Cost-Effectiveness Analysis of Routine Use of 15-Valent Pneumococcal Conjugate Vaccine in the US Pediatric Population
Abstract
:1. Introduction
2. Methods
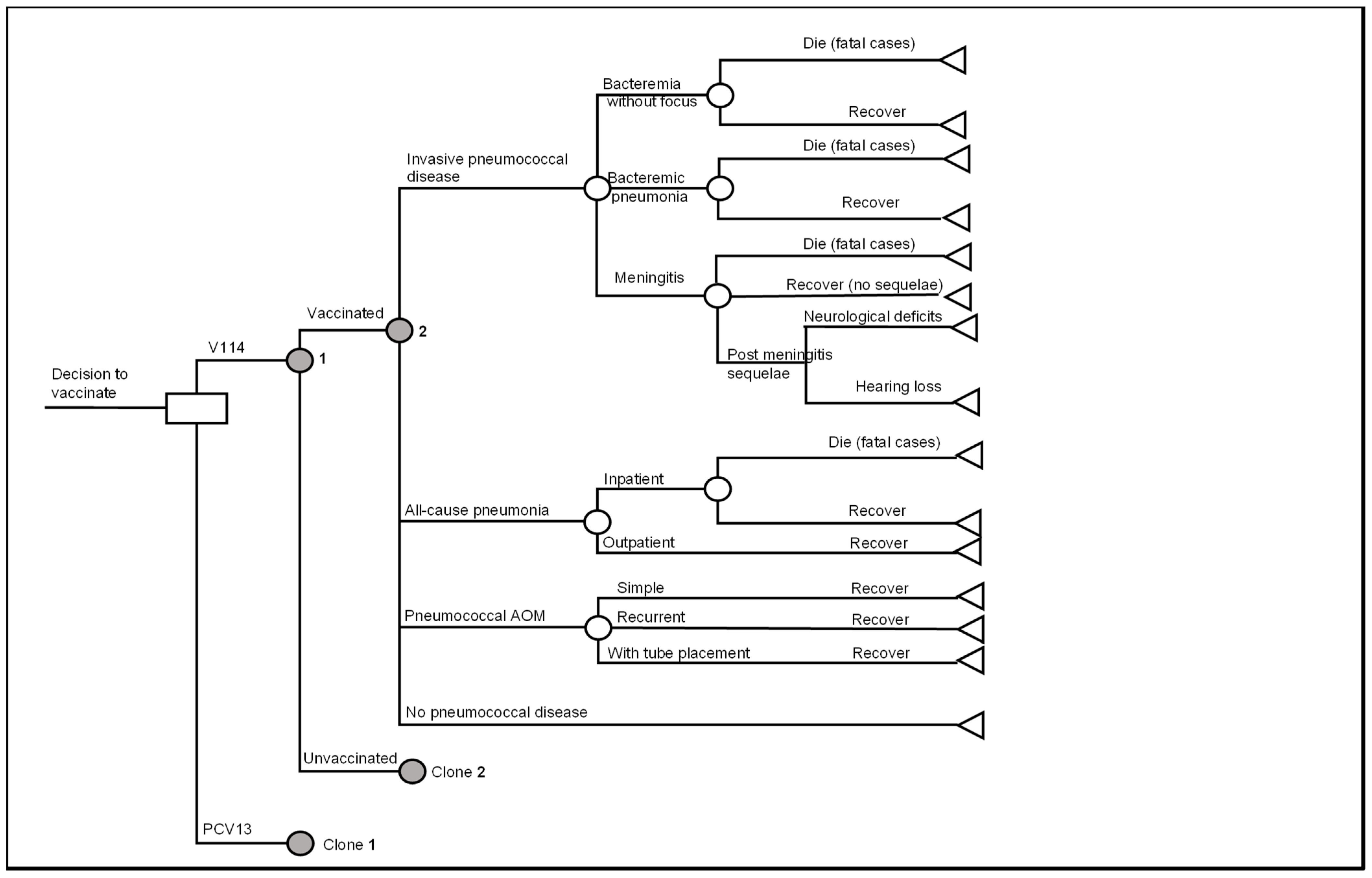
2.1. Model Overview
2.2. Model Structure
2.3. Model Inputs
2.3.1. Target Population Size, Background Mortality and Vaccine Coverage
2.3.2. Baseline PD Incidence and Case-Fatality Rates
2.3.3. Vaccine Effectiveness
IPD
All-Cause Pneumonia
AOM and Tube Placement
Vaccine Effect Waning
Herd Immunity on IPD
2.3.4. Utility Inputs
2.3.5. Cost Inputs
Vaccine Acquisition and Administration Costs
Direct Medical Costs
Direct Non-Medical Costs and Indirect Costs
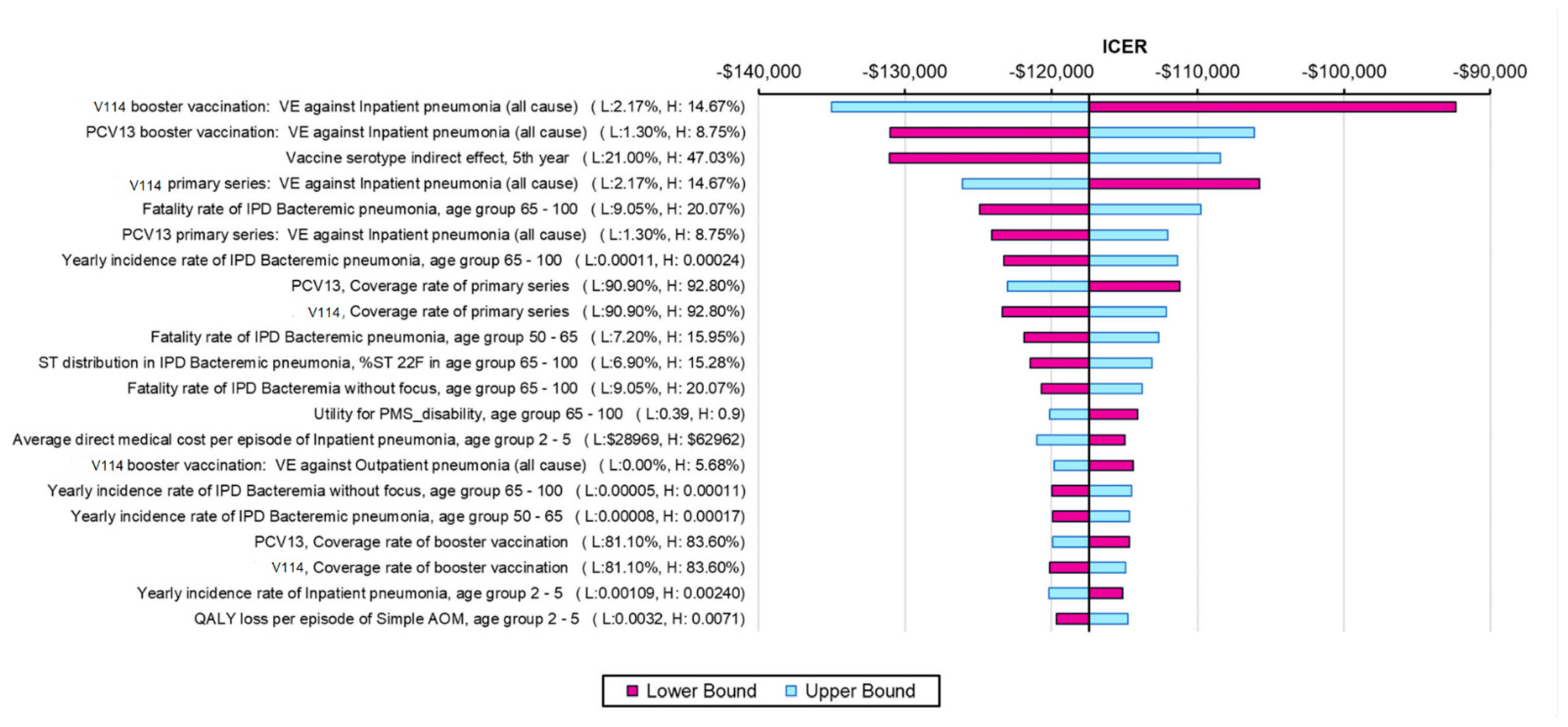
2.4. Sensitivity Analyses
3. Results
3.1. Base Case
3.2. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogaert, D.; De Groot, R.; Hermans, P.W. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Anderson, R. Recent advances in the epidemiology and prevention of Streptococcus pneumoniae infections. F1000Research 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A. The prevention of pneumococcal disease by vaccines: Promises and challenges. Infect. Dis. Clin. N. Am. 2001, 15, 97–122. [Google Scholar] [CrossRef] [PubMed]
- Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000, 49, 1–35. [Google Scholar]
- Tan, T.Q. Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin. Microbiol. Rev. 2012, 25, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Myint, T.T.; Madhava, H.; Balmer, P.; Christopoulou, D.; Attal, S.; Menegas, D.; Sprenger, R.; Bonnet, E. The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: A literature review. Adv. Ther. 2013, 30, 127–151. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, I.; Shea, K.M.; Pelton, S.I. Pneumococcal Disease in the Era of Pneumococcal Conjugate Vaccine. Infect. Dis. Clin. N. Am. 2015, 29, 679–697. [Google Scholar] [CrossRef] [Green Version]
- Schiess, N.; Groce, N.E.; Dua, T. The Impact and Burden of Neurological Sequelae Following Bacterial Meningitis: A Narrative Review. Microorganisms 2021, 9, 900. [Google Scholar] [CrossRef]
- Weycker, D.; Farkouh, R.A.; Strutton, D.R.; Edelsberg, J.; Shea, K.M.; Pelton, S.I. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv. Res. 2016, 16, 182. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.S.; Johnson, K.M.; Ray, G.T.; Wroe, P.; Lieu, T.A.; Moore, M.R.; Zell, E.R.; Linder, J.A.; Grijalva, C.G.; Metlay, J.P.; et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 2011, 29, 3398–3412. [Google Scholar] [CrossRef] [Green Version]
- Suaya, J.A.; Gessner, B.D.; Fung, S.; Vuocolo, S.; Scaife, J.; Swerdlow, D.L.; Isturiz, R.E.; Arguedas, A.G. Acute otitis media, antimicrobial prescriptions, and medical expenses among children in the United States during 2011–2016. Vaccine 2018, 36, 7479–7486. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Amand, C.; Kieffer, A.; Kyaw, M.H. Trends in healthcare utilization and costs associated with pneumonia in the United States during 2008-2014. BMC Health Serv. Res. 2018, 18, 715. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Pneumococcal Disease Surveillance and Reporting. Available online: https://www.cdc.gov/pneumococcal/surveillance.html (accessed on 6 September 2022).
- Hu, T.; Sarpong, E.; Song, Y.; Done, N.; Orvis, E.; Signorovitch, J.; Petigara, T. 1479. Incidence of Acute Otitis Media in Children in the United States before and after the introduction of Pneumococcal Conjugate Vaccines (PCV7 and PCV13) during 1998-2018. Open Forum Infect. Dis. 2020, 7, S740–S741. [Google Scholar] [CrossRef]
- Hu, T.; Sarpong, E.; Song, Y.; Done, N.; Liu, Q.; Signorovitch, J.; Petigara, T. 1480. Incidence of Non-Invasive Pneumococcal Pneumonia in Children in the United States before and after Introduction Pneumococcal Conjugate Vaccines (PCV7 and PCV13) during 1998-2018. Open Forum Infect. Dis. 2020, 7, S741–S742. [Google Scholar] [CrossRef]
- Nuorti, J.P.; Whitney, C.G. Prevention of pneumococcal disease among infants and children—Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2010, 59, 1–18. [Google Scholar] [PubMed]
- Wasserman, M.; Chapman, R.; Lapidot, R.; Sutton, K.; Dillon-Murphy, D.; Patel, S.; Chilson, E.; Snow, V.; Farkouh, R.; Pelton, S. Twenty-Year Public Health Impact of 7- and 13-Valent Pneumococcal Conjugate Vaccines in US Children. Emerg. Infect. Dis. 2021, 27, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.M.; Deloria-Knoll, M.; Kassa, H.T.; O’Brien, K.L. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: Review of evidence on indirect effects. Vaccine 2013, 32, 133–145. [Google Scholar] [CrossRef]
- Poehling, K.K.M. Introduction of the Pneumococcal Working Group. Available online: www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-10/pneumo-01-Poehling-Kobayashi-508.pdf (accessed on 15 March 2022).
- Linley, E.; Bell, A.; Gritzfeld, J.F.; Borrow, R. Should Pneumococcal Serotype 3 Be Included in Serotype-Specific Immunoassays? Vaccines 2019, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Balsells, E.; Guillot, L.; Nair, H.; Kyaw, M.H. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0177113. [Google Scholar] [CrossRef] [Green Version]
- Pichichero, M.; Kaur, R.; Scott, D.A.; Gruber, W.C.; Trammel, J.; Almudevar, A.; Center, K.J. Effectiveness of 13-valent pneumococcal conjugate vaccination for protection against acute otitis media caused by Streptococcus pneumoniae in healthy young children: A prospective observational study. Lancet Child. Adolesc. Health 2018, 2, 561–568. [Google Scholar] [CrossRef]
- Pilishvili, T. 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Effects on Disease Caused by Serotype 3. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2019-02-508.pdf (accessed on 31 May 2022).
- Food and Drug Administration. Label for Pneumococcal 15-Valent Conjugate Vaccine. Available online: https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/1158fa93-ef41-4a29-8252-9251f94c53c8/spl-doc?hl=Pneumococcal%2015-valent%20Conjugate%20Vaccine (accessed on 6 September 2022).
- Merck & Co., Inc. European Commission Expands Merck’s VAXNEUVANCE™ (Pneumococcal 15-Valent Conjugate Vaccine) Indication to Include Infants, Children and Adolescents. Available online: https://www.merck.com/news/european-commission-expands-mercks-vaxneuvance-pneumococcal-15-valent-conjugate-vaccine-indication-to-include-infants-children-and-adolescents/ (accessed on 24 October 2022).
- Varghese, J.; Chochua, S.; Tran, T.; Walker, H.; Li, Z.; Snippes Vagnone, P.M.; Lynfield, R.; McGee, L.; Li, Y.; Metcalf, B.J.; et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin. Microbiol. Infect. 2020, 26, e511–e512. [Google Scholar] [CrossRef] [PubMed]
- Merck & Co., Inc. U.S. FDA Approves Merck’s VAXNEUVANCE™ (Pneumococcal 15-Valent Conjugate Vaccine) for the Prevention of Invasive Pneumococcal Disease in Infants and Children. Available online: https://www.merck.com/news/u-s-fda-approves-mercks-vaxneuvance-pneumococcal-15-valent-conjugate-vaccine-for-the-prevention-of-invasive-pneumococcal-disease-in-infants-and-children/ (accessed on 24 October 2022).
- Lupinacci, R.R.R.; Wittawatmongkol, O.; Jones, J.; Quiñones, J.; Ulukol, B.; Dagan, R.; Richmond, P.; Stek, J.; Romero, L.; Koseoglu, S.; et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate concomitant administration of pediatric vaccines with a 4-dose regimen of V114 in healthy infants (PNEU-PED). In Proceedings of the 12th Biennial International Symposium on Pneumococci & Pneumococcal Diseases (ISPPD), Toronto, ON, Canada, 19–23 June 2022. [Google Scholar]
- Kobayashi, M.; Farrar, J.L.; Gierke, R.; Leidner, A.J.; Campos-Outcalt, D.; Morgan, R.L.; Long, S.S.; Poehling, K.A.; Cohen, A.L.; Kelman, J.; et al. Use of 15-Valent Pneumococcal Conjugate Vaccine Among U.S. Children: Updated Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Gravelle, H.; The Practice of Discounting Economic Evaluation of Health Care Interventions. The University of York. Available online: https://www.york.ac.uk/che/pdf/tp19.pdf (accessed on 15 March 2022).
- Edmond, K.; Clark, A.; Korczak, V.S.; Sanderson, C.; Griffiths, U.K.; Rudan, I. Global and regional risk of disabling sequelae from bacterial meningitis: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 317–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The United States Census Bureau. National Population by Characteristics 2010–2019. Available online: https://www.census.gov/data/tables/time-series/demo/popest/2010s-national-detail.html#par_textimage_98372960 (accessed on 15 March 2022).
- Arias, E.; Xu, J.Q. United States Life Tables. 2018. Available online: https://www.cdc.gov/nchs/data/nvsr/nvsr69/nvsr69-12-508.pdf (accessed on 15 March 2022).
- Hill, H.A.; Elam-Evans, L.D.; Yankey, D.; Singleton, J.A.; Kang, Y. Vaccination Coverage Among Children Aged 19–35 Months—United States, 2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1123–1128. Available online: https://www.cdc.gov/mmwr/volumes/67/wr/mm6740a4.htm (accessed on 6 November 2022). [CrossRef]
- Centers for Disease Control and Prevention. Bacterial Core Surveillance Report, Emerging Infections Program Network. Centers for Disease Control and Prevention. Available online: http://www.cdc.gov/abcs/reports-findings/survreports/spneu18.pdf (accessed on 15 March 2022).
- Jit, M. The risk of sequelae due to pneumococcal meningitis in high-income countries: A systematic review and meta-analysis. J. Infect. 2010, 61, 114–124. [Google Scholar] [CrossRef]
- Hu, T.; Song, Y.; Done, N.; Liu, Q.; Sarpong, E.M.; Lemus-Wirtz, E.; Signorovitch, J.; Mohanty, S.; Weiss, T. Incidence of invasive pneumococcal disease in children with commercial insurance or Medicaid coverage in the United States before and after the introduction of 7- and 13-valent pneumococcal conjugate vaccines during 1998–2018. BMC Public Health 2022, 22, 1677. [Google Scholar] [CrossRef]
- Hu, T.; Done, N.; Petigara, T.; Mohanty, S.; Song, Y.; Liu, Q.; Lemus-Wirtz, E.; Signorovitch, J.; Sarpong, E.; Weiss, T. Incidence of acute otitis media in children in the United States before and after the introduction of 7- and 13-valent pneumococcal conjugate vaccines during 1998–2018. BMC Infect. Dis. 2022, 22, 294. [Google Scholar] [CrossRef]
- Kaur, R.; Fuji, N.; Pichichero, M.E. Dynamic changes in otopathogens colonizing the nasopharynx and causing acute otitis media in children after 13-valent (PCV13) pneumococcal conjugate vaccination during 2015-2019. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 37–44. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Centers for Disease Control ABC Surveillance. In Centers for Disease Control and Prevention, ed. Data on File. 2010–2019. Available online: https://www.cdc.gov/ (accessed on 6 November 2022).
- Olarte, L.; Barson, W.J.; Barson, R.M.; Lin, P.L.; Romero, J.R.; Tan, T.Q.; Givner, L.B.; Bradley, J.S.; Hoffman, J.A.; Hultén, K.G.; et al. Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis in US Children. Clin. Infect. Dis. 2015, 61, 767–775. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Lexau, C.; Bennett, N.M.; Petit, S.; Zansky, S.M.; Harrison, L.H.; Reingold, A.; et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: Analysis of multisite, population-based surveillance. Lancet Infect. Dis. 2015, 15, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Wuerth, B.A.; Bonnewell, J.P.; Wiemken, T.L.; Arnold, F.W. Trends in Pneumonia Mortality Rates and Hospitalizations by Organism, United States, 2002–2011(1). Emerg. Infect. Dis. 2016, 22, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Whitney, C.G.; Pilishvili, T.; Farley, M.M.; Schaffner, W.; Craig, A.S.; Lynfield, R.; Nyquist, A.C.; Gershman, K.A.; Vazquez, M.; Bennett, N.M.; et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: A matched case-control study. Lancet 2006, 368, 1495–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Holtzman, C.; Harrison, L.H.; Zansky, S.M.; Rosen, J.B.; Reingold, A.; Scherzinger, K.; et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: A matched case-control study. Lancet Respir Med. 2016, 4, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Eskola, J.; Kilpi, T.; Palmu, A.; Jokinen, J.; Haapakoski, J.; Herva, E.; Takala, A.; Käyhty, H.; Karma, P.; Kohberger, R.; et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 2001, 344, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, S.; Shinefield, H.; Fireman, B.; Lewis, E.; Ray, P.; Hansen, J.R.; Elvin, L.; Ensor, K.M.; Hackell, J.; Siber, G.; et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect. Dis. J. 2000, 19, 187–195. [Google Scholar] [CrossRef]
- Kaur, R.; Morris, M.; Pichichero, M.E. Epidemiology of Acute Otitis Media in the Postpneumococcal Conjugate Vaccine Era. Pediatrics 2017, 140, e20170181. [Google Scholar] [CrossRef] [Green Version]
- Joloba, M.L.; Windau, A.; Bajaksouzian, S.; Appelbaum, P.C.; Hausdorff, W.P.; Jacobs, M.R. Pneumococcal conjugate vaccine serotypes of Streptococcus pneumoniae isolates and the antimicrobial susceptibility of such isolates in children with otitis media. Clin. Infect. Dis. 2001, 33, 1489–1494. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.C.; Jackson, M.; Yu, O.; Whitney, C.G.; Bounds, L.; Bittner, R.; Zavitkovsky, A.; Jackson, L.A. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine 2008, 26, 4947–4954. [Google Scholar] [CrossRef]
- Treskova, M.; Scholz, S.M.; Kuhlmann, A. Cost Effectiveness of Elderly Pneumococcal Vaccination in Presence of Higher-Valent Pneumococcal Conjugate Childhood Vaccination: Systematic Literature Review with Focus on Methods and Assumptions. Pharmacoeconomics 2019, 37, 1093–1127. [Google Scholar] [CrossRef]
- Lexau, C.A.; Lynfield, R.; Danila, R.; Pilishvili, T.; Facklam, R.; Farley, M.M.; Harrison, L.H.; Schaffner, W.; Reingold, A.; Bennett, N.M.; et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2005, 294, 2043–2051. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease--United States, 1998–2003. MMWR Morb Mortal Wkly. Rep. 2005, 54, 893–897. [Google Scholar]
- Stoecker, C.; Hampton, L.M.; Link-Gelles, R.; Messonnier, M.L.; Zhou, F.; Moore, M.R. Cost-effectiveness of using 2 vs. 3 primary doses of 13-valent pneumococcal conjugate vaccine. Pediatrics 2013, 132, e324–e332. [Google Scholar] [CrossRef] [PubMed]
- Szende, A.; Janssen, B.; Cabases, J. Self-Reported Population Health: An International Perspective Based on EQ-5D; Springer Nature: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef] [Green Version]
- Rubin, J.L.; McGarry, L.J.; Strutton, D.R.; Klugman, K.P.; Pelton, S.I.; Gilmore, K.E.; Weinstein, M.C. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine 2010, 28, 7634–7643. [Google Scholar] [CrossRef] [PubMed]
- Mangen, M.J.; Rozenbaum, M.H.; Huijts, S.M.; van Werkhoven, C.H.; Postma, D.F.; Atwood, M.; van Deursen, A.M.; van der Ende, A.; Grobbee, D.E.; Sanders, E.A.; et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur. Respir. J. 2015, 46, 1407–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bureau of Labor Statistics. CPI for All Urban Consumers (CPI-U): Medical Care in U.S. City Average, All Urban Consumers, Not Seasonally Adjusted. Available online: https://www.bls.gov/news.release/archives/cpi_01122022.pdf (accessed on 15 March 2022).
- Ray, G.T.; Whitney, C.G.; Fireman, B.H.; Ciuryla, V.; Black, S.B. Cost-effectiveness of pneumococcal conjugate vaccine: Evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr. Infect. Dis. J. 2006, 25, 494–501. [Google Scholar] [CrossRef]
- Hu, T. Economic Burden of Pneumococcal Disease in Children in the United States after the Introduction of 13-Valent Pneumococcal Conjugate Vaccines during 2014–2018. BMC Health Serv. Res. 2022; under review. [Google Scholar]
- Stoecker, C. Economic Assessment of PCV15 & PCV20. Available online: https://stacks.cdc.gov/view/cdc/109109 (accessed on 15 March 2022).
- McLaughlin, J.M.; McGinnis, J.J.; Tan, L.; Mercatante, A.; Fortuna, J. Estimated Human and Economic Burden of Four Major Adult Vaccine-Preventable Diseases in the United States, 2013. J. Prim. Prev. 2015, 36, 259–273. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment—United States, 2003. MMWR Morb. Mortal. Wkly. Rep. 2004, 53, 57–59. [Google Scholar]
- Bureau of Labor Statistics. Median Usual Weekly Earnings of Full-Time Wage and Salary Workers by Age, Race, Hispanic or Latino Ethnicity, and Sex, Fourth Quarter 2021 Averages, Not Seasonally Adjusted. Bureau of Labor Statistics. Available online: https://www.bls.gov/news.release/wkyeng.t03.htm (accessed on 15 March 2022).
- Bureau of Labor Statistics. Civilian Labor Force Participation Rate by Age, Sex, Race, and Ethnicity. Bureau of Labor Statistics. Available online: https://www.bls.gov/emp/tables/civilian-labor-force-participation-rate.htm (accessed on 15 March 2022).
- Ryman, J.; Weaver, J.; Yee, K.L.; Sachs, J.R. Predicting effectiveness of the V114 vaccine against invasive pneumococcal disease in children. Expert Rev. Vaccines 2022, 21, 1515–1521. [Google Scholar] [CrossRef]
- Tang, Z.; Matanock, A.; Jeon, S.; Leidner, A.J. A review of health-related quality of life associated with pneumococcal disease: Pooled estimates by age and type of disease. J. Public Health 2022, 44, e234–e240. [Google Scholar] [CrossRef]
- Whitney, C.G.; Farley, M.M.; Hadler, J.; Harrison, L.H.; Bennett, N.M.; Lynfield, R.; Reingold, A.; Cieslak, P.R.; Pilishvili, T.; Jackson, D.; et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 2003, 348, 1737–1746. [Google Scholar] [CrossRef] [Green Version]
- Grijalva, C.G.; Nuorti, J.P.; Arbogast, P.G.; Martin, S.W.; Edwards, K.M.; Griffin, M.R. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: A time-series analysis. Lancet 2007, 369, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Shefer, A.; Kong, Y.; Nuorti, J.P. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997–2004. Pediatrics 2008, 121, 253–260. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, E.D.; Conway, P.; Willingham, J.; Hollingsworth, R.; Lloyd, A. Pneumococcal pneumonia in the UK—How herd immunity affects the cost-effectiveness of 7-valent pneumococcal conjugate vaccine (PCV). Vaccine 2005, 23, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Lieu, T.A.; Ray, G.T.; Black, S.B.; Butler, J.C.; Klein, J.O.; Breiman, R.F.; Miller, M.A.; Shinefield, H.R. Projected cost-effectiveness of pneumococcal conjugate vaccination of healthy infants and young children. Jama 2000, 283, 1460–1468. [Google Scholar] [CrossRef]
- Ray, G.T.; Pelton, S.I.; Klugman, K.P.; Strutton, D.R.; Moore, M.R. Cost-effectiveness of pneumococcal conjugate vaccine: An update after 7 years of use in the United States. Vaccine 2009, 27, 6483–6494. [Google Scholar] [CrossRef]
- Blank, P.R.; Szucs, T.D. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine in Switzerland. Vaccine 2012, 30, 4267–4275. [Google Scholar] [CrossRef] [Green Version]
- Earnshaw, S.R.; McDade, C.L.; Zanotti, G.; Farkouh, R.A.; Strutton, D. Cost-effectiveness of 2 + 1 dosing of 13-valent and 10-valent pneumococcal conjugate vaccines in Canada. BMC Infect. Dis. 2012, 12, 101. [Google Scholar] [CrossRef] [Green Version]
- Klok, R.M.; Lindkvist, R.M.; Ekelund, M.; Farkouh, R.A.; Strutton, D.R. Cost-effectiveness of a 10- versus 13-valent pneumococcal conjugate vaccine in Denmark and Sweden. Clin. Ther. 2013, 35, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Strutton, D.R.; Farkouh, R.A.; Earnshaw, S.R.; Hwang, S.; Theidel, U.; Kontodimas, S.; Klok, R.; Papanicolaou, S. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine: Germany, Greece, and The Netherlands. J. Infect. 2012, 64, 54–67. [Google Scholar] [CrossRef]
- Chen, C.; Wood, J.G.; Beutels, P.; Menzies, R.; MacIntyre, C.R.; Dirmesropian, S.; Reyes, J.F.; McIntyre, P.; Newall, A.T. The role of timeliness in the cost-effectiveness of older adult vaccination: A case study of pneumococcal conjugate vaccine in Australia. Vaccine 2018, 36, 1265–1271. [Google Scholar] [CrossRef]
- Stoecker, C.; Kim, L.; Gierke, R.; Pilishvili, T. Incremental Cost-Effectiveness of 13-valent Pneumococcal Conjugate Vaccine for Adults Age 50 Years and Older in the United States. J. Gen. Intern. Med. 2016, 31, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gierke, R. Current Epidemiology of Pneumococcal Disease, United States—2019 Updates. Centers for Disease Control and Prevention, Advisory Committee on Immunication Practices. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/02-Pneumococcal-Gierke-508.pdf (accessed on 15 March 2022).
- Lo, S.W.; Gladstone, R.A.; van Tonder, A.J.; Lees, J.A.; du Plessis, M.; Benisty, R.; Givon-Lavi, N.; Hawkins, P.A.; Cornick, J.E.; Kwambana-Adams, B.; et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: An international whole-genome sequencing study. Lancet Infect. Dis. 2019, 19, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Bajema, K.L.; Gierke, R.; Farley, M.M.; Schaffner, W.; Thomas, A.; Reingold, A.L.; Harrison, L.H.; Lynfield, R.; Burzlaff, K.E.; Petit, S.; et al. Impact of Pneumococcal Conjugate Vaccines on Antibiotic-Nonsusceptible Invasive Pneumococcal Disease in the United States. J. Infect. Dis. 2022, 226, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.A.; Finkelstein, J.A.; Ray, G.T.; Moore, M.R.; Huang, S.S. Attributable healthcare utilization and cost of pneumonia due to drug-resistant streptococcus pneumonia: A cost analysis. Antimicrob. Resist. Infect. Control 2014, 3, 16. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Age Group (Years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1 | 2–4 | 5–17 | 18–34 | 35–49 | 50–64 | 65–74 | 75–84 | 85+ | |
| Annual incidence rates (per 100, 000) | ||||||||||
| IPD a | 13.3 | 9.5 | 4.3 | 1.7 | 2.8 | 7.1 | 16.6 | 24.0 | 24 | 24 |
| % IPD that is meningitis a | 7.2 | |||||||||
| % IPD that is bacteremia without focus a | 21.4 | |||||||||
| % IPD that is bacteremic pneumonia a | 71.4 | |||||||||
| Inpatient pneumonia (All-cause) b,c | 339 | 339 | 168 | 45 | 58 | 58 | 204 | 723 | 1852 | 3694 |
| Outpatient pneumonia (All-cause) b,c | 2906 | 2906 | 3413 | 1333 | 622 | 622 | 1106 | 1917 | 3408 | 5876 |
| Simple AOM (All-cause) d | 54,200 | 54,200 | 33,800 | 8400 | - | - | - | - | - | - |
| Recurrent AOM (All-cause) d | 19,600 | 19,600 | 6900 | 500 | - | - | - | - | - | - |
| AOM tube placement (All-cause) d | 4340 | 4340 | 1740 | 200 | - | - | - | - | - | - |
| % AOM and tube placement attributable to S. pneumoniae e | 23.8 | |||||||||
| Case fatality rates (%) | ||||||||||
| Meningitis f, h | 10.00 | 10.00 | 10.00 | 10.00 | 6.90 | 7.30 | 11.20 | 14.11 | 14.11 | 14.11 |
| Bacteremia without focus/bacteremic pneumonia g, h | 3.00 | 3.00 | 3.00 | 3.00 | 6.90 | 7.30 | 11.20 | 14.11 | 14.11 | 14.11 |
| Inpatient pneumonia (All-cause) i, j | 0.29 | 0.29 | 0.29 | 0.51 | 1.7/2.4 * | 3.5/4.9 * | 4.9/5.5 * | 6.9 | 8.2 | 11.6/15.4 * |
| PMS k | ||||||||||
| % meningitis cases leading to neurological deficits | 12.2 | 12.2 | 12.2 | 12.2 | 31.7 | 31.7 | 31.7 | 31.7 | 31.7 | 31.7 |
| % meningitis cases leading to hearing loss | 8.2 | 8.2 | 8.2 | 8.2 | - | - | - | - | - | - |
| Disease | Vaccine Type | Serotype-Specific Effectiveness | ||||||||||||||
| Serotype | 1 | 4 | 5 | 6B | 7F | 9V | 14 | 18C | 19F | 23F | 3 | 6A | 19A | 22F | 33F | |
| IPD a | PCV13 | 87% | 93% | 87% | 94% | 97% | 100% | 94% | 97% | 87% | 98% | 80% | 86% | 86% | - | - |
| V114 | 87% | 93% | 87% | 94% | 97% | 100% | 94% | 97% | 87% | 98% | 80% | 86% | 86% | 86% | 86% | |
| AOM b (pneumococcal) | PCV13 | 86% | 57% | 86% | 57% | 86% | 57% | 57% | 57% | 57% | 57% | 15% | 100% | 91% | - | - |
| V114 | 86% | 57% | 86% | 57% | 86% | 57% | 57% | 57% | 57% | 57% | 15% | 100% | 91% | 57% | 57% | |
| AOMtube placement c (pneumococcal) | PCV13 | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | - | - |
| V114 | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | 69% | |
| Disease | Vaccine type | Effectiveness | ||||||||||||||
| Inpatient pneumonia d (all-cause) | PCV13 | 5.5% | ||||||||||||||
| V114 | 9.2% | |||||||||||||||
| Outpatient pneumonia d (all-cause) | PCV13 | 1.9% | ||||||||||||||
| V114 | 3.1% | |||||||||||||||
| Parameter | Age Group (Years) | |||||||
| <18 | 18–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75+ | |
| Health State Utility Values | ||||||||
| Baseline (general population without PD) a | 0.92 | 0.92 | 0.91 | 0.89 | 0.85 | 0.83 | 0.81 | 0.75 |
| PMS, neurological deficits b | 0.68 | |||||||
| PMS, hearing loss b | 0.73 | |||||||
| QALY decrements related to each episode of PD events c | Age group (years) | |||||||
| <18 | 18+ | |||||||
| Meningitis | 0.023 | 0.071 | ||||||
| Bacteremia without focus/bacteremic pneumonia | 0.008 | 0.071 | ||||||
| Inpatient pneumonia (All-cause) | 0.006 | 0.071 | ||||||
| Outpatient pneumonia (All-cause) | 0.004 | 0.005 | ||||||
| AOM and tube placement | 0.005 | - | ||||||
| Cost Component | Input Value | ||||||||
| Vaccine Costs (in 2021 USD) | |||||||||
| Vaccine acquisition costs (per dose) a | |||||||||
| PCV13 | 176.54 | ||||||||
| V114 | 176.54 | ||||||||
| Vaccine administration costs (per dose) b | |||||||||
| PCV13 | 15.5 | ||||||||
| V114 | 15.5 | ||||||||
| Costs per episode of PD events (in 2021 USD) | Age group (years) | ||||||||
| <2 | 2–4 | 5–17 | 18–34 | 35–49 | 50–64 | 65–74 | 75–84 | 85+ | |
| Direct medical costs c | |||||||||
| Meningitis | 65,419 | 65,419 | 65,419 | 57,657 | 57,657 | 58,890 | 28,217 | 28,217 | 28,217 |
| Bacteremia without focus | 46,909 | 46,909 | 46,909 | 57,657 | 57,657 | 58,890 | 28,217 | 28,217 | 28,217 |
| Bacteremic pneumonia | 58,774 | 58,774 | 58,774 | 57,657 | 57,657 | 58,890 | 28,217 | 28,217 | 28,217 |
| Inpatient pneumonia (All-cause) | 42,708 | 42,708 | 42,708 | 25,814 | 25,814 | 27,797 | 18,400 | 18,400 | 18,400 |
| Outpatient pneumonia (All-cause) | 525 | 525 | 525 | 812 | 812 | 774 | 671 | 671 | 671 |
| Simple AOM | 291 | 291 | 291 | - | - | - | - | - | - |
| Recurrent AOM | 711 | 711 | 711 | - | - | - | - | - | - |
| AOM tube placement | 2653 | 2653 | 2653 | - | - | - | - | - | - |
| Direct non-medical and indirect costs d | |||||||||
| Meningitis | 3416 | 983 | 983 | 3345 | 3345 | 3345 | 1342 | 892 | 806 |
| Bacteremia without focus | 652 | 983 | 983 | 3345 | 3345 | 3345 | 1342 | 892 | 806 |
| Bacteremic pneumonia | 652 | 983 | 983 | 3345 | 3345 | 3345 | 1342 | 892 | 806 |
| Inpatient pneumonia (All-cause) | 487 | 983 | 983 | 2702 | 2702 | 2702 | 1084 | 720 | 652 |
| Outpatient pneumonia (All-cause) | 487 | 487 | 487 | 1286 | 1286 | 1286 | 517 | 344 | 310 |
| Simple AOM | 193 | 193 | 193 | - | - | - | - | - | - |
| Recurrent AOM | 193 | 193 | 193 | - | - | - | - | - | - |
| AOM tube placement | 482 | 482 | 482 | - | - | - | - | - | - |
| Annual costs of PMS and premature death (in 2021 USD) | Age group (years) | ||||||||
| <16 | 16–19 | 20–24 | 25–34 | 35–44 | 45–54 | 55 -64 | 64–74 | 75+ | |
| Direct medical costs e | |||||||||
| PMS, neurological deficits | 8116 | ||||||||
| PMS, hearing loss | 1521 | ||||||||
| Direct non-medical and indirect costs | |||||||||
| PMS, neurological deficits f | 6820 | 31,850 | 66,069 | 106,986 | 125,566 | 125,838 | 107,502 | 48,166 | 21,937 |
| PMS, hearing loss f | 8335 | 16,908 | 28,628 | 42,641 | 49,005 | 49,098 | 42,818 | 22,496 | 13,513 |
| Annual earnings g | - | 10,154 | 24,036 | 40,635 | 48,172 | 48,283 | 40,844 | 16,773 | 6133 |
| Outcomes | V114 | PCV13 | Incremental Outcomes (V114 versus PCV13) |
|---|---|---|---|
| Clinical outcomes (undiscounted) | |||
| IPD cases | 3,383,921 | 3,569,631 | −185,711 |
| All-cause pneumonia cases | 580,425,873 | 581,413,600 | −987,727 |
| Pneumococcal AOM cases | 289,559,830 | 300,711,303 | −11,151,473 |
| PMS cases | 68,141 | 71,733 | −3592 |
| IPD deaths | 391,677 | 411,874 | −20,197 |
| Pneumonia deaths | 11,004,703 | 11,004,744 | −41 |
| LYs and QALYs (discounted) | |||
| Total LYs | 10,042,394,010 | 10,042,303,984 | 90,026 |
| Total QALYs | 8,703,494,217 | 8,703,398,161 | 96,056 |
| Cost outcomes (2021 USD, discounted) | |||
| Vaccine acquisition costs | $68,689,837,125 | $68,689,813,959 | $23,166 |
| Vaccine administration costs | $6,030,885,213 | $6,030,883,179 | $2034 |
| IPD treatment costs | $49,825,494,002 | $52,463,715,243 | −$2,638,221,241 |
| All-cause pneumonia treatment costs | $979,125,959,933 | $981,375,224,621 | −$2,249,264,688 |
| AOM treatment costs | $44,096,603,184 | $45,853,332,383 | −$1,756,729,199 |
| PMS treatment costs | $3,035,895,936 | $3,191,739,537 | −$155,843,601 |
| Direct non-medical/indirect costs | 208,629,466,353 | $210,638,743,324 | −$2,009,276,972 |
| Costs of premature death | 470,698,119,691 | $472,706,362,297 | −$2,008,242,605 |
| Total Costs | $1,830,132,261,437 | $1,840,949,814,543 | −$10,817,553,106 |
| ICER | V114 dominant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Hu, T.; Weaver, J.; Owusu-Edusei, K.; Elbasha, E. Cost-Effectiveness Analysis of Routine Use of 15-Valent Pneumococcal Conjugate Vaccine in the US Pediatric Population. Vaccines 2023, 11, 135. https://doi.org/10.3390/vaccines11010135
Huang M, Hu T, Weaver J, Owusu-Edusei K, Elbasha E. Cost-Effectiveness Analysis of Routine Use of 15-Valent Pneumococcal Conjugate Vaccine in the US Pediatric Population. Vaccines. 2023; 11(1):135. https://doi.org/10.3390/vaccines11010135
Chicago/Turabian StyleHuang, Min, Tianyan Hu, Jessica Weaver, Kwame Owusu-Edusei, and Elamin Elbasha. 2023. "Cost-Effectiveness Analysis of Routine Use of 15-Valent Pneumococcal Conjugate Vaccine in the US Pediatric Population" Vaccines 11, no. 1: 135. https://doi.org/10.3390/vaccines11010135
APA StyleHuang, M., Hu, T., Weaver, J., Owusu-Edusei, K., & Elbasha, E. (2023). Cost-Effectiveness Analysis of Routine Use of 15-Valent Pneumococcal Conjugate Vaccine in the US Pediatric Population. Vaccines, 11(1), 135. https://doi.org/10.3390/vaccines11010135





