A Comprehensive Review of the Antitumor Activity of Olive Compounds: The Case of Olive Oil, Pomace, and Leaf Extracts, Phenolic Alcohols, Secoiridoids, and Triterpenes
Abstract
:1. Introduction
2. Methodology
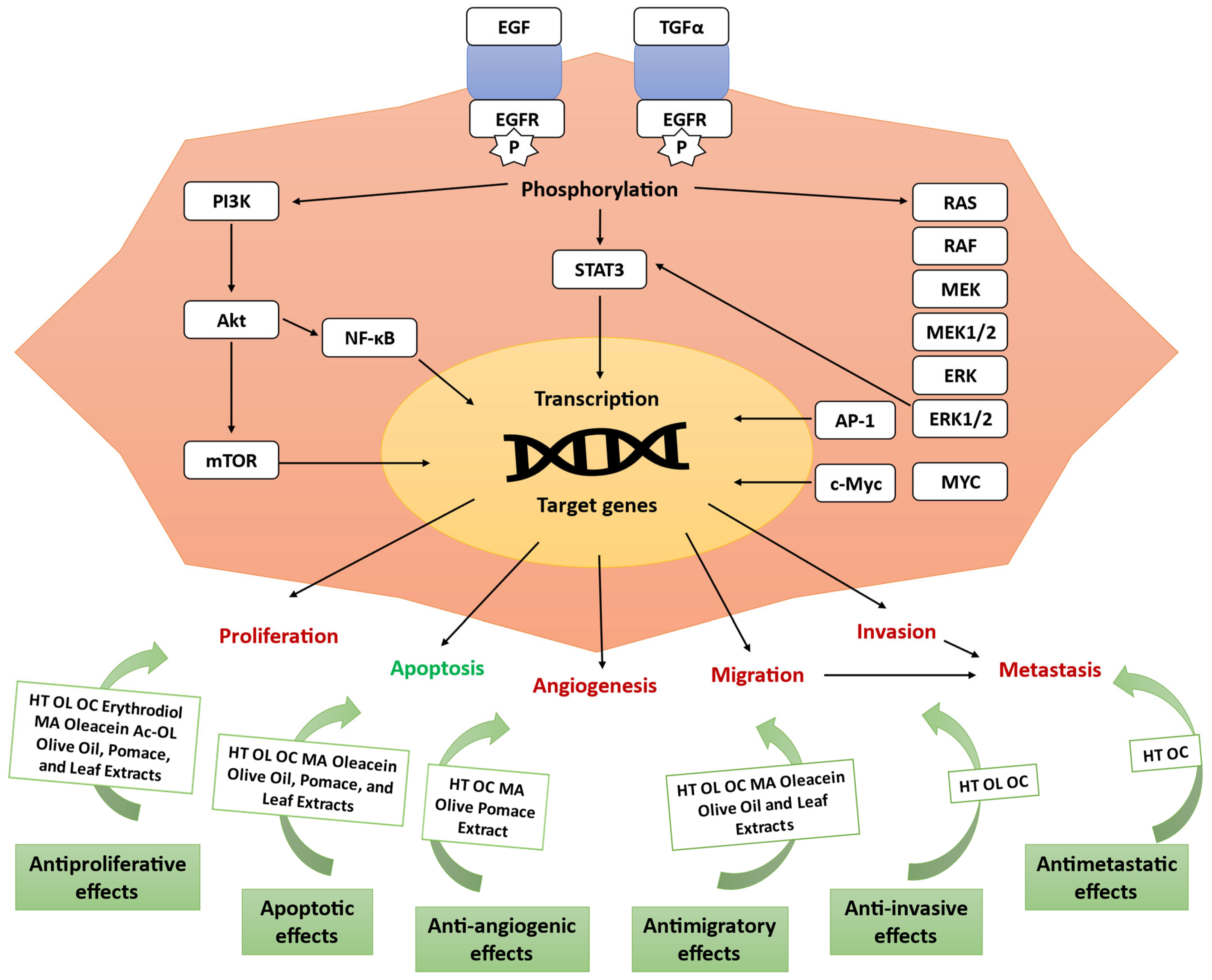
3. Antitumor Activity of Olive Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization. Cancer. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 6 May 2024).
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Torić, J.; Marković, A.K.; Brala, C.J.; Barbarić, M. Anticancer effects of olive oil polyphenols and their combinations with anticancer drugs. Acta Pharm. 2019, 69, 461–482. [Google Scholar] [CrossRef]
- Quero, J.; Ballesteros, L.F.; Ferreira-Santos, P.; Velderrain-Rodriguez, G.R.; Rocha, C.M.R.; Pereira, R.N.; Teixeira, J.A.; Martin-Belloso, O.; Osada, J.; Rodríguez-Yoldi, M.J. Unveiling the Antioxidant Therapeutic Functionality of Sustainable Olive Pomace Active Ingredients. Antioxidants 2022, 11, 828. [Google Scholar] [CrossRef]
- Ramos, P.; Santos, S.A.O.; Guerra, Â.R.; Guerreiro, O.; Felício, L.; Jerónimo, E.; Silvestre, A.J.D.; Neto, C.P.; Duarte, M. Valorization of olive mill residues: Antioxidant and breast cancer antiproliferative activities of hydroxytyrosol-rich extracts derived from olive oil by-products. Ind. Crops Prod. 2013, 46, 359–368. [Google Scholar] [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; D’Amore, S.; Gnoni, A.; Pellegrino, M.; Storelli, C.; De Caterina, R.; Palasciano, G.; Carluccio, M.A. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. J. Nutr. Biochem. 2016, 28, 19–29. [Google Scholar] [CrossRef]
- Xie, P.; Cecchi, L.; Bellumori, M.; Balli, D.; Giovannelli, L.; Huang, L.; Mulinacci, N. Phenolic compounds and triterpenes in different olive tissues and olive oil by-products, and cytotoxicity on human colorectal cancer cells: The case of frantoio, moraiolo and leccino cultivars (Olea europaea L.). Foods 2021, 10, 2823. [Google Scholar] [CrossRef]
- International Olive Council. World Market of Olive Oil and Table Olives—Data from December 2024. Available online: https://www.internationaloliveoil.org/world-market-of-olive-oil-and-table-olives-data-from-december-2024/?utm_source=chatgpt.com (accessed on 7 January 2025).
- Ferreira, D.M.; Barreto-Peixoto, J.; Andrade, N.; Machado, S.; Silva, C.; Lobo, J.C.; Nunes, M.A.; Álvarez-Rivera, G.; Ibáñez, E.; Cifuentes, A.; et al. Comprehensive analysis of the phytochemical composition and antitumoral activity of an olive pomace extract obtained by mechanical pressing. Food Biosci. 2024, 61, 104759. [Google Scholar] [CrossRef]
- Ferreira, D.M.; de Oliveira, N.M.; Chéu, M.H.; Meireles, D.; Lopes, L.; Oliveira, M.B.; Machado, J. Updated Organic Composition and Potential Therapeutic Properties of Different Varieties of Olive Leaves from Olea europaea. Plants 2023, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Siracusa, L.; Carrubba, M.; Parafati, L.; Proetto, I.; Pesce, F.; Fallico, B. Olive Leaves, a Promising Byproduct of Olive Oil Industry: Assessment of Metabolic Profiles and Antioxidant Capacity as a Function of Cultivar and Seasonal Change. Agronomy 2022, 12, 2007. [Google Scholar] [CrossRef]
- Natac. Revolutionising the Olive Industry: OLEAF4VALUE Unveils Market-Ready, Bio-Based Products from Olive Leaf Valorisation. Available online: https://natacgroup.com/news/revolutionising-the-olive-industry-oleaf4value-unveils-market-ready-bio-based-products-from-olive-leaf-valorisation/?utm_source=chatgpt.com (accessed on 7 January 2025).
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical composition and antimicrobial activity of a new olive pomace functional ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Muñoz-Ollero, P.; Forbes-Hernández, T.Y.; Esteban-Muñoz, A.; Giampieri, F.; Delgado Noya, I.; Bullón, P.; Vera-Ramírez, L.; et al. An Olive-Derived Extract 20% Rich in Hydroxytyrosol Prevents β-Amyloid Aggregation and Oxidative Stress, Two Features of Alzheimer Disease, via SKN-1/NRF2 and HSP-16.2 in Caenorhabditis elegans. Antioxidants 2022, 11, 629. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Vera-Ramírez, L.; Forbes-Hernández, T.J.; Esteban-Muñoz, A.; Giampieri, F.; Bullón, P.; Battino, M.; Sánchez-González, C.; et al. An oleuropein rich-olive (Olea europaea L.) leaf extract reduces β-amyloid and tau proteotoxicity through regulation of oxidative- and heat shock-stress responses in Caenorhabditis elegans. Food Chem. Toxicol. 2022, 162, 112914. [Google Scholar] [CrossRef]
- Alam, M.; Alam, S.; Shamsi, A.; Adnan, M.; Elasbali, A.M.; Al-Soud, W.A.; Alreshidi, M.; Hawsawi, Y.M.; Tippana, A.; Pasupuleti, V.R. Bax/Bcl-2 cascade is regulated by the EGFR pathway: Therapeutic targeting of non-small cell lung cancer. Front. Oncol. 2022, 12, 869672. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2020, 9, 84. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; Maiuolo, J.; D’Agostino, M.; Oliverio, M.; Procopio, A.; Filetti, S.; Russo, D. Antioxidant and antigrowth action of peracetylated oleuropein in thyroid cancer cells. J. Mol. Endocrinol. 2013, 51, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Pachón-Peña, G.; Lizárraga, D.; Rufino-Palomares, E.E.; Cascante, M.; Lupiáñez, J.A. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 2011, 11, 154. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol induces apoptosis in human colon cancer cells through ROS generation. Food Funct. 2014, 5, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G. Oleuropein induces apoptosis via abrogating NF-κB activation cascade in estrogen receptor–negative breast cancer cells. J. Cell. Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- Yan, C.M.; Chai, E.Q.; Cai, H.Y.; Miao, G.Y.; Ma, W. Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3-kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Mol. Med. Rep. 2015, 11, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, A.; Balasus, D.; Azzolina, A.; Augello, G.; Emma, M.R.; Di Sano, C.; Gramignoli, R.; Strom, S.C.; McCubrey, J.A.; Montalto, G. Oleocanthal exerts antitumor effects on human liver and colon cancer cells through ROS generation. Int. J. Oncol. 2017, 51, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Allouche, Y.; Warleta, F.; Campos, M.; Sánchez-Quesada, C.; Uceda, M.; Beltrán, G.; Gaforio, J.J. Antioxidant, Antiproliferative, and Pro-apoptotic Capacities of Pentacyclic Triterpenes Found in the Skin of Olives on MCF-7 Human Breast Cancer Cells and Their Effects on DNA Damage. J. Agric. Food Chem. 2011, 59, 121–130. [Google Scholar] [CrossRef]
- Albogami, S.; Hassan, A.M. Assessment of the Efficacy of Olive Leaf (Olea europaea L.) Extracts in the Treatment of Colorectal Cancer and Prostate Cancer Using In Vitro Cell Models. Molecules 2021, 26, 4069. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vazquez-Martin, A.; Colomer, R.; Brunet, J.; Carrasco-Pancorbo, A.; Garcia-Villalba, R.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Olive oil’s bitter principle reverses acquired autoresistance to trastuzumab (Herceptin™) in HER2-overexpressing breast cancer cells. BMC Cancer 2007, 7, 80. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vazquez-Martin, A.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Oliveras-Ferraros, C.; Fernandez-Gutierrez, A.; Segura-Carretero, A. tabAnti-HER2 (erbB-2) oncogene effects of phenolic compounds directly isolated from commercial Extra-Virgin Olive Oil (EVOO). BMC Cancer 2008, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Analyzing effects of extra-virgin olive oil polyphenols on breast cancer-associated fatty acid synthase protein expression using reverse-phase protein microarrays. Int. J. Mol. Med. 2008, 22, 433–439. [Google Scholar] [CrossRef]
- Fu, S.; Arráez-Roman, D.; Segura-Carretero, A.; Menéndez, J.A.; Menéndez-Gutiérrez, M.P.; Micol, V.; Fernández-Gutiérrez, A. Qualitative screening of phenolic compounds in olive leaf extracts by hyphenated liquid chromatography and preliminary evaluation of cytotoxic activity against human breast cancer cells. Anal. Bioanal. Chem. 2010, 397, 643–654. [Google Scholar] [CrossRef]
- Goulas, V.; Exarchou, V.; Troganis, A.N.; Psomiadou, E.; Fotsis, T.; Briasoulis, E.; Gerothanassis, I.P. Phytochemicals in olive-leaf extracts and their antiproliferative activity against cancer and endothelial cells. Mol. Nutr. Food Res. 2009, 53, 600–608. [Google Scholar] [CrossRef]
- Di Francesco, A.; Falconi, A.; Di Germanio, C.; Micioni Di Bonaventura, M.V.; Costa, A.; Caramuta, S.; Del Carlo, M.; Compagnone, D.; Dainese, E.; Cifani, C.; et al. Extravirgin olive oil up-regulates CB1 tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J. Nutr. Biochem. 2015, 26, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Polini, B.; Digiacomo, M.; Carpi, S.; Bertini, S.; Gado, F.; Saccomanni, G.; Macchia, M.; Nieri, P.; Manera, C.; Fogli, S. Oleocanthal and oleacein contribute to the In Vitro therapeutic potential of extra virgin oil-derived extracts in non-melanoma skin cancer. Toxicol. In Vitro 2018, 52, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M. Olive Leaf Extract and Its Main Component Oleuropein Prevent Chronic Ultraviolet B Radiation-Induced Skin Damage and Carcinogenesis in Hairless Mice. J. Nutr. 2009, 139, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Kugić, A.; Dabelić, S.; Brala, C.J.; Dabelić, N.; Barbarić, M. Extra Virgin Olive Oil Secoiridoids Modulate the Metabolic Activity of Dacarbazine Pre-Treated and Treatment-Naive Melanoma Cells. Molecules 2022, 27, 3310. [Google Scholar] [CrossRef]
- Anter, J.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Demyda-Peyras, S.; Moreno-Millán, M.; Alonso-Moraga, Á.; Muñoz-Serrano, A.; Luque de Castro, M.D. A pilot study on the DNA-protective, cytotoxic, and apoptosis-inducing properties of olive-leaf extracts. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 723, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Anter, J.; Tasset, I.; Demyda-Peyrás, S.; Ranchal, I.; Moreno-Millán, M.; Romero-Jimenez, M.; Muntané, J.; Luque de Castro, M.D.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Evaluation of potential antigenotoxic, cytotoxic and proapoptotic effects of the olive oil by-product “alperujo”, hydroxytyrosol, tyrosol and verbascoside. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 772, 25–33. [Google Scholar] [CrossRef]
- De Stefanis, D.; Scimè, S.; Accomazzo, S.; Catti, A.; Occhipinti, A.; Bertea, C.M.; Costelli, P. Anti-Proliferative Effects of an Extra-Virgin Olive Oil Extract Enriched in Ligstroside Aglycone and Oleocanthal on Human Liver Cancer Cell Lines. Cancers 2019, 11, 1640. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.; Sakoff, J.; Stathopoulos, C.; Roach, P.; Scarlett, C. Cytotoxicity of Methanol and Aqueous Olive Pomace Extracts Towards Pancreatic Cancer Cells In Vitro. J. Nat. Prod. Cancer Chemo Prev. Agents 2018, 2, 1–6. Available online: https://www.unitedscientificgroup.org/journals/ets/articles/v1n1/jnpcpt-02-chloe-desiree-goldsmith.pdf (accessed on 22 December 2024).
- Papakonstantinou, A.; Koumarianou, P.; Diamantakos, P.; Melliou, E.; Magiatis, P.; Boleti, H. A Systematic Ex-Vivo Study of the Anti-Proliferative/Cytotoxic Bioactivity of Major Olive Secoiridoids’ Double Combinations and of Total Olive Oil Phenolic Extracts on Multiple Cell-Culture Based Cancer Models Highlights Synergistic Effects. Nutrients 2023, 15, 2538. [Google Scholar] [CrossRef]
- Sakavitsi, M.E.; Breynaert, A.; Nikou, T.; Lauwers, S.; Pieters, L.; Hermans, N.; Halabalaki, M. Availability and Metabolic Fate of Olive Phenolic Alcohols Hydroxytyrosol and Tyrosol in the Human GI Tract Simulated by the In Vitro GIDM-Colon Model. Metabolites 2022, 12, 391. [Google Scholar] [CrossRef]
- Calahorra, J.; Martínez-Lara, E.; De Dios, C.; Siles, E. Hypoxia modulates the antioxidant effect of hydroxytyrosol in MCF-7 breast cancer cells. PLoS ONE 2018, 13, e0203892. [Google Scholar] [CrossRef]
- Cruz-Lozano, M.; González-González, A.; Marchal, J.A.; Muñoz-Muela, E.; Molina, M.P.; Cara, F.E.; Brown, A.M.; García-Rivas, G.; Hernández-Brenes, C.; Lorente, J.A.; et al. Hydroxytyrosol inhibits cancer stem cells and the metastatic capacity of triple-negative breast cancer cell lines by the simultaneous targeting of epithelial-to-mesenchymal transition, Wnt/β-catenin and TGFβ signaling pathways. Eur. J. Nutr. 2019, 58, 3207–3219. [Google Scholar] [CrossRef] [PubMed]
- El-azem, N.; Pulido-Moran, M.; Ramirez-Tortosa, C.L.; Quiles, J.L.; Cara, F.E.; Sanchez-Rovira, P.; Granados-Principal, S.; Ramirez-Tortosa, M. Modulation by hydroxytyrosol of oxidative stress and antitumor activities of paclitaxel in breast cancer. Eur. J. Nutr. 2019, 58, 1203–1211. [Google Scholar] [CrossRef]
- Calahorra, J.; Martínez-Lara, E.; Granadino-Roldán, J.M.; Martí, J.M.; Cañuelo, A.; Blanco, S.; Oliver, F.J.; Siles, E. Crosstalk between hydroxytyrosol, a major olive oil phenol, and HIF-1 in MCF-7 breast cancer cells. Sci. Rep. 2020, 10, 6361. [Google Scholar] [CrossRef]
- Corona, G.; Deiana, M.; Incani, A.; Vauzour, D.; Dessì, M.A.; Spencer, J.P. Hydroxytyrosol inhibits the proliferation of human colon adenocarcinoma cells through inhibition of ERK1/2 and cyclin D1. Mol. Nutr. Food Res. 2009, 53, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Donnini, S.; Giachetti, A.; Iñiguez, M.A.; Fresno, M.; Melillo, G.; Ziche, M. Inhibition of hypoxia inducible factor-1alpha by dihydroxyphenylethanol, a product from olive oil, blocks microsomal prostaglandin-E synthase-1/vascular endothelial growth factor expression and reduces tumor angiogenesis. Clin. Cancer Res. 2010, 16, 4207–4216. [Google Scholar] [CrossRef]
- Terzuoli, E.; Giachetti, A.; Ziche, M.; Donnini, S. Hydroxytyrosol, a product from olive oil, reduces colon cancer growth by enhancing epidermal growth factor receptor degradation. Mol. Nutr. Food Res. 2016, 60, 519. [Google Scholar] [CrossRef]
- Terzuoli, E.; Nannelli, G.; Frosini, M.; Giachetti, A.; Ziche, M.; Donnini, S. Inhibition of cell cycle progression by the hydroxytyrosol-cetuximab combination yields enhanced chemotherapeutic efficacy in colon cancer cells. Oncotarget 2017, 8, 83207–83224. [Google Scholar] [CrossRef]
- Hormozi, M.; Salehi Marzijerani, A.; Baharvand, P. Effects of Hydroxytyrosol on Expression of Apoptotic Genes and Activity of Antioxidant Enzymes in LS180 Cells. Cancer Manag. Res. 2020, 12, 7913–7919. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fuccelli, R.; Sepporta, M.V.; Fabiani, R. In Vitro chemopreventive activities of hydroxytyrosol: The main phenolic compound present in extra-virgin olive oil. Food Funct. 2016, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.; Bhardwaj, A.; Ahmad, A.; Srivastava, S.K.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Hydroxytyrosol Induces Apoptosis and Cell Cycle Arrest and Suppresses Multiple Oncogenic Signaling Pathways in Prostate Cancer Cells. Nutr. Cancer 2017, 69, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Tutino, V.; Caruso, M.G.; Messa, C.; Perri, E.; Notarnicola, M. Antiproliferative, antioxidant and anti-inflammatory effects of hydroxytyrosol on human hepatoma HepG2 and Hep3B cell lines. Anticancer Res. 2012, 32, 5371–5377. [Google Scholar]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef]
- Bongiorno, D.; Di Stefano, V.; Indelicato, S.; Avellone, G.; Ceraulo, L. Bio-phenols determination in olive oils: Recent mass spectrometry approaches. Mass Spectrom. Rev. 2023, 42, 1462–1502. [Google Scholar] [CrossRef]
- Karousi, P.; Kontos, C.K.; Papakotsi, P.; Kostakis, I.K.; Skaltsounis, A.-L.; Scorilas, A. Next-generation sequencing reveals altered gene expression and enriched pathways in triple-negative breast cancer cells treated with oleuropein and oleocanthal. Funct. Integr. Genom. 2023, 23, 299. [Google Scholar] [CrossRef] [PubMed]
- Abtin, M.; Alivand, M.R.; Khaniani, M.S.; Bastami, M.; Zaeifizadeh, M.; Derakhshan, S.M. Simultaneous downregulation of miR-21 and miR-155 through oleuropein for breast cancer prevention and therapy. J. Cell. Biochem. 2018, 119, 7151–7165. [Google Scholar] [CrossRef]
- Elamin, M.H.; Elmahi, A.B.; Daghestani, M.H.; Al-Olayan, E.M.; Al-Ajmi, R.A.; Alkhuriji, A.F.; Hamed, S.S.; Elkhadragy, M.F. Synergistic anti-breast-cancer effects of combined treatment with oleuropein and doxorubicin in vivo. Altern. Ther. Health Med. 2019, 25, 17–24. [Google Scholar]
- Przychodzen, P.; Kuban-Jankowska, A.; Wyszkowska, R.; Barone, G.; Bosco, G.L.; Celso, F.L.; Kamm, A.; Daca, A.; Kostrzewa, T.; Gorska-Ponikowska, M. PTP1B phosphatase as a novel target of oleuropein activity in MCF-7 breast cancer model. Toxicol. In Vitro 2019, 61, 104624. [Google Scholar] [CrossRef]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.-Y.; Sayed, K.A.E. Olive phenolics as c-Met inhibitors:(-)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models. PLoS ONE 2014, 9, e97622. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.M.; Siddique, A.B.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. The olive oil phenolic (-)-oleocanthal modulates estrogen receptor expression in luminal breast cancer in vitro and in vivo and synergizes with tamoxifen treatment. Eur. J. Pharmacol. 2017, 810, 100–111. [Google Scholar] [CrossRef]
- Diez-Bello, R.; Jardin, I.; Lopez, J.J.; El Haouari, M.; Ortega-Vidal, J.; Altarejos, J.; Salido, G.M.; Salido, S.; Rosado, J.A. (−)-Oleocanthal inhibits proliferation and migration by modulating Ca2+ entry through TRPC6 in breast cancer cells. BBA Mol. Cell Res. 2019, 1866, 474–485. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ebrahim, H.Y.; Akl, M.R.; Ayoub, N.M.; Goda, A.A.; Mohyeldin, M.M.; Nagumalli, S.K.; Hananeh, W.M.; Liu, Y.-Y.; Meyer, S.A.; et al. (−)-Oleocanthal Combined with Lapatinib Treatment Synergized against HER-2 Positive Breast Cancer In Vitro and In Vivo. Nutrients 2019, 11, 412. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ayoub, N.M.; Tajmim, A.; Meyer, S.A.; Hill, R.A.; El Sayed, K.A. (−)-Oleocanthal Prevents Breast Cancer Locoregional Recurrence After Primary Tumor Surgical Excision and Neoadjuvant Targeted Therapy in Orthotopic Nude Mouse Models. Cancers 2019, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- Qusa, M.H.; Siddique, A.B.; Nazzal, S.; El Sayed, K.A. Novel olive oil phenolic (−)-oleocanthal (+)-xylitol-based solid dispersion formulations with potent oral anti-breast cancer activities. Int. J. Pharm. 2019, 569, 118596. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ebrahim, H.; Mohyeldin, M.; Qusa, M.; Batarseh, Y.; Fayyad, A.; Tajmim, A.; Nazzal, S.; Kaddoumi, A.; El Sayed, K. Novel liquid-liquid extraction and self-emulsion methods for simplified isolation of extra-virgin olive oil phenolics with emphasis on (-)-oleocanthal and its oral anti-breast cancer activity. PLoS ONE 2019, 14, e0214798. [Google Scholar] [CrossRef]
- Elnagar, A.; Sylvester, P.; El Sayed, K. Oleocanthal as a c-Met inhibitor for the control of metastatic breast and prostate cancer. Planta Medica 2011, 77, 1013–1019. [Google Scholar] [CrossRef]
- Khanfar, M.A.; Bardaweel, S.K.; Akl, M.R.; El Sayed, K.A. Olive oil-derived oleocanthal as potent inhibitor of mammalian target of rapamycin: Biological evaluation and molecular modeling studies. Phytother. Res. 2015, 29, 1776–1782. [Google Scholar] [CrossRef]
- Goren, L.; Zhang, G.; Kaushik, S.; Breslin, P.A.; Du, Y.-C.N.; Foster, D.A. (-)-Oleocanthal and (-)-oleocanthal-rich olive oils induce lysosomal membrane permeabilization in cancer cells. PLoS ONE 2019, 14, e0216024. [Google Scholar] [CrossRef]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Cerdá-Nicolás, J.M.; Giner, R.M. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol. Nutr. Food Res. 2016, 60, 242–255. [Google Scholar] [CrossRef]
- Sepporta, M.V.; Fuccelli, R.; Rosignoli, P.; Ricci, G.; Servili, M.; Fabiani, R. Oleuropein Prevents Azoxymethane-Induced Colon Crypt Dysplasia and Leukocytes DNA Damage in A/J Mice. J. Med. Food 2016, 19, 983–989. [Google Scholar] [CrossRef]
- Khanal, P.; Oh, W.-K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.-G.; Kwon, S.-M.; Choi, H.-K.; Choi, H.S. p-HPEA-EDA, a phenolic compound of virgin olive oil, activates AMP-activated protein kinase to inhibit carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Sherif, I.O.; Al-Gayyar, M.M.H. Oleuropein potentiates anti-tumor activity of cisplatin against HepG2 through affecting proNGF/NGF balance. Life Sci. 2018, 198, 87–93. [Google Scholar] [CrossRef]
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R.; Sun, B.; Pan, S.; Liang, D.; Liu, L. (−)-Oleocanthal inhibits growth and metastasis by blocking activation of STAT3 in human hepatocellular carcinoma. Oncotarget 2016, 7, 43475. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; La Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Arena, C.; Carpi, S.; Polini, B.; Bertini, S.; Digiacomo, M.; Gado, F.; Saba, A.; Saccomanni, G.; Breschi, M.C. Cytotoxic activity of oleocanthal isolated from virgin olive oil on human melanoma cells. Nutr. Cancer 2016, 68, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, J.; Peng, L. (-)-Oleocanthal exerts anti-melanoma activities and inhibits STAT3 signaling pathway. Oncol. Rep. 2017, 37, 483–491. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Zhang, Q.; Li, X.; Zhu, X.; Wang, Q.; Cao, S.; Du, L. Mitochondria-mediated apoptosis was induced by oleuropein in H1299 cells involving activation of p38 MAP kinase. J. Cell. Biochem. 2019, 120, 5480–5494. [Google Scholar] [CrossRef]
- Siddique, A.B.; Kilgore, P.C.S.R.; Tajmim, A.; Singh, S.S.; Meyer, S.A.; Jois, S.D.; Cvek, U.; Trutschl, M.; Sayed, K.A.E. (−)-Oleocanthal as a Dual c-MET-COX2 Inhibitor for the Control of Lung Cancer. Nutrients 2020, 12, 1749. [Google Scholar] [CrossRef]
- Seçme, M.; Eroğlu, C.; Dodurga, Y.; Bağcı, G. Investigation of anticancer mechanism of oleuropein via cell cycle and apoptotic pathways in SH-SY5Y neuroblastoma cells. Gene 2016, 585, 93–99. [Google Scholar] [CrossRef]
- Ünsal, Ü.Ü.; Mete, M.; Aydemir, I.; Duransoy, Y.K.; Umur, A.Ş.; Tuglu, M.I. Inhibiting effect of oleocanthal on neuroblastoma cancer cell proliferation in culture. Biotech. Histochem. 2020, 95, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Celano, M.; Lombardo, G.E.; Maggisano, V.; Procopio, A.; Russo, D.; Navarra, M. Oleacein inhibits STAT3, activates the apoptotic machinery, and exerts anti-metastatic effects in the SH-SY5Y human neuroblastoma cells. Food Funct. 2020, 11, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Miceli, C.; Nediani, C.; Berti, A.; Cascella, R.; Pantano, D.; Nardiello, P.; Luccarini, I.; Casamenti, F.; Stefani, M. Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: A mechanistic insight. Oncotarget 2015, 6, 35344. [Google Scholar] [CrossRef]
- Przychodzen, P.; Wyszkowska, R.; Gorzynik-Debicka, M.; Kostrzewa, T.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Anticancer potential of oleuropein, the polyphenol of olive oil, with 2-methoxyestradiol, separately or in combination, in human osteosarcoma cells. Anticancer Res. 2019, 39, 1243–1251. [Google Scholar] [CrossRef]
- Potočnjak, I.; Škoda, M.; Pernjak-Pugel, E.; Peršić, M.P.; Domitrović, R. Oral administration of oleuropein attenuates cisplatin-induced acute renal injury in mice through inhibition of ERK signaling. Mol. Nutr. Food Res. 2016, 60, 530–541. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Leal, A.S.; Alho, D.P.S.; Gonçalves, B.M.F.; Valdeira, A.S.; Mendes, V.I.S.; Jing, Y. Chapter 2—Highlights of Pentacyclic Triterpenoids in the Cancer Settings. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 41, pp. 33–73. [Google Scholar]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Mokhtari, K.; Medina, P.P.; Lupiáñez, J.A.; Peragón, J. Maslinic acid, a triterpenic anti-tumoural agent, interferes with cytoskeleton protein expression in HT29 human coloncancer cells. J. Proteom. 2013, 83, 15. [Google Scholar] [CrossRef]
- Sánchez-Tena, S.; Reyes-Zurita, F.J.; Díaz-Moralli, S.; Vinardell, M.P.; Reed, M.; García-García, F.; Dopazo, J.; Lupiáñez, J.A.; Günther, U.; Cascante, M. Maslinic acid-enriched diet decreases intestinal tumorigenesis in ApcMin/+ mice through transcriptomic and metabolomic reprogramming. PLoS ONE 2013, 8, e59392. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, B.; Li, P.; Wen, X.; Yang, J. Maslinic Acid Inhibits Colon Tumorigenesis by the AMPK–mTOR Signaling Pathway. J. Agric. Food Chem. 2019, 67, 4259–4272. [Google Scholar] [CrossRef]
- Juan, M.E.; Wenzel, U.; Daniel, H.; Planas, J. Erythrodiol, a natural triterpenoid from olives, has antiproliferative and apoptotic activity in HT-29 human adenocarcinoma cells. Mol. Nutr. Food Res. 2008, 52, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, D.; Zhang, X.; Shan, L.; Liu, Z. Maslinic acid induced apoptosis in bladder cancer cells through activating p38 MAPK signaling pathway. Mol. Cell. Biochem. 2014, 392, 281. [Google Scholar] [CrossRef]
- Thakor, P.; Song, W.; Subramanian, R.B.; Thakkar, V.R.; Vesey, D.A.; Gobe, G.C. Maslinic acid inhibits proliferation of renal cell carcinoma cell lines and suppresses angiogenesis of endothelial cells. J. Kidney Cancer VHL 2017, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Alowaiesh, B.F.; Alhaithloul, H.A.S.; Saad, A.M.; Hassanin, A.A. Green Biogenic of Silver Nanoparticles Using Polyphenolic Extract of Olive Leaf Wastes with Focus on Their Anticancer and Antimicrobial Activities. Plants 2023, 12, 1410. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, H. Olive leaf extract green-formulated manganese nanoparticles: Characterization and antioxidant, cytotoxicity and anti-breast carcinoma properties. Inorg. Chem. Commun. 2024, 163, 112376. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Zhu, J.-S.; Zhang, Z.; Shen, W.-J.; Jiang, S.; Long, Y.-F.; Wu, B.; Ding, T.; Huan, F.; Wang, S.-L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion of MDA-MB-231 Triple-Negative Breast Cancer Cell via Induction of Autophagy. Anti Cancer Agents Med. Chem. 2019, 19, 1983–1990. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Zhu, J.-S.; Xie, J.; Zhang, Z.; Zhu, J.; Jiang, S.; Shen, W.-J.; Wu, B.; Ding, T.; Wang, S.-L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion via Induction of Autophagy in ER-Positive Breast Cancer Cell Lines (MCF7 and T47D). Nutr. Cancer 2021, 73, 350–360. [Google Scholar] [CrossRef]
- Busnena, B.A.; Foudah, A.I.; Melancon, T.; El Sayed, K.A. Olive secoiridoids and semisynthetic bioisostere analogues for the control of metastatic breast cancer. Bioorg. Med. Chem. 2013, 21, 2117–2127. [Google Scholar] [CrossRef]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; Lastra, C.A.d.l. Oleuropein, a Secoiridoid Derived from Olive Tree, Inhibits the Proliferation of Human Colorectal Cancer Cell Through Downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef]
- Hazas, M.-C.L.d.l.; Piñol, C.; Macià, A.; Motilva, M.-J. Hydroxytyrosol and the Colonic Metabolites Derived from Virgin Olive Oil Intake Induce Cell Cycle Arrest and Apoptosis in Colon Cancer Cells. J. Agric. Food Chem. 2017, 65, 6467–6476. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of Hydroxytyrosol-Enriched Fractions from Olea europaea L. Byproducts and Evaluation of the In Vitro Effects on a Model of Colorectal Cancer Cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Ben Saad, A.; Zgheib, A.; Annabi, B. Olive oil compounds inhibit the paracrine regulation of TNF-α-induced endothelial cell migration through reduced glioblastoma cell cyclooxygenase-2 expression. J. Nutr. Biochem. 2016, 27, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Banim, P.J.R.; Luben, R.; Khaw, K.-T.; Hart, A.R. Dietary oleic acid is inversely associated with pancreatic cancer—Data from food diaries in a cohort study. Pancreatology 2018, 18, 655–660. [Google Scholar] [CrossRef]


| Comp. | Cancer | Effects | Study | Model | Dose | Ref. |
|---|---|---|---|---|---|---|
| Elenolic acid, HT, OL aglycone, TYR | Breast | Antiproliferative, apoptotic, OL aglycone synergy w/trastuzumab | In vitro | MCF-7, SKBR3, SKBR3/Tzb100 | 6.25–100 μM, 1–5 d | [28] |
| Elenolic acid, HT, OL derivatives, TYR, etc. | Antiproliferative, apoptotic, cytotoxic | MCF-7, SKBR3 | 25–100 μM, 6 h–4 d | [29] | ||
| FAS inhibition | 50 μM, 48 h | [30] | ||||
| HT, HT glucoside, OL, OL derivatives, VER | Antiproliferative, HT synergy w/5-fluorouracil | MDA-MB-231 | Extracts (0.1–20 μg/mL), HT (162–2595 μM), 48 h | [5] | ||
| OL, OL derivatives, oleanolic acid, etc. | Cytotoxic | JIMT-1, MCF-7, SKBR3 | 10–1000 μg/mL, 72 h | [31] | ||
| HT, HT acetate, LUT, LUT derivatives, OL | Breast, bladder, endothelial | Antiproliferative | BBCE, MCF-7, T-24 | 0–50 μM, 2 d | [32] | |
| EVOO’s compounds, HT | Colorectal | Antiproliferative | In vitro, in vivo | Caco-2, NCM460; Sprague Dawley rats | 50 μM, 24–48 h; 250 μL/300 g of EVOO: single intake at day 10 or daily for 10 d | [33] |
| Apigenin, caffeic acid, HT, OL, quercetin, TYR, etc. | Antiproliferative, apoptotic, selective cytotoxicity, protective | In vitro | Caco-2 | 1.5 mg/mL, 72 h | [4] | |
| Erythrodiol, HT, MA, OC, OL aglycone, oleacein, etc. | Cytotoxic | HCT-116 | 88.25–875.5 μg/mL, 72 h | [7] | ||
| Gallic acid, catechin, caffeic acid, kaempferol, etc. | Colorectal, prostate | Cytotoxic, antimigratory, apoptotic | HT-29, PC3 | 198.6–535.3 μg/mL, 12–72 h | [27] | |
| OOE’s compounds, HT, OC, oleacein, TYR | Skin | Antiproliferative, antimigratory, apoptotic | A431, HaCat | 1–200 μM, 72–144 h | [34] | |
| OLE’s compounds, OL | Anticarcinogenic, anti-inflammatory | In vivo | Albino hairless mice | 10–1000 mg/kg, 2x/d, 30 wk, oral | [35] | |
| EVOO’s compounds, OC, oleacein | Melanoma | Antiproliferative, selective cytotoxicity | In vitro | A375, A375M, HaCaT | 0.025–0.4% (v/v), 24–48 h | [36] |
| OLE’s compounds, OL, LUT | Leukemia | Antigenotoxic, antimutagenic, apoptotic, cytotoxic, protective | In vitro, in vivo | HL60; D. melanogaster | 3.75–160 μL/mL, 2–640 μM, 72 h | [37] |
| OPE’s compounds, HT, TYR, VER | Antigenotoxic, antimutagenic, apoptotic, antiproliferative, protective | 3.75–320 μL/mL, 6.25–480 μM, 72 h | [38] | |||
| Elenolic acid, ligstroside aglycone, OC, OL aglycone | Liver | Antiproliferative, apoptotic, cytotoxic, autophagy activation | In vitro | Hep3B, HepG2, Huh7 | 4.81–9.62 μg/mL, 24–72 h | [39] |
| OPE’s compounds | Breast, colon, glioblastoma, lung, neuroblastoma, ovarian, pancreatic, prostate, skin | Antiproliferative, selective cytotoxicity | MIA PaCa-2, HT-29, A2780, H460, A431, Du145, BE2-C, MCF-7, U-87, SJ-G2, SMA, MCF-10A, HPDE | 0–200 μg/mL, 72 h | [40] | |
| OC, oleacein, ligstroside aglycone, OL aglycone, oleomissional | Breast, cervix, colon, gastric, liver, lung, melanoma, pancreas | Antiproliferative, selective cytotoxicity | MDA-MB-231, SK-BR-3, MCF-7, SK-MEL-28, A2058, HT-29, AGS, HepG2, PANC-1, H1299, Hela, HaCaT, MCF-10A | Half of the EC50, 72 h | [41] | |
| Comselogoside, elenolic acid, HT, LUT, MA, VER, etc. | Breast, pancreatic, colorectal | Antiproliferative, cytotoxic, anti-angiogenic | MCF-7, AsPC-1, HT-29, Caco-2 | 100 mg/mL, 24 h | [9] |
| Comp. | Cancer | Effects | Study | Model | Dose | Ref. |
|---|---|---|---|---|---|---|
| HT | Breast | Antiproliferative, cytotoxic | In vitro | MCF-7 | 5–600 μM, 16 h | [43] |
| Anti-invasive, antimigratory, antiproliferative, antimetastatic | BT549, Hs578T, MDA-MB-231, SBE-HEK293, SUM159PT | 0.5–100 μM, 72 h | [44] | |||
| Antiproliferative, antitumorigenic, protective, synergy w/paclitaxel | In vitro, in vivo | MCF-7, MDA-MB-231; Sprague Dawley rats | 10–100 μM, 72 h; 0.5–2 mg/kg/d, 6 wk, oral | [45] | ||
| Antitumorigenic | In vitro, in silico | MCF-7 | 5–400 μM, 16 h | [46] | ||
| Colorectal | Antiproliferative | In vitro | Caco-2 | HT (5–162.5 µM, 15 m-96 h) | [47] | |
| Anti-angiogenic, anti-inflammatory, antitumorigenic | In vitro, in vivo | HT-29, WiDr; mice w/HT-29 xenograft | 50–100 μM, 3–48 h; 10 mg/kg/d, 14 d | [48] | ||
| Apoptotic, selective cytotoxicity | In vitro | CRL1807, DLD1 | 50–200 μM, 24, 48 h | [22] | ||
| HT, TYR | Selective cytotoxicity, antiproliferative, antitumorigenic | In vitro, in vivo | Caco-2, CCD-18Co, HT-29, WiDr; mice w/HT-29 xenograft | 1–300 μM, 2–48 h; 10 mg/kg/d, 14 d, intraperitoneal | [49] | |
| HT | Antiproliferative, selective cytotoxicity, synergy w/cetuximab, activated autophagy | In vitro | Caco-2, CCD-18Co, HaCaT, HT-29, WiDr | 1–300 μM, 8–48 h | [50] | |
| Apoptotic | LS180 | 50–150 μM, 24 h | [51] | |||
| Breast, colorectal, prostate | Antiproliferative, protective | HCT, LNCAP, MCF-7, MDA, PBMC, PC3, SW480 | 10–100 μM, 0.5–24 h | [52] | ||
| Prostate | Antiproliferative, apoptotic, selective cytotoxicity | C4-2, LNCaP, RWPE1, RWPE2 | 10–400 μM, 24–72 h | [53] | ||
| Liver | Anti-inflammatory, antiproliferative | Hep3B, HepG2 | 30–200 μM, 48–72 h | [54] | ||
| Anti-angiogenic, antiproliferative, apoptotic, selective cytotoxicity, antitumorigenic | In vitro, in vivo | Hep3B, HepG2, HL-7702, Huh-7, SK-HEP-1; HCC mice | 100–400 μM, 48–72 h; 10–20 mg/kg/d, 3 wk, intraperitoneal | [55] |
| Comp. | Cancer | Effects | Study | Model | Dose | Ref. |
|---|---|---|---|---|---|---|
| OC, OL | Breast | Antiproliferative, apoptotic | In vitro | MDA-MB-231, MDA-MB-468 | OC: 250 μM, OL: 500 μM, 12–48 h | [57] |
| OL | Antimigratory, antiproliferative, apoptotic, cytotoxic | MCF-7 | 150–2400 μg/mL, 24–72 h | [58] | ||
| Antiproliferative, apoptotic, selective cytotoxicity, antitumorigenic, synergy w/doxorubicin | In vitro, in vivo | MCF-10A, MDA-MB-231; mice injected w/MDA-MB-231 | 50 mg/kg, 72 h; 50 mg/kg, 4 wk, 1X/wk, intraperitoneal | [59] | ||
| Anti-invasive, antimigratory, apoptotic | In vitro | MCF-7, MDA-MB-231 | 12.5–100 μM, 24–72 h | [23] | ||
| Antiproliferative, cytostatic | In vitro, in silico | MCF-7 | 0.98–250 μM, 24–48 h | [60] | ||
| OC | Anti-invasive, antimigratory, antiproliferative, apoptotic, selective cytotoxicity | In vitro, in vivo | BT-474, MCF-7, MCF-10A, MDA-MB-231, mice w/MDA-MB-231 xenograft | 5–15 μM, 24–72 h; 5 mg/kg, 3X/wk, intraperitoneal | [61] | |
| Antiproliferative, antitumorigenic, selective toxicity, synergy w/tamoxifen | In vitro, in vivo, in silico | BT-474, MCF-7, T-47D, mice w/BT-474 xenograft | 5–60 μM, 24–48 h; 5–10 mg/kg, 3X/wk, 43 d, intraperitoneal | [62] | ||
| Antimigratory, antiproliferative, selective cytotoxicity | In vitro | MCF-7, MCF-10A, MDA-MB-231 | 10–20 μM, 24–72 h | [63] | ||
| Anti-invasive, antimigratory, antiproliferative, selective cytotoxicity, antitumorigenic, synergy w/lapatinib | In vitro, in vivo | BT-474, MCF-12A, SKBR3, mice w/BT-474 xenograft | 5–80 μM, 24–48 h, 10 mg/kg, 3X/wk, intraperitoneal | [64] | ||
| Antitumorigenic, selective toxicity | In vivo | Mice w/BT-474 or MDA-MB-231 xenograft | 10 mg/kg/d, 40 d, oral | [65] | ||
| In vivo, in silico | Mice w/MDA-MB-231 xenograft | 10 mg/kg/d, 15–70 d, oral | [66] | |||
| Antiproliferative, selective cytotoxicity, antitumorigenic | In vitro, in vivo | MDA-MB-231, MDA-MB-468, BT-474, MCF-7, MCF10A; Foxn1nu/Foxn1+ mice | 2.5–80 μM; 10 mg/kg, 7X/wk, 40 d, oral | [67] | ||
| OC | Breast, prostate | Anti-angiogenic, anti-invasive, antimigratory, antiproliferative | In vitro, in silico | MCF-7, MDA-MB-231, PC-3 | 2–100 μM, 24 h | [68] |
| Breast, cervical, colorectal | Antiproliferative | Caco-2, HeLa, MCF-7, MDA-MB-231, T47D | 0.1–50 μM, 24–72 h | [69] | ||
| Breast, pancreas, prostate | Antiproliferative, selective cytotoxicity, antitumorigenic | In vitro, in vivo | BJ-hTert, MCF-7, MCF-10A, MDA-MB-231, HEK-293T, PC3, PNET mice | 1–100 μM, 1–24 h; 5 mg/kg/d, 5 wk, intraperitoneal | [70] | |
| OL | Colorectal | Preventive against colitis | In vivo | C57BL/6 mice | 50–100 mg/kg, 63 d, oral | [71] |
| Preventive against carcinogen (azoxymethane) | A/J mice w/azoxymethane-induced tumors | 125 mg/kg, 6 wk, 1X/wk, intraperitoneal | [72] | |||
| p-HPEA-EDA | Apoptotic, antitumorigenic, inhibited colony formation | In vitro, in vivo | HCT-116, HT-29, JB6Cl41, SKBR3; chicken embryo chorioallantoic membrane | 0.1–10 μg/mL, 12–48 h; 50 μg/mL, 3 d | [73] | |
| OL | Colorectal, liver | Antiproliferative, apoptotic | In vitro | HepG2, Huh7, RKO | 10–80 μM, 24 h | [24] |
| OC | Antiproliferative, apoptotic, selective cytotoxicity | Hep3B, HepG2, HT-29, Huh7, PLC/PRF/5, SW480 | 0.78–100 μM, 24–72 h | [25] | ||
| OL | Liver | Synergy w/cisplatin, cytotoxic | HepG2 | 100–400 μM, 24–48 h | [74] | |
| OC | Anti-invasive, antimigratory, antiproliferative, apoptotic, selective cytotoxicity, antitumorigenic, antimetastatic | In vitro, in vivo | HCCLM3, HepG2, Huh-7, LO2; HCC mice | 10–80 μM, 24–72 h; 5–10 mg/kg/d, 5 wk, intraperitoneal | [75] | |
| OL | Melanoma | Antiproliferative, apoptotic, cytotoxic, synergy w/dacarbazine and everolimus | In vitro | A375, M21, WM266–4 | 250–800 μM, 24–72 h | [76] |
| OC | Antiproliferative, apoptotic, selective cytotoxicity | A375, 501Mel, HDFa | 0.01–50 μM, 72 h | [77] | ||
| OC | Melanoma, lung | Anti-angiogenic, anti-invasive, antimigratory, antiproliferative, apoptotic, antitumorigenic, antimetastatic | In vitro, in vivo | A2058, A375, HaCaT, HUVEC; mice w/A375 xenograft; lung metastasis model | 5–60 μM, 24–48 h; 10 mg/kg/d, 3 wk, intraperitoneal; 15 mg/kg/d, 6 wk | [78] |
| OL | Lung | Antiproliferative, apoptotic | In vitro | H1299 | 50–200 μM, 24 h | [79] |
| OC | Antimigratory, antiproliferative, selective cytotoxicity, antitumorigenic, antimetastatic | In vitro, in vivo | A549, HMVEC, NCI-H322M; Foxn1nu/Foxn1+ mice injected w/A549 | 1–60 μM, 24–72 h; 10 mg/kg/d, 8 wk, oral | [80] | |
| OL | Neuroblastoma | Anti-invasive, antimigratory, antiproliferative, apoptotic | In vitro | SH-SY5Y | 25–800 μM, 24–72 h | [81] |
| OC | Apoptotic, selective cytotoxicity | BMDN, NB2a | 0.1–1000 μM, 24 h | [82] | ||
| Oleacein | Antimigratory, antiproliferative, apoptotic, selective cytotoxicity | SH-SY5Y, WI-38 | 1–50 μM, 24–72 h | [83] | ||
| OL aglycone | Autophagy induction | In vitro, in vivo | SH-SY5Y; TgCRND8 mice | 50 μM, 5 m-5 h; 50 mg/kg of diet, 8 wk, oral | [84] | |
| OL | Osteosarcoma | Antimigratory, antiproliferative, autophagy induction, synergy w/2-methoxyestradiol | In vitro | 143B | 1–250 μM, 24–60 h | [85] |
| OL | - | Protective effect against acute renal injury caused by cisplatin | In vivo | BALB/cN mice | 20 mg/kg, oral gavage | [86] |
| OL, Ac-OL | Thyroid | Antiproliferative, selective cytotoxicity | In vitro | BCPAP, TAD-2, TPC-1 | 10–100 μM, 24–48 h | [19] |
| Comp. | Cancer | Effects | Study | Model | Dose | Ref. |
|---|---|---|---|---|---|---|
| MA | Colorectal | Apoptotic, clastogenic, cytotoxic | In vitro | HT-29 | 61 μM, 12–72 h | [20] |
| Antiproliferative, cytotoxic | 3.75–30 μM, 3–72 h | [88] | ||||
| Antitumorigenic, chemopreventive, protective, apoptotic | In vivo | ApcMin/+ mice | 100 mg/kg of feed, 6 wk, oral | [89] | ||
| Antimigratory, antiproliferative, apoptotic | In vitro, in vivo | HCT116, SW480; AOM/DSS mice; BALB/c mice w/HCT116 xenograft | 5–20 μM, 12–36 h; 10–30 mg/kg every 2 d, 17 d, oral gavage | [90] | ||
| Erythrodiol | Antiproliferative, apoptotic | In vitro | HT-29 | 10–150 μM, 24–72 h | [91] | |
| MA | Bladder | Apoptotic, selective cytotoxicity, antitumorigenic | In vitro, in vivo | 253J, L-02, MRC-5, RT4, T24, TCCSUP, PBC-1, PBC-2; BALB/c mice xenograft model | 1–1000 μM, 48–96 h; 5–20 mg/kg every other day, intraperitoneal | [92] |
| Kidney | Anti-angiogenic, antiproliferative, selective cytotoxicity | In vitro | ACHN, Caki-1, HUVEC, PTEC, SN12K1 | 47.11–97.04 μM, 24 h | [93] | |
| Erythrodiol, MA, oleanolic acid, uvaol | Breast, lymphoma | Antiproliferative, apoptotic, cytotoxic | MCF-7, MDA-MB-231, U937 | 12.5–100 μM, 24–120 h | [26] |
| Comp. | Cancer | Effects | Study | Model | Dose | Ref. |
|---|---|---|---|---|---|---|
| OC, ligstroside aglycone, TYR derivatives | Breast | Anti-invasive, antimigratory, antiproliferative, selective cytotoxicity | In vitro, in silico | MDA-MB-231, MCF-10A | 10–100 μM, 24–48 h | [98] |
| HT, OL | Anti-invasive, antimigratory, antiproliferative | In vitro | MDA-MB-231 | 0–150 μM, 24–48 h | [96] | |
| MCF-7, T47D | [97] | |||||
| Colorectal | Antiproliferative, apoptotic | HT-29 | 200–800 μM, 24–72 h | [99] | ||
| HT, HT butanoate, octanoate and oleate | Antiproliferative | HCT8-β8n | 5–50 μM, 20–80 h | [101] | ||
| HT, catechol, PA, PP, HPP, DHPP | Antiproliferative, apoptotic, cytotoxic | Caco-2, HT-29 | 100–200 μM, 8–48 h | [100] | ||
| HT, TYR, OL, oleic acid | Glioblastoma | Anti-angiogenic, anti-inflammatory, antimigratory | HBMEC, U-87 | 100 μM, 24 h | [104] | |
| HT, OL | Pancreatic | Antiproliferative, apoptotic, selective cytotoxicity | ASPC-1, BxPC-3, CFPAC-1, HPDE, MIA PaCa-2 | 0–300 μM, 24–48 h | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo Ferreira, D.; Oliveira, M.B.P.P.; Alves, R.C. A Comprehensive Review of the Antitumor Activity of Olive Compounds: The Case of Olive Oil, Pomace, and Leaf Extracts, Phenolic Alcohols, Secoiridoids, and Triterpenes. Antioxidants 2025, 14, 237. https://doi.org/10.3390/antiox14020237
Melo Ferreira D, Oliveira MBPP, Alves RC. A Comprehensive Review of the Antitumor Activity of Olive Compounds: The Case of Olive Oil, Pomace, and Leaf Extracts, Phenolic Alcohols, Secoiridoids, and Triterpenes. Antioxidants. 2025; 14(2):237. https://doi.org/10.3390/antiox14020237
Chicago/Turabian StyleMelo Ferreira, Diana, Maria Beatriz P. P. Oliveira, and Rita Carneiro Alves. 2025. "A Comprehensive Review of the Antitumor Activity of Olive Compounds: The Case of Olive Oil, Pomace, and Leaf Extracts, Phenolic Alcohols, Secoiridoids, and Triterpenes" Antioxidants 14, no. 2: 237. https://doi.org/10.3390/antiox14020237
APA StyleMelo Ferreira, D., Oliveira, M. B. P. P., & Alves, R. C. (2025). A Comprehensive Review of the Antitumor Activity of Olive Compounds: The Case of Olive Oil, Pomace, and Leaf Extracts, Phenolic Alcohols, Secoiridoids, and Triterpenes. Antioxidants, 14(2), 237. https://doi.org/10.3390/antiox14020237








