Polymer Nanocomposites. Eng. Sci. 2020, 10, 35–50. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Fang, Z.; Huang, Y.; Xu, H.; Liu, Y.; Wu, D.; Zhuang, J.; Sun, J. Recent Advances in Preparation, Mechanisms, and Applications of Thermally Conductive Polymer Composites: A Review. J. Compos. Sci. 2020, 4, 180. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Li, D.; Yang, X.; Wu, D.; Sun, J. Thermal Conductivity Enhancement Via Conductive network Conversion from “Sand-like” to “Stone-like” in the Polydimethylsiloxane Composites. Compos. Commun. 2020, 22, 100509. [Google Scholar] [CrossRef]
- Idumah, C.I. Emerging Advancements in Flame Retardancy of Polypropylene Nanocomposites. J. Thermoplast. Compos. 2020, 0892705720930782. [Google Scholar] [CrossRef]
- Bar, M.; Alagirusamy, R.; Das, A. Flame Retardant Polymer Composites. Fibers Polym. 2015, 16, 705–717. [Google Scholar] [CrossRef]
- Salmeia, K.; Fage, J.; Liang, S.; Gaan, S. An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Wang, J.; Wang, B.; Wang, X.; Zhou, X.; Cai, W.; Mu, X.; Hou, Y.; Hu, Y.; Song, L. Large-Scale Production of Simultaneously Exfoliated and Functionalized Mxenes as Promising Flame Retardant for Polyurethane. Compos. Part B Eng. 2019, 179, 107486. [Google Scholar] [CrossRef]
- Amarnath, N.; Appavoo, D.; Lochab, B. Eco-Friendly Halogen-Free Flame Retardant Cardanol Polyphosphazene Polybenzoxazine Networks. ACS Sustain. Chem. Eng. 2017, 6, 389–402. [Google Scholar] [CrossRef]
- Zou, W.; Basharat, M.; Dar, S.U.; Zhang, S.; Abbas, Y.; Liu, W.; Wu, Z.; Zhang, T. Preparation and Performances of Novel Polyphosphazene-Based Thermally Conductive Composites. Compos. Part A Appl. Sci. Manuf. 2019, 119, 145–153. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant Surface Treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Chan, S.Y.; Si, L.; Lee, K.I.; Ng, P.F.; Chen, L.; Yu, B.; Hu, Y.; Yuen, R.K.; Xin, J.H.; Fei, B. A Novel Boron–Nitrogen Intumescent Flame Retardant Coating on Cotton with Improved Washing Durability. Cellulose 2017, 25, 843–857. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, D.; Carosio, F.; Camino, G.; Bergström, L. Tuning the Nanocellulose–Borate Interaction To Achieve Highly Flame Retardant Hybrid Materials. Chem. Mater. 2016, 28, 1985–1989. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, G.; Xiao, Z.; Malik, O.P. Feature Extraction Based on Adaptive Multiwavelets and LTSA for Rotating Machinery Fault Diagnosis. Shock. Vib. 2019, 2019, 1201084. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Chai, Y.; Ni, L.; Lyu, W. Flame Retardant Properties and Thermal Decomposition Kinetics of Wood Treated with Boric Acid Modified Silica Sol. Materials 2020, 13, 4478. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, A.; Li, W.; Li, Z. Synthesis of Carborane Acrylate and Flame Retardant Modification on Silk Fabric Via Graft Copolymerization with Phosphate-Containing Acrylate. Fire Mater. 2019, 43, 880–891. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Wu, Y.-X.; Huang, Y.-T.; Jiang, J.-R.; Xu, J.-T.; Guan, J.-P. Synthesis of a Reactive Boron-Based Flame Retardant to Enhance the Flame Retardancy of Silk. React. Funct. Polym. 2020, 156, 104731. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Biodegradable Flame Retardants for Biodegradable Polymer. Biomolecules 2020, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C. Recent Advances in Bio-Based Flame Retardant Additives for Synthetic Polymeric Materials. Polymers 2019, 11, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, P.; Tretsiakova-McNally, S. Melt-Flow Behaviours of Thermoplastic Materials under Fire Conditions: Recent Experimental Studies and Some Theoretical Approaches. Materials 2015, 8, 8793–8803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baby, A.; Tretsiakova-McNally, S.; Arun, M.; Joseph, P.; Zhang, J. Reactive and Additive Modifications of Styrenic Polymers with Phosphorus-Containing Compounds and Their Effects on Fire Retardance. Molecules 2020, 25, 3779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lei, Y.; Li, X.; Shao, H.; Xu, W.; Li, D. A Facile, Environmentally and Friendly Flame-Retardant: Synergistic Flame Retardant Property of Polyurethane Rigid foam. Mater Lett. 2020, 267, 127542. [Google Scholar] [CrossRef]
- Wichman, I.S. Material Flammability, Combustion, Toxicity and Fire Hazard in Transportation. Prog. Energy Combust. Sci. 2003, 29, 247–299. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent Developments in the Fire Retardancy of Polymeric Materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New Prospects in Flame Retardant Polymer Materials: From Fundamentals to Nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Jimenez, M.; Duquesne, S.; Bourbigot, S. Intumescent Fire Protective Coating: Toward a Better Understanding of Their Mechanism of Action. Thermochim. Acta 2006, 449, 16–26. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Pornwannchai, W.; Hu, Y.; Kandola, B. The Effect of Graphene presence in Flame Retarded Epoxy Resin Matrix on the Mechanical and Flammability Properties of Glass Fiber-Reinforced Composites. Compos. Part A Appl. Sci. Manuf. 2013, 53, 88–96. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Yang, H.; Xing, W.; Kandola, B.; Hu, Y. Simultaneous Reduction and Surface Functionalization of Graphene Oxide with POSS for Reducing Fire Hazards in Epoxy Composites. J. Mater. Chem. 2012, 22, 22037–22043. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, W.; Wang, S.; Shen, Z.; Wang, Y.; Zhang, Q. A Novel Transposon, Mediated Transfer of Variant in ESBL-Producing Aeromonas veronii. Infect. Drug Resist. 2020, 13, 893–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Kalali, E.N.; Wan, J.-T.; Wang, D.-Y. Carbon-Family Materials for Flame Retardant Polymeric Materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Morgan, A.B. The Future of Flame Retardant Polymers–Unmet Needs and Likely New Approaches. Polym. Rev. 2019, 59, 25–54. [Google Scholar] [CrossRef]
- Vahabi, H.; Laoutid, F.; Mehrpouya, M.; Saeb, M.R.; Dubois, P. Flame Retardant Polymer Materials: An Update and the Future for 3D Printing Developments. Mater. Sci. Eng. R Rep. 2021, 144, 100604. [Google Scholar] [CrossRef]
- Shahari, S.; Fathullah, M.; Abdullah, M.M.A.B.; Shayfull, Z.; Mia, M.; Budi Darmawan, V.E. Recent Developments in Fire Retardant Glass Fibre Reinforced Epoxy Composite and Geopolymer as a Potential Fire-Retardant Material: A review. Constr. Build. Mater. 2021, 277, 122246. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, Z.; Chu, F.; Gui, Z.; Song, L.; Hu, Y.; Hu, W. A Review on Metal-Organic Hybrids as Flame Retardants for Enhancing Fire Safety of Polymer Composites. Compos. Part B Eng. 2021, 221, 109014. [Google Scholar] [CrossRef]
- Ferry, L.; Lopez Cuesta, J.M.; Chivas, C.; Mac Way Hoy, G.; Dvir, H. Incorporation of a Grafted Brominated Monomer in Glass Fiber Reinforced Polypropylene to Improve the Fire Resistance. Polym. Degrad. Stab. 2001, 74, 449–456. [Google Scholar] [CrossRef]
- Li, X.-M.; Yang, R.-J. Study on Blooming of Tetrabromobisphenol a Bis (2,3-Dibromopropyl Ether) in Blends with Polypropylene. J. Appl. Polym. Sci. 2006, 101, 20–24. [Google Scholar] [CrossRef]
- Chen, X.-S.; Yu, Z.-Z.; Liu, W.; Zhang, S. Synergistic Effect of Decabromodiphenyl Ethane and Montmorillonite on Flame Retardancy of Polypropylene. Polym. Degrad. Stab. 2009, 94, 1520–1525. [Google Scholar] [CrossRef]
- Montalbano, A.M.; Albano, G.D.; Anzalone, G.; Moscato, M.; Gagliardo, R.; Di Sano, C.; Bonanno, A.; Ruggieri, S.; Cibella, F.; Profita, M. Cytotoxic and Genotoxic Effects of the Flame Retardants (PBDE-47, PBDE-99 and PBDE-209) in Human Bronchial Epithelial Cells. Chemosphere 2020, 245, 125600. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, S.; Jeon, H.; Kim, A.H.; Lee, W.; Lee, Y.; Yang, S.; Yun, J.; Jung, Y.S.; Lee, J. Tetrabromobisphenol A-Induced Apoptosis in Neural Stem Cells Through Oxidative Stress and Mitochondrial Dysfunction. Neurotox. Res. 2020, 38, 74–85. [Google Scholar] [CrossRef]
- Simond, A.E.; Houde, M.; Lesage, V.; Michaud, R.; Verreault, J. Metabolomic Profiles of the Endangered St. Lawrence Estuary Beluga Population and Associations with Organohalogen Contaminants. Sci. Total Environ. 2020, 717, 137204. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, M.; Harrad, S.; Abou-Elwafa Abdallah, M.; Drage, D.S.; Berresheim, H. Phasing-Out of Legacy Brominated Flame Retardants: The UNEP Stockholm Convention and Other Legislative Action Worldwide. Environ. Int. 2020, 144, 106041. [Google Scholar] [CrossRef]
- Fang, Z.; Guo, Z.; Ma, H.; Chen, Q.; Wu, Y. Study of a Halogen-Free Flame-Retarded Polypropylene Composition with Balanced Strength and Toughness. Int. J. Mater. Prod. Technol. 2010, 37, 350–357. [Google Scholar] [CrossRef]
- Qi, Z.-L.; Li, D.-H.; Yang, R.-J. Preparation of Ammonium Polyphosphate Coated with Aluminium Hydroxide and Its Application in Polypropylene as Flame Retardant. J. Inorg. Mater. 2015, 30, 1267–1272. [Google Scholar] [CrossRef]
- Lewin, M.; Endo, M. Catalysis of Intumescent Flame Retardancy of Polypropylene by Metallic Compounds. Polym. Advan. Technol. 2003, 14, 3–11. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wang, Q. Preparation of High Loading Magnesium Hydroxide Flame Retardant Polypropylene by Solid State Shear Milling. J. Compos. Mater. 2016, 41, 1995–2003. [Google Scholar] [CrossRef]
- Marosfoi, B.B.; Garas, S.; Bodzay, B.; Zubonyai, F.; Marosi, G. Flame Retardancy Study on Magnesium Hydroxide Associated with Clays of Different Morphology in Polypropylene Matrix. Polym. Advan. Technol. 2008, 19, 693–700. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Lu, S.; Wu, H.; Guo, S.; Luo, Z. Combustion Characteristics of Polypropylene/Magnesium Hydroxide/Expandable Graphite Composites. J. Macromol. Sci. Part B 2009, 48, 1081–1092. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Zhang, Z.; Wang, Q. Preparation of Ammonium Polyphosphate and Dye Co-Intercalated LDH/Polypropylene Composites with Enhanced Flame Retardant and UV Resistance Properties. Chemosphere 2021, 277, 130370. [Google Scholar] [CrossRef]
- Yan, J.; Xu, P.; Zhang, P.; Fan, H. Surface-Modified Ammonium Polyphosphate for Flame-Retardant and Reinforced Polyurethane Composites. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127092. [Google Scholar] [CrossRef]
- Cui, M.; Li, J.; Qin, D.; Sun, J.; Chen, Y.; Xiang, J.; Yan, J.; Fan, H. Intumescent Flame Retardant Behavior of Triazine Group and Ammonium Polyphosphate in Waterborne Polyurethane. Polym. Degrad. Stab. 2021, 183, 109439. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Li, D.F.; Fu, T.; He, L.; Wang, X.L.; Wang, Y.Z. Biomimetic Construction Peanut-Leaf Structure on Ammonium Polyphosphate Surface: Improving Its Compatibility with Poly (Lactic Acid) and Flame-Retardant Efficiency Simultaneously. Chem. Eng. J. 2021, 412, 128737. [Google Scholar] [CrossRef]
- Rabe, S.; Chuenban, Y.; Schartel, B. Exploring the Modes of Action of Phosphorus-Based Flame Retardants in Polymeric Systems. Materials 2017, 10, 455. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Wang, S.; Dewhurst, R.D.; Ignat’ev, N.V.; Finze, M.; Braunschweig, H. Boron: Its Role in Energy-Related Processes and Applications. Angew. Chem. Int. Ed. 2020, 59, 8800–8816. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.K. Boron-Based Flame Retardants in Non-Halogen-Based Polymers. In Non-Halogenated Flame Retardant Handbook; John Wiley & Sons: New York, NY, USA, 2014; pp. 201–241. [Google Scholar]
- Li, Y.-M.; Hu, S.-L.; Wang, D.-Y. Polymer-Based Ceramifiable Composites for Flame Retardant Applications: A Review. Compos. Commun. 2020, 21, 100405. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, Y.; Liang, D.; Liu, S.; Chi, Z.; Xu, J. Influence of Zinc Borate on the Flame Retardancy and Thermal Stability of Intumescent Flame Retardant Polypropylene Composites. J. Anal. Appl. Pyrolysis 2015, 115, 224–232. [Google Scholar] [CrossRef]
- Camargo, Á.; Ibañez, C.M. Initial Study of Micronized Zinc Borate as Flame Retardant in Eucalyptus Grandis from Uruguay. MRS Adv. 2018, 3, 3551–3556. [Google Scholar] [CrossRef]
- Geng, Y.-J.; Liu, Z.-H. Preparation and Thermodynamic Characterization of 2CaO·B2O3·H2O Nanomaterials with Enhanced Flame Retardant Properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 563–568. [Google Scholar] [CrossRef]
- Tawiah, B.; Yu, B.; Yang, W.; Yuen, R.K.K.; Fei, B. Facile Flame Retardant Finishing of Cotton Fabric with Hydrated Sodium Metaborate. Cellulose 2019, 26, 4629–4640. [Google Scholar] [CrossRef]
- Durrani, H.; Sharma, V.; Bamboria, D.; Shukla, A.; Basak, S.; Ali, W. Exploration of Flame Retardant Efficacy of Cellulosic Fabric Using in-Situ Synthesized Zinc Borate Particles. Cellulose 2020, 27, 9061–9073. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, W.; Zhang, M.; Qu, J.; Shi, L.; Qu, H.; Xu, J. Hydrothermal Synthesis of 4ZnO·B2O3 H2O/RGO Hybrid Material and Its Flame Retardant Behavior in Flexible PVC and Magnesium Hydroxide Composites. Appl. Surf. Sci. 2017, 425, 896–904. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Liu, Y.; Wang, Q. Properties and Mechanisms of Different Guanidine Flame Retardant Wood Pulp Paper. J. Anal. Appl. Pyrolysis 2017, 128, 224–231. [Google Scholar] [CrossRef]
- Wan, C.; Liu, S.; Chen, Y.; Zhang, F. Facile, One–Pot, Formaldehyde-Free Synthesis of Reactive NP Flame Retardant for a Biomolecule of Cotton. Int. J. Biol. Macromol. 2020, 163, 1659–1668. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Rahman, M.Z.; Song, L.; Hu, Y. An Environmentally Friendly Approach to Fabricating Flame Retardant, Antibacterial and Antifungal Cotton Fabrics Via Self-Assembly of Guanazole-Metal Complex. J. Clean. Prod. 2020, 273, 122832. [Google Scholar] [CrossRef]
- Wu, K.; Wang, X.; Xu, Y.; Guo, W. Flame Retardant Efficiency of Modified Para-Aramid Fiber Synergizing with Ammonium Polyphosphate on PP/EPDM. Polym. Degrad. Stab. 2020, 172, 109065. [Google Scholar] [CrossRef]
- Reuter, J.; Greiner, L.; Kukla, P.; Döring, M. Efficient Flame Retardant Interplay of Unsaturated Polyester Resin Formulations Based on Ammonium Polyphosphate. Polym. Degrad. Stab. 2020, 178, 109134. [Google Scholar] [CrossRef]
- Gao, X.; Lin, Y.; Li, J.; Xu, Y.; Qian, Z.; Lin, W. Spatial Pattern Analysis Reveals Multiple Sources of Organophosphorus Flame Retardants in Coastal Waters. J. Hazard. Mater. 2021, 417, 125882. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.C.; Castro, V.; Quintana, J.B.; Rodil, R.; Beiras, R.; Vidal-Liñán, L. Bioaccumulation of Organophosphorus Flame Retardants in the Marine Mussel Mytilus Galloprovincialis. Sci. Total Environ. 2021, 805, 150384. [Google Scholar] [CrossRef]
- Zhong, W.; Cui, Y.; Li, R.; Yang, R.; Li, Y.; Zhu, L. Distribution and Sources of Ordinary Monomeric and Emerging Oligomeric Organophosphorus Flame Retardants in Haihe Basin, China. Sci. Total Environ. 2021, 785, 147274. [Google Scholar] [CrossRef]
- Shi, Q.; Guo, W.; Shen, Q.; Han, J.; Lei, L.; Chen, L.; Yang, L.; Feng, C.; Zhou, B. In vitro Biolayer Interferometry Analysis of Acetylcholinesterase as a Potential Target of Aryl-Organophosphorus Flame-Retardants. J. Hazard. Mater. 2021, 409, 124999. [Google Scholar] [CrossRef]
- Albaqami, M.D.; Shaikh, S.F.; Nafady, A. Utilization of Hybrid Silicone Rubber/Exolit AP 422 Composite for the Fabrication of Mechanically Flexible, Flame-Retardant and Superhydrophobic Polyurethane Foams. Mater. Chem. Phys. 2021, 273, 125133. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Song, S. Preparation of a Novel Type of Flame Retardant Diatomite and Its Application in Silicone Rubber Composites. Adv. Powder Technol. 2019, 30, 1567–1575. [Google Scholar] [CrossRef]
- Beaugendre, A.; Lemesle, C.; Bellayer, S.; Degoutin, S.; Duquesne, S.; Casetta, M.; Pierlot, C.; Jaime, F.; Kim, T.; Jimenez, M. Flame Retardant and Weathering Resistant Self-layering Epoxy-Silicone Coatings for Plastics. Prog. Org. Coat. 2019, 136, 105269. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.; Choi, J.; Lee, J.; Yu, K.; Baeck, S.H.; Shim, S.E.; Qian, Y. Valorization of Fly Ash as a Harmless Flame Retardant Via Carbonation Treatment for Enhanced Fire-Proofing Performance and Mechanical Properties of Silicone Composites. J. Hazard. Mater. 2021, 404, 124202. [Google Scholar] [CrossRef]
- Huang, N.H.; Chen, Z.J.; Wang, J.Q.; Wei, P. Synergistic Effects of Sepiolite on Intumescent Flame Retardant Polypropylene. Express Polym. Lett. 2010, 4, 743–752. [Google Scholar] [CrossRef]
- Villamil Watson, D.A.; Schiraldi, D.A. Biomolecules as Flame Retardant Additives for Polymers: A Review. Polymers 2020, 12, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, C.; Chen, X. Flammability and Thermal Degradation of Intumescent Flame-Retardant Polypropylene Composites. Polym. Eng. Sci. 2010, 50, 767–772. [Google Scholar] [CrossRef]
- Peng, H.-Q.; Zhou, Q.; Wang, D.-Y.; Chen, L.; Wang, Y.-Z. A Novel Charring Agent Containing Caged Bicyclic Phosphate and Its Application in Intumescent Flame Retardant Polypropylene Systems. J. Ind. Eng. Chem. 2008, 14, 589–595. [Google Scholar] [CrossRef]
- Dong, X.; Yang, J.; Hua, X.; Nie, S.; Kong, F. Synthesis of a Novel Char-Forming Agent (PEIC): Improvement in Flame Retardancy, Thermal Stability, and Smoke Suppression for Intumescent Flame-Retardant Polypropylene Composites. J. Appl. Polym. Sci. 2019, 137, 48296. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Yang, Q.; Liao, X.; Li, G. Synthesis and Characterization of a Novel Charring Agent and Its Application in Intumescent Flame Retardant Polypropylene System. J. Appl. Polym. Sci. 2012, 123, 1636–1644. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, J. Regulating Effects of Nitrogenous Bases on the Char Structure and Flame Retardancy of Polypropylene/Intumescent Flame Retardant Composites. ACS Sustain. Chem. Eng. 2017, 5, 2375–2383. [Google Scholar] [CrossRef]
- Liu, J.-C.; Xu, M.-J.; Lai, T.; Li, B. Effect of Surface-Modified Ammonium Polyphosphate with KH550 and Silicon Resin on the Flame Retardancy, Water Resistance, Mechanical and Thermal Properties of Intumescent Flame Retardant Polypropylene. Ind. Eng. Chem. Res. 2015, 54, 9733–9741. [Google Scholar] [CrossRef]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame Retardant Polymeric Nanocomposites through the Combination of Nanomaterials and Conventional Flame Retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Lu, S.; Hong, W.; Chen, X. Nanoreinforcements of Two-Dimensional Nanomaterials for Flame Retardant Polymeric Composites: An Overview. Adv. Polym. Technol. 2019, 2019, 4273253. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Zhao, X.; Li, Z.; Yu, L.; Li, X.; Zhang, Z. Bismuth Oxychloride Nanosheets for Improvement of Flexible Poly (Vinyl Chloride) Flame Retardancy. J. Mater. Sci. 2019, 55, 631–643. [Google Scholar] [CrossRef]
- Yurddaskal, M.; Celik, E. Effect of Halogen-Free Nanoparticles on the Mechanical, Structural, Thermal and Flame Retardant Properties of Polymer Matrix Composite. Compos. Struct. 2018, 183, 381–388. [Google Scholar] [CrossRef]
- Tawiah, B.; Yu, B.; Yuen, R.K.; Hu, Y.; Wei, R.; Xin, J.H.; Fei, B. Highly Efficient Flame Retardant and Smoke Suppression Mechanism of Boron Modified Graphene Oxide/Poly(Lactic Acid) Nanocomposites. Carbon 2019, 150, 8–20. [Google Scholar] [CrossRef]
- Cavallaro, G.; Micciulla, S.; Chiappisi, L.; Lazzara, G. Chitosan-Based Smart Hybrid Materials: A Physico-Chemical Perspective. J. Mater. Chem. B 2021, 9, 594–611. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, J.H.; Lee, D.H.; Kim, M.D.; Kong, J.H.; Lee, Y.K.; Nam, J.D. Flame Retardancy of Polypropylene/Montmorillonite Nanocomposites with Halogenated Flame Retardants. Polym. Korea 2003, 27, 569–575. [Google Scholar]
- Yu, H.; Jiang, Z.; Gilman, J.W.; Kashiwagi, T.; Liu, J.; Song, R.; Tang, T. Promoting Carbonization of Polypropylene during Combustion through Synergistic Catalysis of a Trace of Halogenated Compounds and Ni2O3 for Improving Flame Retardancy. Polymer 2009, 50, 6252–6258. [Google Scholar] [CrossRef]
- Dvir, H. Optimization of a Flame-Retarded Polypropylene Composite. Compos. Sci. Technol. 2003, 63, 1865–1875. [Google Scholar] [CrossRef]
- Tai, C.M.; Li, R.K.Y. Mechanical Properties of Flame Retardant Filled Polypropylene Composites. J. Appl. Polym. Sci. 2001, 80, 2718–2728. [Google Scholar] [CrossRef]
- Zhai, W.; Zhong, Y.; Wei, X. Processing Renewable Corks into Excellent Thermally Stable, Flame-Retardant and Smoke-Suppressant Composite Materials by Respiratory Impregnation Method. Ind. Crop. Prod. 2020, 157, 112932. [Google Scholar] [CrossRef]
- Kalali, E.N.; Zhang, L.; Shabestari, M.E.; Croyal, J.; Wang, D.-Y. Flame-Retardant Wood Polymer Composites (WPCs) as Potential Fire Safe Bio-Based Materials for Building Products: Preparation, Flammability and Mechanical Properties. Fire Saf. J. 2019, 107, 210–216. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Ye, Q.; Shen, L.; Lin, H. Flame-Retardant Ethylene Vinyl Acetate Composite Materials by Combining Additions of Aluminum Hydroxide and Melamine Cyanurate: Preparation and Characteristic Evaluations. J. Colloid. Interface. Sci. 2021, 589, 525–531. [Google Scholar] [CrossRef]
- Cinausero, N.; Azema, N.; Lopez-Cuesta, J.M.; Cochez, M.; Ferriol, M. Synergistic Effect between Hydrophobic Oxide Nanoparticles and Ammonium Polyphosphate on Fire Properties of Poly (Methyl Methacrylate) and Polystyrene. Polym. Degrad. Stabil. 2011, 96, 1445–1454. [Google Scholar] [CrossRef]
- Nazaré, S.; Kandola, B.K.; Horrocks, A.R. Flame-Retardant Unsaturated Polyester Resin Incorporating Nanoclays. Polym. Advan. Technol. 2006, 17, 294–303. [Google Scholar] [CrossRef]
- Jiang, Y.; Hao, Z.; Luo, H.; Shao, Z.; Yu, Q.; Sun, M.; Ke, Y.; Chen, Y. Synergistic Effects of Boron-Doped Silicone Resin and a Layered Double Hydroxide Modified with Sodium Dodecyl Benzenesulfonate for Enhancing the Flame Retardancy of Polycarbonate. Rsc Adv. 2018, 8, 11078–11086. [Google Scholar] [CrossRef] [Green Version]
- Tawiah, B.; Yu, B.; Cheung, W.Y.; Chan, S.Y.; Yang, W.; Fei, B. Synthesis and Application of Synergistic Azo-Boron-BPA/Polydopamine as Efficient Flame Retardant for Poly (Lactic Acid). Polym. Degrad. Stabil. 2018, 152, 64–74. [Google Scholar] [CrossRef]
- Lv, Q.; Huang, J.Q.; Chen, M.J.; Zhao, J.; Tan, Y.; Chen, L.; Wang, Y.Z. An Effective Flame Retardant and Smoke Suppression Oligomer for Epoxy Resin. Ind. Eng. Chem. Res. 2013, 52, 9397–9404. [Google Scholar] [CrossRef]
- Li, M.E.; Wang, S.X.; Han, L.X.; Yuan, W.J.; Cheng, J.B.; Zhang, A.N.; Zhao, H.B.; Wang, Y.Z. Hierarchically Porous SiO2/Polyurethane Foam Composites towards Excellent Thermal Insulating, Flame-Retardant and Smoke-Suppressant Performances. J. Hazard. Mater. 2019, 375, 61–69. [Google Scholar] [CrossRef]
- Luo, C.; Zuo, J.; Wang, F.; Yuan, Y.; Lin, F.; Huang, H.; Zhao, J. High Refractive Index and Flame Retardancy of Epoxy Thermoset Cured by Tris (2-Mercaptoethyl) Phosphate. Polym. Degrad. Stab. 2016, 129, 7–11. [Google Scholar] [CrossRef]
- Jian, R.-K.; Ai, Y.-F.; Xia, L.; Zhao, L.-J.; Zhao, H.-B. Single Component Phosphamide-Based Intumescent Flame Retardant with Potential Reactivity towards Low Flammability and Smoke Epoxy Resins. J. Hazard. Mater. 2019, 371, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.N.; Chen, L.; Fu, T.; Zhao, H.B.; Guo, D.M.; Wang, X.L.; Wang, Y.Z. New Application for Aromatic Schiff Base: High Efficient Flame-Retardant and Anti-Dripping Action for Polyesters. Chem. Eng. J. 2018, 336, 622–632. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, J.; Wang, X.-L.; Wang, Y.-Z. Highly Thermostable and Durably Flame-Retardant Unsaturated Polyester Modified by a Novel Polymeric Flame Retardant Containing Schiff Base and Spirocyclic Structures. Chem. Eng. J. 2018, 344, 419–430. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Ma, S.; Yuan, W.; Li, Q.; Feng, J.; Zhu, J. Vanillin-Derived Phosphorus-Containing Compounds and Ammonium Polyphosphate as Green Fire-Resistant Systems for Epoxy Resins with Balanced Properties. Polym. Advan. Technol. 2019, 30, 264–278. [Google Scholar] [CrossRef]
- El Gouri, M.; El Bachiri, A.; Hegazi, S.E.; Rafik, M.; El Harfi, A. Thermal Degradation of a Reactive Flame Retardant Based on Cyclotriphosphazene and Its Blend with DGEBA Epoxy Resin. Polym. Degrad. Stab. 2009, 94, 2101–2106. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, C.-L.; Zhao, J.; Wang, J.-S.; Chen, L.; Wang, Y.-Z. An Efficiently Halogen-Free Flame-Retardant Long-Glass-Fiber-Reinforced Polypropylene System. Polym. Degrad. Stab. 2011, 96, 363–370. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Han, Z.; Wang, Q. A Coating Method Combined with Bulk Addition for Efficient Flame Retardant Thermoplastic Polyolefin Sheet Material. Polym. Degrad. Stab. 2020, 174, 109093. [Google Scholar] [CrossRef]
- Yen, Y.-Y.; Wang, H.-T.; Guo, W.-J. Synergistic Effect of Aluminum Hydroxide and Nanoclay on Flame Retardancy and Mechanical Properties of EPDM Composites. J. Appl. Polym. Sci. 2013, 130, 2042–2048. [Google Scholar] [CrossRef]
- Laachachi, A.; Leroy, E.; Cochez, M.; Ferriol, M.; Lopez Cuesta, J.M. Use of Oxide Nanoparticles and Organoclays to Improve Thermal Stability and Fire Retardancy of Poly (Methyl Methacrylate). Polym. Degrad. Stabil. 2005, 89, 344–352. [Google Scholar] [CrossRef]
- Mishra, S.; Sonawane, S.H.; Singh, R.P.; Bendale, A.; Patil, K. Effect of Nano-Mg(OH)2 on the Mechanical and Flame-Retarding Properties of Polypropylene Composites. J. Appl. Polym. Sci. 2004, 94, 116–122. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Bonnaud, L.; Gallos, A.; Fontaine, G.; Bourbigot, S.; Dubois, P. The Production and Properties of Polylactide Composites Filled with Expanded Graphite. Polym. Degrad. Stabil. 2010, 95, 889–900. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Adelnia, H.; Gudarzi, M.M. Intumescent Flame Retardant Polyurethane/Reduced Graphene Oxide Composites with Improved Mechanical, Thermal, and Barrier Properties. J. Mater. Sci. 2013, 49, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Wang, X.; Qian, X.; Xing, W.; Yang, H.; Ma, L.; Lin, Y.; Jiang, S.; Song, L.; Hu, Y.; et al. Functionalized Graphene Oxide/Phosphoramide Oligomer Hybrids Flame Retardant Prepared Via in Situ Polymerization for Improving the Fire Safety of Polypropylene. Rsc Adv. 2014, 4, 31782. [Google Scholar] [CrossRef]
- Chen, M.; Xu, Y.; Chen, X.; Ma, Y.; He, W.; Yu, J.; Zhang, Z. Thermal Stability and Combustion Behavior of Flame-Retardant Polypropylene with Thermoplastic Polyurethane-Microencapsulated Ammonium Polyphosphate. High Perform. Polym. 2014, 26, 445–454. [Google Scholar] [CrossRef]
- Lim, K.S.; Bee, S.T.; Sin, L.T.; Tee, T.T.; Ratnam, C.T.; Hui, D.; Rahmat, A.R. A Review of Application of Ammonium Polyphosphate as Intumescent Flame Retardant in Thermoplastic Composites. Compos. Part B Eng. 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Suppakarn, N.; Jarukumjorn, K. Mechanical Properties and Flammability of Sisal/PP Composites: Effect of Flame Retardant Type and Content. Compos. Part B Eng. 2009, 40, 613–618. [Google Scholar] [CrossRef]
- Sain, M.; Park, S.H.; Suhara, F.; Law, S. Flame Retardant and Mechanical Properties of Natural Fibre–PP Composites Containing Magnesium Hydroxide. Polym. Degrad. Stabil. 2004, 83, 363–367. [Google Scholar] [CrossRef]
- Manfredi, L.B.; Rodríguez, E.S.; Wladyka-Przybylak, M.; Vázquez, A. Thermal Degradation and Fire Resistance of Unsaturated Polyester, Modified Acrylic Resins and Their Composites with Natural Fibres. Polym. Degrad. Stabil. 2006, 91, 255–261. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Anwar, Z.; Muhammad, B. Recent Developments in Different Types of Flame Retardants and Effect on Fire Retardancy of Epoxy Composite. Polym. Plast. Technol. Eng. 2016, 55, 1512–1535. [Google Scholar] [CrossRef]
- Baysal, E.; Yalinkilic, M.K.; Altinok, M.; Sonmez, A.; Peker, H.; Colak, M. Some Physical, Biological, Mechanical, and Fire Properties of Wood Polymer Composite (WPC) Pretreated with Boric Acid and Borax Mixture. Constr. Build. Mater. 2007, 21, 1879–1885. [Google Scholar] [CrossRef]
- Fernandes, V.J.; Araujo, A.S.; Fonseca, V.M.; Fernandes, N.S.; Silva, D.R. Thermogravimetric Evaluation of Polyester/Sisal Flame Retarded Composite. Thermochim. Acta 2002, 392–393, 71–77. [Google Scholar] [CrossRef]
- Jones, M.; Bhat, T.; Huynh, T.; Kandare, E.; Yuen, R.; Wang, C.H.; John, S. Waste-Derived Low-Cost Mycelium Composite Construction Materials with Improved Fire Safety. Fire Mater. 2018, 42, 816–825. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alhazmi, M.; Alatiah, K.A.; Alshatwi, A.A. Synthesis of Biogenic Silica Nanoparticles from Rice Husks for Biomedical Applications. Ceram. Int. 2015, 41, 275–281. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Khass, T.; Mostafa, M.E. Thermo-Physical and Kinetics Parameters Determination and Gases Emissions of Self-ignition of Sieved Rice Husk of Different Sizes on a Hot Plate. Asia Pac. J. Chem. Eng. 2017, 12, 536–550. [Google Scholar] [CrossRef]
- Zuo, Y.; Xiao, J.; Wang, J.; Liu, W.; Li, X.; Wu, Y. Preparation and Characterization of Fire Retardant Straw/Magnesium Cement Composites with an Organic-Inorganic Network Structure. Constr. Build. Mater. 2018, 171, 404–413. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, H.; Sheng, C. Self-Heating of Agricultural Residues During Storage and Its Impact on Fuel Properties. Energy Fuels 2017, 32, 4227–4236. [Google Scholar] [CrossRef]


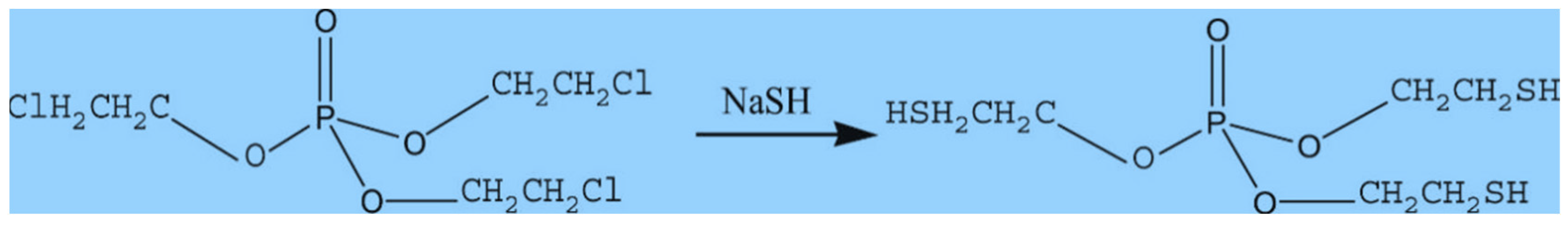
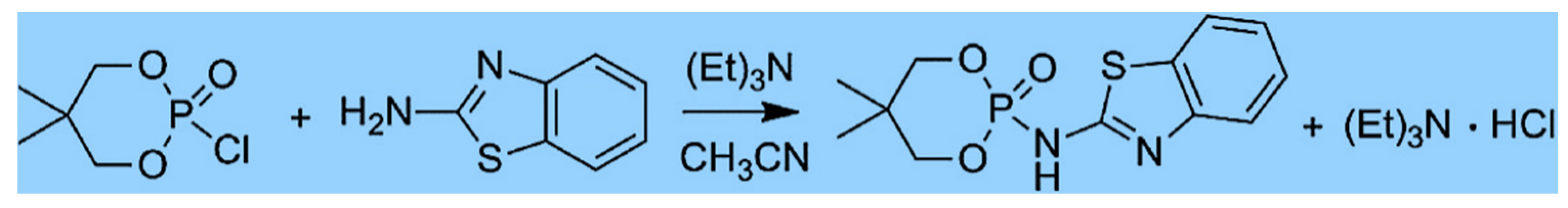
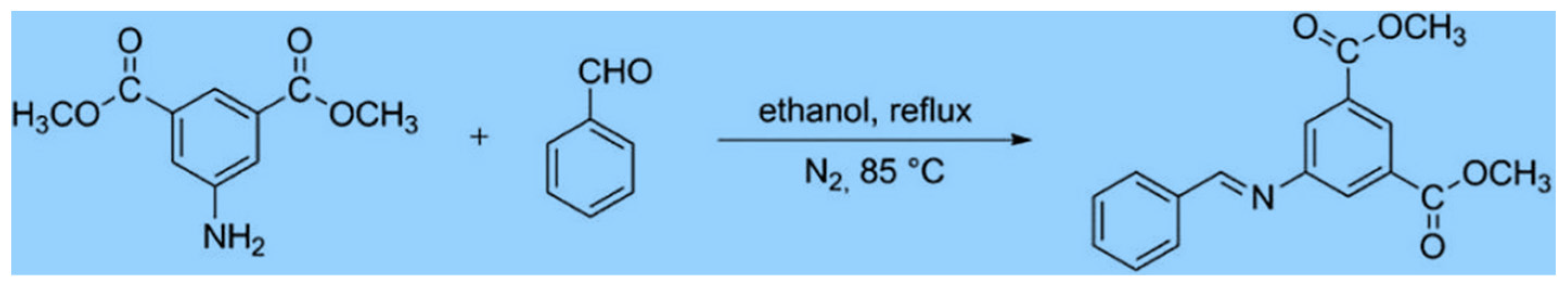

| Composites | Characteristics | References |
|---|---|---|
| Decabromodiphenylethane and brominated trimethylphenyl | Thermal stability, low permeability and excellent impact resistance | [98] |
| Pentabromobenzyl acrylate | Better flame retardant performance | [100] |
| Phosphate bromide/antimony trioxide/PP | Low filler content and high flame retardancy | [101] |
| Composites | Characteristics | References |
|---|---|---|
| Cosiae-SP and Cosiae-VP | Excellent thermal stability and smoke suppression ability | [102] |
| WPCs | Better flame retardant performance, no pollution and environmental protection | [103] |
| EVA-ATH-MCA composite | Wide range of applications | [104] |
| APP/PS and APP/PMMA MP/ATH | Low PHRR and smoke suppression ability | [105,106] |
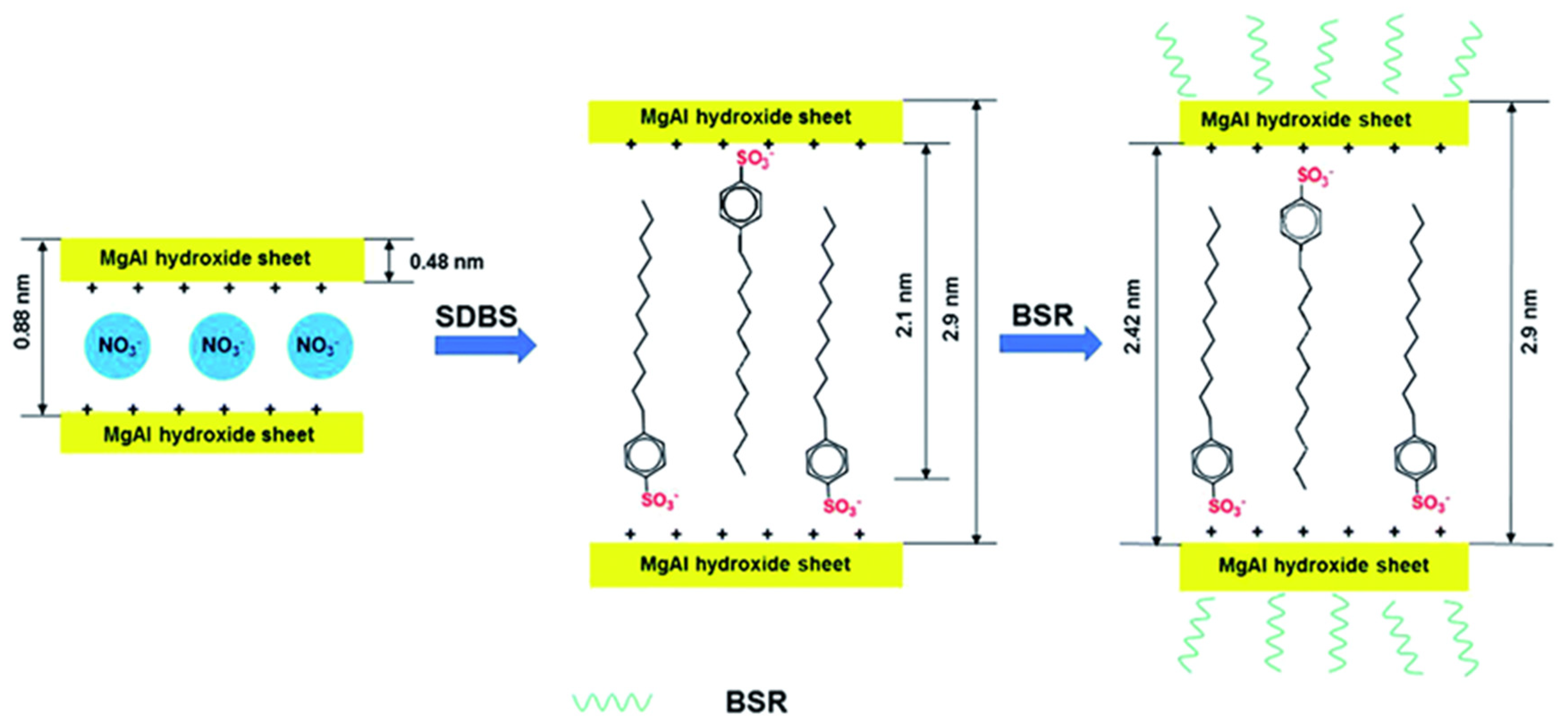
| DBS-LDH/BSR | No pollution and low PHRR | [107] |
| Composites | Characteristics | References |
|---|---|---|
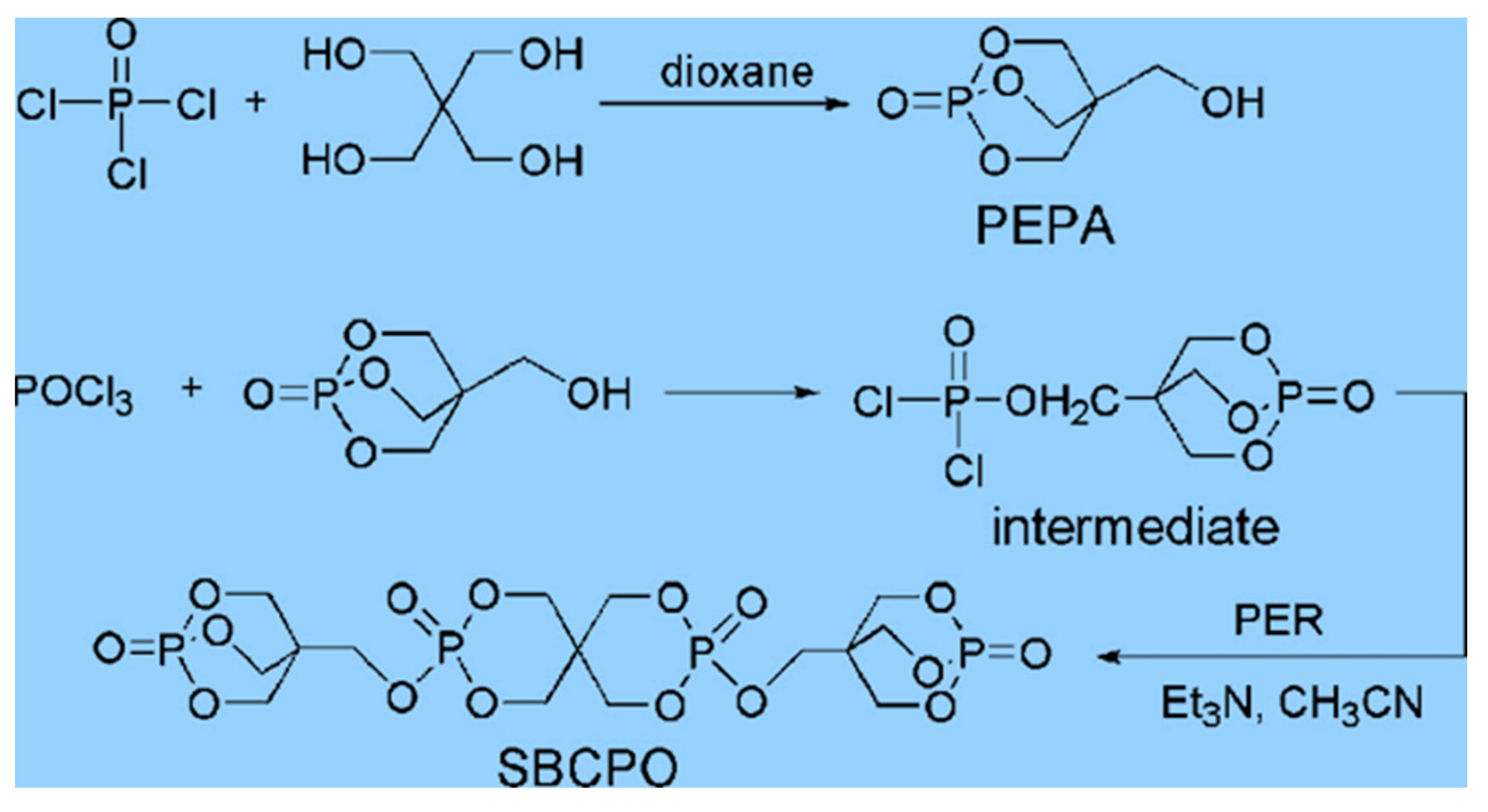
| SBCPO | Low content and high flame retardancy | [101] |
| PMPC | Blending and synergistic effect | [109] |
| APP/SiO2 | Processing convenience and excellent flame retardant properties | [110] |
| TMEP | Excellent flame retardant properties | [111] |
| DOP-ABZ | Good thermal stability and less smoke | [112] |
| BA/PET | Better flame retardant properties | [113] |
| PPLSP | Good durability and excellent water resistance | [114] |
| MP/APP/EP | Synergistic flame retardant properties | [115] |
| HGCP/EP | Excellent thermal stability and smoke suppression ability | [116] |
| Composites | Characteristics | References |
|---|---|---|
| CA/APP/OMMT | Low content and high flame retardancy | [117] |
| Intumescent flame retardant PP | Metal ions | [53] |
| TPO | Processing convenience and low PHRR | [118] |
| Composites | Characteristics | References |
|---|---|---|
| Nano-clay/polymer composites | Improve the flame retardant properties of composites | [34] |
| Nano-clays/EPDM/ATH | Processing convenience | [119] |
| Titanium oxide and iron oxide nano-fillers | Processing convenience and low PHRR | [120] |
| Nano-MDH/PP | Improved flame retardancy | [121] |
| PLA/CaSO4/OMLS | Improved flame retardancy | [122] |
| IFRPU | Excellent droplet resistance and low PHRR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Liang, J.; Lin, X.; Lin, H.; Yu, J.; Wang, S. The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering. Polymers 2022, 14, 82. https://doi.org/10.3390/polym14010082
Shen J, Liang J, Lin X, Lin H, Yu J, Wang S. The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering. Polymers. 2022; 14(1):82. https://doi.org/10.3390/polym14010082
Chicago/Turabian StyleShen, Jingjing, Jianwei Liang, Xinfeng Lin, Hongjian Lin, Jing Yu, and Shifang Wang. 2022. "The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering" Polymers 14, no. 1: 82. https://doi.org/10.3390/polym14010082
APA StyleShen, J., Liang, J., Lin, X., Lin, H., Yu, J., & Wang, S. (2022). The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering. Polymers, 14(1), 82. https://doi.org/10.3390/polym14010082







