Biopolymer: A Sustainable Material for Food and Medical Applications
Abstract
:1. Introduction
1.1. Need for Biopolymers
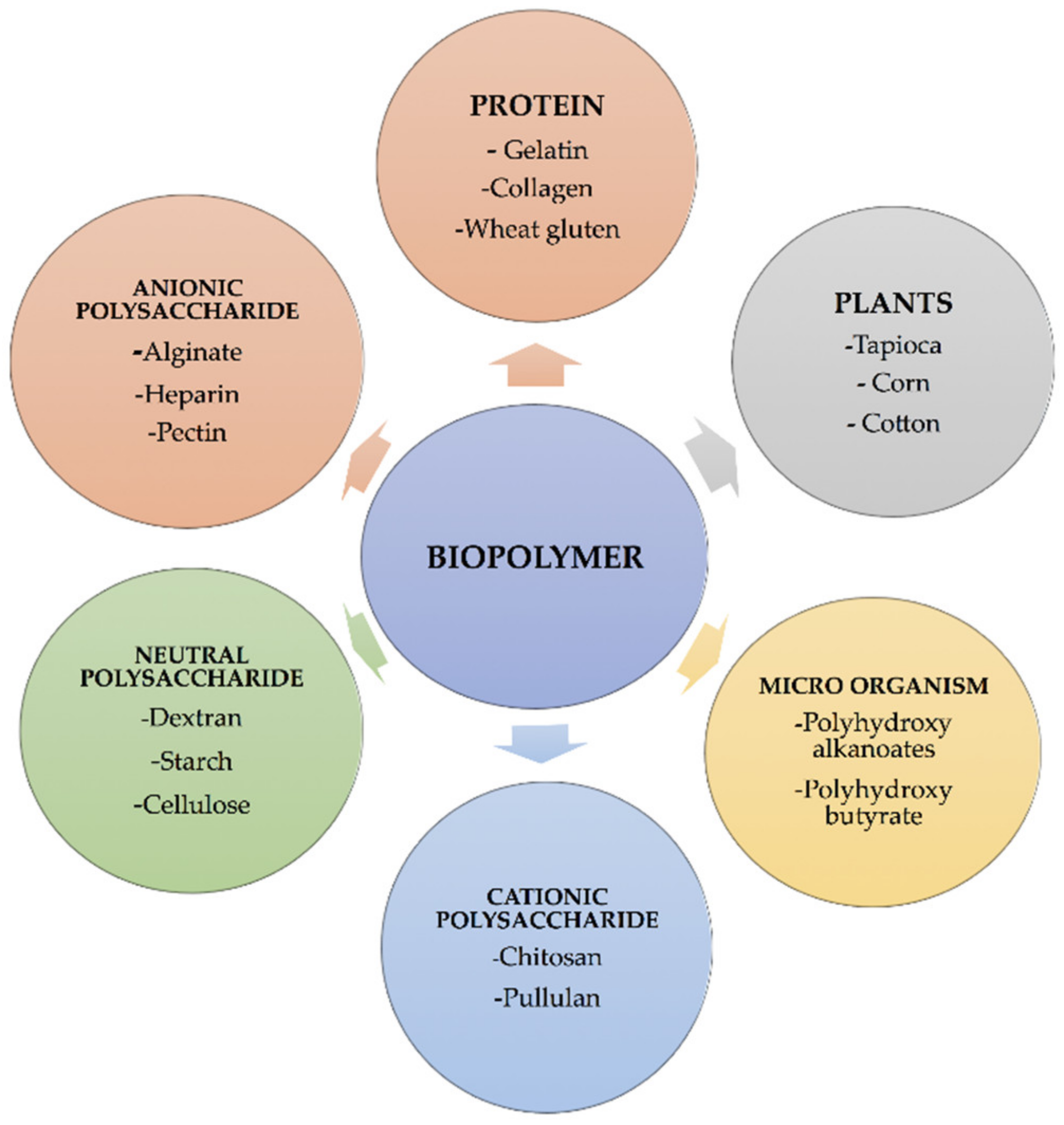
1.2. Sources of Biopolymers
1.2.1. As a Bio-Resource: Lignin
1.2.2. Carbohydrate Based Biopolymers: Polysaccharides
2. Biopolymers: State of Art
3. Biopolymers for Medical Applications
3.1. Biomedical Applications of Protein-Based Biopolymers
| Nanoparticles | Properties | Ref. |
|---|---|---|
| Chitosan | Non-toxic, blood viable, antitumor, antioxidant, antimicrobial, inexpensive, and biodegradable | [86] |
| Superparamagnetic iron oxide nanoparticles | Superparamagnetic, paramagnetic | [87] |
| Poly-L-lysine | High loading capacity, biodegradable, targeted delivery | [88] |
| Poly-D-L-lactide-co-glycolide | Biocompatible, non-toxic by-products | [89] |
| Liposomes | Biocompatible, carries hydrophobic material | [90] |
| Alginate | Water-soluble, biocompatible | [91] |
| Gold | Biocompatible, hyperthermia | [92] |
| Micelles | Capable of carrying water-soluble drug | [93] |
3.2. Chitosan in Medical Applications
4. Biopolymers for Food Applications
4.1. Nanostructured Coatings on Fruits during COVID-19
4.2. Microbial Polysaccharides in Food Industry
4.3. The Role of Dietary Fibers in Contemporary Food Production
4.4. The Functionality of Starch Derivatives in Bakery and Confectionery Products
4.5. Polymer for Food Sector: Guar Gum
4.6. Chitosan Application in Food Industry
5. Biopolymer Industry
5.1. Market Overview
5.2. Market Outlook
5.3. Major Growth Drivers
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ezeoha, S.L. Production of Biodegradable Plastic Packaging Film from Cassava Starch. IOSR J. Eng. 2013, 3, 14–20. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Kratochvíl, P.; Stepto, R.F.T.; Suter, U.W. Glossary of basic terms in polymer science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Stryer, L.; Berg, J.M.; Tymoczko, J.L. Biochemistry And Study Guide, 5th ed.; W.H. Freeman: New York, NY, USA, 2002. [Google Scholar]
- Gullapalli, S.; Wong, M.S. Nanotechnology: A Guide to Nano-Objects. Chem. Eng. Prog. 2011, 107, 28–32. [Google Scholar]
- Ruso, J.M.; Messina, P.V. Biopolymers for Medical Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Awad, Y.M.; Blagodatskaya, E.; Ok, Y.S.; Kuzyakov, Y. Effects of polyacrylamide, biopolymer and biochar on the decomposition of14C-labelled maize residues and on their stabilization in soil aggregates. Eur. J. Soil Sci. 2013, 64, 488–499. [Google Scholar] [CrossRef]
- Karmanov, A.P.; Kanarsky, A.V.; Kocheva, L.S.; Belyy, V.A.; Semenov, E.I.; Rachkova, N.G.; Bogdanovich, N.I.; Pokryshkin, S.A. Chemical structure and polymer properties of wheat and cabbage lignins–Valuable biopolymers for biomedical applications. Polymer 2021, 220, 123571. [Google Scholar] [CrossRef]
- Tanamool, V.; Imai, T.; Danvirutai, P.; Kaewkannetra, P. An alternative approach to the fermentation of sweet sorghum juice into biopolymer of poly-β-hydroxyalkanoates (PHAs) by newly isolated, Bacillus aryabhattai PKV01. Biotechnol. Bioprocess Eng. 2013, 18, 65–74. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Pérez, E.; Guzmán, R.; Tapia, M.S.; Famá, L. Physicochemical and Functional Properties of Native and Modified by Crosslinking, Dark-Cush-Cush Yam (Dioscorea trifida) and Cassava (Manihot esculenta) Starch. J. Polym. Biopolym. Phys. Chem. 2014, 2, 1–5. [Google Scholar] [CrossRef]
- Perotti, G.F.; Tronto, J.; Bizeto, M.A.; Izumi, C.M.S.; Temperini, M.L.A.; Lugão, A.B.; Parra, D.F.; Constantino, V.R.L. Biopolymer-Clay Nanocomposites: Cassava Starch and Synthetic Clay Cast Films. J. Braz. Chem. Soc. 2013, 25, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Borah, P.P.; Das, P.; Badwaik, L.S. Ultrasound treated potato peel and sweet lime pomace based biopolymer film development. Ultrason. Sonochem. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Redondo-Gómez, C.; Rodríguez Quesada, M.; Vallejo Astúa, S.; Murillo Zamora, J.P.; Lopretti, M.; Vega-Baudrit, J.R. Biorefinery of Biomass of Agro-Industrial Banana Waste to Obtain High-Value Biopolymers. Molecules 2020, 25, 3829. [Google Scholar] [CrossRef]
- Abral, H.; Dalimunthe, M.H.; Hartono, J.; Efendi, R.P.; Asrofi, M.; Sugiarti, E.; Sapuan, S.M.; Park, J.-W.; Kim, H.-J. Characterization of Tapioca Starch Biopolymer Composites Reinforced with Micro Scale Water Hyacinth Fibers. Starch-Stärke 2018, 70, 1700287. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Inverse vulcanization of octenyl succinate-modified corn starch as a route to biopolymer–sulfur composites. Mater. Adv. 2021, 2, 2391–2397. [Google Scholar] [CrossRef]
- Aguilar, N.M.; Arteaga-Cardona, F.; de Anda Reyes, M.E.; Gervacio-Arciniega, J.J.; Salazar-Kuri, U. Magnetic bioplastics based on isolated cellulose from cotton and sugarcane bagasse. Mater. Chem. Phys. 2019, 238. [Google Scholar] [CrossRef]
- Yu, P. Molecular chemical structure of barley proteins revealed by ultra-spatially resolved synchrotron light sourced FTIR microspectroscopy: Comparison of barley varieties. Biopolymers 2007, 85, 308–317. [Google Scholar] [CrossRef]
- Flaris, V.; Singh, G. Recent developments in biopolymers. J. Vinyl Addit. Technol. 2009, 15, 1–11. [Google Scholar] [CrossRef]
- Sharma, V.; Kundu, P.P. Addition polymers from natural oils—A review. Prog. Polym. Sci. 2006, 31, 983–1008. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Sabbagh, F.; Abdul Karim, N. Polyhydroxyalkanoates: A Valuable Secondary Metabolite Produced in Microorganisms and Plants. In Plant Secondary Metabolites, 1st ed.; Apple Academic Press: Oakville, MO, USA, 2017; Volume 3, pp. 205–234. [Google Scholar]
- Mojaveryazdi, F.S.; Muhamad, I.I.; Rezania, S.; Benham, H. Importance of Glucose and Pseudomonas in Producing Degradable Plastics. J. Teknol. 2014, 69, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Sivamani, S.; Sivakumar, N.; Maran, J.P.; Hosseini-Bandegharaei, A. Ecofriendly biopolymers and composites: Preparation and their applications in water-treatment. Biotechnol. Adv. 2021, 52, 107815. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Satitsri, S.; Muanprasat, C. Chitin and Chitosan Derivatives as Biomaterial Resources for Biological and Biomedical Applications. Molecules 2020, 25, 5961. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int. J. Biol. Macromol. 2020, 154, 1050–1073. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.J.F. Starch: Major Sources, Properties and Applications as Thermoplastic Materials. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 321–342. [Google Scholar] [CrossRef]
- Li, Z.; Wang, M.; Wang, F.; Gu, Z.; Du, G.; Wu, J.; Chen, J. γ-Cyclodextrin: A review on enzymatic production and applications. Appl. Microbiol. Biotechnol. 2007, 77, 245–255. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 85–126. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Lignin Structure, Properties, and Applications. In Biopolymers; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–63. [Google Scholar] [CrossRef]
- Donaldson, L.; Hague, J.; Snell, R. Lignin Distribution in Coppice Poplar, Linseed and Wheat Straw. Holzforschung 2001, 55, 379–385. [Google Scholar] [CrossRef]
- Kato, Y.; Onishi, H.; Machida, Y. Application of Chitin and Chitosan Derivatives in the Pharmaceutical Field. Curr. Pharm. Biotechnol. 2003, 4, 303–309. [Google Scholar] [CrossRef]
- Gangalla, R.; Gattu, S.; Palaniappan, S.; Ahamed, M.; Macha, B.; Thampu, R.K.; Fais, A.; Cincotti, A.; Gatto, G.; Dama, M.; et al. Structural Characterisation and Assessment of the Novel Bacillus amyloliquefaciens RK3 Exopolysaccharide on the Improvement of Cognitive Function in Alzheimer’s Disease Mice. Polymers 2021, 13, 2842. [Google Scholar] [CrossRef]
- Deniz, A.A.; Mukhopadhyay, S.; Lemke, E.A. Single-molecule biophysics: At the interface of biology, physics and chemistry. J. R. Soc. Interface 2007, 5, 15–45. [Google Scholar] [CrossRef]
- Alessandrini, A.; Facci, P. AFM: A versatile tool in biophysics. Meas. Sci. Technol. 2005, 16, R65–R92. [Google Scholar] [CrossRef]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic Molecular Motors and Mechanical Machines. Angew. Chem. Int. Ed. 2007, 46, 72–191. [Google Scholar] [CrossRef]
- Schatz, C.; Lecommandoux, S. Polysaccharide-Containing Block Copolymers: Synthesis, Properties and Applications of an Emerging Family of Glycoconjugates. Macromol. Rapid Commun. 2010, 31, 1664–1684. [Google Scholar] [CrossRef]
- Cushen, J.D.; Otsuka, I.; Bates, C.M.; Halila, S.; Fort, S.; Rochas, C.; Easley, J.A.; Rausch, E.L.; Thio, A.; Borsali, R.; et al. Oligosaccharide/Silicon-Containing Block Copolymers with 5 nm Features for Lithographic Applications. ACS Nano 2012, 6, 3424–3433. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Miles, C.; DeVetter, L.; Ghimire, S.; Hayes, D.G. Suitability of Biodegradable Plastic Mulches for Organic and Sustainable Agricultural Production Systems. HortScience 2017, 52, 10–15. [Google Scholar] [CrossRef]
- Gutierrez Cisneros, C.; Bloemen, V.; Mignon, A. Synthetic, Natural, and Semisynthetic Polymer Carriers for Controlled Nitric Oxide Release in Dermal Applications: A Review. Polymers 2021, 13, 760. [Google Scholar] [CrossRef]
- Neuendorf, R.E.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Adhesion between biodegradable polymers and hydroxyapatite: Relevance to synthetic bone-like materials and tissue engineering scaffolds. Acta Biomater. 2008, 4, 1288–1296. [Google Scholar] [CrossRef]
- Chen, Y.; Hung, S.-T.; Chou, E.; Wu, H.-S. Review of Polyhydroxyalkanoates Materials and other Biopolymers for Medical Applications. Mini Rev. Org. Chem. 2018, 15, 105–121. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Wang, P.-Y.; Lin, I.C.; Huang, H.; Liu, G.-S.; Tseng, C.-L. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-B.; Lih, E.; Park, K.-S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Yahya, E.B.; Amirul, A.A.; Khalil, H.P.S.A.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; Atty Sofea, A.K.; Adnan, A.S. Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Matthew, H.; Kohn, J.; Mikos, A.G.; Prestwich, G.D.; Yip, C.M. Biomaterials and Scaffolds in Reparative Medicine. Ann. N. Y. Acad. Sci. 2002, 961, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Lima, A.C.; Mano, J.F. Bioinspired methodology to fabricate hydrogel spheres for multi-applications using superhydrophobic substrates. Soft Matter 2010, 6, 5868–5871. [Google Scholar] [CrossRef]
- Hook, A.L.; Anderson, D.G.; Langer, R.; Williams, P.; Davies, M.C.; Alexander, M.R. High throughput methods applied in biomaterial development and discovery. Biomaterials 2010, 31, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Verdinelli, V.; Ruso, J.M.; Messina, P.V. Assessing structure and dynamics of fibrinogen films on silicon nanofibers: Towards hemocompatibility devices. Soft Matter 2012, 8, 6582–6592. [Google Scholar] [CrossRef]
- Pattanashetti, N.A.; Heggannavar, G.B.; Kariduraganavar, M.Y. Smart Biopolymers and their Biomedical Applications. Procedia Manuf. 2017, 12, 263–279. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, V.K. Biopolymers for Biomedical and Pharmaceutical Applications: Recent Advances and Overview of Alginate Electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 17. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I. Production of poly-hydroxyalkanoate as secondary metabolite with main focus on sustainable energy. Renew. Sustain. Energy Rev. 2017, 72, 95–104. [Google Scholar] [CrossRef]
- Tripathi, D.; Rastogi, K.; Tyagi, P.; Rawat, H.; Mittal, G.; Jamini, A.; Singh, H.; Tyagi, A. Comparative Analysis of Collagen and Chitosan-based Dressing for Haemostatic and Wound Healing Application. AAPS PharmSciTech 2021, 22, 76. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Aguilar, J.M.; Bengoechea, C.; López-Castejón, M.L.; Guerrero, A. Rheology and Water Absorption Properties of Alginate–Soy Protein Composites. Polymers 2021, 13, 1807. [Google Scholar] [CrossRef]
- Dulińska-Litewka, J.; Dykas, K.; Felkle, D.; Karnas, K.; Khachatryan, G.; Karewicz, A. Hyaluronic Acid-Silver Nanocomposites and Their Biomedical Applications: A Review. Materials 2021, 15, 234. [Google Scholar] [CrossRef]
- Nelson, D.W.; Gilbert, R.J. Extracellular Matrix-Mimetic Hydrogels for Treating Neural Tissue Injury: A Focus on Fibrin, Hyaluronic Acid, and Elastin-Like Polypeptide Hydrogels. Adv. Healthc. Mater. 2021, 10, 2101329. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A. How to Improve Physico-Chemical Properties of Silk Fibroin Materials for Biomedical Applications?—Blending and Cross-Linking of Silk Fibroin—A Review. Materials 2021, 14, 1510. [Google Scholar] [CrossRef]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Khaliq, T.; Sohail, M.; Minhas, M.U.; Ahmed Shah, S.; Jabeen, N.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Rashid, H. Self-crosslinked chitosan/κ-carrageenan-based biomimetic membranes to combat diabetic burn wound infections. Int. J. Biol. Macromol. 2022, 197, 157–168. [Google Scholar] [CrossRef]
- Klavert, J.; van der Eerden, B.C.J. Fibronectin in Fracture Healing: Biological Mechanisms and Regenerative Avenues. Front. Bioeng. Biotechnol. 2021, 9, 274. [Google Scholar] [CrossRef]
- Goswami, M.; Rekhi, P.; Debnath, M.; Ramakrishna, S. Microbial Polyhydroxyalkanoates Granules: An Approach Targeting Biopolymer for Medical Applications and Developing Bone Scaffolds. Molecules 2021, 26, 860. [Google Scholar] [CrossRef]
- Sarangthem, V.; Singh, T.D.; Dinda, A.K. Emerging Role of Elastin-Like Polypeptides in Regenerative Medicine. Adv. Wound Care 2021, 10, 257–269. [Google Scholar] [CrossRef]
- Sellappan, L.K.; Anandhavelu, S.; Doble, M.; Perumal, G.; Jeon, J.-H.; Vikraman, D.; Kim, H.-S. Biopolymer film fabrication for skin mimetic tissue regenerative wound dressing applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 196–207. [Google Scholar] [CrossRef]
- Varma, K.; Gopi, S. Biopolymers and their role in medicinal and pharmaceutical applications. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–191. [Google Scholar] [CrossRef]
- Yadav, P. Biomedical Biopolymers, their Origin and Evolution in Biomedical Sciences: A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE21. [Google Scholar] [CrossRef]
- Bhatia, M.; Girdhar, A.; Tiwari, A.; Nayarisseri, A. Implications of a novel Pseudomonas species on low density polyethylene biodegradation: An in vitro to in silico approach. SpringerPlus 2014, 3, 497. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, Z.; Du, B.; Han, C.; Hou, Z. Effect of Fullerene Nanoparticle on Tuning Trap Level Distribution of Fullerene/Polyethylene Nanocomposites. In Proceedings of the 2019 2nd International Conference on Electrical Materials and Power Equipment (ICEMPE), Guangzhou, China, 7–10 April 2019; pp. 333–336. [Google Scholar]
- Peters, D.; Kastantin, M.; Kotamraju, V.R.; Karmali, P.P.; Gujraty, K.; Tirrell, M.; Ruoslahti, E. Targeting atherosclerosis by using modular, multifunctional micelles. Proc. Natl. Acad. Sci. USA 2009, 106, 9815–9819. [Google Scholar] [CrossRef] [Green Version]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in Medical Implants: A Brief Review. Procedia Eng. 2017, 200, 236–243. [Google Scholar] [CrossRef]
- Nitta, S.; Numata, K. Biopolymer-Based Nanoparticles for Drug/Gene Delivery and Tissue Engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, S.; Radhakrishnan, S.; Kalkura, S.N.; Balme, S.; Miele, P.; Bechelany, M. Overview of Protein-Based Biopolymers for Biomedical Application. Macromol. Chem. Phys. 2019, 220, 1900126. [Google Scholar] [CrossRef]
- Nagarajan, S.; Abessolo Ondo, D.; Gassara, S.; Bechelany, M.; Balme, S.; Miele, P.; Kalkura, N.; Pochat-Bohatier, C. Porous Gelatin Membrane Obtained from Pickering Emulsions Stabilized by Graphene Oxide. Langmuir 2018, 34, 1542–1549. [Google Scholar] [CrossRef]
- Biscarat, J.; Bechelany, M.; Pochat-Bohatier, C.; Miele, P. Graphene-like BN/gelatin nanobiocomposites for gas barrier applications. Nanoscale 2015, 7, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridhar, R.; Lakshminarayanan, R.; Madhaiyan, K.; Amutha Barathi, V.; Lim, K.H.C.; Ramakrishna, S. Electrosprayed nanoparticles and electrospun nanofibers based on natural materials: Applications in tissue regeneration, drug delivery and pharmaceuticals. Chem. Soc. Rev. 2015, 44, 790–814. [Google Scholar] [CrossRef] [Green Version]
- Jammalamadaka, U.; Tappa, K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Gaspar-Pintiliescu, A.; Stefan, L.M.; Anton, E.D.; Berger, D.; Matei, C.; Negreanu-Pirjol, T.; Moldovan, L. Physicochemical and Biological Properties of Gelatin Extracted from Marine Snail Rapana venosa. Mar. Drugs 2019, 17, 589. [Google Scholar] [CrossRef] [Green Version]
- Thandapani, G.; Prasad, S.; Sudha, P.N.; Sukumaran, A. Size optimization and in vitro biocompatibility studies of chitosan nanoparticles. Int. J. Biol. Macromol. 2017, 104, 1794–1806. [Google Scholar] [CrossRef]
- Ahmed, H.; Hashim, A. Structure, Optical, Electronic and Chemical Characteristics of Novel (PVA-CoO) Structure Doped with Silicon Carbide. Silicon 2020, 13, 4331–4344. [Google Scholar] [CrossRef]
- Kodama, Y.; Kuramoto, H.; Mieda, Y.; Muro, T.; Nakagawa, H.; Kurosaki, T.; Sakaguchi, M.; Nakamura, T.; Kitahara, T.; Sasaki, H. Application of biodegradable dendrigraft poly-l-lysine to a small interfering RNA delivery system. J. Drug Target. 2016, 25, 49–57. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [Green Version]
- Ekici, S.; Ilgin, P.; Butun, S.; Sahiner, N. Hyaluronic acid hydrogel particles with tunable charges as potential drug delivery devices. Carbohydr. Polym. 2011, 84, 1306–1313. [Google Scholar] [CrossRef]
- Ilkhanizadeh, S.; Khalafy, J.; Dekamin, M.G. Sodium alginate: A biopolymeric catalyst for the synthesis of novel and known polysubstituted pyrano[3,2-c]chromenes. Int. J. Biol. Macromol. 2019, 140, 605–613. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable Gold Nanovesicles with an Ultrastrong Plasmonic Coupling Effect for Photoacoustic Imaging and Photothermal Therapy. Angew. Chem. 2013, 125, 14208–14214. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Bronich, T.K.; Vetro, J.A.; Kabanov, A.V. Polymer Micelles as Drug Carriers. In Nanoparticulates as Drug Carriers; Imperial College Pres: London, UK, 2006; pp. 57–93. [Google Scholar] [CrossRef] [Green Version]
- Ak, H.P.S.; Saurabh, C.K.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.M.K.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Khaksar, S.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Salahshour, P.; Sadeghi, F.; Rostamnia, S.; Vahdat, S.M. Advancements in Fabrication and Application of Chitosan Composites in Implants and Dentistry: A Review. Biomolecules 2022, 12, 155. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [Green Version]
- Laine, C.; Harlin, A.; Hartman, J.; Hyvärinen, S.; Kammiovirta, K.; Krogerus, B.; Pajari, H.; Rautkoski, H.; Setälä, H.; Sievänen, J.; et al. Hydroxyalkylated xylans–Their synthesis and application in coatings for packaging and paper. Ind. Crops Prod. 2013, 44, 692–704. [Google Scholar] [CrossRef]
- Moraga, G.; Talens, P.; Moraga, M.J.; Martínez-Navarrete, N. Implication of water activity and glass transition on the mechanical and optical properties of freeze-dried apple and banana slices. J. Food Eng. 2011, 106, 212–219. [Google Scholar] [CrossRef]
- Bankura, K.P.; Maity, D.; Mollick, M.M.R.; Mondal, D.; Bhowmick, B.; Bain, M.K.; Chakraborty, A.; Sarkar, J.; Acharya, K.; Chattopadhyay, D. Synthesis, characterization and antimicrobial activity of dextran stabilized silver nanoparticles in aqueous medium. Carbohydr. Polym. 2012, 89, 1159–1165. [Google Scholar] [CrossRef]
- Kanmani, P.; Lim, S.T. Synthesis and characterization of pullulan-mediated silver nanoparticles and its antimicrobial activities. Carbohydr. Polym. 2013, 97, 421–428. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zhong, L.; Wu, G. Preparation of high-stable silver nanoparticle dispersion by using sodium alginate as a stabilizer under gamma radiation. Radiat. Phys. Chem. 2009, 78, 251–255. [Google Scholar] [CrossRef]
- Islam, M.S.; Yeum, J.H. Electrospun pullulan/poly(vinyl alcohol)/silver hybrid nanofibers: Preparation and property characterization for antibacterial activity. Colloids Surf. Physicochem. Eng. Asp. 2013, 436, 279–286. [Google Scholar] [CrossRef]
- Venkatesan, J.; Singh, S.; Anil, S.; Kim, S.-K.; Shim, M. Preparation, Characterization and Biological Applications of Biosynthesized Silver Nanoparticles with Chitosan-Fucoidan Coating. Molecules 2018, 23, 1429. [Google Scholar] [CrossRef] [Green Version]
- Youssef, A.M.; Abou-Yousef, H.; El-Sayed, S.M.; Kamel, S. Mechanical and antibacterial properties of novel high performance chitosan/nanocomposite films. Int. J. Biol. Macromol. 2015, 76, 25–32. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications–A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef] [Green Version]
- Chitrakar, B.; Zhang, M.; Bhandari, B. Improvement strategies of food supply chain through novel food processing technologies during COVID-19 pandemic. Food Control 2021, 125, 108010. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, A.K. Potential of engineered nanostructured biopolymer based coatings for perishable fruits with Coronavirus safety perspectives. Prog. Org. Coat. 2022, 163, 106632. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. Fruits: A Source of Polyphenols and Health Benefits. In Natural and Artificial Flavoring Agents and Food Dyes; Academic Press: Cambridge, MA, USA, 2018; pp. 189–228. [Google Scholar] [CrossRef]
- Mehany, T.; Khalifa, I.; Barakat, H.; Althwab, S.A.; Alharbi, Y.M.; El-Sohaimy, S. Polyphenols as promising biologically active substances for preventing SARS-CoV-2: A review with research evidence and underlying mechanisms. Food Biosci. 2021, 40, 100891. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Revel, J.S.; Stevens, J.F. Potential use of polyphenols in the battle against COVID-19. Curr. Opin. Food Sci. 2020, 32, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, Y.A.G.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent Advancements in Microbial Polysaccharides: Synthesis and Applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.K.; Sandford, P.A.; Cottrell, I.W. Industrial Applications of Some New Microbial Polysaccharides. Biotechnology 1983, 1, 778–783. [Google Scholar] [CrossRef]
- Aluwihare, L.I.; Repeta, D.J. A comparison of the chemical characteristics of oceanic DOM and extracellular DOM produced by marine algae. Mar. Ecol. Prog. Ser. 1999, 186, 105–117. [Google Scholar] [CrossRef]
- Kimmel, S.A.; Roberts, R.F.; Ziegler, G.R. Optimization of Exopolysaccharide Production by Lactobacillus delbrueckii subsp. bulgaricus RR Grown in a Semidefined Medium. Appl. Environ. Microbiol. 1998, 64, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Nwodo, U.; Green, E.; Okoh, A. Bacterial Exopolysaccharides: Functionality and Prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [Green Version]
- Özcan, E.; Öner, E.T. Microbial Production of Extracellular Polysaccharides from Biomass Sources. In Polysaccharides; Springer: Cham, Switzerland, 2015; pp. 161–184. [Google Scholar] [CrossRef]
- Višić, K.; Pušić, T.; Čurlin, M. Carboxymethyl Cellulose and Carboxymethyl Starch as Surface Modifiers and Greying Inhibitors in Washing of Cotton Fabrics. Polymers 2021, 13, 1174. [Google Scholar] [CrossRef]
- Mirzaei, M.; Alimi, M.; Shokoohi, S.; Golchoobi, L. Synergistic interactions between konjac-mannan and xanthan/tragacanth gums in tomato ketchup: Physical, rheological, and textural properties. J. Texture Stud. 2018, 49, 586–594. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Yang, B.; Weng, Y. Hemicellulose-Based Film: Potential Green Films for Food Packaging. Polymers 2020, 12, 1775. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 2009, 37, 47–73. [Google Scholar] [CrossRef]
- Santana, Á.L.; Meireles, M.A.A. New Starches are the Trend for Industry Applications: A Review. Food Public Health 2014, 4, 229–241. [Google Scholar] [CrossRef]
- BeMiller, J.N. Xanthan. In Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands, 2019; pp. 261–269. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan production from agro-industrial waste and its applications in food industry: A review. Carbohydr. Polym. 2019, 217, 46–57. [Google Scholar] [CrossRef]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Ruelas-Chacon, X.; Aguilar-González, A.; de la Luz Reyes-Vega, M.; Peralta-Rodríguez, R.D.; Corona-Flores, J.; Rebolloso-Padilla, O.N.; Aguilera-Carbo, A.F. Bioactive Protecting Coating of Guar Gum with Thyme Oil to Extend Shelf Life of Tilapia (Oreoschromis niloticus) Fillets. Polymers 2020, 12, 3019. [Google Scholar] [CrossRef]
- BeMiller, J.N. Gum Arabic and Other Exudate Gums. In Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–321. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Omoto, T.; Uno, Y.; Asai, I. The latest technologies for the application of gellan gum. In Physical Chemistry and Industrial Application of Gellan Gum; Springer: Berlin/Heidelberg, Germany, 1999; pp. 123–126. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Classification, Technological Properties, and Sustainable Sources. In Dietary Fiber: Properties, Recovery, and Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 27–58. [Google Scholar] [CrossRef]
- Peltzer, M.; Delgado, J.F.; Salvay, A.G.; Wagner, J.R. β-Glucan, a Promising Polysaccharide for Bio-based Films Developments for Food Contact Materials and Medical Applications. Curr. Org. Chem. 2018, 22, 1249–1254. [Google Scholar] [CrossRef]
- Felton, L.A. Mechanisms of polymeric film formation. Int. J. Pharm. 2013, 457, 423–427. [Google Scholar] [CrossRef]
- Thammakiti, S.; Suphantharika, M.; Phaesuwan, T.; Verduyn, C. Preparation of spent brewer’s yeast beta-glucans for potential applications in the food industry. Int. J. Food Sci. Technol. 2004, 39, 21–29. [Google Scholar] [CrossRef]
- Konusova, V.; Vorbeychikov, E.; Shamtsyan, M. Potential role of mushroom beta-glucans in immunity and inflammation in viral infections and COVID-19. J. Food Bioact. 2021, 16, 8–18. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. β(1-3)-D-glucan affects adipogenesis, wound healing and inflammation. Orient. Pharm. Exp. Med. 2011, 11, 169–175. [Google Scholar] [CrossRef]
- Pisanello, D.; Caruso, G. Novel Foods in the European Union, 1st ed.; Springer International Publishing: Basel, Switzerland, 2018. [Google Scholar]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal B-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial Nanocellulose—A Biobased Polymer for Active and Intelligent Food Packaging Applications: Recent Advances and Developments. Polymers 2020, 12, 2209. [Google Scholar] [CrossRef]
- Huijbrechts, A.M. Multifunctional Starch Derivatives: Synthesis, Characterization and Properties; Wageningen University and Research: Wageningen, The Netherlands, 2008. [Google Scholar]
- Stephen, A.M.; Phillips, G.O. Food Polysaccharides and Their Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Eerlingen, R.C.; Delcour, J.A. Formation, analysis, structure and properties of type III enzyme resistant starch. J. Cereal Sci. 1995, 22, 129–138. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Wang, Z.; Chen, H.; Fan, D. Renewable bio-based adhesive fabricated from a novel biopolymer and soy protein. RSC Adv. 2021, 11, 11724–11731. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Introduction to food hydrocolloids. In Handbook of Hydrocolloids; Woodhead Publishing: Sawston, UK, 2021; pp. 3–26. [Google Scholar] [CrossRef]
- Wilderjans, E.; Luyts, A.; Goesaert, H.; Brijs, K.; Delcour, J.A. A model approach to starch and protein functionality in a pound cake system. Food Chem. 2010, 120, 44–51. [Google Scholar] [CrossRef]
- Baranwal, K.; Dwivedi, L.M.; Shehala; Singh, V. Guar gum mediated synthesis of NiO nanoparticles: An efficient catalyst for reduction of nitroarenes with sodium borohydride. Int. J. Biol. Macromol. 2018, 120, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Funazukuri, T. Noncatalytic hydrolysis of guar gum under hydrothermal conditions. Carbohydr. Res. 2006, 341, 870–877. [Google Scholar] [CrossRef]
- Dea, I.C.M.; Morrison, A. Chemistry and Interactions of Seed Galactomannans. In Advances in Carbohydrate Chemistry and Biochemistry; Academic Press: Cambridge, MA, USA, 1975; Volume 31, pp. 241–312. [Google Scholar] [CrossRef]
- Tripathy, S.; Das, M.K. Guar Gum: Present Status and Applications. J. Pharm. Sci. Innov. 2013, 4, 24–28. [Google Scholar] [CrossRef]
- Wielinga, W.C. Galactomannans. In Handbook of Hydrocolloids; Woodhead Publishing: Sawston, UK, 2009; pp. 228–251. [Google Scholar] [CrossRef]
- Shen, Y.; Babu, K.S.; Amamcharla, J.; Li, Y. Emulsifying properties of pea protein/guar gum conjugates and mayonnaise application. Int. J. Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Bhat, V.G.; Narasagoudr, S.S.; Masti, S.P.; Chougale, R.B.; Vantamuri, A.B.; Kasai, D. Development and evaluation of Moringa extract incorporated Chitosan/Guar gum/Poly (vinyl alcohol) active films for food packaging applications. Int. J. Biol. Macromol. 2022, 200, 50–60. [Google Scholar] [CrossRef]
- Singh, D.; Viswakarma, P.; Kumar, B. A review of guar gum processing, properties, and its food applications. Asian J. Multidimens. Res. 2021, 10, 965–969. [Google Scholar] [CrossRef]
- Goemaere, O.; De Ketelaere, B.; Hanskens, J.; Masijn, Q.; Pérez Santaescolastica, C.; Fraeye, I. Comparison of the Technological Application Potential of Functional Ingredients for the Meat Industry Based upon a Novel Fast Screening Tool. Foods 2021, 10, 2078. [Google Scholar] [CrossRef]
- Adimule, V.; Kerur, S.S.; Chinnam, S.; Yallur, B.C.; Nandi, S.S. Guar Gum and its Nanocomposites as Prospective Materials for Miscellaneous Applications: A Short Review. Top. Catal. 2022, 1–14. [Google Scholar] [CrossRef]
- Hesarinejad, M.A.; Lorenzo, J.M.; Rafe, A. Influence of gelatin/guar gum mixture on the rheological and textural properties of restructured ricotta cheese. Carbohydr. Polym. Technol. Appl. 2021, 2, 100162. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Akhila, K.; Ramakanth, D.; Gaikwad, K.K. Guar gum/carboxymethyl cellulose based antioxidant film incorporated with halloysite nanotubes and litchi shell waste extract for active packaging. Int. J. Biol. Macromol. 2022, 201, 1–13. [Google Scholar] [CrossRef]
- Lee, Y.; Chang, Y.H. Influence of guar gum addition on physicochemical, microbial, rheological and sensory properties of stirred yoghurt. Int. J. Dairy Technol. 2016, 69, 356–363. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A Review. J. Food Sci. Technol. 2011, 51, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.; Ladero, V.; del Rio, B.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Biofilm-Forming Capacity in Biogenic Amine-Producing Bacteria Isolated from Dairy Products. Front. Microbiol. 2016, 7, 162. [Google Scholar] [CrossRef]
- Baranwal, K.; Dwivedi, L.M.; Siddique, S.; Tiwari, S.; Singh, V. Chitosan Grown Copper Doped Nickel Oxide Nanoparticles: An Excellent Catalyst for Reduction of Nitroarenes. J. Cluster Sci. 2020, 32, 937–947. [Google Scholar] [CrossRef]
- Li, B.; Elango, J.; Wu, W. Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application. Appl. Sci. 2020, 10, 4719. [Google Scholar] [CrossRef]
- Karthik, R.; Manigandan, V.; Saravanan, R.; Rajesh, R.P.; Chandrika, B. Structural characterization and in vitro biomedical activities of sulfated chitosan from Sepia pharaonis. Int. J. Biol. Macromol. 2016, 84, 319–328. [Google Scholar] [CrossRef]
- Wan, A.; Xu, Q.; Sun, Y.; Li, H. Antioxidant Activity of High Molecular Weight Chitosan and N,O-Quaternized Chitosans. J. Agric. Food Chem. 2013, 61, 6921–6928. [Google Scholar] [CrossRef]
- Manigandan, V.; Karthik, R.; Ramachandran, S.; Rajagopal, S. Chitosan Applications in Food Industry. In Biopolymers for Food Design; Elsevier: Cambridge, MA, USA, 2018; pp. 469–491. [Google Scholar] [CrossRef]
- EUBIA. Biopolymers. Available online: https://www.eubia.org/cms/wiki-biomass/bio-based-products/biopolymers/ (accessed on 1 February 2022).
- Biodegradable Plastics Market by Type (PLA, Starch Blends, PHA, Biodegradable Polyesters), End Use Industry (Packaging, Consumer Goods, Textile, Agriculture & Horticulture), and Region (APAC, Europe, North America & RoW)–Global Forecast to 2026. Available online: https://www.marketsandmarkets.com/Market-Reports/biodegradable-plastics-93.html (accessed on 10 January 2022).
- A.R.C. Biopolymers Market–Forecast (2022–2027). Available online: https://www.industryarc.com/Report/11739/biopolymers-market.html (accessed on 15 January 2022).



| Biopolymers | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Natural Biopolymers | Biologically renewable, biodegradable, biocompatible, non-toxic, bioadhesive material, biofuctional. | Less stable, low melting point, high surface tension, structurally more complex. | [2] |
| Synthetic Biopolymers | Biocompatibility, higher reproducibility, better mechanical, and chemical stability | Toxic, non-biodegradable, expensive synthesis procedure. | [4] |
| Biopolymers | Sources | Structure | Reference (Ref.) |
|---|---|---|---|
| Chitin | Corals, horseshoe worms, lamp shells, sponges, squid, cuttlefish, and clams are examples of aquatic species | 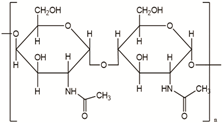 | [24,25] |
| Chitosan | Fungi, mollusks, algae, crustaceans, and insects | 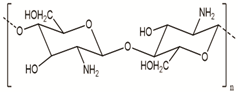 | [24,26] |
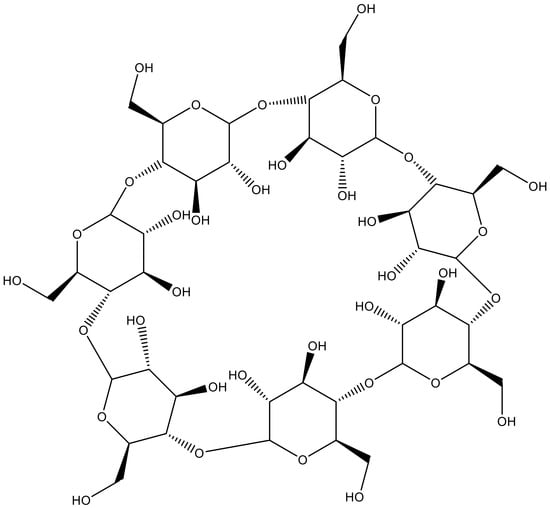
| Cellulose | Agricultural trashes, such as Seaweed, rice husk, and sugarcane bagasse. Plant sources like wood, bamboo, sugarbeet, banana rachis, potato tubers, cotton, fique, kapok, agave, jute, kenaf, flax, hemp, vine, sisal, coconut, grass, wheat, rice, and barley |  | [27] |
| Alginate | Seawood |  | [28] |
| Starch | Potatoes, maize, cassava, rice, sorghum, banana wheat, yams | 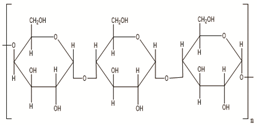 | [29] |
| Cyclodextrin | Starch sources like tapioca, potato, wheat, rice, and corn |  | [30] |
| Polycaprolactone | Polycondensation of ε -caprolactone | 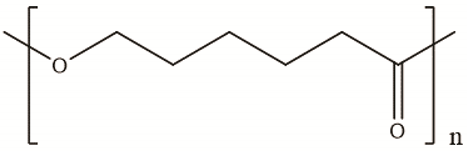 | [31] |
| Biopolymer | Medical Application | Ref. |
|---|---|---|
| Collagen | Surface coating for tissue culture plates | [58] |
| Simple gels for cell culture | ||
| Alginate | Regenerative medicine | [59] |
| Tissue engineering | ||
| Hyaluronic acid | Treatment and lubrication of damaged joints | [60] |
| Cutaneous and corneal wound healing | ||
| Fibrin | Blood clotting, wound healing, and tumor growth | [61] |
| Hemostatic agent, sealant, and surgical glue | ||
| Silk fibroin | Regenerative medicine Treatment of wounds, bioengineering of tissues | [62] |
| Agarose | Skeletal tissues regeneration, kidney and fibroblast encapsulation | [63] |
| Carrageenan | Skeletal tissues regeneration, cell delivery system | [64] |
| Fibronectin | Wound healing, cardiac repair, bone regeneration | [65] |
| PHAs | Drug delivery systems, one tissue regeneration, | [66] |
| Elastin | soft-tissue reconstruction, orthopedics and cell encapsulation | [67] |
| Keratin | Cornea tissue engineering, skin regeneration | [68] |
| Starch | Bone and cartilage regeneration, spinal cord injury treatment | [69] |
| Biopolymers | Properties | Applications | Ref. |
|---|---|---|---|
| Carboxymethyl- cellulose | Coating, Emulsifying agent | Confectionary Salad dressing | [117] [118] |
| Hemicellulose | Binding agent | Pet foods | [119] |
| Pectins | Adhesive | Icings and glazes | [120] |
| Starch | Stabilizer | Ice cream, salad dressing | [121] |
| Xanthan gum | Foam stabilizer | Beer | [122] |
| Pullulan | Film formation | Protective coating | [123] |
| Alginate | Gelling agent | Confectionary milk-based desserts, jellies | [124] |
| Guar gum | Thickening agent | Jams, syrups, and pie fillings | [125] |
| Gum karaya | Syneresis inhibitor | Frozen foods, cheeses | [126] |
| Agar | Swelling agent | Processed meat products | [127] |
| Gellan | Inhibitor | Frozen foods, sugar syrups | [128] |
| Properties | Applications | Ref. |
|---|---|---|
| Improving Textures | Stabilizer, thickener, gluten-free noodles, emulsifier, reducing oil uptake during fry | [150] |
| Beverage Industry | Thickener, stabilizer, dietary fiber | [151] |
| Dairy Products | Viscosifier, improving texture and mouthfeel, foam stabilization, preventing ice crystal growth in ice creams | [152] |
| Meat Products | Edible films, fat replacer, thickener | [153] |
| Soluble type of dietary fiber | Prebiotic, reducing blood, sugar, and cholesterol, treating constipation and diarrhea | [154] |
| Bakery industry | Frozen dough improvement, gluten-free products, texture, and physical property improvement | [155] |
| Others | Biodegradable films, flavor encapsulation | [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. https://doi.org/10.3390/polym14050983
Baranwal J, Barse B, Fais A, Delogu GL, Kumar A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers. 2022; 14(5):983. https://doi.org/10.3390/polym14050983
Chicago/Turabian StyleBaranwal, Jaya, Brajesh Barse, Antonella Fais, Giovanna Lucia Delogu, and Amit Kumar. 2022. "Biopolymer: A Sustainable Material for Food and Medical Applications" Polymers 14, no. 5: 983. https://doi.org/10.3390/polym14050983
APA StyleBaranwal, J., Barse, B., Fais, A., Delogu, G. L., & Kumar, A. (2022). Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers, 14(5), 983. https://doi.org/10.3390/polym14050983