Journal Description
International Journal of Neonatal Screening
International Journal of Neonatal Screening
is an international, peer-reviewed, open access journal on neonatal screening and neonatal medicine published quarterly online by MDPI. The journal is owned by the International Society for Neonatal Screening (ISNS). The International Society for Neonatal Screening (ISNS), German Society for Neonatal Screening (DGNS), the Japanese Society for Neonatal Screening (JSNS), the Association of Public Health Laboratories (APHL), the UK Newborn Screening Laboratory Network (UKNSLN) and more societies are affiliated with IJNS and their members receive discounts on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), PubMed, PMC, Embase, and other databases.
- Journal Rank: JCR - Q1 (Genetics and Heredity) / CiteScore - Q1 (Pediatrics, Perinatology and Child Health)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 25.3 days after submission; acceptance to publication is undertaken in 5.6 days (median values for papers published in this journal in the second half of 2024).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
4.0 (2023);
5-Year Impact Factor:
3.6 (2023)
Latest Articles
The Establishment of Expanded Newborn Screening in Rural Areas of a Developing Country: A Model from Health Regions 7 and 8 in Thailand
Int. J. Neonatal Screen. 2025, 11(2), 26; https://doi.org/10.3390/ijns11020026 (registering DOI) - 12 Apr 2025
Abstract
►
Show Figures
Expanded newborn screening (NBS) programs are essential for early detection and treatment. This study highlights the implementation of an expanded NBS program for inborn errors of metabolism (IEMs) and congenital hypothyroidism (CH) in rural Thailand, focusing on Health Regions 7 and 8 as
[...] Read more.
Expanded newborn screening (NBS) programs are essential for early detection and treatment. This study highlights the implementation of an expanded NBS program for inborn errors of metabolism (IEMs) and congenital hypothyroidism (CH) in rural Thailand, focusing on Health Regions 7 and 8 as a model for resource-limited settings. Using the KKU-IEM web-based platform, the program streamlined workflows, integrating logistics, real-time sample tracking, and electronic data management. Regular training sessions, continuous feedback, and systematic monitoring improved outcomes. Starting from October 2022, the program covered 98.6% of 123,692 live births, identifying 101 CH cases (1 in 1208 live births) and 20 IEM cases (1 in 6100 live births). The CH incidence was slightly higher than Thailand’s national average, while the IEM incidence was double that found in a previous Bangkok pilot study. Six cases highlighted maternal conditions affecting outcomes. Process improvements reduced the average reporting time from 9.13 days in 2023 to 8.4 days in 2024, with a 19% reduction in Bueng Kan Province. Efficiencies were driven by electronic ordering, real-time tracking, and stakeholder collaboration. This program demonstrates a scalable model for rural settings, emphasizing technology integration, collaboration, and quality control. Future efforts should refine diagnostics, expand disease coverage, and enhance long-term outcomes.
Full article
Open AccessCase Report
Rethinking Newborn Screening: A Case of GALM Deficiency
by
Eva M. M. Hoytema van Konijnenburg, Silvia Radenkovic, Klaas Koop, Hubertus C. M. T. Prinsen and Monique de Sain-van der Velden
Int. J. Neonatal Screen. 2025, 11(2), 25; https://doi.org/10.3390/ijns11020025 - 11 Apr 2025
Abstract
►▼
Show Figures
Galactosemia is a group of hereditary disorders of galactose metabolism. A new type of galactosemia was discovered, caused by a deficiency in galactose mutarotase (GALM), which catalyzes the epimerization between beta- and alpha-D-galactose. All GALM-deficient patients reported in the literature (n = 44)
[...] Read more.
Galactosemia is a group of hereditary disorders of galactose metabolism. A new type of galactosemia was discovered, caused by a deficiency in galactose mutarotase (GALM), which catalyzes the epimerization between beta- and alpha-D-galactose. All GALM-deficient patients reported in the literature (n = 44) had abnormal newborn screening (NBS) results or did not receive NBS (n = 2). We present the first patient with GALM deficiency who had negative NBS in the Netherlands and was identified at age 1.5 years during broad metabolic screening because of her global developmental delay, nystagmus, and a history of jaundice. Biochemical evaluation showed a significantly increased excretion of galactose (13,167 mmol/mol creatinine, upper limit of normal (ULN) 326) and galactitol (427 mmol/mol creatinine, ULN 71). Whole exome sequencing showed homozygous variants in GALM (c.424G>A p.(Gly142Arg)). A galactose-restricted diet was started, resulting in biochemical normalization. We present a comprehensive review of GALM-deficient patients, NBS data, and treatment. Different designs of galactosemia screening may lead to overlooking patients with GALM deficiency. Although the effects of lactose-restricted diet are largely unknown, a diet might prevent cataract in some patients.
Full article
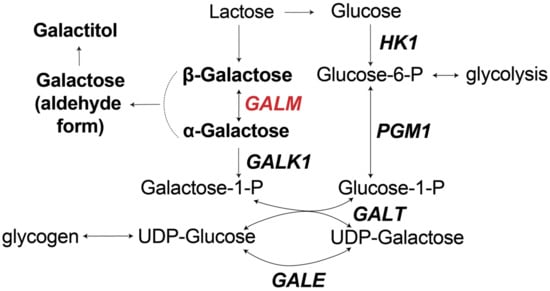
Figure 1
Open AccessGuidelines
Cystic Fibrosis Newborn Screening: A Systematic Review-Driven Consensus Guideline from the United States Cystic Fibrosis Foundation
by
Meghan E. McGarry, Karen S. Raraigh, Philip Farrell, Faith Shropshire, Karey Padding, Cambrey White, M. Christine Dorley, Steven Hicks, Clement L. Ren, Kathryn Tullis, Debra Freedenberg, Q. Eileen Wafford, Sarah E. Hempstead, Marissa A. Taylor, Albert Faro, Marci K. Sontag and Susanna A. McColley
Int. J. Neonatal Screen. 2025, 11(2), 24; https://doi.org/10.3390/ijns11020024 - 2 Apr 2025
Abstract
Newborn screening for cystic fibrosis (CF) has been universal in the US since 2010; however, there is significant variation among newborn screening algorithms. Systematic reviews were used to develop seven recommendations for newborn screening program practices to improve timeliness, sensitivity, and equity in
[...] Read more.
Newborn screening for cystic fibrosis (CF) has been universal in the US since 2010; however, there is significant variation among newborn screening algorithms. Systematic reviews were used to develop seven recommendations for newborn screening program practices to improve timeliness, sensitivity, and equity in diagnosing infants with CF: (1) The CF Foundation recommends the use of a floating immunoreactive trypsinogen (IRT) cutoff over a fixed IRT cutoff; (2) The CF Foundation recommends using a very high IRT referral strategy in CF newborn screening programs whose variant panel does not include all CF-causing variants in CFTR2 or does not have a variant panel that achieves at least 95% sensitivity in all ancestral groups within the state; (3) The CF Foundation recommends that CF newborn screening algorithms should not limit CFTR variant detection to the F508del variant or variants included in the American College of Medical Genetics-23 panel; (4) The CF Foundation recommends that CF newborn screening programs screen for all CF-causing CFTR variants in CFTR2; (5) The CF Foundation recommends conducting CFTR variant screening twice weekly or more frequently as resources allow; (6) The CF Foundation recommends the inclusion of a CFTR sequencing tier following IRT and CFTR variant panel testing to improve the specificity and positive predictive value of CF newborn screening; (7) The CF Foundation recommends that both the primary care provider and the CF specialist be notified of abnormal newborn screening results. Through implementation, it is anticipated that these recommendations will result in improved sensitivity, equity, and timeliness of CF newborn screening, leading to improved health outcomes for all individuals diagnosed with CF following newborn screening and a decreased burden on families.
Full article
(This article belongs to the Special Issue Advances in Cystic Fibrosis Newborn Screening: From Laboratory Testing to Diagnosis)
►▼
Show Figures

Figure 1
Open AccessArticle
A Review of Newborn Screening for VLCADD: The Wisconsin Experience
by
Breanna Mitchell, Jessica Scott-Schwoerer, Ashley Kuhl, Kristina Garcia and Patrice Held
Int. J. Neonatal Screen. 2025, 11(2), 23; https://doi.org/10.3390/ijns11020023 - 26 Mar 2025
Abstract
Very-long-chain acyl-CoA dehydrogenase deficiency (VLCADD) is due to a defect in metabolism of long-chain fatty acids. Infants with VLCADD may experience cardiomyopathy, hypoglycemia, or even death; thus, early detection and intervention is crucial. The spectrum of disease and natural variation in newborn metabolism,
[...] Read more.
Very-long-chain acyl-CoA dehydrogenase deficiency (VLCADD) is due to a defect in metabolism of long-chain fatty acids. Infants with VLCADD may experience cardiomyopathy, hypoglycemia, or even death; thus, early detection and intervention is crucial. The spectrum of disease and natural variation in newborn metabolism, however, lead to overlap in acylcarnitine values between affected and unaffected individuals, which contributes to the difficulty in identifying true positive cases while minimizing false positive cases. VLCADD was added to the state of Wisconsin’s newborn screening (NBS) panel in 2000. A previous retrospective review of VLCADD screen positive cases identified between 2000 and 2014 resulted in a change to the screening algorithm. Following implementation, a reduction in the percentage of false positive screens from 25.3% to 20.4% was observed between 2015 and 2021. The overall PPV also decreased, from 37.2% to 28%, due to an increase in the number of carriers identified (27.5% of cases in 2000–2014 and 51.8% of cases in 2015–2021). A data review also identified three long-chain acylcarnitine elevations (C14:1, C14:1/C16, and C14:1/C2) that had statistically significant differences in concentrations in true positive populations versus false positive populations. Utilization of the C14:1, C14:1/C16, and C14:1/C2 values in newborn screening may provide clearer distinction between true positive and carrier populations and additionally increase the PPV of this screen.
Full article
Open AccessArticle
Assessment of Long-Read Sequencing-Based Congenital Adrenal Hyperplasia Genotyping Assay for Newborns in Fujian, China
by
Xudong Wang, Xingxiu Lu, Faming Zheng, Kun Lin, Minjuan Liao, Yi Dong, Tiantian Chen, Ying He, Mei Lu, Jing Chen, Yanfang Li and Yulin Zhou
Int. J. Neonatal Screen. 2025, 11(1), 22; https://doi.org/10.3390/ijns11010022 - 13 Mar 2025
Abstract
►▼
Show Figures
Long-read sequencing (LRS) provides comprehensive genetic information, but research of LRS applied to congenital adrenal hyperplasia (CAH) newborn screening is limited. This study aimed to evaluate the clinical utility of LRS in genetic diagnosis and second-tier newborn screening. Neonates born between January 2017
[...] Read more.
Long-read sequencing (LRS) provides comprehensive genetic information, but research of LRS applied to congenital adrenal hyperplasia (CAH) newborn screening is limited. This study aimed to evaluate the clinical utility of LRS in genetic diagnosis and second-tier newborn screening. Neonates born between January 2017 and December 2022 in Fujian, China, were recruited for biochemical and LRS-based genetic screening assay. The LRS covers the entire gene regions and exon–intron boundary regions for CYP21A2, CYP11B1, CYP17A1, HSD3B2, and StAR. In this retrospective study, 1,774,555 newborns received 17α-OHP screening, yielding a screening positive rate of 0.20%. Of these high-risk neonates, 3411 were successfully recalled for re-evaluation. Finally, 66 neonates were diagnosed with CAH, with a positive predictive value of 28.82%. Based on this data, the overall prevalence of CAH in Fujian was estimated to be 1:26,883. LRS was performed on 57 neonates with 21-hydroxylase deficiency (21-OHD) and 109 variant alleles were identified. The c.293-13C>G variant (31.19%) was the most prevalent in Fujian. Additionally, 647 neonates with suspected positive results were genotyped, and 41 were identified as carriers, with carrier frequencies of 1:18 for CYP21A2, 1:162 for HSD3B2, and 1:324 for CYP17A1 in Xiamen. Therefore, LRS can provide comprehensive genotypes in approximately 1.5 days at a cost of less than $20 USD per sample, and effectively improve screening efficiency, reduce anxiety of parents during newborn screening (NBS), and shorten the time to referral of CAH patients (approximately 10 days). Such a combined screening strategy is worthy to be recommended for NBS programs in the future.
Full article
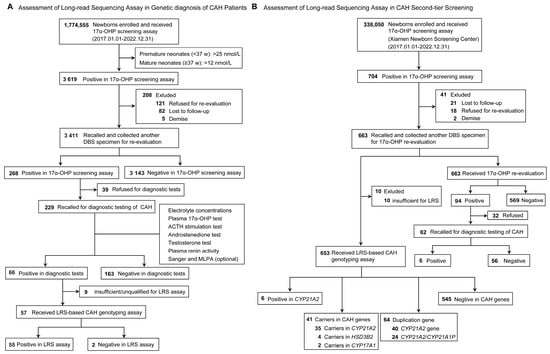
Figure 1
Open AccessConference Report
Oral and Poster Abstracts of the 13th ISNS European Regional Meeting
by
Kate Hall, Peter C. J. I. Schielen and Dimitris Platis
Int. J. Neonatal Screen. 2025, 11(1), 21; https://doi.org/10.3390/ijns11010021 - 10 Mar 2025
Abstract
This Abstract Book contains abstracts of oral and poster presentations of the 13th ISNS European Regional Meeting in Luxembourg, held from 23 to 26 March 2025.
Full article
(This article belongs to the Special Issue Selected Papers from 13th ISNS European Regional Meeting—Celebrating 10 Years of IJNS)
Open AccessArticle
Evaluation of the Florida Newborn Screening Program Education Campaign
by
Mirine Richey, Cynthia B. Wilson, Minna Jia and Travis Galbraith
Int. J. Neonatal Screen. 2025, 11(1), 20; https://doi.org/10.3390/ijns11010020 - 10 Mar 2025
Abstract
►▼
Show Figures
Florida’s Newborn Screening Program campaign aims to increase the awareness and participation of birthing facilities, providers, and parents. This evaluation aimed to determine the effectiveness and reach of the Newborn Screening Program (NBS) Statewide Educational Campaign to pregnant women through surveys and focus
[...] Read more.
Florida’s Newborn Screening Program campaign aims to increase the awareness and participation of birthing facilities, providers, and parents. This evaluation aimed to determine the effectiveness and reach of the Newborn Screening Program (NBS) Statewide Educational Campaign to pregnant women through surveys and focus groups. The online survey, conducted throughout Florida in English, Spanish, and Haitian Creole, evaluated the reach and effectiveness of educational materials such as paid advertisements and brochures. The surveys also served to recruit participants for in-person focus groups throughout the state. The findings showed that 85.3% of the mothers had discussions with health professionals about the screening program, while others did not hear about it from health professionals. More than 50% of the respondents learned about the program through health facilities, with additional exposure from media platforms such as television, radio, and friends. This study shows the need for increased outreach of the campaign and better communication and education from medical professionals to increase awareness.
Full article

Figure A1
Open AccessArticle
Insights from the Newborn Screening Program for Very Long-Chain Acyl-CoA Dehydrogenase (VLCAD) Deficiency in Kuwait
by
Hind Alsharhan, Amir A. Ahmed, Marwa Abdullah, Moudhi Almaie, Makia J. Marafie, Ibrahim Sulaiman, Reem M. Elshafie, Ahmad Alahmad, Asma Alshammari, Parakkal Xavier Cyril, Usama M. Elkazzaz, Samia M. Ibrahim, Mohamed Elghitany, Ayman M. Salloum, Fahmy Yassen, Rasha Alsafi, Laila Bastaki and Buthaina Albash
Int. J. Neonatal Screen. 2025, 11(1), 19; https://doi.org/10.3390/ijns11010019 - 28 Feb 2025
Abstract
►▼
Show Figures
Newborn screening for very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency in Kuwait was initiated in October 2014. Over a 7-year period (January 2015 to December 2021), 43 newborns were diagnosed with VLCAD deficiency out of 356,819 screened, corresponding to an incidence of 1:8290 and
[...] Read more.
Newborn screening for very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency in Kuwait was initiated in October 2014. Over a 7-year period (January 2015 to December 2021), 43 newborns were diagnosed with VLCAD deficiency out of 356,819 screened, corresponding to an incidence of 1:8290 and 1:5405 among only Kuwaiti newborns. This study represents the first comprehensive review of newborn screening for VLCAD deficiency in Kuwait. The screening process begins with the detection of elevated blood C14:1 levels in dried blood spots, followed by confirmatory testing using dried blood spots acylcarnitine profiling, with or without molecular testing. Furthermore, this study demonstrates that incorporating the C14:1/C2 ratio as a supplementary marker in first-tier testing alongside C14:1 improves the positive predictive value (PPV) of the current newborn screening for VLCAD deficiency. Adding molecular genetic testing for known VLCAD variants as a second-tier strategy to the national program is also recommended to further enhance specificity and improve PPV. Our findings provide evidence that the expanded newborn screening program in Kuwait has successfully facilitated the early detection of VLCAD deficiency, preventing death and disability in affected infants.
Full article

Figure 1
Open AccessArticle
International Survey on Phenylketonuria Newborn Screening
by
Domen Trampuž, Peter C. J. I. Schielen, Rolf H. Zetterström, Maurizio Scarpa, François Feillet, Viktor Kožich, Trine Tangeraas, Ana Drole Torkar, Matej Mlinarič, Daša Perko, Žiga Iztok Remec, Barbka Repič Lampret, Tadej Battelino, ISNS Study Group on PKU, Francjan J. van Spronsen, James R. Bonham and Urh Grošelj
Int. J. Neonatal Screen. 2025, 11(1), 18; https://doi.org/10.3390/ijns11010018 - 26 Feb 2025
Abstract
►▼
Show Figures
Newborn screening for Phenylketonuria enables early detection and timely treatment with a phenylalanine-restricted diet to prevent severe neurological impairment. Although effective and in use for 60 years, screening, diagnostic, and treatment practices still vary widely across countries and centers. To evaluate the Phenylketonuria
[...] Read more.
Newborn screening for Phenylketonuria enables early detection and timely treatment with a phenylalanine-restricted diet to prevent severe neurological impairment. Although effective and in use for 60 years, screening, diagnostic, and treatment practices still vary widely across countries and centers. To evaluate the Phenylketonuria newborn screening practices internationally, we designed a survey with questions focusing on the laboratory aspect of the screening system. We analyzed 24 completed surveys from 23 countries. Most participants used the same sampling age range of 48–72 h; they used tandem mass spectrometry and commercial non-derivatized kits to measure phenylalanine (Phe), and had non-negative cut-off values (COV) set mostly at 120 µmol/L of Phe. Participants mostly used genetic analysis of blood and detailed amino acid analysis from blood plasma as their confirmatory methods and set the COV for the initiation of dietary therapy at 360 µmol/L of Phe. There were striking differences in practice as well. While most participants reported a 48–72 h range for age at sampling, that range was overall quite diverse Screening COV varied as well. Additional screening parameters, e.g., the phenylalanine/tyrosine ratio were used by some participants to determine the screening result. Some participants included testing for tetrahydrobiopterin deficiency, or galactosemia in their diagnostic process. Results together showed that there is room to select a best practice from the many practices applied. Such a best practice of PKU-NBS parameters and post-screening parameters could then serve as a generally applicable guideline.
Full article

Figure 1
Open AccessArticle
Does Early Diagnosis and Treatment Alter the Clinical Course of Wolman Disease? Divergent Trajectories in Two Siblings and a Consideration for Newborn Screening
by
Maria Jose de Castro Lopez, Fiona J. White, Victoria Holmes, Jane Roberts, Teresa H. Y. Wu, James A. Cooper, Heather J. Church, Gemma Petts, Robert F. Wynn, Simon A. Jones and Arunabha Ghosh
Int. J. Neonatal Screen. 2025, 11(1), 17; https://doi.org/10.3390/ijns11010017 - 25 Feb 2025
Abstract
Wolman disease (WD) is a lethal disorder defined by the deficiency of the lysosomal acid lipase enzyme. Patients present with intestinal failure, malnutrition, and hepatosplenomegaly. Enzyme replacement therapy (ERT) with dietary substrate reduction (DSR) significantly improves survival. We sought to determine the outcomes
[...] Read more.
Wolman disease (WD) is a lethal disorder defined by the deficiency of the lysosomal acid lipase enzyme. Patients present with intestinal failure, malnutrition, and hepatosplenomegaly. Enzyme replacement therapy (ERT) with dietary substrate reduction (DSR) significantly improves survival. We sought to determine the outcomes of two siblings with WD treated after the onset of symptoms (sibling 1) and presymptomatic (sibling 2). A chart review was conducted on two siblings with WD treated with ERT and DSR at 4 months of age (sibling 1) and immediately after birth (sibling 2) to determine clinical outcomes based on survival, laboratory results, growth, dietary records, and gut biopsies. Sibling 1 presented with hepatosplenomegaly and liver dysfunction and developed hemophagocytic lymphohistiocytosis despite treatment. She received a bone marrow transplant at 8 months of age but died at 13 months. Sibling 2 is alive at 16 months of age with height, weight, and MUAC above the 95th centile, fully orally fed, with no gastrointestinal symptoms, normal liver function, and normal oxysterols. Sibling 2 duodenal biopsies show normal villus architecture with no foamy macrophage infiltration. Initiation of treatment prior to the onset of symptoms can prevent clinical manifestations and increase survival. The divergent trajectory in these siblings raises the question of WD’s candidacy for newborn screening.
Full article
(This article belongs to the Special Issue Neonatal Screening in Europe: On the Brink of a New Era)
►▼
Show Figures

Figure 1
Open AccessArticle
Five-Year Outcomes of Patients with Pompe Disease Identified by the Pennsylvania Newborn Screen
by
Hayley A. Ron, Owen Kane, Rose Guo, Caitlin Menello, Nicole Engelhardt, Shaney Pressley, Brenda DiBoscio, Madeline Steffensen, Sanmati Cuddapah, Kim Ng, Can Ficicioglu and Rebecca C. Ahrens-Nicklas
Int. J. Neonatal Screen. 2025, 11(1), 16; https://doi.org/10.3390/ijns11010016 - 24 Feb 2025
Abstract
►▼
Show Figures
Pennsylvania started newborn screening for Pompe disease (PD) in 2016. As a result, the prevalence of PD has increased with early detection, primarily of late-onset Pompe disease (LOPD). No clear guidelines exist regarding if and when to initiate enzyme replacement therapy (ERT) in
[...] Read more.
Pennsylvania started newborn screening for Pompe disease (PD) in 2016. As a result, the prevalence of PD has increased with early detection, primarily of late-onset Pompe disease (LOPD). No clear guidelines exist regarding if and when to initiate enzyme replacement therapy (ERT) in patients identified through a newborn screen (NBS). To help define the natural history and indications for starting ERT, we present the long-term follow-up data of 45 patients identified through NBS from 2016 to 2021. These patients were evaluated at regular intervals through our multi-disciplinary clinic at the Children’s Hospital of Philadelphia (CHOP) with physical examinations, physical therapy evaluations, muscle biomarkers including creatine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and hexosaminidase 4 levels (Hex4), as well as cardiac evaluation at certain points in time. We found that newborn screening of acid alpha-glucosidase (GAA) enzyme detected primarily LOPD. One case of infantile-onset PD (IOPD) was detected. Muscle biomarkers in LOPD were elevated at birth and showed a general downward trend over time. NBS GAA levels and initial CK levels helped to differentiate LOPD cases from unaffected infants (carriers, pseudodeficiency alleles), while Hex4 was not a meaningful discriminator. On repeat NBS, there was a significant difference between mean GAA levels for the unaffected vs. compound heterozygote groups and unaffected vs. homozygote groups for the common splice site pathogenic variant (c.-32-13T>G). Echocardiogram and electrocardiogram (EKG) are essentially normal at the first evaluation in LOPD. One LOPD patient was started on ERT at age 4.5 months. Continued data collection on these patients is critical for developing management guidelines, including timing of ERT and improved genotype–phenotype correlation.
Full article

Figure 1
Open AccessArticle
Non-Uptake of Newborn Screening in Planned Homebirth Is Associated with Preventive Health Practices for Infants: A Retrospective Case-Control Study
by
Chen Stein-Zamir, Hanna Shoob, Sandra Katan, Gina Verbov and Shlomo Almashanu
Int. J. Neonatal Screen. 2025, 11(1), 15; https://doi.org/10.3390/ijns11010015 - 21 Feb 2025
Abstract
►▼
Show Figures
Universal Newborn Screening (NBS) programs (for endocrine, immunologic and metabolic disorders) are effective in reducing child morbidity and mortality. Despite available health services, NBS is not carried out for some newborns. The contributing factors for this should be explored. In high-income settings, homebirth
[...] Read more.
Universal Newborn Screening (NBS) programs (for endocrine, immunologic and metabolic disorders) are effective in reducing child morbidity and mortality. Despite available health services, NBS is not carried out for some newborns. The contributing factors for this should be explored. In high-income settings, homebirth generally refers to planned birth at home, attended by skilled health professionals. We aimed to assess trends and characteristics of planned homebirths and the uptake of NBS and infant health practices. A retrospective case-control study including 3246 infants compared planned homebirth (cases) to age-matched hospital birth controls. During 2016–2023, 0.56% of livebirths (1623/290,458) in the Jerusalem District (JD), Israel, were planned homebirths. The rate has increased since 2020 (COVID-19 pandemic), 0.45% in 2016–2019 vs. 0.67% in 2020–2023. Homebirth infants had a higher birthweight, lower firstborn rate and higher socioeconomic rank. The overall NBS uptake in homebirths was significantly lower (73.7% vs. 99.5% in hospital births) and declined over time (81.1% in 2016–2019 vs. 68.7% in 2020–2023). Regarding preventive health practices for homebirth infants, the registration rate to Mother and Child Health Clinics (MCHCs) was lower (47.1% vs. 92.8% in hospital births), and routine immunization rates were decreased (DTaP-IPV-HiB3 90.7% vs. 60.1%). The NBS uptake among homebirth infants was significantly associated with MCHC registration and routine immunizations (RR = 4.15, 95%CI 3.3–5.3). NBS uptake in homebirths is considerably lower and is associated with subsequent patterns of preventive health practices. Notably, the national NBS program data also indicate a trend of increase in non-uptake rates. Barriers to NBS for homebirths should be identified and targeted interventions implemented. The trends in national NBS non-uptake necessitate further follow-up, and evidence from successful outreach programs should be reviewed and translated into guidelines for health organizations.
Full article

Figure 1
Open AccessArticle
Newborn Screening for Gaucher Disease: Parental Stress and Psychological Burden
by
Chiara Cazzorla, Vincenza Gragnaniello, Giacomo Gaiga, Daniela Gueraldi, Andrea Puma, Christian Loro, Giada Benetti, Rossana Schiavo, Elena Porcù, Alessandro P. Burlina and Alberto B. Burlina
Int. J. Neonatal Screen. 2025, 11(1), 14; https://doi.org/10.3390/ijns11010014 - 14 Feb 2025
Abstract
In the last few decades, neonatal screening (NBS) has expanded to include lysosomal storage diseases, allowing for the early identification of both symptomatic and asymptomatic cases. However, neonatal diagnosis of late-onset disorders can cause parental stress and affect family well-being, possibly leading to
[...] Read more.
In the last few decades, neonatal screening (NBS) has expanded to include lysosomal storage diseases, allowing for the early identification of both symptomatic and asymptomatic cases. However, neonatal diagnosis of late-onset disorders can cause parental stress and affect family well-being, possibly leading to overmedicalization. The impact of a positive NBS for Gaucher disease type 1 (GD1) can have an important impact on parental psychological well-being and psychosocial functioning. This study aims to study parental stress in parents of newborns who had a positive result for Gaucher disease in an NBS program in Northeastern Italy. Fourteen parents (7 fathers and 7 mothers) of seven children with confirmed GD1 (86% boys) completed the Parenting Stress Index—Short Form (PSI-SF) at diagnosis (T0), 12 months (T1), and 36 months (T2). A control group of fourteen parents (7 fathers and 7 mothers) whose children had normal NBS results was included. Interviews were conducted for the GD1 group at T2 to investigate the usefulness of the NBS program. At T0, higher parental stress was assessed in GD1 parents compared to the healthy controls. Subsequently, the parents of GD1 children reported significant reductions in Parental Distress at T1 compared to T0. Mothers showed further reductions at T2, while the fathers’ distress decreased but not significantly. GD1 mothers had significantly higher distress scores than the controls at T1, but this difference diminished over time. Our study highlights the psychological impact of NBS on GD1, emphasizing the need for better multidisciplinary communication to reduce parental stress throughout the diagnostic and treatment process.
Full article
Open AccessTechnical Note
Seventh ISNS Reference Preparation for Neonatal Screening for Thyroid Stimulating Hormone, Phenylalanine, and 17α-Hydroxyprogesterone in Blood Spots
by
Peter C. J. I. Schielen, Dianne Webster, J. Gerard Loeber and James R. Bonham
Int. J. Neonatal Screen. 2025, 11(1), 13; https://doi.org/10.3390/ijns11010013 - 9 Feb 2025
Abstract
The International Society for Neonatal Screening (ISNS) has supported the standardization of the measurement of key biochemical markers for the neonatal screening of diseases: thyroid-stimulating hormone (TSH) for congenital hypothyroidism, phenylalanine (PHE) for phenylketonuria, and 17α-hydroxyprogesterone (17OHP) for congenital adrenal hyperplasia. These diseases
[...] Read more.
The International Society for Neonatal Screening (ISNS) has supported the standardization of the measurement of key biochemical markers for the neonatal screening of diseases: thyroid-stimulating hormone (TSH) for congenital hypothyroidism, phenylalanine (PHE) for phenylketonuria, and 17α-hydroxyprogesterone (17OHP) for congenital adrenal hyperplasia. These diseases are commonly a part of neonatal screening panels worldwide. The ISNS provides a series of secondary reference materials to the manufacturers of neonatal screening reagents to assist in the production of calibration materials for kits. This technical note describes the manufacture of the seventh combined dried blood spot reference preparation for neonatal screening (RPNS) for these analytes.
Full article
Open AccessCommunication
Clinical Utility of the Addition of Molecular Genetic Testing to Newborn Screening for Hemoglobinopathies for Confirmation of Alpha-Thalassemia Trait
by
Lisa M. Shook, Deidra Haygood and Charles T. Quinn
Int. J. Neonatal Screen. 2025, 11(1), 12; https://doi.org/10.3390/ijns11010012 - 7 Feb 2025
Abstract
►▼
Show Figures
Hemoglobinopathies are commonly detected by newborn screening (NBS). One of the most difficult to accurately diagnose is alpha-thalassemia, which is indicated by the presence of hemoglobin (Hb) Barts on NBS. This mixed methods study incorporated (1) an implementation and quality improvement project to
[...] Read more.
Hemoglobinopathies are commonly detected by newborn screening (NBS). One of the most difficult to accurately diagnose is alpha-thalassemia, which is indicated by the presence of hemoglobin (Hb) Barts on NBS. This mixed methods study incorporated (1) an implementation and quality improvement project to demonstrate the clinical utility of genetic testing added to standard procedures for likely alpha-thalassemia trait and (2) a qualitative study to determine the related educational needs of primary care providers (PCPs). During a two-year period, we attempted to perform alpha-globin genetic testing for all newborns with an abnormal NBS result (an “FA + Barts” pattern). We conducted semi-structured interviews with seven PCPs for thematic abstraction. In sixty neonates with presumed Hb Barts on initial NBS who had genetic testing, three (5%) did not have alpha-thalassemia. The remaining 57 (95%) had an alpha-thalassemia trait genotype. Non-deletion alpha-thalassemia occurred in 5%. Eight (13%) had genotypes that substantially altered genetic counseling for the individual and family members. Race and ethnicity were poor surrogates for genotype. PCPs expressed a willingness to participate in NBS follow up but had little specific knowledge about alpha-thalassemia. The addition of genetic testing for likely alpha-thalassemia trait to NBS had very high clinical utility, supporting its use in standard clinical care. Whenever possible, education and genetic counseling should not be provided based on the detection of possible Hb Barts alone without subsequent specific genetic verification. Educational and outreach programs for both PCPs and families about the importance of testing and trait counseling are needed for ongoing improvement.
Full article

Figure 1
Open AccessArticle
Neonatal Screening for Spinal Muscular Atrophy and Severe T- and B-Cell Lymphopenias in Andalusia: A Prospective Study
by
Beatriz De Felipe, Carmen Delgado-Pecellin, Mercedes Lopez-Lobato, Peter Olbrich, Pilar Blanco-Lobo, Josefina Marquez-Fernandez, Carmen Salamanca, Beatriz Mendoza, Rocio Castro-Serrano, Cristina Duque, Mariana Moreno-Prieto, Marcos Madruga-Garrido, Jose M. Lucena, Raquel M. Fernandez, Maria Ruiz-Camacho, Alberto Varona and Olaf Neth
Int. J. Neonatal Screen. 2025, 11(1), 11; https://doi.org/10.3390/ijns11010011 - 30 Jan 2025
Abstract
►▼
Show Figures
Spinal muscular atrophy (SMA) and severe T- and/or B-cell lymphopenias (STBCL) in the form of severe combined immunodeficiencies (SCID) or X-linked agammaglobulinemia (XLA) are rare but potentially fatal pathologies. In January 2021, we initiated the first pilot study in Spain to evaluate the
[...] Read more.
Spinal muscular atrophy (SMA) and severe T- and/or B-cell lymphopenias (STBCL) in the form of severe combined immunodeficiencies (SCID) or X-linked agammaglobulinemia (XLA) are rare but potentially fatal pathologies. In January 2021, we initiated the first pilot study in Spain to evaluate the efficacy of a very early detection technique for SMA and SCID. RT–PCR was performed on prospectively collected dried blood spots (DBSs) from newborns in Western Andalusia (Spain). Internal and external controls (SCID, XLA and SMA) were included. The determination of SMA was relative (positive/negative) and that of TRECs and KRECs was quantitative (copies/punch). A total of 14.035 prospective samples were analysed. All controls were correctly identified while no cases of SMA or SCID/XLA were prospectively identified. DBS analysis of infants with suspected SMA or STBCL that presented to our centre showed pathological values in two cases each for SMA and SCID and one for XLA, all of them being subsequently confirmed genetically. In this prospective pilot study, no infants with SMA or STBCL were detected; however, the technique applied here was shown to be reliable and fast, further supporting the benefits and need to include SMA and SCID in national newborn screening (NBS) programs, as it will allow early supportive and curative therapy.
Full article

Figure 1
Open AccessArticle
Newborn Screening for Sickle Cell Disease: Results from a Pilot Study in the Portuguese Population
by
Diogo Rodrigues, Ana Marcão, Lurdes Lopes, Ana Ventura, Teresa Faria, Anabela Ferrão, Carolina Gonçalves, Paula Kjöllerström, Ana Castro, Sofia Fraga, Marta Almeida, Tabita Maia, João Gomes, Ana Lachado, Isabel Guerra, Fátima Ferreira, Fernanda Trigo, Celeste Bento and Laura Vilarinho
Int. J. Neonatal Screen. 2025, 11(1), 10; https://doi.org/10.3390/ijns11010010 - 27 Jan 2025
Abstract
►▼
Show Figures
The Portuguese Newborn Screening Program currently includes 28 pathologies: congenital hypothyroidism, cystic fibrosis, 24 inborn errors of metabolism, sickle cell disease and spinal muscular atrophy. This pilot study for sickle cell disease newborn screening, including 188,217 samples, was performed between May 2021 and
[...] Read more.
The Portuguese Newborn Screening Program currently includes 28 pathologies: congenital hypothyroidism, cystic fibrosis, 24 inborn errors of metabolism, sickle cell disease and spinal muscular atrophy. This pilot study for sickle cell disease newborn screening, including 188,217 samples, was performed between May 2021 and December 2023, with phase I, including 24,130 newborns, in the Lisbon and Setubal districts and phase II, including 164,087 newborns, in the whole country. DBS samples were analyzed through capillary electrophoresis. In phase I, a high birth incidence of sickle cell disease was found (1:928 NBs), resulting from the identification of 24 HbSS and 2 HbSC patients. This birth incidence decreased but remained significant when the pilot study for sickle cell disease newborn screening was expanded to a national level, with the identification of 67 sickle cell disease patients (59 HbSS and 8 HbSC), revealing a birth incidence of 1:2449 NBs. These data suggest that this condition is becoming increasingly relevant in Portugal, thus reflecting a general European trend, where sickle cell disease is already recognized as a public health problem. Therefore, it highlights the importance of its integration into the Portuguese National Newborn Screening Program panel in January 2024, thus allowing the early identification and clinical follow-up of these patients.
Full article

Figure 1
Open AccessSystematic Review
Sudden Death of a Four-Day-Old Newborn Due to Mitochondrial Trifunctional Protein/Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiencies and a Systematic Literature Review of Early Deaths of Neonates with Fatty Acid Oxidation Disorders
by
Ana Drole Torkar, Ana Klinc, Ziga Iztok Remec, Branislava Rankovic, Klara Bartolj, Sara Bertok, Sara Colja, Vanja Cuk, Marusa Debeljak, Eva Kozjek, Barbka Repic Lampret, Matej Mlinaric, Tinka Mohar Hajnsek, Daša Perko, Katarina Stajer, Tine Tesovnik, Domen Trampuz, Blanka Ulaga, Jernej Kovac, Tadej Battelino, Mojca Zerjav Tansek and Urh Groseljadd
Show full author list
remove
Hide full author list
Int. J. Neonatal Screen. 2025, 11(1), 9; https://doi.org/10.3390/ijns11010009 - 26 Jan 2025
Abstract
►▼
Show Figures
Mitochondrial trifunctional protein (MTP) and long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiencies have been a part of the Slovenian newborn screening (NBS) program since 2018. We describe a case of early lethal presentation of MTPD/LCHADD in a term newborn. The girl was born after an
[...] Read more.
Mitochondrial trifunctional protein (MTP) and long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiencies have been a part of the Slovenian newborn screening (NBS) program since 2018. We describe a case of early lethal presentation of MTPD/LCHADD in a term newborn. The girl was born after an uneventful pregnancy and delivery, and she was discharged home at the age of 3 days, appearing well. At the age of 4 days, she was found without signs of life. Resuscitation was not successful. The NBS test performed using tandem mass spectrometry (MS/MS) showed a positive screen for MTPD/LCHADD. Genetic analysis performed on a dried blood spot (DBS) sample identified two heterozygous variants in the HADHA gene: a nucleotide duplication introducing a premature termination codon (p.Arg205Ter) and a nucleotide substitution (p.Glu510Gln). Post-mortem studies showed massive macro-vesicular fat accumulation in the liver and, to a smaller extent, in the heart, consistent with MTPD/LCHADD. A neonatal acute cardiac presentation resulting in demise was suspected. We conducted a systematic literature review of early neonatal deaths within 14 days postpartum attributed to confirmed fatty acid oxidation disorders (FAODs), which are estimated to account for 5% of sudden infant deaths. We discuss the pitfalls of the NBS for MTPD/LCHADD.
Full article

Figure 1
Open AccessArticle
The Training and Evaluation of the “Dual-Index” Screening Method for Neonatal Congenital Heart Disease: A Multi-Center Study in China
by
Panpan Huang, Qing Gu, Xiaoting Zhu, Ijaz ul Haq, Liling Li, Xiaojing Hu and Guoying Huang
Int. J. Neonatal Screen. 2025, 11(1), 8; https://doi.org/10.3390/ijns11010008 - 14 Jan 2025
Abstract
►▼
Show Figures
Background: This study aimed to enhance the scope of neonatal congenital heart disease (CHD) screening by evaluating the effectiveness of training personnel in CHD screening using the “dual-index” method, combining pulse oximetry with cardiac murmur auscultation. Methods: From 2019 to 2022, a total
[...] Read more.
Background: This study aimed to enhance the scope of neonatal congenital heart disease (CHD) screening by evaluating the effectiveness of training personnel in CHD screening using the “dual-index” method, combining pulse oximetry with cardiac murmur auscultation. Methods: From 2019 to 2022, a total of 2374 screening personnel from the Xinjiang, Yunnan, Hainan, Fujian, and Anhui provinces underwent training in neonatal CHD screening using the “dual-index” method, which involves pulse oximetry and cardiac murmur auscultation. Pre- and post-training assessments were conducted using a neonatal CHD screening knowledge questionnaire, distributed through the Questionnaire Star platform, to evaluate the impact of the training. The annual neonatal CHD screening rates were consistently recorded in these five provinces during the same period to assess the increase in screening coverage. Results: After the training, the screening personnel exhibited a significantly improved understanding of the neonatal CHD screening method (p < 0.001). Additionally, the professional background (t = −8.007, p < 0.001) and years of experience (t = 2.839, p = 0.005) of the screening personnel were identified as independent factors influencing their screening knowledge. During the same period, there was consistent linear growth in the screening coverage rate for neonatal CHD across the five provinces (χ2 = 121065.416, p < 0.001). Conclusion: Standardized training in the “dual-index” method, incorporating pulse oximetry and cardiac murmur auscultation, for screening personnel significantly enhances their screening knowledge, thereby playing a critical role in expanding the coverage of neonatal CHD screening.
Full article

Figure 1
Open AccessArticle
Outcomes of a Pilot Newborn Screening Program for Spinal Muscular Atrophy in the Valencian Community
by
Alba Berzal-Serrano, Belén García-Bohórquez, Elena Aller, Teresa Jaijo, Inmaculada Pitarch-Castellano, Dolores Rausell, Gema García-García and José M. Millán
Int. J. Neonatal Screen. 2025, 11(1), 7; https://doi.org/10.3390/ijns11010007 - 14 Jan 2025
Abstract
►▼
Show Figures
Spinal muscular atrophy (SMA) is a degenerative neuromuscular condition resulting from a homozygous deletion of the survival motor neuron 1 (SMN1) gene in 95% of patients. A timely diagnosis via newborn screening (NBS) and initiating treatment before the onset of symptoms
[...] Read more.
Spinal muscular atrophy (SMA) is a degenerative neuromuscular condition resulting from a homozygous deletion of the survival motor neuron 1 (SMN1) gene in 95% of patients. A timely diagnosis via newborn screening (NBS) and initiating treatment before the onset of symptoms are critical for improving health outcomes in affected individuals. We carried out a screening test by quantitative PCR (qPCR) to amplify the exon seven of SMN1 using dried blood spot (DBS) samples. From October 2021 to August 2024, a total of 31,560 samples were tested in the Valencian Community (Spain) and 4 of them were positive for SMA, indicating an incidence of 1/7890. Genetic confirmation was performed using multiplex ligation-dependent probe amplification (MLPA) and AmplideX PCR/CE SMN1/2 Plus kit, in parallel obtaining concordant results in survival motor neuron 2 (SMN2) gene copy number. Within the first few weeks of their lives, two of the four patients detected by NBS showed signs of severe hypotonia, becoming ineligible for treatment. The other two patients were the first presymptomatic patients with two copies of SMN2 to receive treatment with Risdiplam in Spain. In order to treat positive cases in their early stages, we conclude that the official deployment of SMA newborn screening is necessary.
Full article

Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics

Conferences
Special Issues
Special Issue in
IJNS
Advances in Cystic Fibrosis Newborn Screening: From Laboratory Testing to Diagnosis
Guest Editors: Susanna A. McColley, Marci K. SontagDeadline: 30 April 2025
Special Issue in
IJNS
Global Updates on the Advancements in CCHD Screening
Guest Editors: Gerard R. Martin, Lisa A. WandlerDeadline: 30 November 2025
Special Issue in
IJNS
Equity Issues in Newborn Screening
Guest Editors: Lynn Wein Bush, Juan Cabello, Amy BrowerDeadline: 31 December 2025
Special Issue in
IJNS
Selected Papers from 13th ISNS European Regional Meeting—Celebrating 10 Years of IJNSGuest Editors: Kate Hall, Peter C. J. I. Schielen, Ralph FingerhutDeadline: 31 December 2025
Topical Collections
Topical Collection in
IJNS
Newborn Screening in Japan
Collection Editors: Toshihiro Tajima, Seiji Yamaguchi







