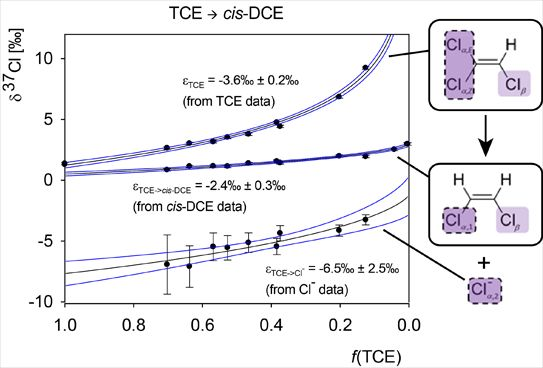
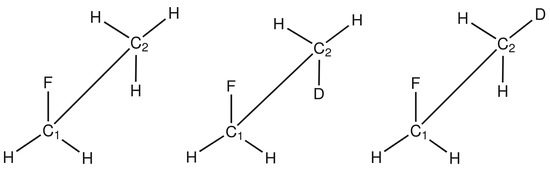
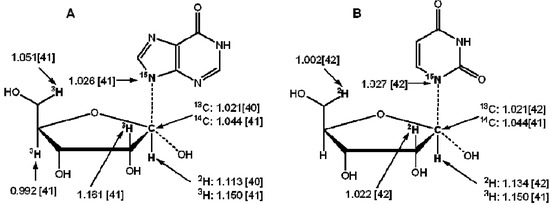
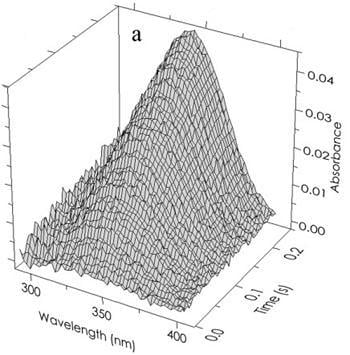
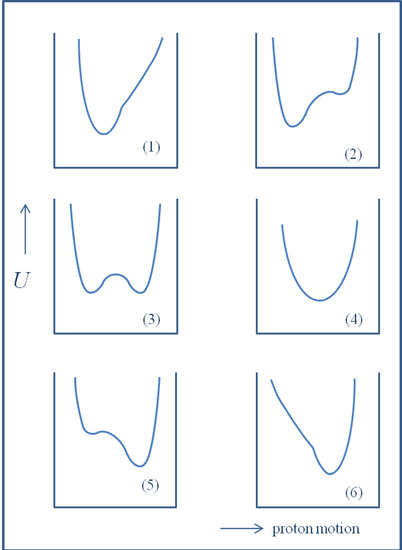
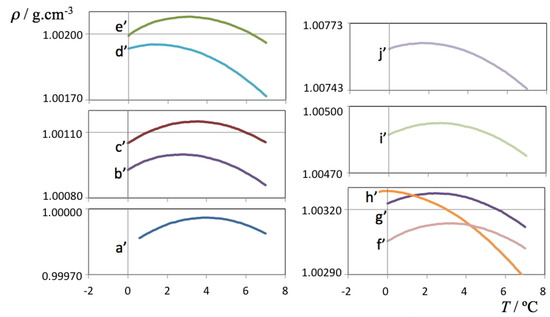
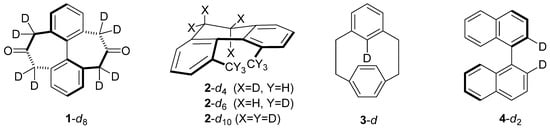
Isotope Effects
A topical collection in Molecules (ISSN 1420-3049).
Viewed by 109536Editor
Interests: isotope effects in biological systems; intramolecular hydrogen bonding; tautomerism; natural products; theoretical calculations of spectroscopic properties
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
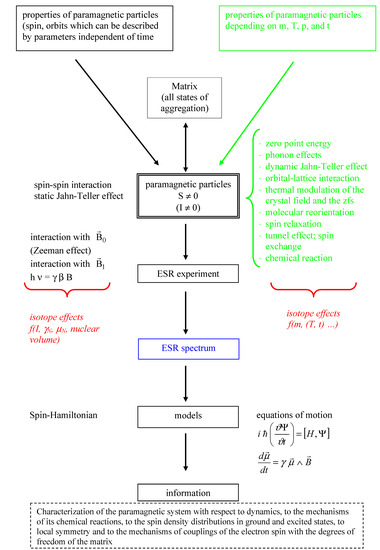
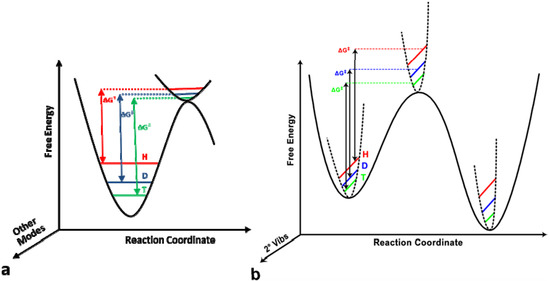
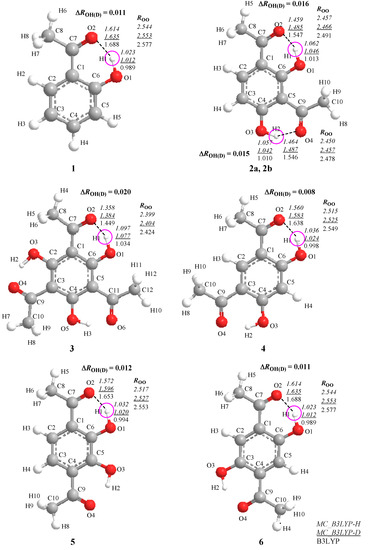
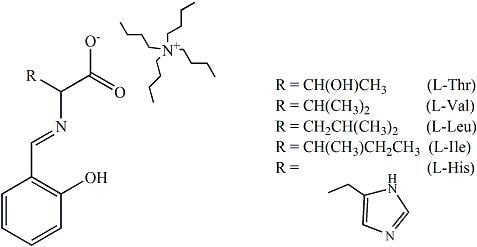
The journal Molecules has asked me to be guest editor on a Special Issue: “Isotope effects”. I have accepted as I find this a good opportunity to follow up on recent excellent books on the subject. It is important to keep the fire burning. No need to tell you how important isotope effects are but it is important to make the subject very visible. Hence this issue.
The volume will have some twenty contributions. The contributions can be either review papers or cover new research. Subjects can typically be kinetic isotope effects, reaction mechanisms, isotope effects in H-bond research, isotope effects as tool in structural studies, use of isotope effects in food research, theoretical calculations of isotope effects, use of isotope effects in environmental studies to mention some areas but papers dealing with all types of isotope effects in chemistry are invited.
Prof. Dr. Poul Erik Hansen
Guest Editor
Submission
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. Papers will be published continuously (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are refereed through a peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Molecules is an international peer-reviewed Open Access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1800 CHF (Swiss Francs).