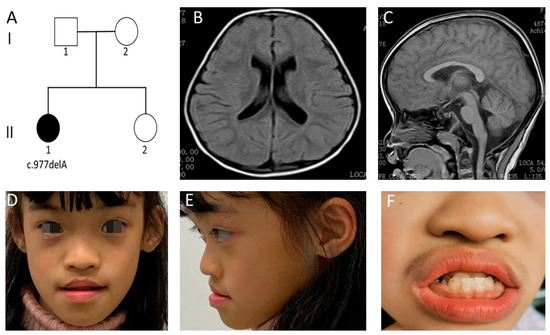
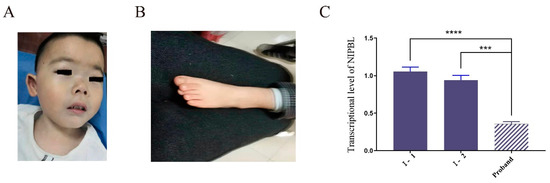
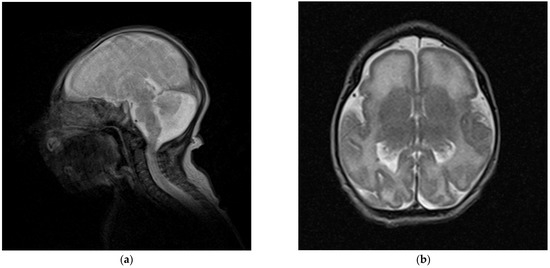
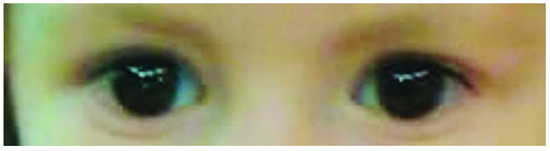
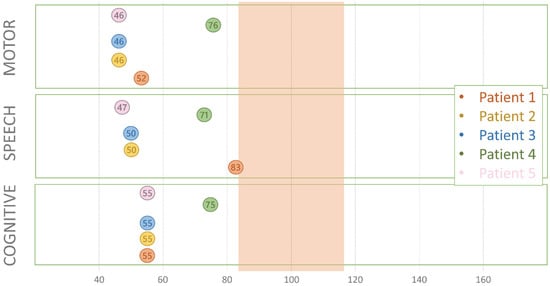
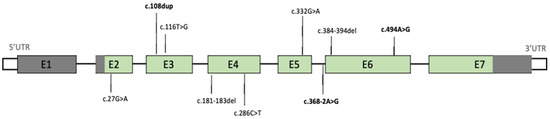
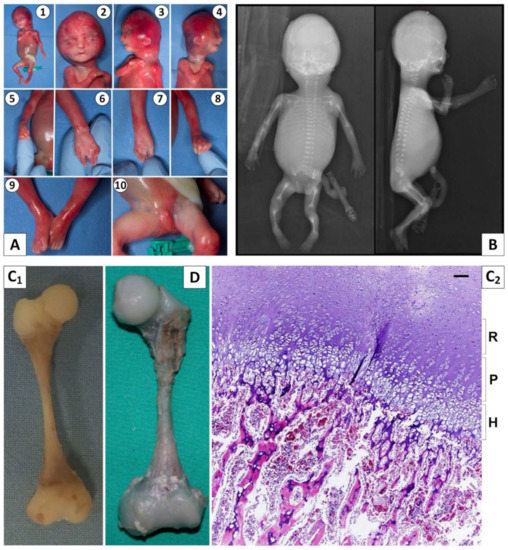
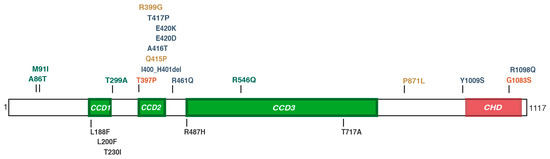
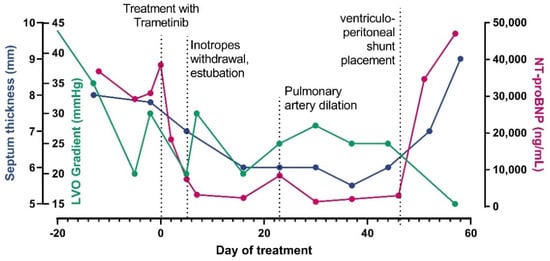
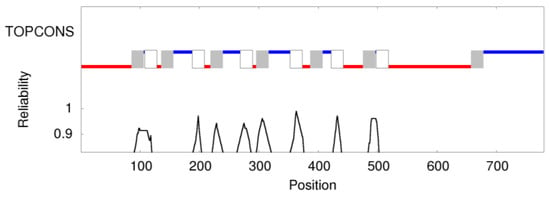
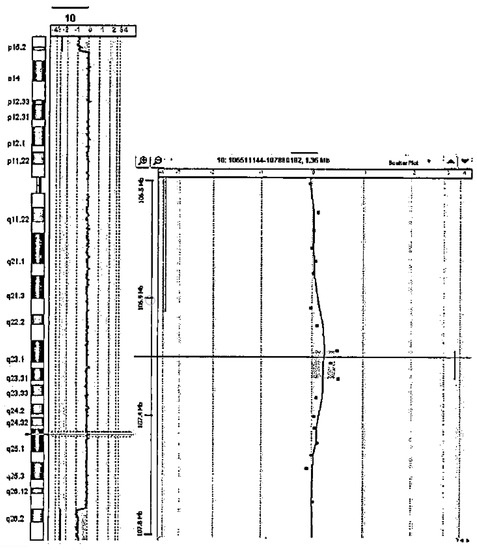
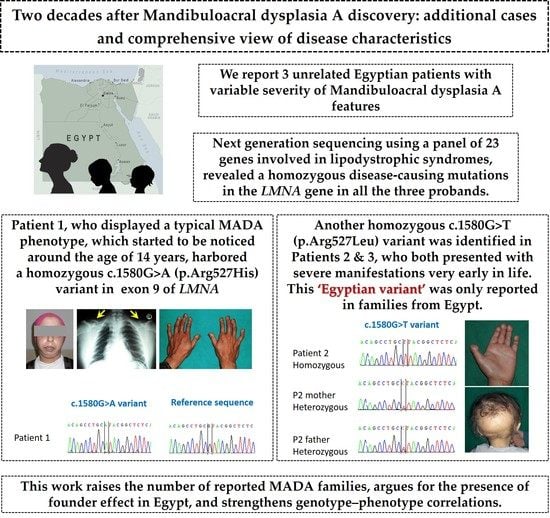
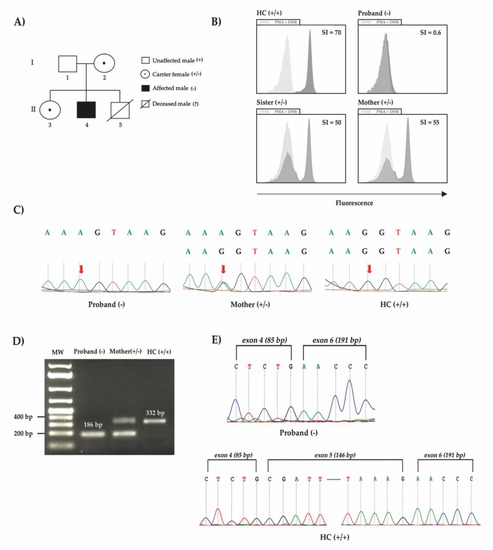
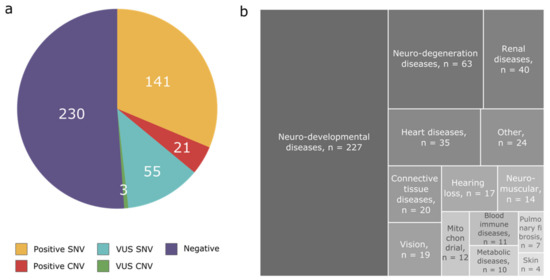
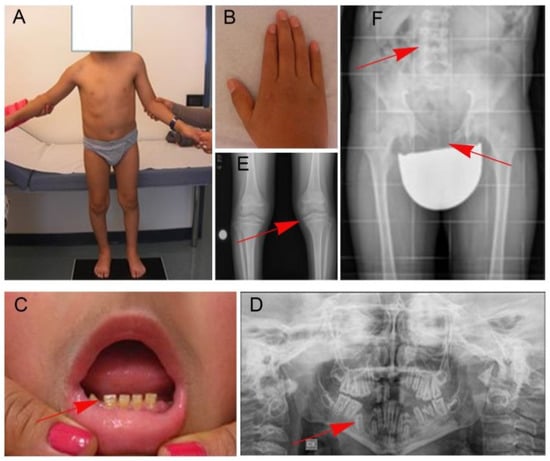
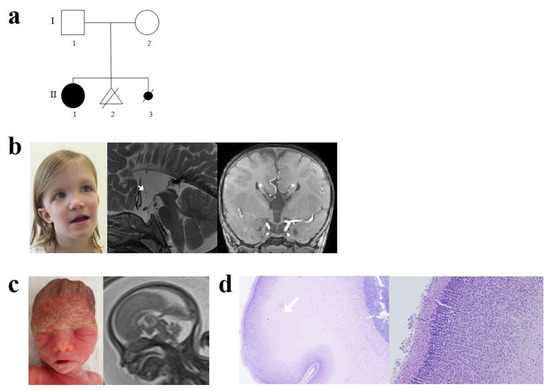
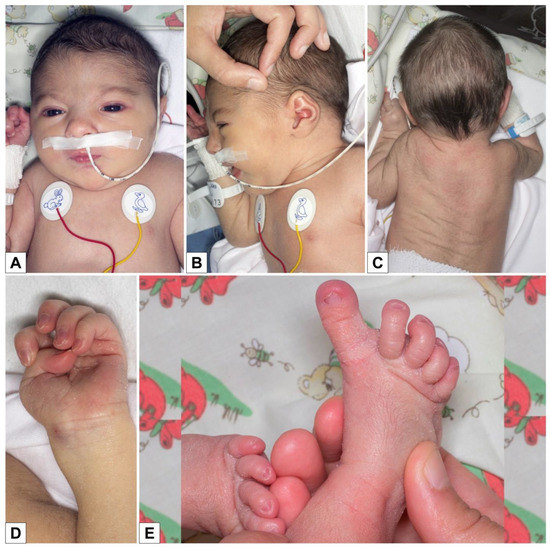
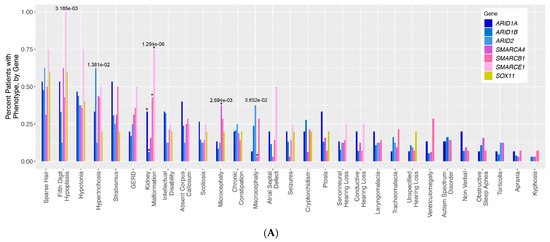
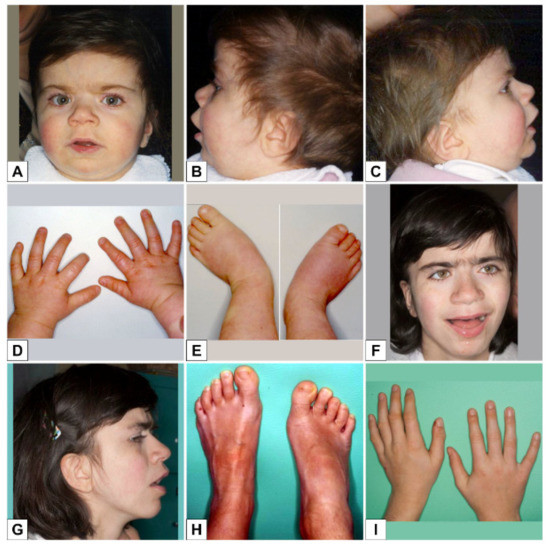
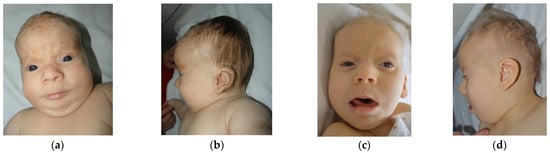
Study on Genotypes and Phenotypes of Pediatric Clinical Rare Diseases
A topical collection in Genes (ISSN 2073-4425). This collection belongs to the section "Human Genomics and Genetic Diseases".
Viewed by 304957Editors
Interests: genetica clinica; sindromi genetiche; malformazioni congenite malattie rare; pediatria
Topical Collection Information
Dear Colleagues,
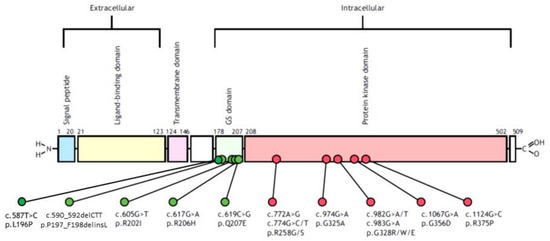
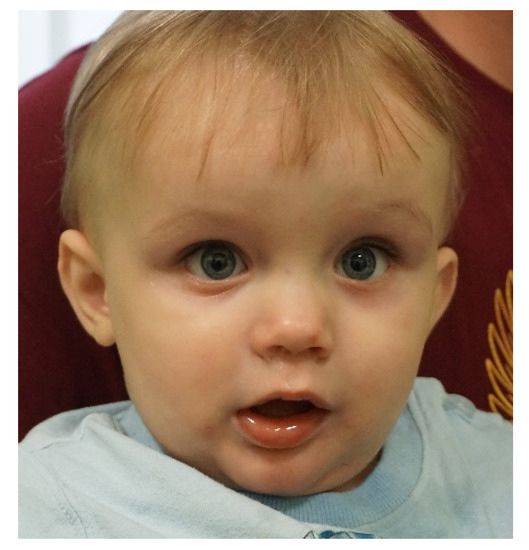
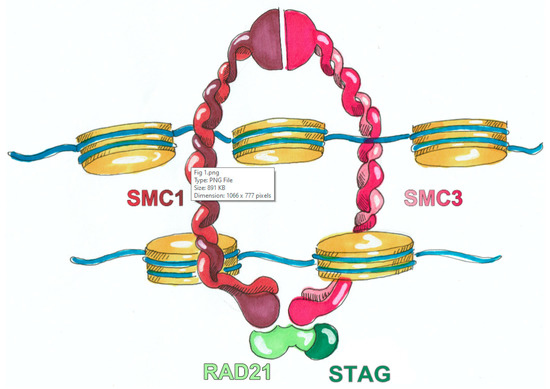
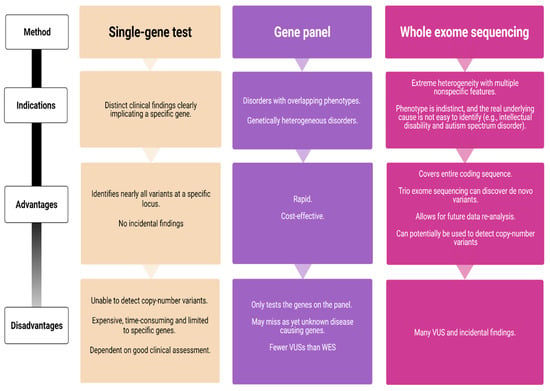
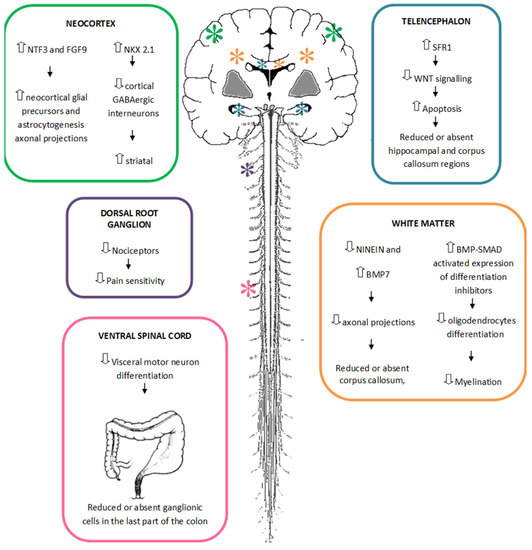
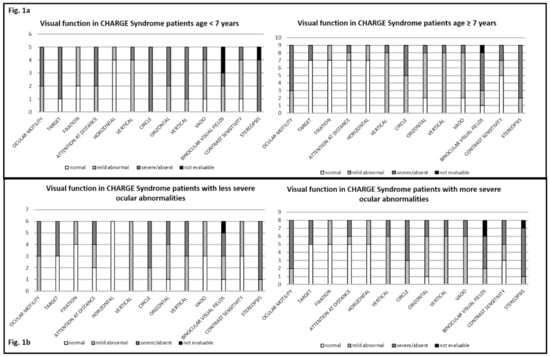
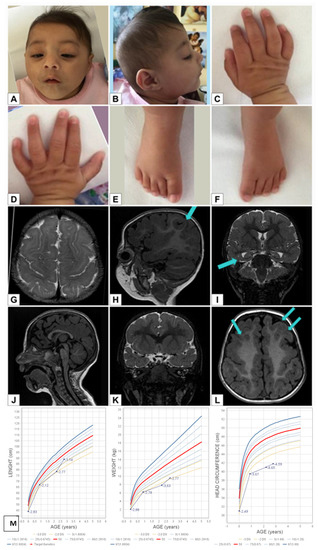
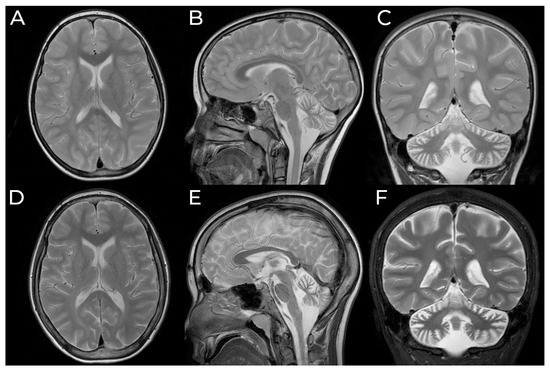
With the arrival and widespread adoption of high-throughput DNA sequencing, genetic discoveries in neurodevelopmental disorders (NDDs) and genetic syndromes are advancing very quickly. The identification of novel genes and of rare, highly penetrant pathogenic variants is helping to enhance our understanding of genotype–phenotype correlations. While most dominant NDD genes are highly intolerant to variation, some exceptions are connected to the presence of variants in transcripts that are not brain expressed and/or genes that demonstrate acquired somatic mosaicism in blood. A large number of NDD genes have been identified in cases where varying phenotypes depend on the type of inheritance (for example, dominant or recessive), the nature (for example missense or truncating) or location of the mutation.
The study of the genotype–phenotype correlation is not simple in recently-described genetic syndromes, with limited numbers of clinical cases, but it is very important for the clinician, who has to interpret the genetic results and organize the follow-up for children with genetic syndromes.
This is the reason why we believe it is important to prepare a Topical Collection with the title “Study on Genotypes and Phenotypes of Pediatric Clinical Rare Diseases”.
It would be an honour for us if you agreed to be one of the authors of this initiative. We would be happy to accept your suggestion for a title on a subject in which you are an expert.
Dr. Livia Garavelli
Dr. Stefano Giuseppe Caraffi
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Genes is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- genotype
- phenotype
- RASopathies
- overgrowth syndromes
- arthrogryposis