Hardness and Roughness of Glass/Epoxy Composite Laminates Subjected to Different Hostile Solutions: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Weight Gain
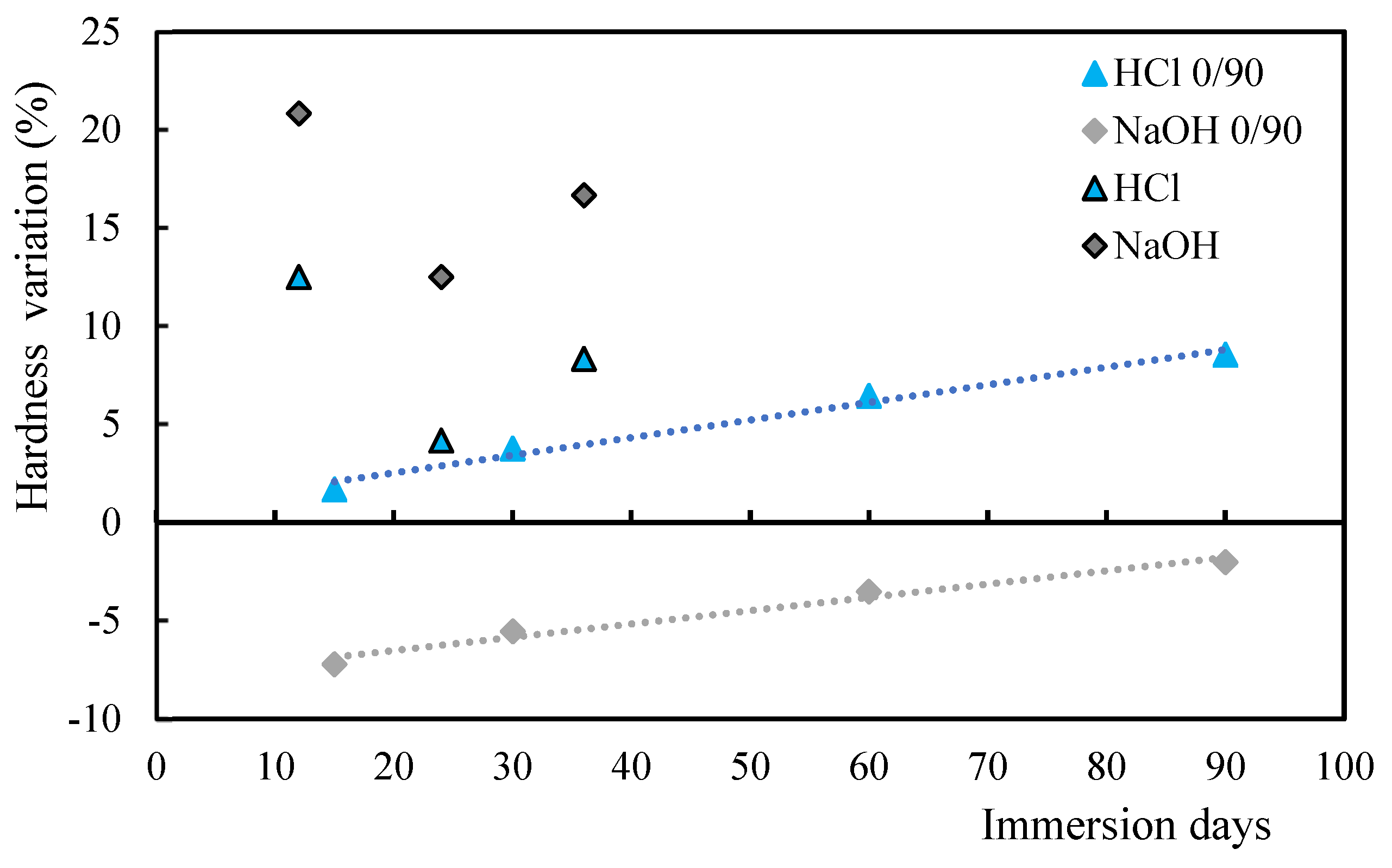
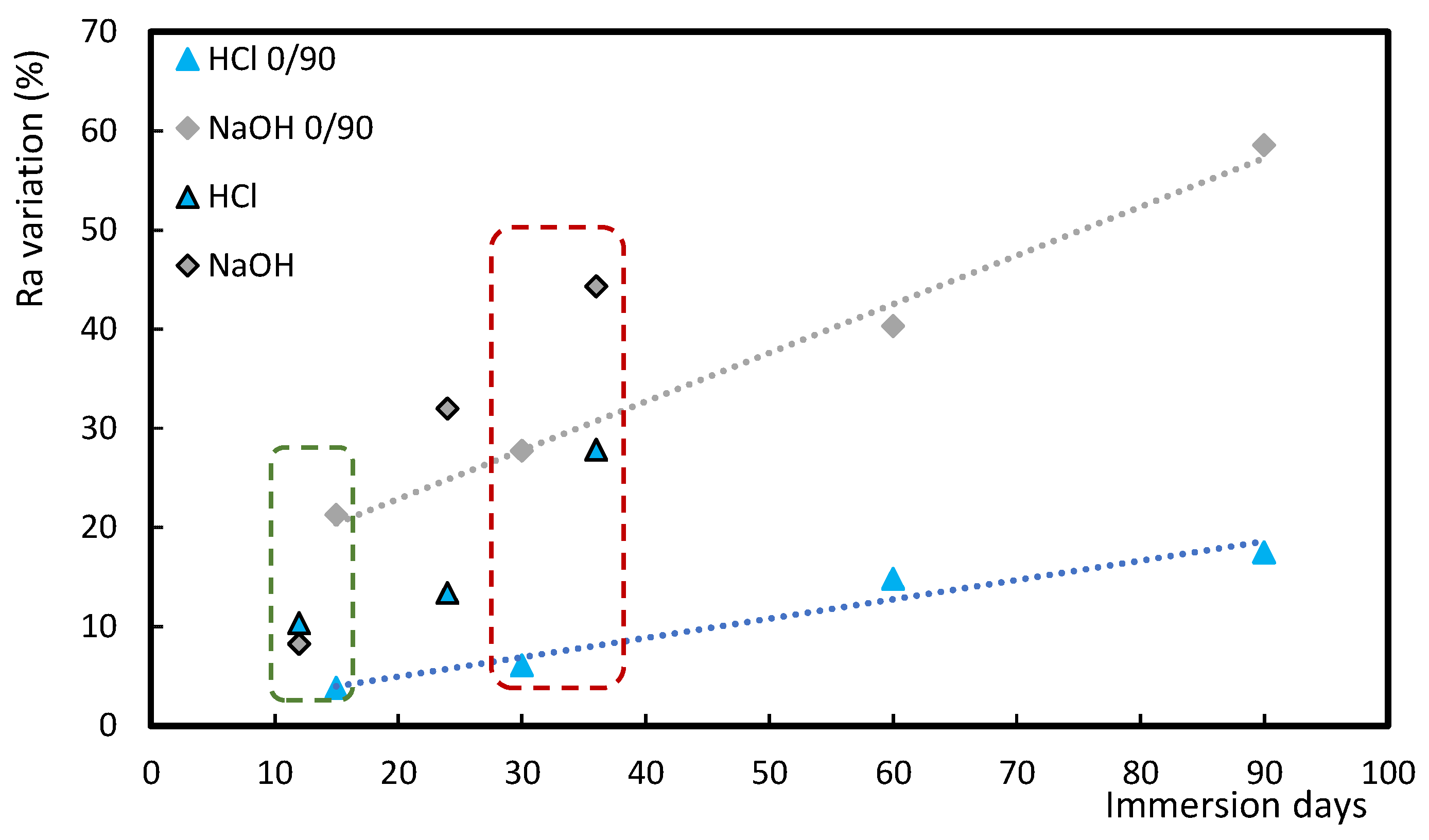
3.2. Comparison Between Acid/Alkaline Solutions and Mortars
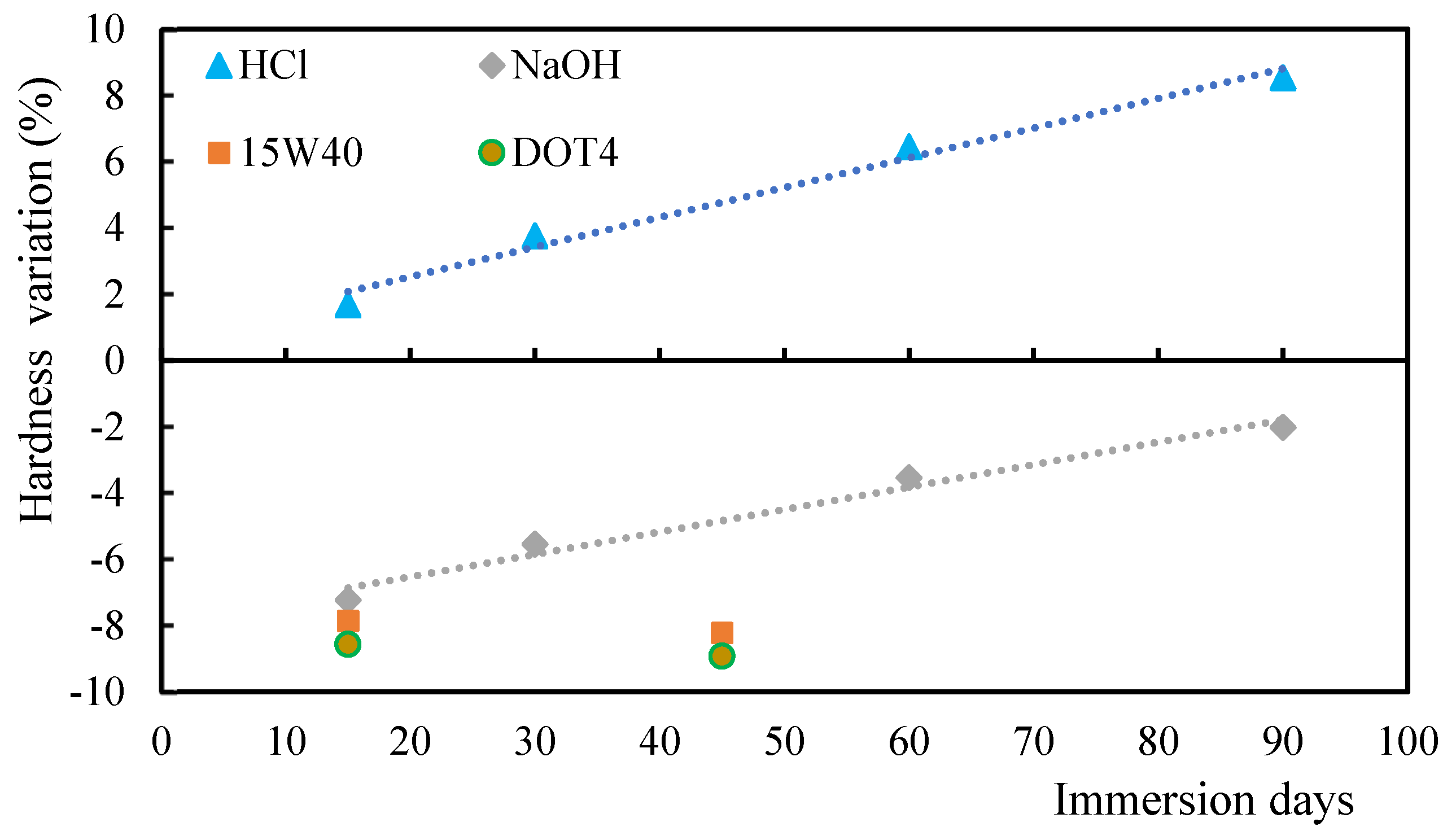
3.3. Comparison Between Acid/Alkaline Solutions and Oils
3.4. Influence of Stacking Sequence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sajan, S.; Philip Selvaraj, D. A review on polymer matrix composite materials and their applications. Mater. Today Proc. 2021, 47, 5493–5498. [Google Scholar] [CrossRef]
- Friedrich, K.; Almajid, A.A. Manufacturing Aspects of Advanced Polymer Composites for Automotive Applications. Appl. Compos. Mater. 2012, 20, 107–128. [Google Scholar]
- Enamul Hossain, M. The current and future trends of composite materials: An experimental study. J. Compos. Mater. 2011, 45, 2133–2144. [Google Scholar] [CrossRef]
- Boparai, K.S.; Singh, R.; Hashmi, M.S.J. Reinforced non-conventional material composites: A comprehensive review. Adv. Mater. Process. Technol. 2020, 7, 333–342. [Google Scholar]
- Kangishwar, S.; Radhika, N.; Sheik, A.A.; Chavali, A.; Hariharan, S.A. Comprehensive Review on Polymer Matrix Composites: Material Selection, Fabrication, and Application. Polym. Bull. 2023, 80, 47–87. [Google Scholar]
- Khan, F.; Hossain, N.; Mim, J.J.; Maksudur Rahman, S.M.; Jayed Iqbal Md Billah, M.; Chowdhury, M.A. Advances of composite materials in automobile applications—A review. J. Eng. Res. 2024; in press. [Google Scholar] [CrossRef]
- Barile, C.; Casavola, C.; De Cillis, F. Mechanical comparison of new composite materials for aerospace applications. Compos. B Eng. 2019, 162, 122–128. [Google Scholar]
- Mosallam, A.S.; Bayraktar, A.; Elmikawi, M.; Pul, S.; Adanur, S. Polymer Composites in Construction: An Overview. SOJ Mater. Sci. Eng. 2013, 2, 25. [Google Scholar]
- Rubino, F.; Nisticò, A.; Tucci, F.; Carlone, P. Marine Application of Fiber Reinforced Composites: A Review. J. Mar. Sci. Eng. 2020, 8, 26. [Google Scholar] [CrossRef]
- Fan, Q.; Duan, H.; Xing, X. A review of composite materials for enhancing support, flexibility and strength in exercise. Alex. Eng. J. 2024, 94, 90–103. [Google Scholar]
- Cheng, X.; Du, B.; He, J.; Long, W.; Su, G.; Liu, J.; Fan, Z.; Chen, L. A Review of thermoplastic composites on wind turbine blades. Compos. B Eng. 2025, 299, 112411. [Google Scholar]
- Ozdil, D.; Aydin, H.M. Polymers for medical and tissue engineering applications. J. Chem. Technol. Biotechnol. 2014, 89, 1793–1810. [Google Scholar] [CrossRef]
- Friedrich, K. Stress corrosion crack propagation in glass fibre reinforced/thermoplastic PET. J. Mater. Sci. 1981, 16, 3292–3302. [Google Scholar] [CrossRef]
- Jones, F.R.; Rock, J.W.; Wheatley, A.R. Stress corrosion cracking and its implications for the long-term durability of E-glass fibre composites. Composites 1983, 14, 262–269. [Google Scholar] [CrossRef]
- Hogg, P.J. Factors affecting the stress corrosion of GRP in acid environments. Composites 1983, 14, 254–261. [Google Scholar] [CrossRef]
- Norwood, L.S.; Hogg, P.J. GRP in contact with acidic environments e a case study. Compos. Sci. Technol. 1984, 2, 1–22. [Google Scholar] [CrossRef]
- Tanaka, H.; Kuraoka, K.; Yamanaka, H.; Yazawa, T. Development and disappearance of microporous structure in acid treated E-glass fiber. J. Non Cryst. Soliids 1997, 215, 262–270. [Google Scholar] [CrossRef]
- Kawada, H.; Srivastava, V.K. The effect of an acidic stress environment on the stress-intensity factor for GRP laminates. Compos. Sci. Technol. 2001, 61, 1109–1114. [Google Scholar] [CrossRef]
- Bazli, M.; Ashrafi, H.; Oskouei, A.V. Effect of harsh environments on mechanical properties of GFRP pultruded profiles. Compos. Part B Eng. 2016, 99, 203–215. [Google Scholar] [CrossRef]
- Haghara, H.; Watanabe, R.; Shimada, T.; Funabashi, M.; Kumioka, M.; Sato, H. Degradation behavior and lifetime estimation of fiber reinforced plastics tanks for hydrochloric acid storage. Compos. Part A Appl. Sci. Manuf. 2018, 112, 126–133. [Google Scholar]
- Mahmoud, M.K.; Tantawi, S.H. Effect of strong acids on mechanical properties of glass/polyester GRP pipe at normal and high temperatures. Polym. Plast. Technol. Eng. 2003, 42, 677–688. [Google Scholar] [CrossRef]
- Won, J.P.; Lee, S.J.; Kim, Y.J.; Jang, C.I.; Lee, S.W. The effect of exposure to alkaline solution and water on the strength–porosity relationship of GFRP rebar. Compos. Part B Eng. 2008, 39, 764–772. [Google Scholar]
- Banna, M.H.; Shirokoff, J.; Molgaard, J. Effects of two aqueous acid solutions on polyester and bisphenol A epoxy vinyl ester resins. Mater. Sci. Eng. A Struct. 2011, 528, 2137–2142. [Google Scholar]
- Benmokrane, B.; Ali, A.H.; Mohamed, H.M.; ElSafty, A.; Manalo, A. Laboratory assessment and durability performance of vinyl-ester, polyester, and epoxy glass-FRP bars for concrete structures. Compos. B Eng. 2017, 114, 163–174. [Google Scholar]
- Stamenovic, M.; Putic, S.; Rakin, M.; Medjo, B.; Cikara, D. Effect of alkaline and acid solutions on the tensile properties of glass-polyester pipes. Mater. Des. 2011, 32, 2456–2461. [Google Scholar]
- Griffiths, R.; Ball, A. An assessment of the properties and degradation behaviour of glass-fibre-reinforced polyester polymer concrete. Compos. Sci. Technol. 2000, 60, 2747e53. [Google Scholar]
- Pai, R.; Kamath, M.S.; Rao, R.M.V.G.K. Acid resistance of glass fibre composites with different layup sequencing: Part I e diffusion studies. J. Reinf. Plast. Compos. 1997, 16, 1002–1012. [Google Scholar] [CrossRef]
- Sindhu, K.; Joseph, K.; Joseph, J.M.; Mathew, T.V. Degradation studies of coir fiber/polyester and glass fiber/polyester composites under different conditions. J. Reinf. Plast. Compos. 2007, 26, 1571–1585. [Google Scholar]
- Tanks, J.D.; Arao, Y.; Kubouchi, M. Diusion kinetics, swelling, and degradation of corrosion-resistant -glass/epoxy woven composites in harsh environments. Compos. Struct. 2018, 202, 686–694. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Shen, H.; Wu, Z. Long-term performance of pultruded basalt fiber reinforced polymer profiles under acidic conditions. J. Mater. Civ. Eng. 2018, 30, 04018096. [Google Scholar]
- Kusano, M.; Kanai, T.; Arao, Y.; Kubouchi, M. Degradation behavior and lifetime estimation of fiber reinforced plastics tanks for hydrochloric acid storage. Eng. Fail. Anal. 2017, 79, 971–979. [Google Scholar] [CrossRef]
- Kanerva, M.; Jokinena, J.; Sarlina, E.; Pärnänena, T.; Lindgren, M.; Järventausta, M.; Vuorinen, J. Lower stiffness of GFRP after sulfuric acid-solution aging is due to degradation of fibre-matrix interfaces? Compos. Struct. 2019, 212, 524–534. [Google Scholar]
- Amaro, A.M.; Reis, P.N.B.; Neto, M.A.; Louro, C. Effects of alkaline and acid solutions on glass/epoxy composites. Polym. Degrad. Stab. 2013, 98, 853–862. [Google Scholar] [CrossRef]
- Amaro, A.M.; Reis, P.N.B.; Neto, M.A.; Louro, C. Effect of different acid solutions on glass/epoxy composites. J. Reinf. Plast. Compos. 2013, 32, 1018–1029. [Google Scholar]
- Mortas, N.; Er, O.; Reis, P.N.B.; Ferreira, J.A.M. Effect of corrosive solutions on composites laminates subjected to low velocity impact loading. Compos. Struct. 2014, 108, 205–211. [Google Scholar] [CrossRef]
- Reis, P.N.B.; Amaro, A.M.; Neto, M.A.; Cirne, J.S. Effect of Hostile Solutions on Composites Laminates Subjected to Low and High Velocity Impact Loads. Fibers Polym. 2019, 20, 158–164. [Google Scholar]
- Hota, G.; Barker, W.; Manalo, A. Degradation mechanism of glass fiber/vinylester-based composite materials under accelerated and natural aging. Constr. Build. Mater. 2020, 256, 119462. [Google Scholar]
- Reis, P.N.B.; Silva, M.P.; Santos, P.; Parente, J.; Valvez, S. Effect of Hostile Solutions on the Residual Fatigue Life of Kevlar/Epoxy Composites after Impact Loading. Molecules 2021, 26, 5520. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.; Santos, P.; Parente, J.; Valvez, S.; Reis, P.N.B. Effect of Harsh Environmental Conditions on the Impact Response of Carbon Composites with Filled Matrix by Cork Powder. Appl. Sci. 2021, 11, 7436. [Google Scholar] [CrossRef]
- Kamal, H.M.; Kadhim, M.J. Effect of chemical solutions on the mechanical properties of nano-silica reinforced (glass/Kevlar) fabrics polyester hybrid composite materials. Int. J. Energy Environ. 2018, 9, 187–194. [Google Scholar]
- Nagendiran, S.; Badghaish, A.; Hussein, I.A.; Shuaib, A.N.; Furquan, S.A.; Al-Mehthel, M.H. Epoxy/oil fly ash composites prepared through in situ polymerization: Enhancement of thermal and mechanical properties. Polym. Compos. 2016, 7, 512–522. [Google Scholar]
- Feng, P.; Wang, J.; Wang, Y.; Loughery, D.; Niu, D. Effect of corrosive environments on properties of pultruded GFRP plates. Compos. B Eng. 2014, 67, 427–433. [Google Scholar]
- Ancas, A.D.; Profire, M.; Cirstolovean, I.L.; Hornet, M.; Cojocaru, G. Lifetime glass reinforced plastic pipes by measuring the stiffness. J. Eng. Appl. Sci. 2018, 8, 7–12. [Google Scholar]
- Ancas, A.D.; Profire, M. Effects of soil pH on mechanical properties of glass reinforced plastic pipes, Environ. Eng. Manag. J. 2018, 17, 1879–1885. [Google Scholar] [CrossRef]
- Reis, P.N.B.; Amaro, A.M.; Neto, M.A. Effect of Fibre Orientation and Hostile Solutions on Stress Relaxation of Glass/Polyamide Composites. Polymers 2020, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.N.B.; Neto, M.A.; Amaro, A.M. Effect of hostile solutions on stress relaxation of carbon/epoxy composites. Polym. Degrad. Stab. 2019, 165, 60–67. [Google Scholar]
- BS EN ISO 62:1999; Plastics—Determination of Water Absorption. ISO: Geneva, Switzerland, 1999.
- Antunes, J.M.; Cavaleiro, A.; Menezes, L.F.; Simões, M.I.; Fernandes, J.V. Ultra-microhardness testing procedure with Vickers indenter. Surf. Coat. Technol. 2002, 149, 27–35. [Google Scholar]
- Amaro, A.M.; Reis, P.N.B.; Neto, M.A.; Louro, C. Effect of different commercial oils on mechanical properties of composite materials. Compos. Struct. 2014, 118, 1–8. [Google Scholar]
- Cousin, P.; Hassan, M.; Vijay, P.; Robert, M.; Benmokrane, B. Chemical resistance of carbon, basalt, and glass fibers used in FRP reinforcing bars. J. Compos. Mater. 2019, 53, 3651–3670. [Google Scholar]
- Zhu, H.-G.; Leung, C.K.Y.; Kim, J.-K.; Liu, M.-Y. Degradation of glass fiber-reinforced plastic composites containing nanoclay in alkaline environment. J. Compos. Mater. 2011, 45, 2147–2156. [Google Scholar]
- Călin, C.; Diniță, A.; Brănoiu, G.; Popovici, D.R.; Tănase, M.; Sirbu, E.-E.; Portoacă, A.-I.; Mihai, S. Assessment of Environmental Impact on Glass-Fiber-Reinforced Polymer Pipes Mechanical and Thermal Properties. Polymers 2024, 16, 1779. [Google Scholar] [CrossRef]
- Tănase, M.; Diniță, A.; Lvov, G.; Portoacă, A.I. Experimental Determination of Circumferential Mechanical Properties of GFRP Pipes Using the Split-Disk Method: Evaluating the Impact of Aggressive Environments. Appl. Sci. 2024, 14, 11845. [Google Scholar] [CrossRef]
- Kojnoková, T.; Nový, F.; Markovičová, L. The Study of Chemical and Thermal Influences of the Environment on the Degradation of Mechanical Properties of Carbon Composite with Epoxy Resin. Polymers 2022, 14, 3245. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A.M.; Pinto, M.I.M.; Reis, P.N.B.; Neto, M.A.; Lopes, S.M.R. Structural integrity of glass/epoxy composites embedded in cement or geopolymer mortars. Compos. Struct. 2018, 206, 509–516. [Google Scholar]



| Stacking Sequence | Hostile Solution | Immersion Time (Days) | Properties |
|---|---|---|---|
| [452, 902, −452, 02]s | HCl | 12/24/36 | Hardness/roughness |
| H2SO4 | |||
| NaOH | |||
| [452, 902, −452, 02]s | Cement | 30/60/90 | Hardness/roughness |
| Cement with water | |||
| Geopolymers | |||
| [02, 902]2s | HCl | 15/30/60/90 | Hardness/roughness |
| NaOH | |||
| [02, 902]2s | 15W40 | 15/45 | Hardness/roughness |
| DOT4 |
| Stacking Sequence | Hostile Solution | Immersion Time (Days) | Weight Gain (%) | Std. Dev. (%) |
|---|---|---|---|---|
| [452, 902, −452, 02]s | HCl | 12 | 1.83 | 0.07 |
| 24 | 4.15 | 0.08 | ||
| 36 | 4.74 | 0.10 | ||
| [452, 902, −452, 02]s | H2SO4 | 12 | 2.78 | 0.03 |
| 24 | 3.68 | 0.05 | ||
| 36 | 5.24 | 0.07 | ||
| [452, 902, −452, 02]s | NaOH | 12 | 1.23 | 0.07 |
| 24 | 3.26 | 0.09 | ||
| 36 | 4.17 | 0.09 | ||
| [452, 902, −452, 02]s | Cement | 30 | 0.18 | 0.02 |
| 60 | 0.13 | 0.01 | ||
| 90 | 0.11 | 0.03 | ||
| [452, 902, −452, 02]s | Cement with water | 30 | 0.30 | 0.02 |
| 60 | 0.55 | 0.03 | ||
| 90 | 0.97 | 0.03 | ||
| [452, 902, −452, 02]s | Geopolymers | 30 | 0.13 | 0.02 |
| 60 | 0.15 | 0.004 | ||
| 90 | 0.22 | 0.003 | ||
| [02, 902]2s | 15W40 | 15 | 0.21 | 0.08 |
| 45 | 0.38 | 0.12 | ||
| [02, 902]2s | DOT4 | 15 | −0.43 | 0.14 |
| 45 | −2.16 | 0.18 | ||
| [02, 902]2s | HCl | 15 | 0.21 | 0.001 |
| 30 | 1.57 | 0.03 | ||
| 60 | 2.19 | 0.13 | ||
| 90 | 3.93 | 0.05 | ||
| [02, 902]2s | NaOH | 15 | 0.16 | 0.05 |
| 30 | 0.64 | 0.02 | ||
| 60 | 1.18 | 0.17 | ||
| 90 | 1.37 | 0.14 |
| Hostile Solution | Immersion Time (Days) | H (GPa) | ER (MPa) | E (GPa) |
|---|---|---|---|---|
| Control | - | 0.24c0.01 | 13.12 ± 0.94 | 11.64 ± 0.91 |
| HCl | 12 | 0.27 ± 0.01 | 7.60 ± 0.20 | 6.73 ± 0.15 |
| 24 | 0.25 ± 0.01 | 7.43 ± 0.21 | 6.53 ± 0.14 | |
| 36 | 0.26 ± 0.01 | 7.10 ± 0.57 | 6.25 ± 0.48 | |
| H2SO4 | 12 | 0.35 ± 0.09 | 9.37 ± 0.75 | 8.27 ± 0.62 |
| 24 | 0.33 ± 0.02 | 8.53 ± 0.93 | 7.57 ± 0.81 | |
| 36 | 0.29 ± 0.03 | 7.85 ± 0.64 | 7.00 ± 0.57 | |
| NaOH | 12 | 0.29 ± 0.01 | 9.30 ± 0.44 | 8.23 ± 0.42 |
| 24 | 0.27 ± 0.01 | 9.15 ± 0.67 | 8.10 ± 0.58 | |
| 36 | 0.28 ± 0.01 | 8.70 ± 0.64 | 7.70 ± 0.58 | |
| Cement | 30 | 0.43 ± 0.02 | 15.08 ± 0.61 | 13.95 ± 0.49 |
| 60 | 0.29 ± 0.01 | 6.89 ± 0.52 | 6.33 ± 0.23 | |
| 90 | 0.26 ± 0.02 | 11.09 ± 0.72 | 10.17 ± 0.42 | |
| Cement with water | 30 | 0.35 ± 0.02 | 13.02 ± 0.68 | 11.98 ± 0.57 |
| 60 | 0.38 ± 0.02 | 15.24 ± 0.75 | 13.80 ± 0.61 | |
| 90 | 0.37 ± 0.03 | 14.50 ± 0.65 | 13.40 ± 0.52 | |
| Geopolymers | 30 | 0.29 ± 0.01 | 10.28 ± 0.56 | 9.45 ± 0.34 |
| 60 | 0.28 ± 0.02 | 8.98 ± 0.43 | 8.20 ± 0.25 | |
| 90 | 0.25 ± 0.02 | 10.02 ± 0.62 | 9.22 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaro, A.M.; Paulino, M.F.; Neto, M.A.; Reis, P.N.B. Hardness and Roughness of Glass/Epoxy Composite Laminates Subjected to Different Hostile Solutions: A Comparative Study. Polymers 2025, 17, 993. https://doi.org/10.3390/polym17070993
Amaro AM, Paulino MF, Neto MA, Reis PNB. Hardness and Roughness of Glass/Epoxy Composite Laminates Subjected to Different Hostile Solutions: A Comparative Study. Polymers. 2025; 17(7):993. https://doi.org/10.3390/polym17070993
Chicago/Turabian StyleAmaro, Ana Martins, M. F. Paulino, Maria Augusta Neto, and Paulo N. B. Reis. 2025. "Hardness and Roughness of Glass/Epoxy Composite Laminates Subjected to Different Hostile Solutions: A Comparative Study" Polymers 17, no. 7: 993. https://doi.org/10.3390/polym17070993
APA StyleAmaro, A. M., Paulino, M. F., Neto, M. A., & Reis, P. N. B. (2025). Hardness and Roughness of Glass/Epoxy Composite Laminates Subjected to Different Hostile Solutions: A Comparative Study. Polymers, 17(7), 993. https://doi.org/10.3390/polym17070993










