The Plant Cell Walls and Their Impact on Plant Physiology (Closed)
A topical collection in International Journal of Molecular Sciences (ISSN 1422-0067). This collection belongs to the section "Molecular Plant Sciences".
Viewed by 14189Editor
Interests: plant; developement; evolution; terestrialisation; cell wall; peroxidase; reactive oxygen species
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
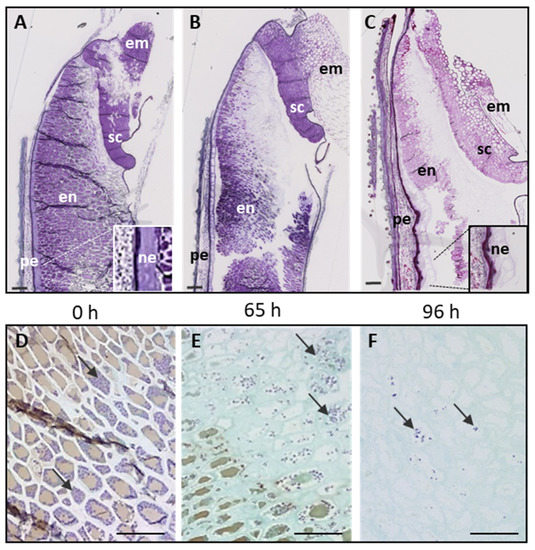
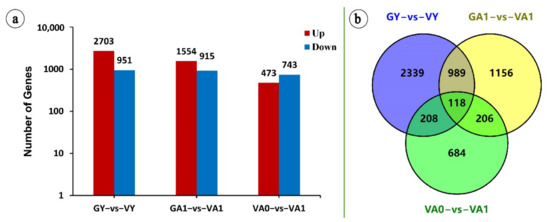
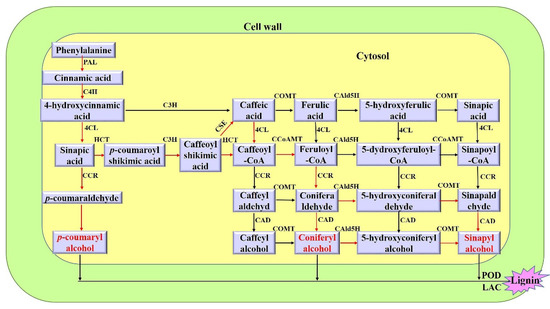
The plant cell wall is an extracellular compartment surrounding cells that play critical roles in plant life like providing an external skeleton, protection against external clues, or a means for cell-to-cell communication. It is a composite structure comprising polymers like polysaccharides (cellulose, pectins, and hemicelluloses), lignin in lignified secondary walls, cell wall proteins, and ions. The pectins and hemicelluloses are two polysaccharide families that contain a large collection of molecules differing by their content in monosaccharides and the type of linkages between them. Since the plant body contains different cell types, the structure and the composition of their walls are diverse, thus, allowing them to fulfill their functions and to respond to environmental constraints. Moreover, the composition of the plant cell wall varies, depending on the plant family and this is well illustrated by the differences between the walls of Poaceae and those of dicotyledonous plants. Altogether, each cell is surrounded by a specific wall that biogenesis requires a whole cascade of finely-tuned regulatory mechanisms, from the regulation of the transcription of the genes encoding the biosynthesis of the elementary bricks to the regulation of the assembly of the supramolecular structure and the modifications of the cell wall architecture during growth and upon interactions with the biotic and abiotic environment.
This Topical Collection of IJMS will gather articles dealing with plant cell wall biology. It will welcome experimental articles, review articles, or commentaries.
Prof. Dr. Christophe Dunand
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Molecular Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. There is an Article Processing Charge (APC) for publication in this open access journal. For details about the APC please see here. Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Adaptation to environmental constraints
- Alga
- Cell wall
- Cell wall architecture
- Cell wall polysaccharides
- Evolution
- Plant
- Polysaccharide biosynthesis
- Primary wall
- Protein/polysaccharide interactions
- Proteomics
- Reactive oxygen species
- Secondary wall
- Signaling
- Systems biology