Simple Summary
Simple Summary: The genetics of sex determination in mammals has been studied mainly in humans and mice. However, some animals develop in other ways, like moles, whose females have mixed gonads that combine two parts: male (testes) and female (ovaries). In other animals and humans, such gonads are known but they are formed due to pathologies and disturb the ability to reproduce up to sterility. We first characterized the structural features of some genes and hypothesized that changes in the structure of the key gene for testis development Sry may be responsible for the ovotestes formation in European moles. Here, we considered the presence of the intron-containing fragments of the retroposon and presumably the existing copies of the Sry gene along with features of the placenta structure.
Abstract
Here, for the first time, the structure of genes involved in sex determination in mammals (full Sry and partial Rspo1, Eif2s3x, and Eif2s3y) was analyzed for the European mole Talpa europaea with ovotestes in females. We confirmed male-specificity for Eif2s3y and Sry. Five exons were revealed for Rspo1 and the deep similarity with the structure of this gene in T. occidentalis was proved. The most intriguing result was obtained for the Sry gene, which, in placental mammals, initiates male development. We described two exons for this canonically single-exon gene: the first (initial) exon is only 15 bp while the second exon includes 450 bp. The exons are divided by an extended intron of about 1894 bp, including the fragment of the LINE retroposon. Moreover, in chromatogram fragments, which correspond to intron and DNA areas, flanking both exons, we revealed double peaks, similar to heterozygous nucleotide sites of autosomal genes. This may indicate the existence of two or more copies of the Sry gene. Proof of copies requires an additional in-depth study. We hypothesize that unusual structure and possible supernumerary copies of Sry may be involved in ovotestes formation.
1. Introduction
Sex determination is a fundamental biological process. The presence of two sexes, male and female, with specific types of gonads that produce two types of gametes, provides an opportunity for the exchange of genetic information; sexual reproduction maintains genetic links between generations and ensures the genetic integrity of a species. The formation of gonads as the first and essential event of sexual differentiation is triggered by the interaction of antagonistic genes in the early developmental pathways [1]. The precise timing, location, and regulation of gene expression are necessary for this process [2,3].
In the early 1990s, the crucial role of a member of the SOX family, the Sry gene (sex-determining region Y) as a trigger of sex determination in placental mammals, was shown [4,5]. This gene consists of a highly conserved HMG box (high-mobility group DNA-binding domain) and a species-specific and rather variable region outside the HMG box. In mammals, activation of the Sry gene located in the Y chromosome initiates the development of testes by upregulation of autosomal Sox9 [6]. In the absence of Sry, such as in the case seen in XX individuals, bipotential gonads differentiate into ovaries probably by initiation of the Wnt4 pathway, which is under positive feedback with Rspo1 [7,8]. Normal development of the female gonads requires proper regulation of a number of genes such as Sox9, Wnt4, Fgf9, Rspo1, and others. These genes, as far as it is known, are localized in the X chromosome or autosomes, i.e., those chromosomes that are also present in males, so the leading role of such factors as a trigger remains problematic. Still, in mammalian species, including humans, some deviations in the gene structure can lead to pathology. If we analyze disorders of gonad development in females with XX, it turns out that few gene mutations lead to the formation of ovotestes, i.e., gonads consisting of tissues characteristic of both ovaries and testes [9]. In the case of the mutations of Rspo1 (R-Spondin 1) gene, ovotestis development was observed in humans with the XX karyotype [10]. In the case of the XY karyotype in mice (the model B6. XY TIR), the absence of Rspo1 repression can lead to sex reversal [11].
Although the key data on the genetics of sex determination were obtained for humans and laboratory mice, there are other natural or pathological cases of unusual gonad development or sex differentiation gene system functioning. The deviations, existing both as a norm and as a pathology, have received poor attention. Two mutually exclusive cases have been described in different groups of mammals, with one involving the presence of normal sex chromosomes along with the formation of mixed gonads (ovotestes) in females, and the other entailing the absence of Sry along with the formation of normal testes in males and ovaries in females. Several species of the Ellobius genus (with X0/X0 or XX/XX sex chromosomes) completely lost the Y chromosome and Sry [12,13,14]; at the same time, for Ellobius fuscocapillus, the only species with standard sex chromosomes in the genus (XX/XY), the presence of the Sry gene was described in females [15]. Some species of the Tokudaia genus (X0) have partial loss of the Y chromosome and no Sry alongside normal testis formation and function in males [16,17].
Alongside these extraordinary rodents, some other mammals obtain unusual gonad structures. Cases of ovotestes presence were reported in freemartin syndrome for bovines in the 18th century [18] and were first explained by Lillie [19] through the hormonal influence of male fetus to female one in monochorionic twins due to vascular anastomosis in early development. Distinct cases of ovotestes formation were described in phenotypically female individuals with the XX genotype (in Delphinus delphis [20], dogs, and cats [21,22]) and in phenotypically male individuals with the XY genotype and the presence of the Sry gene (in domestic cat [23]). In contrast to the listed cases of pathological gonad development, there are naturally evolved exceptions [24,25]. At least eight mole species of the Talpidae family (XX/XY) possess normal testes in males and ovotestes in females: Talpa occidentalis, T. romana, T. europaea, T. stankovici, Galemys pyrenaicus, Condylura cristata, Neurotrichus gibbsii, and Mogera wogura [26,27,28,29,30]. To date, it is considered that female gametogenesis occurs in the ovarian part of ovotestis (despite the late formation of the follicular structure and postnatal meiosis), while its testicular part includes androgen-producing Leydig cells, typical for male gonads [31,32,33]. In this case, two sexes are still required for sexual reproduction and no background for hermaphroditism occurs.
We hypothesized that a specific structure of some genes might be a reason for the change in the timeline and chain of genetic events in the embryogenesis of the European mole Talpa europaea that led to turning the ovary into ovotestis. We aimed to confirm the genetic structure for two main players—Sry and Rspo1—and compare specificity with species with typical gonads. For the control of sex and species specificity, we intend to analyze two orthologous genes Eif2s3 (eukaryotic translation initiation factor 2, subunit 3, structural gene) from X and Y chromosomes, namely Eif2s3x and Eif2s3y, respectively.
2. Materials and Methods
2.1. Samples
In total, eight European moles were collected in the Ivanovo and Moscow regions of Russia in 2016–2023. Information about the studied animals and the collection localities is presented in Table S1. Animals were treated according to conventional international protocols according to the Guidelines for Humane Endpoints for Animals Used in Biomedical Research. All the experimental protocols were approved by the Ethics Committee for Animal Research of the Koltzov Institute of Developmental Biology RAS in accordance with the Regulations for Laboratory Practice in the Russian Federation, the most recent protocol being № 37-25.06.2020. Every possible care was taken to reduce the animal’s suffering during capturing and sampling. Tissue samples and chromosome suspensions were deposited to the Large-Scale Research Facility “Collection of wildlife tissues for genetic research” IDB RAS, state registration number 3579666.
2.2. Karyotyping and Molecular Genetic Analysis
To check the reliability of sex determination in moles, we additionally conducted the chromosome analysis in two specimens from our sample (T23-16 and T23-17, see Table S1). Chromosomes were prepared from bone marrow according to the Ford and Hamerton method [34].
For all specimens, total DNA was extracted from ground heart samples, which were stored in alcohol. A DNA extraction was carried out after treatment with proteinase K; double phenol-chloroform deproteinization with intermediate incubation with ribonuclease A occurred after the first deproteinization phase; and the final precipitation in isopropanol [35].
For our own primer design, for determination of the exon–intron structure, and for interspecific comparisons of the studied genes, we used whole genome sequences from GenBank as well as predicted transcripts and protein-coding DNA fragments for several species of the Talpidae, Soricidae, and Erinaceidae families; sequences of the house mouse Eif2s3x and Eif2s3y genes were used too (Table S2). Primers for amplification and sequencing of the entire Sry gene (including flanking areas and the internal intron); three non-overlapping fragments of the Rspo1 gene, which contain all exons along with short introns and flanking areas; and one fragment of the Eif2s3x and Eif2s3y genes, are listed in Table 1. A polymerase chain reaction (PCR) was carried out in a mixture containing 35 ng of DNA, 2 μL of 10× Taq buffer, 1.6 μL of 2.5 mM dNTP (Sileks, Moscow, Russia), 4 pM of each primer, 1 unit of Taq polymerase (Syntol, Moscow, Russia), and deionized water to a final volume of 20 μL. Amplification was conducted in a TERTSIK thermal cycler (DNA-Technology, Moscow, Russia). PCR included preheating at 94 °C (3 min) and then 35 cycles as follows: 30 s at 94 °C; 1 min at 60–67 °C, depending on the used primer combination (see the exact annealing temperatures in Table 2); 1 min at 72 °C; finally, an extension of the PCR products was performed at 72 °C (6 min). Sanger sequencing was carried out using the ABI PRISM® BigDyeTM Terminator v. 3.1 Kit (Applied Biosystems, Foster City, CA, USA) in the AB 3500 genetic analyzer (Applied Biosystems, Foster City, CA, USA) at the Core Centrum of Koltzov Institute of Developmental Biology, Russian Academy of Sciences.

Table 1.
Primers designed by us and used in the study.

Table 2.
Primer combinations, which were used in this study.
Sequence alignments were performed in Mega X [36]. After alignment, the lengths of three non-overlapping Rspo1 gene fragments analyzed in all specimens were equal to 251 bp, 485 bp, and ≈1885 bp (an approximate length of the last fragment is due to the difficulty of precisely establishing the number of multiply repeating the same nucleotide in one region, see Alignment S1,a–c); the fragments of the Eif2s3x and Eif2s3y genes were 247 bp and 231 bp, respectively (Alignment S2); and the total Sry gene together with adjacent DNA fragments comprised ≈3584 bp (its approximate length is due to the difficulty of precisely establishing the number of multiply repeating the same nucleotide in two regions, see Alignment S3,a).
The RepeatMasker web tool (https://www.repeatmasker.org/cgi-bin/WEBRepeatMasker, accessed on 9 March 2024) was applied for searching retroposons in obtained sequences. Additional analysis for gene structure was made using the UCSC browser [37]. Sequences of all the genes that we have analyzed have been deposited in GenBank; their accession numbers are presented in Table S1.
3. Results
3.1. Karyotyping Results
In karyotypes of both studied specimens of the European mole, we revealed 34 chromosomes, including sex chromosomes. The female T23-17 had two middle-sized metacentric X while the male T23-16 had one X and one dot-like Y (Table S1). Thus, the karyotype structure in these specimens was very similar to that described previously [38].
3.2. The Rspo1 Gene Analysis
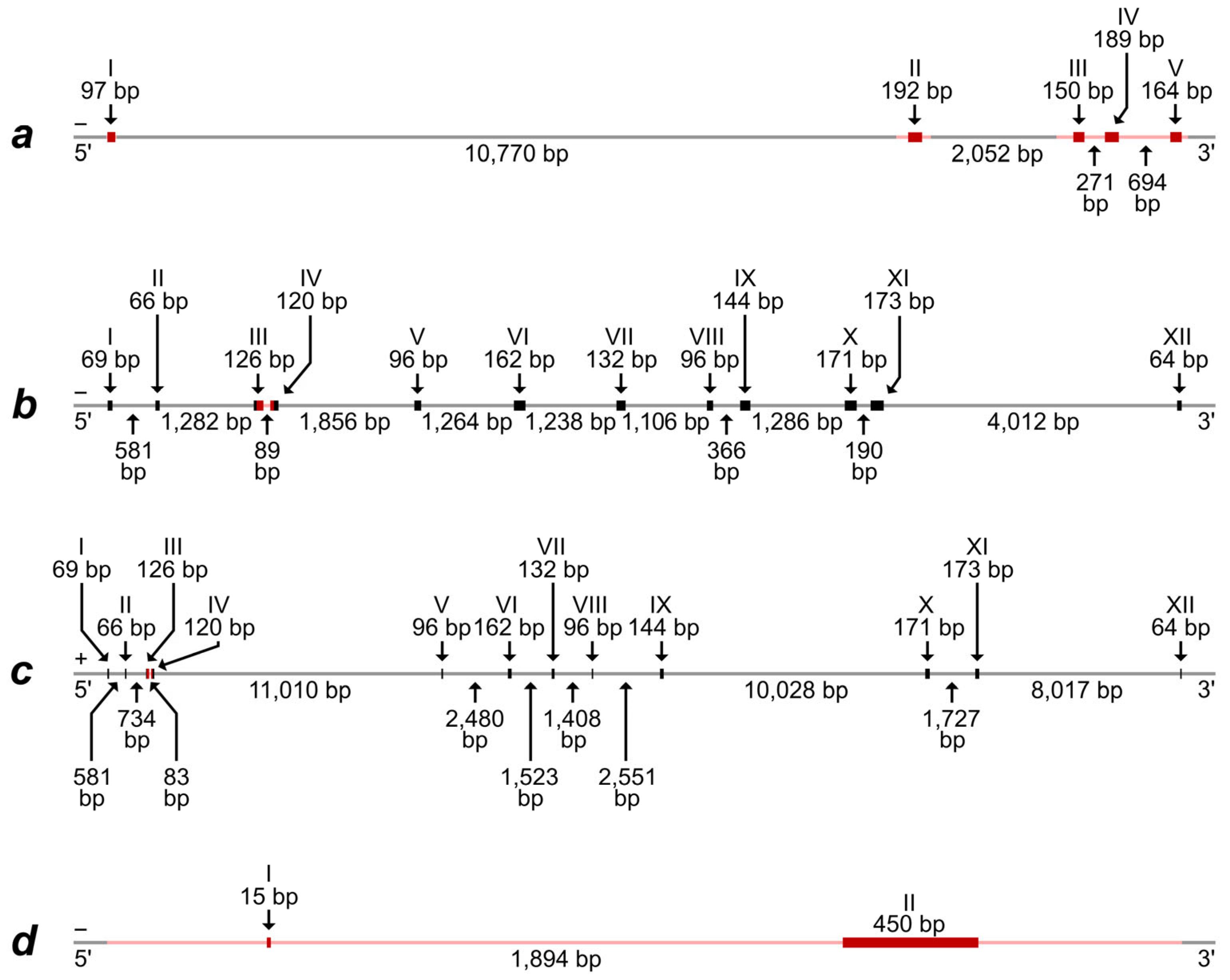
Alignment of the predicted mRNA and protein-coding part of the Iberian mole (T. occidentalis) Rspo1 gene with a corresponding whole genome fragment of the same species demonstrate the presence of five exons, which are divided by introns with lengths from 271 bp to more than 10,700 bp (Figure 1a and Figure S3).
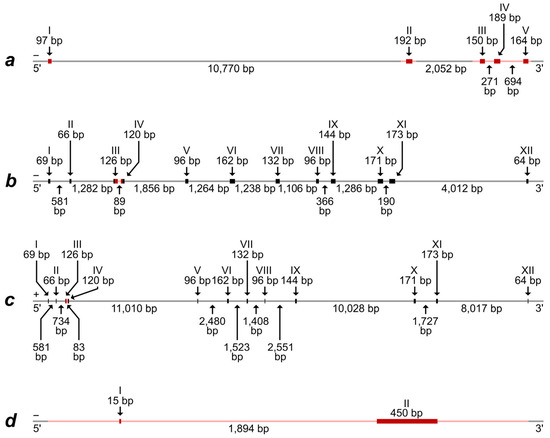
Figure 1.
The schemes of exon–intron structure of (a)—the Rspo1 gene; (b)—the Eif2s3x gene; (c)—the Eif2s3y gene; and (d)—the Sry gene. The schemes are based on whole genome sequences, predicted mRNA, and protein-coding DNA areas of T. occidentalis. The “positive” and “negative” DNA chains are designated by symbols “+” and “−”, respectively. Exon and intron boundaries are determined approximately because of the same short nucleotide motifs and single nucleotides at the 3′-ends of some exons and adjacent introns. Exons are designated by Roman numerals, with thicker lines and richer shades. Exon and intron lengths are represented above and below the scheme, respectively. DNA areas, corresponding to the sequence in T. europaea specimens, are marked by red shades.
A comparison of sequences obtained by us for European moles demonstrated their high similarity, excluding one heterozygotic site in one intron of two specimens (Alignment S1,b). High correspondence of both exons and introns is obvious for the genus Talpa species, T. europaea, and T. occidentalis (Alignments S1,a–c). More significant differences are observed when comparing genera and families, although the presence of five exons and their homology is quite obvious, for the exclusion of Suncus etruscus (Alignment S1,d). Therefore, we note the maintenance of a general structure of the Rspo1 gene in species, which have ovotestis (the species of the Talpa genus and Condylura cristata) and do not have it (the species of Sorex genus); at the same time, significant differences are revealed between species without ovotestis (Suncus etruscus and the Sorex genus). These results indicate that differences in the Rspo1 gene can hardly determine the ovotestis development in moles.
3.3. Analysis of the Eif2s3x and Eif2s3y Genes
The alignment of two predicted different mRNA types, which were preliminarily identified as “EIF2S3” and “EIF2S3, X-linked-like”, with the whole genome fragments of the Iberian mole revealed the most similarity of the mRNA with areas from two different linkage groups. Both alignments demonstrate the presence of 12 exons, which are divided by introns with lengths from 83 bp to more than 11,000 bp (Figure 1b,c). The exons from these two different gene types exhibit a high homology with some nucleotide substitutions, which, along with the house mouse Eif2s3x and Eif2s3y genes, were used by us for the design of primers allowing us to specifically amplify the fragments of these homologous sequences in moles. Amplified fragments comprised parts of the third and fourth exons and the total intron between them. As a result, the DNA fragment, corresponding to the “EIF2S3” mRNA type, was successfully amplified and sequenced in all European moles while the DNA fragment, corresponding to the “EIF2S3, X-linked-like” mRNA type, was successful in males only (Figure S1, Alignment S2). Thus, the latter should really be identified as a fragment of the Eif2s3y gene localized in the Y chromosome in mammals. This assumption is also supported by the fact that the DNA fragment, corresponding to the “EIF2S3, X-linked-like” mRNA type, is revealed in the same whole genome contig (RCFO01000018) where the Sry gene is (see Table S2 and the text below).
Primers designed by us for the Eif2s3y gene fragment amplification may be used for the determination of sex in moles, from which only limited material (skin, fixed tissues, cells, carcasses, etc.) is available or whose gonads cannot be reliably identified.
3.4. The Sry Gene Analysis
Alignment of predicted mRNA and the protein-coding part of the Iberian mole Sry gene with a corresponding whole genome fragment of the same species demonstrated the presence of two exons. The first (initial) exon is very short and consists of only 15 nucleotides while the second exon includes 450 nucleotides. The exons are divided by an extended intron of 1894 bp (Figure 1d).
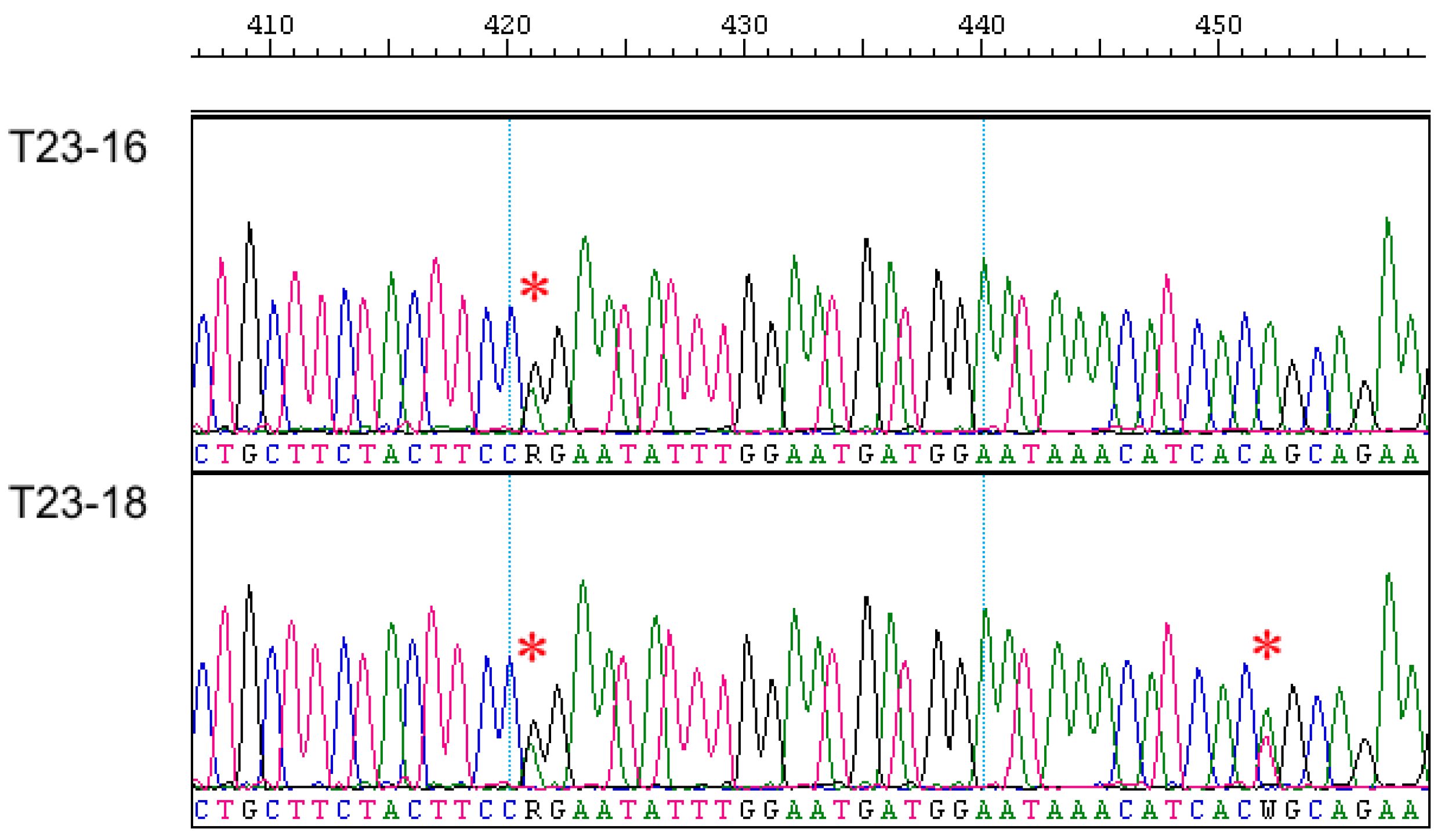
As in the case of the Eif2s3y gene, the Sry gene was successfully amplified and sequenced by us, only in males of the European mole (Figure S2); the analysis of this entire gene in T. europaea was first conducted. In general, the Sry gene appeared to be similar in European and Iberian moles both in exon and intron parts. However, when analyzing chromatogram parts, which correspond to the intron and DNA areas flanking both exons, we unexpectedly revealed double peaks similar to heterozygous sites of autosomal gene sequences (Figure 2). These double peaks are consistently reproduced in chromatograms regardless of the used primers and their combinations. Two of such double peaks were different in two studied T. europaea males despite their origin from the same locality; additionally, one more transition (G/T) was revealed inside the intron in these specimens (Alignment S3,a). There were no such double peaks within exons.
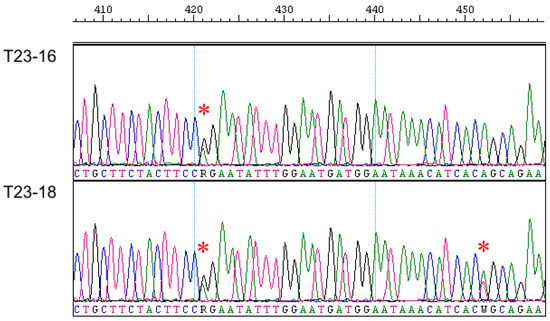
Figure 2.
Fragments of the Sry gene chromatograms of studied European mole males. Sites with double peaks are marked by an asterisk.
A check of the Sry gene sequences of European and Iberian moles by means of the RepeatMasker tool revealed fragments of the LINE-L1 retroposon on both sides of the initial exon (Alignment S3,a). In the Iberian mole model, fragments of LINE-L1 and LTR-ERV1 retroposons were also determined in more distant DNA areas, flanking both exons (Figure S4).
A comparison of previously published sequences [39], which correspond to short fragment (158 bp) of the Sry gene second exon, demonstrated their complete identity in several species of moles (Alignment S3,b). However, it is surprising that this fragment appeared to be the same also in species that belong to Talpidae and Soricidae families; only the North African hedgehog, Erinaceus algirus, has a highly different Sry variant. These unexpected results seem to need additional studies of the Sry gene in representatives of the Soricidae family.
4. Discussion
Through the analysis of the structure of genes presumably involved in gonad formation, we have shown that in Talpa europaea characteristics of Rspo1 and studied fragments of two orthologous genes of X and Y chromosomes, Eif2s3x and Eif2s3y genes, are rather consistent with other Talpidae and furthermore with the majority of other mammals.
The most intriguing result was obtained for the Sry gene. We described the two-exon structure for this canonical single-exon gene (Figure 1d). The exons are divided by an extended intron; fragments of LINE-L1 retroposon are detected in both sides of the initial exon. Moreover, LINE и LTR retroposons were detected in more distant DNA areas, flanking both exons in T. occidentalis. The presence of numerous consistently reproduced double peaks, which were located in chromatograms in the intron part of the gene and in adjacent DNA areas to both exons, is difficult to explain because the Sry gene is linked with the Y chromosome, which occurs in each cell in a single copy. The phenomenon needs additional analysis, which should be based on both studying European moles from a number of localities and applying special molecular genetic approaches (DNA cloning etc.). Now, we can propose a preliminary hypothesis that the unusual chromatogram pattern might be indicative of the existence of several copies of the Sry similar to Arvicanthis nairobae, Lemniscomys barbarus, L. massaicus, L. rosalia, Pelomys fallax, and Rhabdomys pumilio [40]. Since we have only been able to amplify this gene in T. europaea males so far and because sequencing of the most extended PCR product did not show any fragmentation, we tend to propose that these presumable Sry gene copies are complete and located in the Y chromosome. It is not excluded that their origin might be due to found retroposons. Several copies of Sry are usually a set of pseudogenes with a single functioning copy of the gene in some rodents [41,42,43] but six copies of Sry in Rattus norvegicus are not pseudogenes and at least a few of them are expressed [44].
Recent studies demonstrate that in mice, the Sry gene possesses two exons as well [45]. Presumably, the second exon is responsible for stabilizing the protein product and preventing its early degradation. The Sry two-exon structure in moles is completely different due to dissimilar segmentation and the specificity of the second exon in mice. In fact, an additional gene fragment with retrotransposon-derived sequences was described as a second exon in mice. In moles, the part of the gene, which was previously considered as a single exon and corresponds to the canonical exon in mice, was split due to the inclusion of an intron saturated with a degenerate mobile element (Figure S4) as in Microtus cabrerae [46].
Whereas the structure of Sry has not previously been studied in detail, the time of the expression in embryonic development and in adult gonads has been demonstrated [33]; several other genes involved in gonad development and their expression patterns were investigated to identify the cause of ovotestes formation in T. occidentalis. The most interesting finding is that Sox9 and Amh expression is completely absent in ovotestes, both in early development and after birth [30,33,47,48]. This does not correspond to the view of normal development of testes in other mammals since it is believed that Sertoli cells (normally expressing Sox9 and Amh) are the first to start differentiation and their formation is critical for maintenance of male-type differentiation of the gonad. Given that in moles the testicular part in the ovotestes grows during the postnatal period and reaches its maximum volume in adult females rather than in early development, it can be concluded that the male pathway is also impaired. In T. occidentalis, in both male and female gonads, Leydig cells begin differentiation with the cessation of Wnt4 expression, suggesting a possible inhibitory role of Wnt4 in Leydig cell differentiation. In normal ovarian development, Wnt4 is also responsible for inhibiting the development of a testis-like vascular system; however, in T. occidentalis, the vascular system of the gonad develops in a testis-like manner in the presence of Wnt4. These findings are also supported by studies in mice, in which double deletion of Wnt4 and Foxl2 shows masculinization of the sexual system and even the onset of spermatogenesis in the absence of Sertoli cells.
Thus, the causes and mechanisms of the development of ovotestes in moles have not been fully clarified.
Based on our data, we hypothesize that the unusual structure and possible presence of supernumerary complete copies of the Sry gene may be involved in the formation of ovotestes in co-twins similar to freemartins. It is known that changes in Sry expression in the differentiating gonadal ridge cause developmental abnormalities in gonads [49]. We believe that the developmental peculiarity established in the Talpidae lineage may be related to the presence of the Sry gene product abundance (presumably because of several functioning copies of this gene) in placental anastomoses, which are characteristic of the early development of moles. The epitheliochorial type of placenta, which is considered to be the most primitive, is known to be typical for Talpa [50]. Similarly, the same type of placenta also arose in Cetartiodactyla, in which ovotestes are known as pathology, in different species of cetaceans, ungulates, and pigs [51]. The mechanism of disturbing development in the freemartins in cattle has remained unclear until now. Jost et al. [52] tried to prove the hypothesis of the hormonal nature, using androgen injections, but failed. So far, the prevailing view is that the developmental disorder in females is due to the delivery through anastomoses of some masculinizing factors produced by the testes of the male twin [53].
The developmental differences in the aforementioned animal groups are significant. Firstly, the number of embryos varies (3–8 for moles, 1–2 for whales, and ungulates). Secondly, for European and Iberian moles, it was shown that up to about day 20 (this is the day when Sry activity was shown), embryos are in a space actually providing the barrier-free exchange of nutrients and other substances due to the formation of syncytial structures. It has been suggested that intrauterine cell transfer between human twins can lead to chimerism [54]. As one of the hypotheses, we believe that the circulation of cells, extracellular vesicles with mRNA, and proteins between embryos due to placental anastomoses [55,56] may be the plausible reason for the change in the trajectory of gonad formation in co-twins with different sexes during a critical time window when Sry triggers the Sox9 expression. From this point of view, the ovotestes in Talpidae might be considered as a natural phenomenon similar to freemartinism in cattle and other animals.
5. Conclusions
Thus, the specific structure and probable existence of several complete functionally active copies of the Sry gene in T. europaea might be an evolutionary advantage favoring greater expression of the gene that, coupled with a specific placenta structure, can change female gonad development to ovotestes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani14152180/s1. Figure S1: The pattern of 1% agarose gel electrophoresis of PCR products (≈60 ng) obtained by amplification of the Eif2s3y gene fragment in studied European moles; Figure S2: The pattern of 1% agarose gel electrophoresis of PCR products (≈60 ng) obtained by amplification of the Sry gene fragment with primers Sry-TFint and Sry-TRint in studied European moles; Figure S3: UCSC browser [37] screenshot of a 13 Mb window of the Iberian mole genome, highlighting the Rspo1 gene structure in T. europaea; Figure S4: UCSC browser [37] screenshot of a 5 Mb window of the Iberian mole genome, highlighting the Sry gene structure in T. europaea; Table S1: Data on European moles used in this work; Table S2: Additional material from the GenBank; Alignment S1,a. Alignment of nucleotide sequences of the Rspo1 gene (the initial fragment I) of T. occidentalis and T. europaea specimens. The exon area is underlined in the upper sequence; Alignment S1,b. Alignment of nucleotide sequences of the Rspo1 gene (the intermediate fragment II) of T. occidentalis and T. europaea specimens. The exon area is underlined in the upper sequence. The heterozygous sites, which were revealed in two of seven European moles, marked by blue; Alignment S1,c. Alignment of nucleotide sequences of the Rspo1 gene (the final fragment III) of T. occidentalis and T. europaea specimens. The exon areas are underlined in the upper sequence; Alignment S1,d. Alignment of nucleotide sequences corresponding to the Rspo1 gene exons of several species of the Talpidae family (Condylura cristata, Talpa occidentalis, and T. europaea) and the Soricidae family (Suncus etruscus, Sorex araneus, Sorex fumeus, and Sorex palustris). Sequence of the predicted protein-coding part of the Suncus etruscus Rspo1 gene is partially represented; Alignment S2. Alignment of nucleotide sequences of the Eif2s3x and Eif2s3y genes of T. occidentalis and T. europaea specimens. The exon areas are underlined in the upper sequence; Alignment S3,a. Alignment of nucleotide sequences of the Sry gene of T. occidentalis and T. europaea specimens. The exon areas are underlined in the upper sequence. The nucleotide sites, in which double peaks were observed in chromatograms, are marked by blue. The sites, demonstrating differences between the analyzed European mole males, are marked by a red asterisk. The area, showing poor homology to LINE-L1 retroposon, is marked by yellow; Alignment S3,b. Alignment of nucleotide sequences of the Sry gene second exon fragment (158 bp) of several species of the Talpidae family (Talpa occidentalis, T. europaea, and T. romana), the Soricidae family (Neomys anomalus and Crocidura suaveolens), and the Erinaceidae family (Erinaceus algirus).
Author Contributions
Conceptualization, A.B., M.S. and I.B.; methodology, A.B., M.S. and I.B.; software, A.B., M.S. and I.B.; validation, A.B., M.S. and I.B.; formal analysis, A.B., M.S. and I.B.; investigation, A.B., M.S. and I.B.; resources, A.B.; data curation, A.B.; writing—original draft preparation, A.B., M.S. and I.B.; writing, A.B., M.S. and I.B.; visualization, A.B. and I.B.; supervision, I.B.; project administration, I.B.; funding acquisition, A.B. and I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the IDB RAS Government basic research program in 2024 N 0088-2024-0011.
Institutional Review Board Statement
Animals were treated according to conventional international protocols according to the Guidelines for Humane Endpoints for Animals Used in Biomedical Research. The animal study protocol was approved by the Ethics Committee for Animal Research of the Koltzov Institute of Developmental Biology RAS in accordance with the Regulations for Laboratory Practice in the Russian Federation, the most recent protocol being № 37-25.06.2020.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequences of all the genes that we have analyzed have been deposited in GenBank; their accession numbers are presented in Table S1.
Acknowledgments
The research was performed using equipment of the Core Centrum of IDB RAS.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Stévant, I.; Nef, S. Genetic control of gonadal sex determination and development. Trends Genet. 2019, 35, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, N.; Neuwald, J.L.; Literman, R. Transcriptional evolution underlying vertebrate sexual development. Dev. Dyn. 2013, 242, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Chakraborty, T.; Paul-Prasanth, B.; Ohta, K.; Nakamura, M. Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiol. Rev. 2021, 101, 1237–1308. [Google Scholar] [CrossRef]
- Gubbay, J.; Collignon, J.; Koopman, P.; Capel, B.; Economou, A.; Münsterberg, A.; Vivian, N.; Goodfellow, P.; Lovell-Badge, R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 1990, 346, 245–250. [Google Scholar] [CrossRef]
- Koopman, P.; Gubbay, J.; Vivian, N.; Goodfellow, P.; Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry. Nature 1991, 351, 117–121. [Google Scholar] [CrossRef]
- Kashimada, K.; Koopman, P. Sry: The master switch in mammalian sex determination. Development 2010, 137, 3921–3930. [Google Scholar] [CrossRef]
- Wilhelm, D.; Palmer, S.; Koopman, P. Sex determination and gonadal development in mammals. Physiol. Rev. 2007, 87, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, D.; Washburn, L.L.; Truong, V.; Fellous, M.; Eicher, E.M.; Koopman, P. Antagonism of the testis- and ovary-determining pathways during ovotestis development in mice. Mech. Dev. 2009, 126, 324–336. [Google Scholar] [CrossRef]
- Syryn, H.; Van De Vijver, K.; Cools, M. Ovotesticular difference of sex development: Genetic background, histological features, and clinical management. Horm. Res. Paediatr. 2023, 96, 180–189. [Google Scholar] [CrossRef]
- Tomaselli, S.; Megiorni, F.; De Bernardo, C.; Felici, A.; Marrocco, G.; Maggiulli, G.; Grammatico, B.; Remotti, D.; Saccucci, P.; Valentini, F.; et al. Syndromic true hermaphroditism due to an R-spondin1 (RSPO1) homozygous mutation. Hum. Mutat. 2008, 29, 220–226. [Google Scholar] [CrossRef]
- Cao, J.; El Mansouri, F.; Reynoso, S.; Liu, Z.; Zhu, J.; Taketo, T. Inefficient Sox9 upregulation and absence of Rspo1 repression lead to sex reversal in the B6. XY TIR mouse gonad. Biol. Reprod. 2024, 110, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Just, W.; Rau, W.; Vogel, W.; Akhverdian, M.; Fredga, K.; Graves, J.A.; Lyapunova, E. Absence of Sry in species of the vole Ellobius. Nat. Genet. 1995, 11, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Just, W.; Baumstark, A.; Süss, A.; Graphodatsky, A.; Rens, W.; Schäfer, N.; Bakloushinskaya, I.; Hameister, H.; Vogel, W. Ellobius lutescens: Sex determination and sex chromosome. Sex. Dev. 2007, 1, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Mulugeta, E.; Wassenaar, E.; Sleddens-Linkels, E.; van IJcken, W.F.; Heard, E.; Grootegoed, J.A.; Just, W.; Gribnau, J.; Baarends, W.M. Genomes of Ellobius species provide insight into the evolutionary dynamics of mammalian sex chromosomes. Genome Res. 2016, 26, 1202–1210. [Google Scholar] [CrossRef]
- Matveevsky, S.; Kolomiets, O.; Bogdanov, A.; Hakhverdyan, M.; Bakloushinskaya, I. Chromosomal evolution in mole voles Ellobius (Cricetidae, Rodentia): Bizarre sex chromosomes, variable autosomes and meiosis. Genes 2017, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Soullier, S.; Hanni, C.; Catzeflis, F.; Berta, P.; Laudet, V. Male sex determination in the spiny rat Tokudaia osimensis (Rodentia: Muridae) is not Sry dependent. Mamm. Genome 1998, 9, 590–592. [Google Scholar] [CrossRef]
- Kuroiwa, A.; Ishiguchi, Y.; Yamada, F.; Shintaro, A.; Matsuda, Y. The process of a Y-loss event in an XO/XO mammal, the Ryukyu spiny rat. Chromosoma 2010, 119, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J. Account of the free martin. Philos. Trans. R. Soc. Lond. 1779, 69, 279–293. [Google Scholar] [CrossRef]
- Lillie, F.R. The theory of the free-martin. Science 1916, 43, 611–613. [Google Scholar] [CrossRef]
- Murphy, S.; Deaville, R.; Monies, R.J.; Davison, N.; Jepson, P.D. True hermaphroditism: First evidence of an ovotestis in a cetacean species. J. Comp. Pathol. 2011, 144, 195–199. [Google Scholar] [CrossRef]
- Meyers-Wallen, V.N. Gonadal and sex differentiation abnormalities of dogs and cats. Sex. Dev. 2012, 6, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Szczerbal, I.; Nowacka-Woszuk, J.; Nizanski, W.; Dzimira, S.; Ligocka, Z.; Jastrzebska, A.; Kabala, B.; Biernacik, M.; Przadka, P.; Switonski, M. Disorders of sex development are an emerging problem in French bulldogs: A description of six new cases and a review of the literature. Sex. Dev. 2020, 13, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Schlafer, D.H.; Valentine, B.; Fahnestock, G.; Froenicke, L.; Grahn, R.A.; Lyons, L.A.; Meyers-Wallen, V.N. A case of SRY-positive 38, XY true hermaphroditism (XY sex reversal) in a cat. Vet. Pathol. 2011, 48, 817–822. [Google Scholar] [CrossRef]
- Jiménez, R.; Barrionuevo, F.J.; Burgos, M. Natural exceptions to normal gonad development in mammals. Sex. Dev. 2013, 7, 147–162. [Google Scholar] [CrossRef]
- Jiménez, R.; Burgos, M.; Barrionuevo, F.J. The biology and evolution of fierce females (moles and hyenas). Annu. Rev. Anim. Biosci. 2023, 11, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, R.; Burgos, M.; Sánchez, A.; Sinclair, A.H.; Alarcón, F.J.; Marín, J.J.; Ortega, E.; Díaz de la Guardia, R. Fertile females of the mole Talpa occidentalis are phenotypic intersexes with ovotestes. Development 1993, 118, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Bullejos, M.; Burgos, M.; Hera, C.; Stamatopoulos, C.; de la Guardia, R.D.; Jiménez, R. Females of four mole species of genus Talpa (Insectivora, Mammalia) are true hermaphrodites with ovotestes. Mol. Reprod. Dev. Inc. Gamete Res. 1996, 44, 289–294. [Google Scholar] [CrossRef]
- Beolchini, F.; Rebecchi, L.; Bertolani, R.; Capanna, E. The gametogenetic cycle of two syntopic populations of moles: Talpa romana and Talpa europaea (Mammalia, Insectivora, Talpidae). Ital. J. Zool. 2003, 70, 109–113. [Google Scholar] [CrossRef]
- Rubenstein, N.M.; Cunha, G.R.; Wang, Y.Z.; Campbell, K.L.; Conley, A.J.; Catania, K.C.; Glickman, S.E.; Place, N.J. Variation in ovarian morphology in four species of New World moles with a peniform clitoris. Reproduction 2003, 126, 713–719. [Google Scholar] [CrossRef]
- Carmona, F.D.; Motokawa, M.; Tokita, M.; Tsuchiya, K.; Jiménez, R.; Sánchez-Villagra, M.R. The evolution of female mole ovotestes evidences high plasticity of mammalian gonad development. J. Exp. Zool. Part B Mol. Dev. Evol. 2008, 310, 259–266. [Google Scholar] [CrossRef]
- Barrionuevo, F.J.; Zurita, F.; Burgos, M.; Jiménez, R. Testis-like development of gonads in female moles. New insights on mammalian gonad organogenesis. Dev. Biol. 2004, 268, 39–52. [Google Scholar] [CrossRef]
- Carmona, F.D.; Lupiáñez, D.G.; Martín, J.E.; Burgos, M.; Jiménez, R.; Zurita, F. The spatio-temporal pattern of testis organogenesis in mammals-insights from the mole. Int. J. Dev. Biol. 2009, 53, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Real, F.M.; Haas, S.A.; Franchini, P.; Xiong, P.; Simakov, O.; Kuhl, H.; Schöpflin, R.; Heller, D.; Moeinzadeh, M.-H.; Heinrich, V.; et al. The mole genome reveals regulatory rearrangements associated with adaptive intersexuality. Science 2020, 370, 208–214. [Google Scholar] [CrossRef]
- Ford, C.E.; Hamerton, J.L. A colchicine, hypotonic citrate, squash sequence for mammalian chromosomes. Stain Technol. 1956, 31, 247–251. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Lab. Press: New York, NY, USA, 1989. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Haeussler, M.; Zweig, A.S.; Tyner, C.; Speir, M.L.; Rosenbloom, K.R.; Raney, B.J.; Lee, C.M.; Lee, B.T.; Hinrichs, A.S.; Gonzalez, J.N. The UCSC Genome Browser database: 2019 update. Nucleic Acids Res. 2019, 47, D853–D858. [Google Scholar] [CrossRef]
- Gornung, E.; Volleth, M.; Capanna, E.; Castiglia, R. Comparative cytogenetics of moles (Eulipotyphla, Talpidae): Chromosomal differences in Talpa romana and T. europaea. Cytogenet. Genome Res. 2008, 121, 249–254. [Google Scholar] [CrossRef]
- Sánchez, A.; Bullejos, M.; Burgos, M.; Hera, C.; Jiménez, R.; Diaz de la Guardia, R. High sequence identity between the SRY HMG box from humans and insectivores. Mamm. Genome 1996, 7, 536–538. [Google Scholar] [CrossRef]
- Lundrigan, B.L.; Tucker, P.K. Evidence for multiple functional copies of the male sex-determining locus, Sry, in African murine rodents. J. Mol. Evol. 1997, 45, 60–65. [Google Scholar] [CrossRef]
- Nagamine, C.M. The testis-determining gene, SRY, exists in multiple copies in Old World rodents. Genet. Res. 1994, 64, 151–159. [Google Scholar] [CrossRef]
- Murata, C.; Yamada, F.; Kawauchi, N.; Matsuda, Y.; Kuroiwa, A. Multiple copies of SRY on the large Y chromosome of the Okinawa spiny rat, Tokudaia muenninki. Chromosome Res. 2010, 18, 623–634. [Google Scholar] [CrossRef]
- Bullejos, M.; Sánchez, A.; Burgos, M.; Hera, C.; Jiménez, R.; Díaz de La Guardia, R. Multiple, polymorphic copies of SRY in both males and females of the vole Microtus cabrerae. Cytogenet. Genome Res. 1997, 79, 167–171. [Google Scholar] [CrossRef]
- Turner, M.E.; Martin, C.; Martins, A.S.; Dunmire, J.; Farkas, J.; Ely, D.L.; Milsted, A. Genomic and expression analysis of multiple Sry loci from a single Rattus norvegicus Y chromosome. BMC Genet. 2007, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, S.; Kuroki, S.; Maeda, R.; Okashita, N.; Koopman, P.; Tachibana, M. The mouse Sry locus harbors a cryptic exon that is essential for male sex determination. Science 2020, 370, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Marchal, J.A.; Acosta, M.J.; Bullejos, M.; de la Guardia, R.D.; Sánchez, A. Origin and spread of the SRY gene on the X and Y chromosomes of the rodent Microtus cabrerae: Role of L1 elements. Genomics 2008, 91, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.D.; Lupiáñez, D.G.; Real, F.M.; Burgos, M.; Zurita, F.; Jiménez, R. SOX9 is not required for the cellular events of testicular organogenesis in XX mole ovotestes. J. Exp. Zool. Part B Mol. Dev. Evol. 2009, 312, 734–748. [Google Scholar] [CrossRef]
- Zurita, F.; Barrionuevo, F.J.; Berta, P.; Ortega, E.; Burgos, M.; Jimenez, R. Abnormal sex-duct development in female moles: The role of anti-Mullerian hormone and testosterone. Int. J. Dev. Biol. 2003, 47, 451–458. [Google Scholar] [PubMed]
- Chen, Y.S.; Racca, J.D.; Phillips, N.B.; Weiss, M.A. Inherited human sex reversal due to impaired nucleocytoplasmic trafficking of SRY defines a male transcriptional threshold. Proc. Natl. Acad. Sci. USA 2013, 110, E3567–E3576. [Google Scholar] [CrossRef] [PubMed]
- Ferner, K.; Siniza, S.; Zeller, U. The placentation of Eulipotyphla—Reconstructing a morphotype of the mammalian placenta. J. Morphol. 2014, 275, 1122–1144. [Google Scholar] [CrossRef]
- Szczerbal, I.; Nowacka-Woszuk, J.; Dzimira, S.; Matuszczyk, A.; Iskrzak, P.; Switonski, M. Elevated incidence of freemartinism in pigs detected by droplet digital PCR and cytogenetic techniques. Livest. Sci. 2019, 219, 52–56. [Google Scholar] [CrossRef]
- Jost, A.; Vigier, B.; Prepin, J. Freemartins in cattle: The first steps of sexual organogenesis. Reproduction 1972, 29, 349–379. [Google Scholar] [CrossRef] [PubMed]
- Lindtke, D.; Seefried, F.R.; Drögemüller, C.; Neuditschko, M. Increased heterozygosity in low-pass sequencing data allows identification of blood chimeras in cattle. Anim. Genet. 2023, 54, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.E.; Konig, T.E.; Verhoeven, M.O.; Schats, R.; Mijatovic, V.; Ket, J.C.; Lambalk, C.B. Unusual twinning resulting in chimerism: A systematic review on monochorionic dizygotic twins. Twin Res. Hum. Genet. 2017, 20, 161–168. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Sánchez-Madrid, F. Intercellular communication: Diverse structures for exchange of genetic information. Nat. Rev. Mol. Cell Biol. 2012, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Morales-Prieto, D.M.; Favaro, R.R.; Markert, U.R. Placental miRNAs in feto-maternal communication mediated by extracellular vesicles. Placenta 2020, 102, 27–33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).