Effect of Alligator Weed (Alternanthera philoxeroides) Supplementation on Production Performance, Immune Response and Antioxidant Function of Improved Rural Chicken
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Ethical Approval
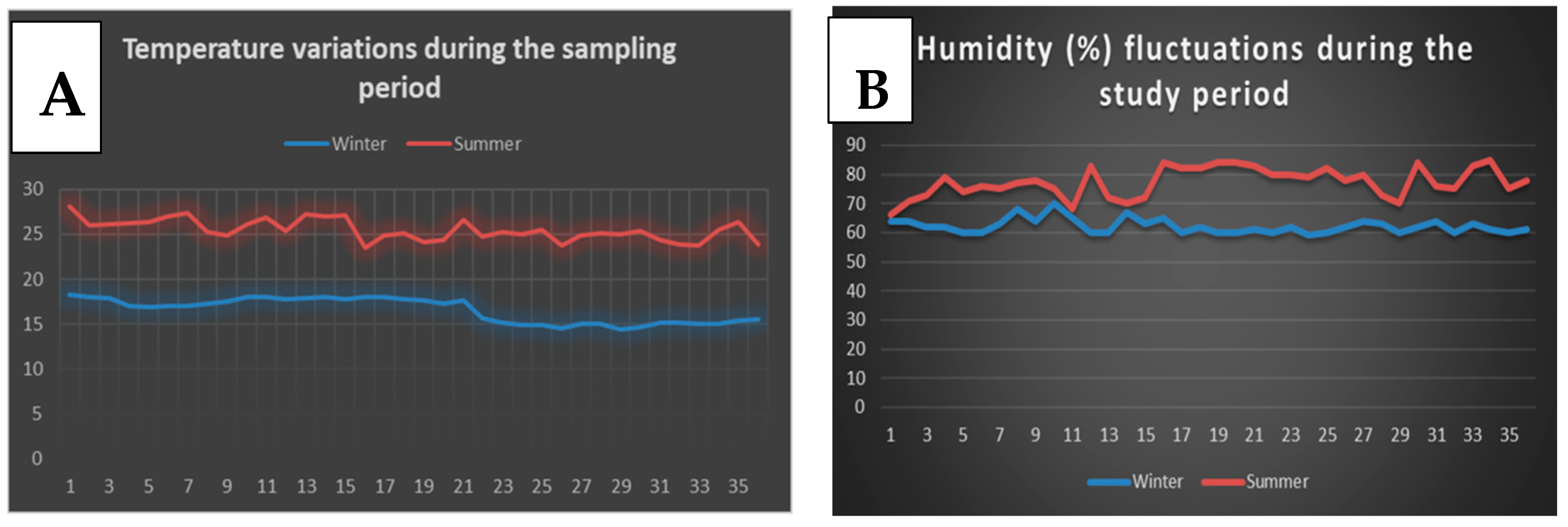
2.3. Experimental Location and Climate Condition
2.4. Processing of Alligator Weed and Proximate Analysis
2.5. Experimental Birds and Study Design
2.6. Phenolic and Flavonoid Estimation
2.7. Production Performances
2.8. Blood Collection and Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
2.9. Estimation of Nitric Oxide (NO) Production—Measure of Oxidative Defence
2.10. Quantitative Reverse Transcriptase–Polymerase Chain Reaction (qRT-PCR)
2.11. Estimation of Antioxidant Status
2.11.1. Superoxide Dismutase (SOD)
2.11.2. Catalase (CAT)
2.12. Liver Function Tests
2.13. Statistical Analysis
3. Results
3.1. Proximate Component Analysis of AW and the Experimental Ration
3.2. Phenolic and Flavonoid Concentration
3.3. Production Performances
3.4. Effect of AW Extract on NO Production and iNOS Gene Expression
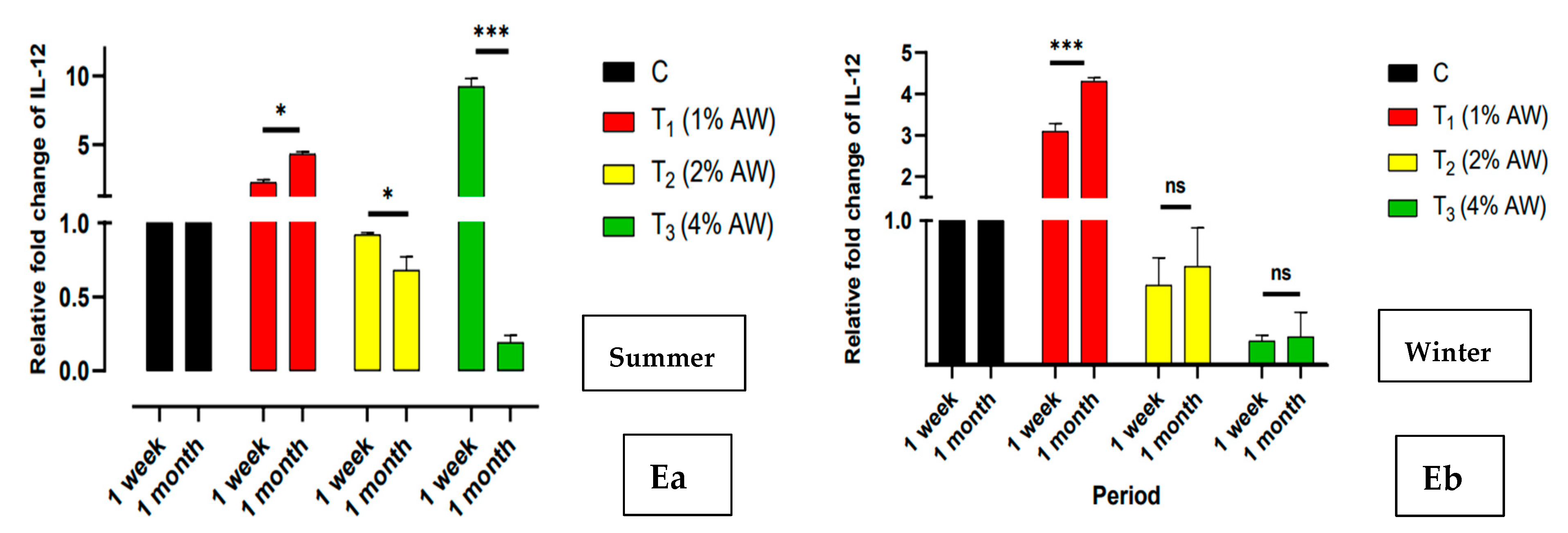
3.5. Relative Expression Profile of Innate Immune Marker Genes
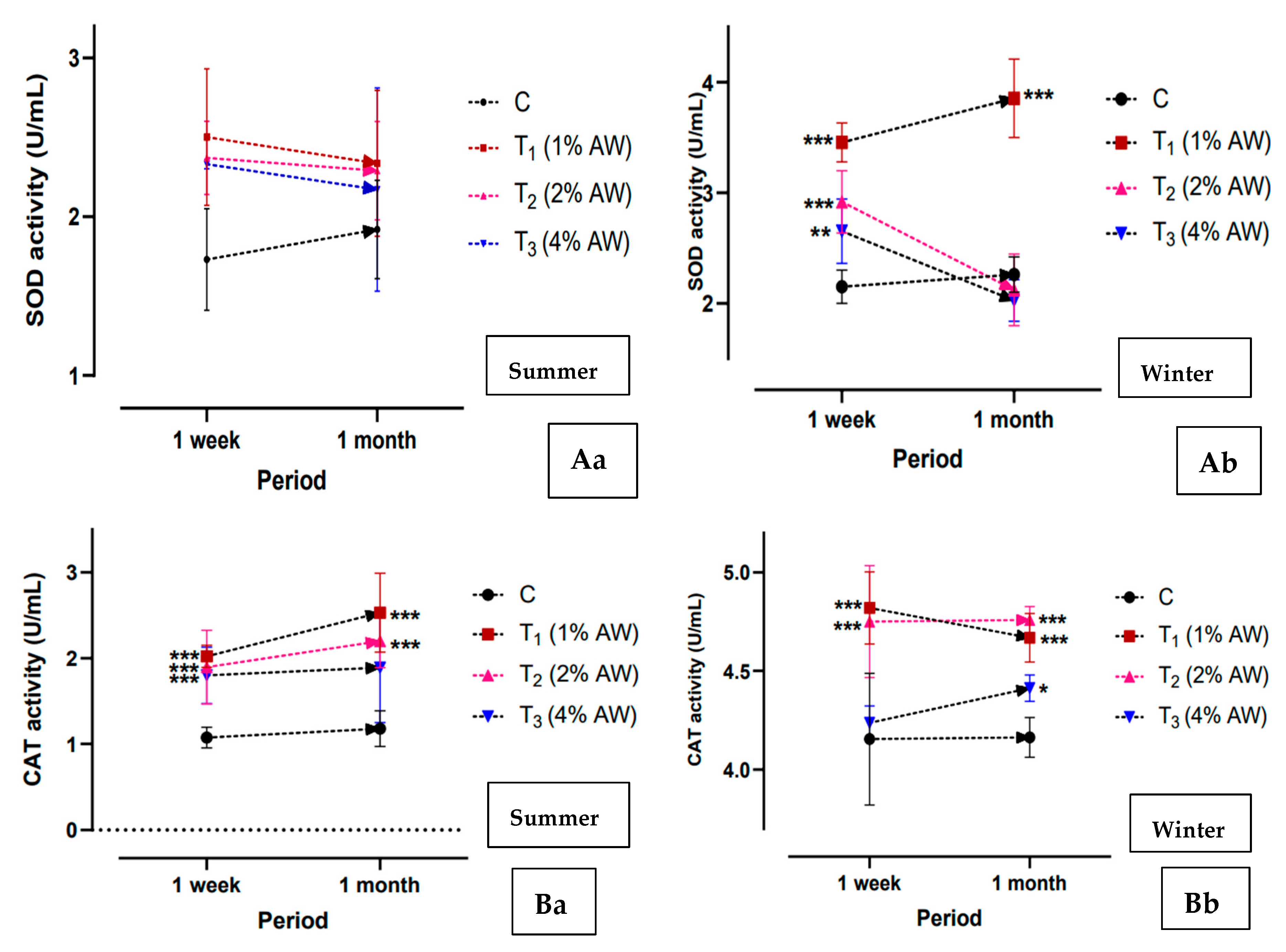
3.6. Antioxidant Activity
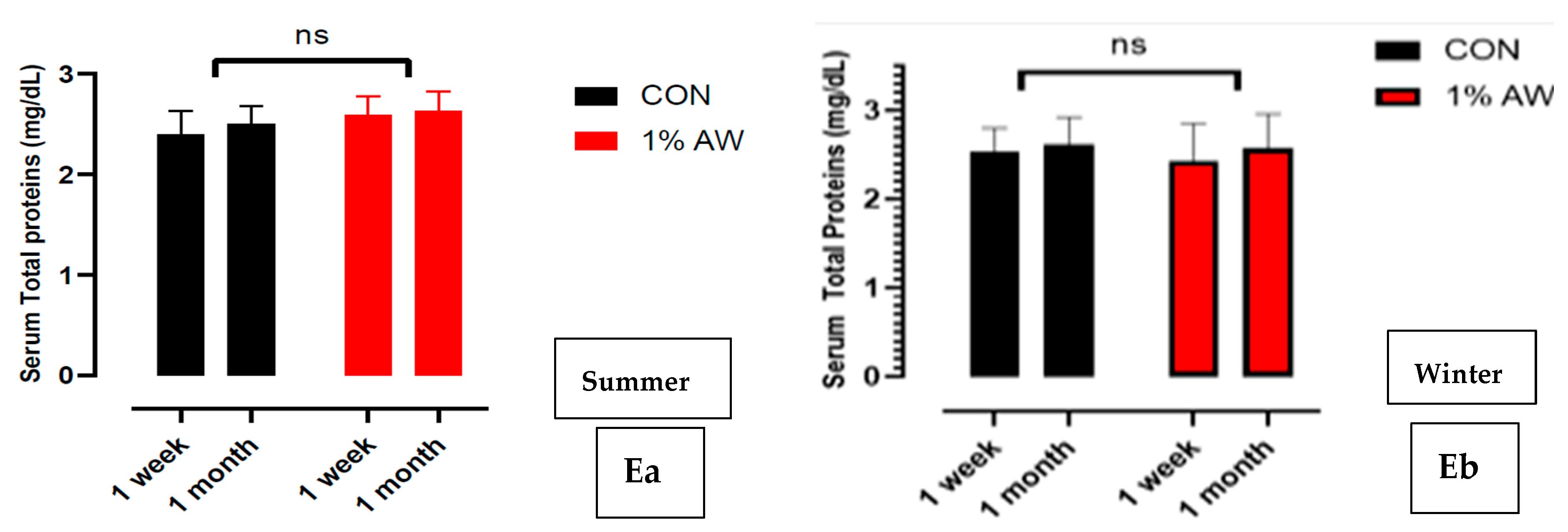
3.7. Liver Function Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehmood, A.; Tanveer, A.; Javed, M.M.; Nadeem, M.A.; Naeem, M.; Abbas, T. Estimation of economic threshold level of alligator weed (Alternanthera philoxeroides (Mart.) Griseb.) to tackle grain quality and yield losses in rice. Arch. Agron. Soil Sci. 2018, 64, 208–218. [Google Scholar] [CrossRef]
- Sushil Kumar, S.S.; Vishwakarma, K. Occurrence of alien alligator weed in India with special reference to its infestation in some districts of Madhya Pradesh. Indian J. Weed Sci. 2009, 41, 185–187. [Google Scholar]
- Maheshwari, J.K. Alligator weed in Indian lakes. Nature 1965, 206, 1270. [Google Scholar] [CrossRef]
- Kumar, S.; Vishwakarma, K. Nutritive value of alligator weed [Alternanthera philoxeroides (Mart.) Griseb.] and its possible utility as a fodder in India. Indian J. Weed Sci. 2005, 37, 152. [Google Scholar]
- Sarkar, S.; Sarkar, U.K.; Ali, S.; Kumari, S.; Puthiyotti, M. Status, ecological services and management of aquatic weeds of floodplain wetlands in India: An overview. Lakes Reserv.: Res. Manag. 2021, 26, 76–91. [Google Scholar] [CrossRef]
- Farooq, N.; Mehmood, A.; Tanveer, A.; Nadeem, M.A.; Sarwar, G.; Abbas, T.; Javaid, M.M.; Matloob, A. Exploiting fodder and nutritive value of alternanthera philoxeroides (alligator weed) at different times of harvest: A potential fodder weed. Pak. J. Bot. 2021, 53, 1101–1106. [Google Scholar] [CrossRef]
- Islam, M.R.; Rahman, S.M.; Rahman, M.M.; Oh, D.H.; Ra, C.S. The effects of biogas slurry on the production and quality of maize fodder. Turk J Agric For. 2010, 34, 91–99. [Google Scholar] [CrossRef]
- Samuil, C.; Vintu, V.; Sirbu, C.; Surmei, G.M. Behaviour of fodder mixtures with alfalfa in North Eastern Romania. Rom. Agric. Res. 2012, 29, 227–235. [Google Scholar]
- Saini, N.; Anmol, A.; Kumar, S.; Bakshi, M.; Dhiman, Z. Exploring phenolic compounds as natural stress alleviators in plants-a comprehensive review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Diaz, P.; Jeong, S.C.; Lee, S.; Khoo, C.; Koyyalamudi, S.R. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin. Med. 2012, 7, 26. [Google Scholar] [CrossRef]
- Serdiati, N.; Islamy, R.A.; Mamat, N.B.; Hasan, V.; Valen, F.S. Nutritional Value of Alligator Weed (Alternanthera philoxeroides) and Its Application for Herbivorous Aquaculture Feed. Int. J. Agric. Biosci. 2024, 13, 318–324. [Google Scholar] [CrossRef]
- Dutta, P. Pharmacognostical evaluation and preliminary phytochemical analysis of Alternanthera philoxeroides. Int. J. MediPharm. Res. 2015, 1, 7–13. [Google Scholar]
- Suthari, S.; Kiran, B.R.; Prasad, M.N.V. Health risks of leafy vegetable Alternanthera philoxeroides (Alligator weed) rich in phytochemicals and minerals. EuroBiotech J. 2017, 1, 293–302. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Mahajan, G.; Chauhan, B.S. Nonconventional weed management strategies for modern agriculture. Weed Sci. 2015, 63, 723–747. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; AOAC International: Arlington, VA, USA, 2006. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- John, B.; Sulaiman, C.T.; George, S.; Reddy, V.R.K. Total Phenolics and Flavonoids in Selected Medicinal Plants from Kerala. Int. J. Pharm. Pharm. Sci. 2014, 6, 406–408. [Google Scholar]
- Yamin, R.; Sartinah, A.; Ihsan, S.; Kasmawati, H.; Suryani, A.; Andriyani, R.; Asma, G.; Adjeng, A.; Arba, M. Radical scavenging assay and determination Flavonoid and Phenolic total of extract and Fractions of Raghu bark (Dracontomelon dao (Blanco) Merr). Res. J. Pharm. Technol. 2020, 13, 2335–2339. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Gautam, A.; Donohue, D.; Hoke, A.; Miller, S.A.; Srinivasan, S.; Sowe, B.; Detwiler, L.; Lynch, J.; Levangie, M.; Hammamieh, R.; et al. Investigating gene expression profiles of whole blood and peripheral blood mononuclear cells using multiple collection and processing methods. PLoS ONE 2019, 14, e0225137. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, M.M.N.; Butt, M.S.; Anjum, F.M.; Nawaz, H. Composition analysis of some selected legumes for protein isolates recovery. J. Anim. Plant Sci. 2012, 22, 1156–1162. [Google Scholar]
- Baneerjee, A.; Matai, S. Composition of Indian Aquatic Plants in Relation to Utilization as Animal Forage. J. Aquat. Plant Manag. 1990, 2, 7–10. [Google Scholar]
- Tanvir, E.M.; Afroz, R.; Karim, N. Antioxidant and antibacterial activities of methanolic extract of BAU kul (Ziziphus mauritiana), an improved variety of fruit from Bangladesh. J. Food Biochem. 2015, 39, 139–147. [Google Scholar] [CrossRef]
- Abhishek, M.; Somashekaraiah, B.; Dharmesh, S. In vivo antidiabetic and antioxidant potential of Psychotria dalzellii in streptozotocin-induced diabetic rats. S. Afr. J. Bot. 2019, 121, 494–499. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Aangamani, A.; Karthishwaran, K.; Cheruth, A. Essential oil from the seeds of Moringa peregrina: Chemical composition and antioxidant potential. S. Afr. J. Bot. 2020, 129, 100–105. [Google Scholar] [CrossRef]
- Goncalves, S.; Moreira, E.; Andrade, P.B.; Valentao, P.; Romano, A. Effect of in vitro gastrointestinal digestion on the total phenolic contents and antioxidant activity of wild Mediterranean edible plant extracts. Eur. Food Res. Technol. 2019, 245, 753–762. [Google Scholar] [CrossRef]
- Materska, M.; Olszowka, K.; Chilczuk, B. Polyphenolic profiles in lettuce (Lactuca sativa L.) after CaCl2 treatment and cold storage. Eur. Food Res. Technol. 2019, 245, 733–744. [Google Scholar] [CrossRef]
- Tukun, A.B.; Shaheen, N.; Banu, C.P.; Mohiduzzaman, M.; Islam, S.; Begum, M. Antioxidant capacity and total phenolic contents in hydrophilic extracts of selected Bangladeshi medicinal plants. Asian Pac. J. Trop. Med. 2014, 7, S568–S573. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, E.M.; Hossen, M.; Hossain, M.; Afroz, R.; Gan, S.H.; Khalil, M.; Karim, N. Antioxidant properties of popular turmeric (Curcuma longa) varieties from Bangladesh. J. Food Qual. 2017, 2017, 8471785. [Google Scholar] [CrossRef]
- Siatka, T.; Kasparova, M. Seasonal variation in total phenolic and flavonoid contents and DPPH scavenging activity of Bellis perennis L. flowers. Molecules 2010, 15, 9450–9461. [Google Scholar] [CrossRef]
- Khandker, S.S.; Alam, M.; Uddin, F.; Shapla, U.M.; Lubna, N.; Mazumder, T.A.; Marzan, M.; Mondal, M.; Khalil, M.I.; Karim, N.; et al. Subchronic Toxicity Study of Alternanthera philoxeroides in Swiss Albino Mice Having Antioxidant and Anticoagulant Activities. J. Toxicol. 2022, 2022, 8152820. [Google Scholar] [CrossRef]
- Pulipati, S.; Babu, P.S. In-vitro antibacterial potential of alternanthera philoxeroides (mart) griseb against multi-drug resistant uropathogens. Int. J. Pharm. Sci. Res. 2020, 11, 3834–3840. [Google Scholar] [CrossRef]
- Kumar, V.; Roy, B.K. Population authentication of the traditional medicinal plant Cassia tora L. based on ISSR markers and FTIR analysis. Sci. Rep. 2018, 8, 10714. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, J.; Wang, S.; Zhang, X.; Hou, J.; Xu, F. Effects of soybean isoflavone and astragalus polysaccharide mixture on colostrum components, serum antioxidant, immune and hormone levels of lactating sows. Animals 2021, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.S.; Cordon, B.; Ma, B.; Xie, J. Nitric oxide: Physiological functions, delivery, and biomedical applications. Adv. Sci. 2023, 10, 2303259. [Google Scholar] [CrossRef]
- Madigan, M.; Zuckerbraun, B. Therapeutic potential of the nitrite-generated no pathway in vascular dysfunction. Front. Immunol. 2013, 4, 174. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A. Pharmacokinetic and pharmacodynamic properties of oral l-citrulline and l-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Harris, M.B.; Blackstone, M.A.; Ju, H.; Venema, V.J.; Venema, R.C. Heat-induced increases in endothelial no synthase expression and activity and endothelial no release. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H333–H440. [Google Scholar] [CrossRef]
- Alisi, C.S.; Asiwe, E.S.; Ene, C.A.; Alisi, P.N. Antioxidant and free radical scavenging properties of aqueous extract of Psidium guajava leaf. FUTO J. Ser. 2018, 4, 222–234. [Google Scholar]
- Teshfam, M.; Brujeni, G.N.; Hassanpour, H. Evaluation of endothelial and inducible nitric oxide synthase mRNA expression in the lung of broiler chickens with developmental pulmonary hypertension due to cold stress. Br. Poult. Sci. 2006, 47, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Lv, Z.H.; Li, J.L.; Li, S.; Xu, S.W.; Wang, X.L. Effects of cold stress on nitric oxide in duodenum of chicks. Poult. Sci. 2011, 90, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2014, 26, 211–219. [Google Scholar]
- Brenner, I.K.M.; Castellani, J.W.; Gabaree, C.; Young, A.J.; Zamecnik, J.; Shephard, R.J.; Shek, P.N. Immune changes in humans during cold exposure: Effects of prior heating and exercise. J. Appl. Physiol. 1999, 87, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.R.; Li, S.Z.; Fang, H.G.; Zhang, X.; Wang, J.F.; Guo, S.; Ji, H.; Zang, L.; Guo, L.; Zhen, L.H. Different duration of cold stress enhances pro-inflammatory cytokines profile and alterations of Th1 and Th2 type cytokines secretion in serum of wistar rats. J. Anim. Vet. Adv. 2012, 11, 1538–1545. [Google Scholar] [CrossRef]
- Rajendran, P.; Chen, Y.F.; Chen, Y.F.; Chung, L.C.; Tamilselvi, S.; Shen, C.Y.; Day, C.H.; Chen, R.J.; Viswanadha, V.P.; Kuo, W.W. The multifaceted link between inflammation and human diseases. J. Cell Physiol. 2018, 233, 6458–6471. [Google Scholar] [CrossRef]
- Bagchi, A.K.; Akolkar, G.; Mandal, S.; Ayyappan, P.; Yang, X.; Singal, P.K. Toll-like receptor 2 dominance over Toll-like receptor 4 in stressful conditions for its detrimental role in the heart. Am. J. Physiol. Heart C. 2017, 312, H1238–H1247. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, J.; Jiang, Y.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Effects of Artemisia argyi flavonoids on growth performance and immune function in broilers challenged with lipopolysaccharide. Anim. Biosci. 2021, 34, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Mahfuz, S.; Piao, X.S. Application of Moringa (Moringa oleifera) as natural feed supplement in poultry diets. Animals 2019, 9, 431. [Google Scholar] [CrossRef]
- Rifat, A.I.; Bormon, C.C.; Akib, M.G.; Ataher, M.S.; Kamruzzaman, M.; Dutta, A.; Mahfuz, S. Dietary inclusion of Moringa oleifera leaf extracts as alternatives to antibiotic growth promoter on live performance, carcase traits, physical meat quality, and health status of broiler chickens. Italian J. Anim. Sci. 2024, 23, 1752–1763. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Alruwaili, N.W.; Al-Baadani, H.H.; Al-Garadi, M.A.; Shamlan, G.; Alhidary, I.A. Investigating the effect of Pulicaria jaubertii as a natural feed additive on the growth performance, blood biochemistry, immunological response, and cecal microbiota of broiler chickens. Animals 2023, 13, 1116. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Jha, R. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019, 6, 60. [Google Scholar] [CrossRef]
- Ognik, K.; Czech, A.; Stachyra, K. Effect of a natural versus a synthetic antioxidant, and sex and age on the redox profile in the blood of growing turkeys. S. Afr. J. Anim. Sci. 2013, 43, 473–481. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Lee, T.T. Potential crosstalk of oxidative stress and immune response in poultry through phytochemicals-A review. Asian Australas J. Anim. Sci. 2019, 32, 309. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperthermia 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Xing, T.; Pan, X.; Zhang, L.; Gao, F. Hepatic oxidative stress, apoptosis, and inflammation in broiler chickens with wooden breast myopathy. Front. Physiol. 2021, 12, 659777. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, B.; Liu, Y.; Wang, Y.; Wang, Z.; Song, Y.; Xue, C. Antioxidants ameliorate oxidative stress in alcoholic liver injury by modulating lipid metabolism and phospholipid homeostasis. Lipids 2023, 58, 229–240. [Google Scholar] [CrossRef] [PubMed]




| Sl No. | Ingredients | Parts |
|---|---|---|
| 1. | Maize | 56 |
| 2. | Rice polish (RP) | 10 |
| 3. | Soya | 16 |
| 4. | Ground nut cake (GNC) | 15 |
| 5. | Mineral mixture (Agrimin ¥: 0.5 kg; Gromin-P €: 2 kg) | 2.5 |
| 6. | Salt | 0.5 |
| Total | 100 |
| Sl. No. | Gene | Sequence | Accession No. |
| 1 | IL-1β | F: GGGACTTTGCTGACAGCGACCTG R: GTCGAAGGACTGTGAGCGGGTGT | AJ245728 |
| 2 | Β-actin | F: GCACCACACTTTCTACAATAG R: ACGACCAGAGGCATACAGG | L08165 |
| 3 | IL-6 | F: GCTCGCCGGCTTCGA R: GGTAGGTCTGAAAGGCGAACAG | AJ250838 |
| 4 | IFN-γ | F: GCCGCACATCAAACACATATCT R: TGAGACTGGCTCCTTTTCCTT | NM_205149 |
| 5 | iNOS | F: AGGCCAAACATCCTGGAGGTC R: TCATAGAGACGCTGCTGCCAG | U46504 |
| 6 | IL-12 | F: TGGTCCACGCTTTGCAGAT R: AAGGTTAAGGCGTGGCTTCTTA | AJ564201 |
| TA | DM | OM | CP | EE | CF | NFE |
|---|---|---|---|---|---|---|
| 18.48 ± 0.40 | 85.47 ± 0.40 | 81.53 ± 0.26 | 14.16 ± 1.42 | 4.83 ± 0.51 | 13.92 ± 1.34 | 48.74 ± 2.55 |
| Particulars (% DM Basis) | C (Control) | T1 (1% AW) | T2 (2% AW) | T3 (4% AW) |
|---|---|---|---|---|
| Moisture | 6.37 a ± 0.13 | 10.58 b ± 0.64 | 10.09 b ± 0.50 | 10.13 b ± 0.10 |
| DM | 93.62 b ± 0.13 | 89.67 a ± 0.89 | 89.90 a ± 0.50 | 89.85 a ± 0.07 |
| TA | 9.20 a ± 0.80 | 9.30 a ± 0.20 | 8.63 a ± 0.58 | 9.37 b ± 0.95 |
| OM | 90.80 a ± 0.80 | 90.70 a ± 0.20 | 91.37 a ± 0.58 | 89.45 a ± 0.95 |
| CP | 20.20 a ± 1.30 | 20.91 a ± 1.08 | 20.35 a ± 1.11 | 19.69 a ± 1.21 |
| Crude fat | 4.55 a ± 0.55 | 4.80 a ± 0.75 | 4.75 a ± 0.15 | 4.63 a ± 0.40 |
| CF | 4.50 ab ± 0.40 | 4.00 a ± 0.30 | 5.50 bc ± 0.15 | 6.50 c ± 0.15 |
| NFE | 61.55 a ± 2.96 | 60.99 a ± 0.79 | 60.77 a ± 2.30 | 59.81 a ± 1.66 |
| Phenolic content: | 2.23 ± 0.32 mg GAE/g fresh weed |
| Flavonoid content: | 1.44 ± 0.19 mg QE/g fresh weed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puro, K.-u.; Abedin, S.N.; Hussain, Z.; Wankhar, J.B.M.; Doley, S.; Aochen, C.; Choudhury, B.U.; Singh, M.; Katiyar, R.; Deori, S. Effect of Alligator Weed (Alternanthera philoxeroides) Supplementation on Production Performance, Immune Response and Antioxidant Function of Improved Rural Chicken. Animals 2025, 15, 742. https://doi.org/10.3390/ani15050742
Puro K-u, Abedin SN, Hussain Z, Wankhar JBM, Doley S, Aochen C, Choudhury BU, Singh M, Katiyar R, Deori S. Effect of Alligator Weed (Alternanthera philoxeroides) Supplementation on Production Performance, Immune Response and Antioxidant Function of Improved Rural Chicken. Animals. 2025; 15(5):742. https://doi.org/10.3390/ani15050742
Chicago/Turabian StylePuro, Kekungu-u, Sayed Nabil Abedin, Zakir Hussain, Jaredth B. M. Wankhar, Sunil Doley, Chubasenla Aochen, Burhan Uddin Choudhury, Mahak Singh, Rahul Katiyar, and Sourabh Deori. 2025. "Effect of Alligator Weed (Alternanthera philoxeroides) Supplementation on Production Performance, Immune Response and Antioxidant Function of Improved Rural Chicken" Animals 15, no. 5: 742. https://doi.org/10.3390/ani15050742
APA StylePuro, K.-u., Abedin, S. N., Hussain, Z., Wankhar, J. B. M., Doley, S., Aochen, C., Choudhury, B. U., Singh, M., Katiyar, R., & Deori, S. (2025). Effect of Alligator Weed (Alternanthera philoxeroides) Supplementation on Production Performance, Immune Response and Antioxidant Function of Improved Rural Chicken. Animals, 15(5), 742. https://doi.org/10.3390/ani15050742








